Abstract
Mammary analogue secretory carcinoma of salivary gland origin (MASC) is a recently described tumor with ETV6 translocation. Akin to secretory breast cancer, MASC expresses S-100 protein, mammaglobin, vimentin, and harbors a t(12;15) (p13;q25) translocation which leads to ETV6-NTRK3 fusion product. Histologically, MASC displays a lobulated growth pattern and is often composed of microcystic, tubular, and solid structures with abundant eosinophilic homogeneous or bubbly secretions. Colloid-like secretory material stains positive for periodic acid-Schiff (PAS) with and without diastase and for Alcian blue. The cells of MASC are devoid of PAS-positive secretory zymogen granules. These features help to exclude the most important differential diagnostic considerations, namely acinic cell carcinoma, low-grade cribriform cystadenocarcinoma, cystadenocarcinoma (not otherwise specified), and low-grade mucoepidermoid carcinoma. To date the presence of the ETV6-NTRK3 fusion gene has not been demonstrated in any other salivary gland tumor than MASC. It is likely that MASC is more common than currently recognized and with further studies, the clinical need for molecular studies of the ETV6-NTRK3 fusion may diminish. However, molecular testing is recommended at this time to arrive at the diagnosis of MASC.
Keywords: Mammary analogue secretory carcinoma, MASC, Salivary gland, ETV6-NTRK3 fusion
Introduction
In recent years, molecular testing has become a standard method in the diagnosis and consequent treatment decisions in many fields of pathology including cancer of the head and neck. Salivary glands may give rise to a wide spectrum of different tumors [1, 2]. They are often diagnostically challenging with interesting and controversial morphological features often overlapping between different entities. Although morphology in combination with immunohistochemical findings still provide the most important clues for diagnosis, recent advances in molecular pathology offer new potential avenues in investigating both differential diagnosis and prognosis in salivary gland oncology [3–5].
Mammary analogue secretory carcinoma (MASC) is a recently described distinctive salivary gland tumor with a known ETV6 gene rearrangement [6]. As the name implies, MASC is characterized by histological and immunohistochemical resemblance to secretory carcinoma of the breast, a rare breast cancer occurring mainly but not exclusively in young patients which carries a favorable clinical outcome [7]. Histologically, both MASC and secretory breast cancer are composed of uniform cells with bland-looking vesicular nuclei and eosinophilic vacuolated cytoplasm, arranged in tubular, microcystic and solid growth patterns with abundant PAS-positive secretions. Furthermore, both tumors are strongly S-100 protein, vimentin, mammaglobin, and cytokeratin positive. Moreover, MASC of salivary glands, similarly to secretory carcinoma of the breast, harbors a recurrent balanced chromosomal translocation t(12;15) (p13;q25) which leads to a fusion gene between the ETV6 gene on chromosome 12 and the NTRK3 gene on chromosome 15 [7].
Inspired by our original report [6], several other groups recently reviewed their salivary gland tumor files and found more cases of MASC and showed that expression of the ETV6-NTRK3 gene fusion is a frequent event in these tumors [8–14] confirming our results [6]. Moreover, 8 new cases of MASC have been documented as case reports within last two years [15–20].
Clinical, Gross and Histological Findings
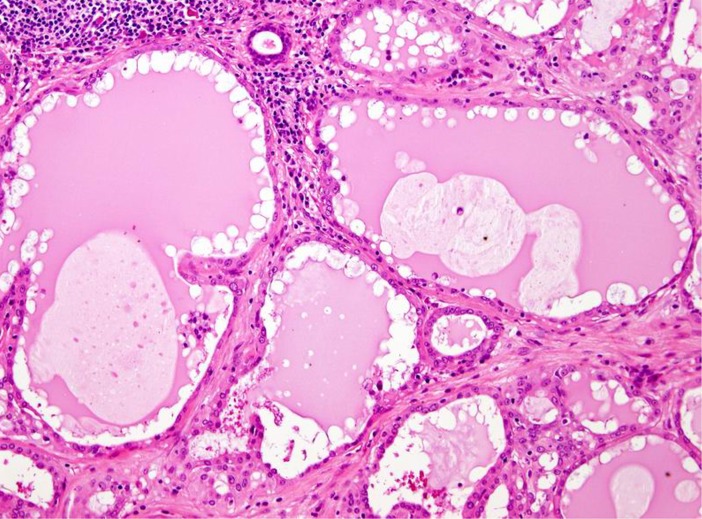
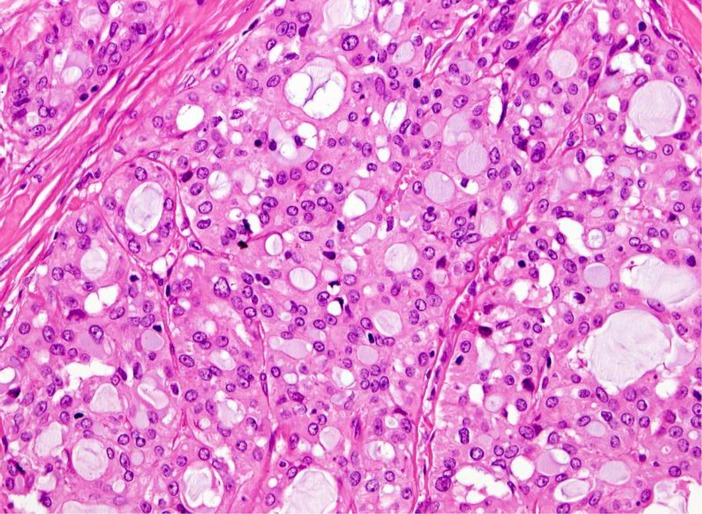
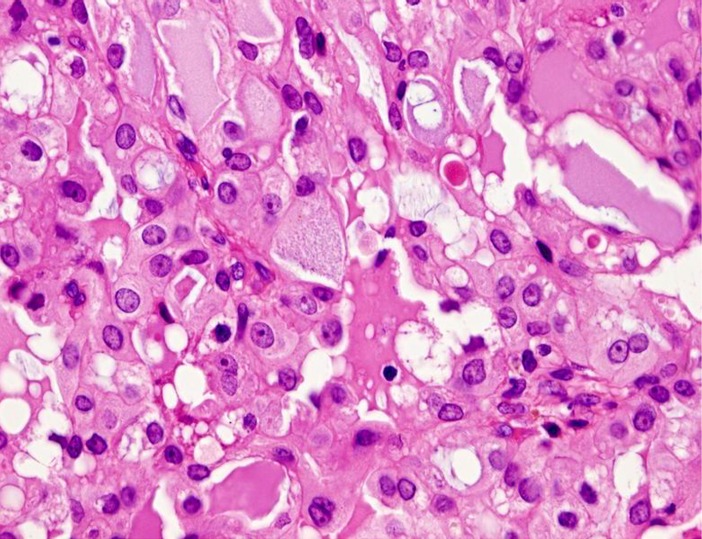
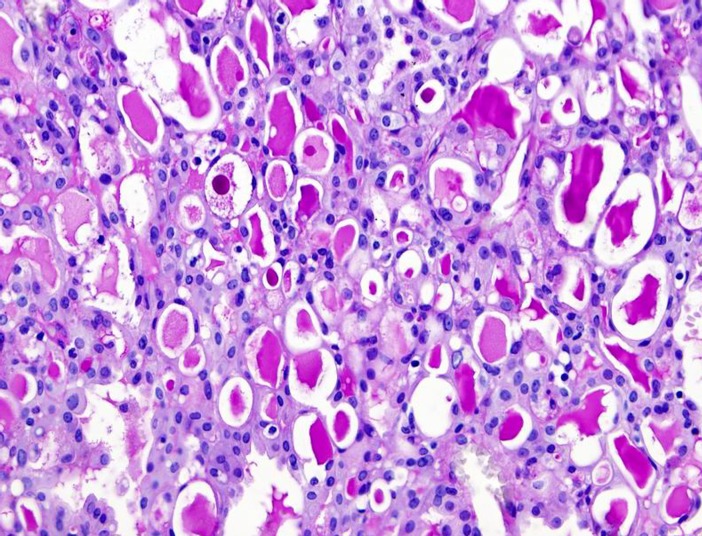
Grossly, the tumors are rubbery, with a white-tan to grey cut surface. Occasionally, on cut surface the tumors may appear cystic, containing yellow-whitish fluid. The borders of the tumors are usually circumscribed but not encapsulated (Fig. 1), and invasion within the salivary gland is often present. Perineural invasion can be present (Fig. 2), but the tumors usually do not have evidence of lymphovascular invasion. Necrosis is typically not identified. Extension into extraglandular tissues can be seen in some cases. Microscopic features of MASC closely mimic intercalated duct cell predominant acinic cell carcinomas (AciCC) showing a variety of architectural patterns. They often have a lobulated growth pattern divided by fibrous septa. In most cases, MASC is composed of microcystic/solid and tubular structures (Fig. 3) with abundant eosinophilic homogeneous or bubbly secretory material (Fig. 4). Less commonly, the tumors are dominated by one large cyst with a multilayered lining, which can display tubular, follicular, macro- and microcystic or papillary architecture, with occasional solid areas (Fig. 5). The tumor cells have low grade vesicular round-to-oval nuclei with finely granular chromatin and distinctive centrally located nucleoli (Fig. 6). The cytoplasm is pale to pink with granular or vacuolated pattern. Cellular atypia is usually mild, and mitotic figures are in most cases rare. Abundant bubbly secretion is present within microcystic and tubular spaces (Fig. 7). The secretory material stains positive for periodic acid-Schiff (PAS) before and after diastase digestion and for Alcian blue (Fig. 8). In contrast to AciCC, serous acinar differentiation is not a feature of MASC, and the cells of MASC are devoid of PAS-positive secretory zymogen cytoplasmic granules.
Fig. 1.
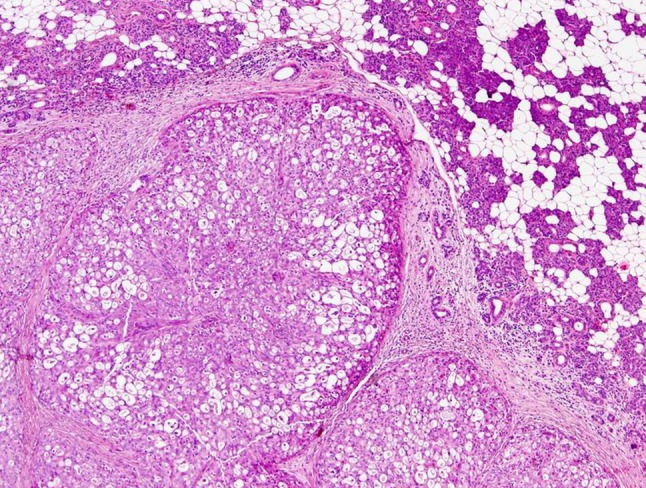
MASCs are usually circumscribed but not encapsulated. Tumors often have a lobulated growth pattern divided by fibrous septa
Fig. 2.
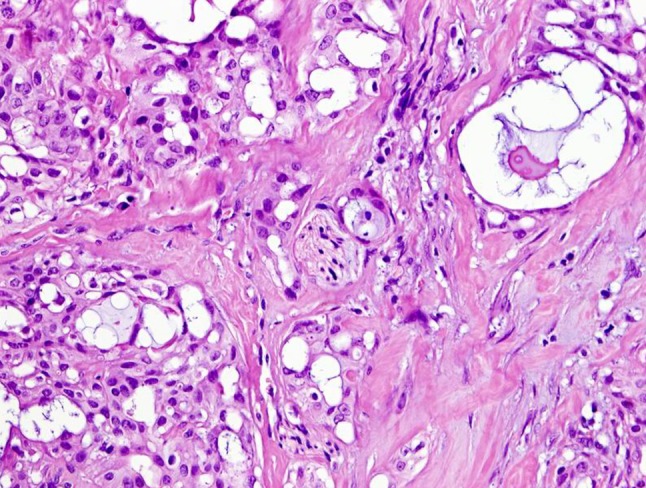
Perineural invasion can be sometimes present
Fig. 3.
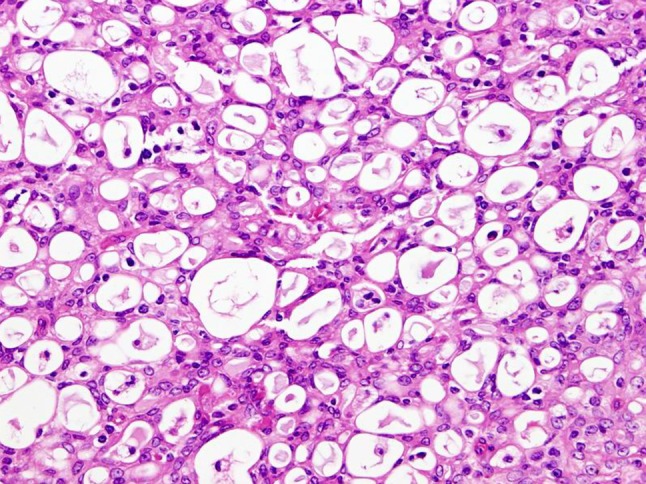
MASC is composed of microcystic/solid and tubular structures in most cases
Fig. 4.
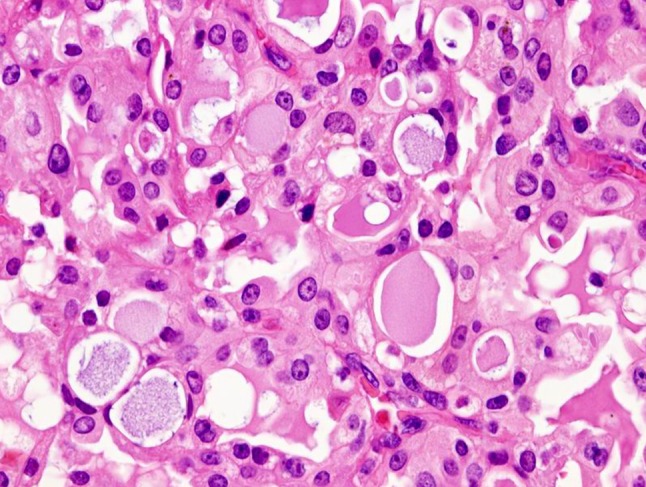
Presence of abundant eosinophilic homogeneous or bubbly secretory material is a typical finding in MASC
Fig. 5.
MASCs may have follicular and macrocystic appearance with resemblance to thyroid gland colloid
Fig. 6.
The tumor cells have low grade vesicular round-to-oval nuclei with finely granular chromatin and distinctive centrally located nucleoli
Fig. 7.
Abundant bubbly secretion is present within microcystic and tubular spaces
Fig. 8.
The secretory material stains positive for periodic acid-Schiff (PAS)
Immunohistochemical Findings
MASC shows diffuse and strong expression of pan–cytokeratin (AE1-AE3 and CAM 5.2), CK7, CK8, CK18, CK19, epithelial membrane antigen (EMA), S-100 protein, and vimentin. The tumor cells also show strong positive expression of STAT5a (signal transducer and activator of transcription 5a) and mammaglobin (secretory material is also positive) in all cases (Fig. 9). In addition, in most cases there is significant positivity for gross cystic disease fluid protein 15 (GCDFP-15) (particularly secretory material stains). Basal cell/myoepithelial cell markers, such as p63, calponin, CK14, smooth muscle actin, and CK5/6 are virtually negative as described in original report [6]. Interestingly, we have recently identified a few cases of MASC, which showed a similar overall morphology and immunohistochemical profile, and also showed features not previously reported in the initial description, such as expression of high-molecular-weight keratins (34betaE12) [11]. Two cases showed very isolated cells with nuclear p63 protein staining, however, the majority of the cells were completely negative [11]. We have also observed, that in some cases, p63 protein highlighted an incomplete rim of non-neoplastic cells surrounding the tumor (tumor cells are invariably negative for p63) [11]. Moreover, recently we have described 2 new cases of MASC with variable expression of some basal/myoepithelial markers [20]. In case 1, we observed very focal but unequivocal nuclear expression of p63 (5 % of tumor cells) and focal cytoplasmic expression of calponin, whereas expression of CD10 was absent [20]. In case 2, the staining of calponin was negative, but there was focal nuclear expression of p63 (10–20 % of tumor cells) and focal cytoplasmic expression of CD10 (5–10 % of tumor cells) [20]. Expression of CK5/6 and SMA was absent in both cases. DOG1 staining profile in MASC is different from AciCC [21]. Most cases of MASC are DOG1 negative, while AciCCs demonstrate intense apical membranous staining around lumina and variable cytoplasmic positivity in most cases.
Fig. 9.
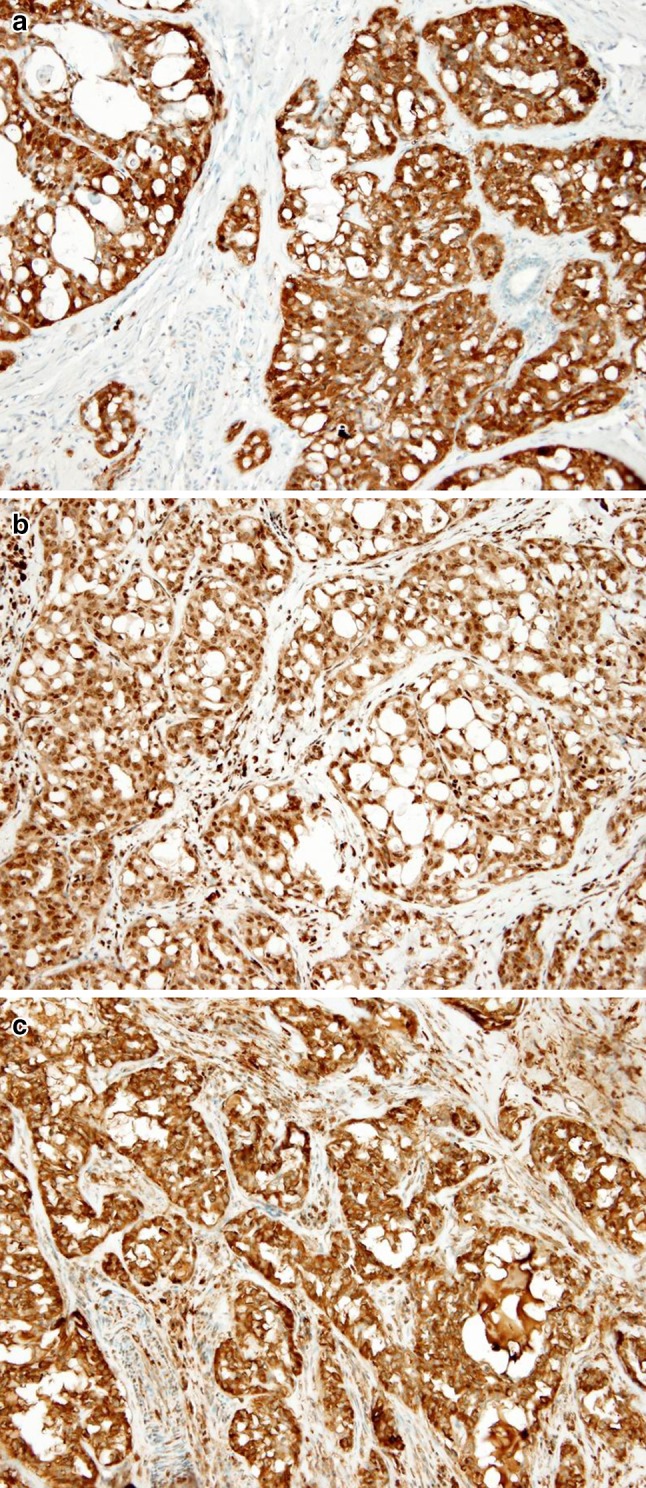
MASCs show diffuse and strong expression of S-100 protein (a), STAT5a (signal transducer and activator of transcription 5a) (b), and mammaglobin (secretory material is also positive) (c) in all cases
While morphological and immunohistochemical features of MASC are now well recognized, the clinical significance of distinguishing MASC remains unclear. In terms of clinical outcome, there is no statistically significant difference in disease-free survival and overall survival between MASC and AciCC. There are, however, some differences with respect to site and gender. In contrast to AciCC, MASC has slight male predilection [6, 13]. MASC is slightly more commonly reported in extra-parotid sites than AciCC. Regarding clinical behavior, MASC appears to have greater potential to develop lymph node metastases [13].
Cytological Features of Mammary Analogue Secretory Carcinoma
In all 7 cases of reported MASC studied on FNA cytology, the smears were extremely cellular and showed sheets and clusters of bland polygonal epithelial cells admixed with bright pink filamentous matrix [18, 22]. The majority of cases demonstrated clusters of cells ranging from tight, small acinar-like structures to larger, papillary arborizing and tubuloglandular patterns. The cells had abundant vacuolated cytoplasm, with the majority having multiple small cytoplasmic vacuoles, some containing mucin. The cytoplasm of other cells was finely granular and very eosinophilic. The majority of cells demonstrated uniform small round eccentrically located nuclei with a small regular distinctive nucleoli. Mitotic figures were not identified in any case. Extracellular material was common and was usually comprised of mucin that in some cases was abundant [22]. Original cytology diagnosis included low grade mucoepidermoid carcinoma (in one cases, ref. [18]), low grade epithelial neoplasm (in 3 cases, ref. [22]), salivary papillary neoplasm (one case, ref. [22]), benign salivary epithelium (one case, ref. [22]) and MASC in one case [22].
Molecular Findings in MASC
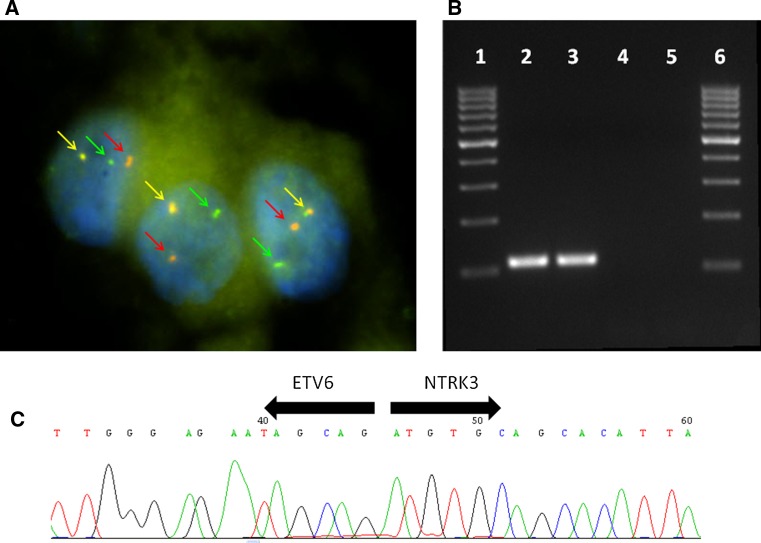
MASC of salivary glands, similar to secretory carcinoma of the breast, harbors a recurrent balanced chromosomal translocation t(12;15) (p13;q25) which leads to a fusion gene between the ETV6 gene on chromosome 12 and the NTRK3 gene on chromosome 15 (Fig. 10). The biological consequence of the translocation is the fusion of the transcriptional regulator (ETV6) with membrane receptor kinase (NTRK3) that activates kinase through ligand independent dimerization and thus promote cell proliferation and survival. The presence of ETV6-NTRK3 fusion gene has not been demonstrated in any other salivary gland tumor so far. The ETV6-NTRK3 translocation is not entirely specific for MASC, as it has been identified in a range of neoplasms including congenital fibrosarcoma, congenital cellular mesoblastic nephroma, and acute myeloid leukemia [23]. Moreover, in the context of breast malignancies, the presence of ETV6-NTRK3 translocation seems to be restricted to secretory breast cancer [7].
Fig. 10.
Fluorescence in situ hybridization (FISH) using LSI ETV6 (TEL) (12p13) Dual Color, Break Apart Rearrangement Probe (VYSIS/Abbott Molecular). Green and red arrows show split signals indicating break of ETV6 gene. Yellow arrows show nonaltered chromosome (Fig. 10a). Expression of ETV6-NTRK3 fusion transcript detected by RT-PCR. 1 marker, 2 positive MASC sample, 3 positive amplification control, 4 negative amplification control, 5 non-template control (Fig. 10b). Sequence analysis of ETV6-NTRK3 fusion transcript. Arrows show the translocation breakpoint (Fig. 10c)
Differential Diagnosis
There is a growing body of evidence, that MASC is not as rare as expected, rather the tumor was not recognized as a distinct tumor type. Chiosea et al. [12], in their most recent study of 81 cases of AciCC, reclassified most cases of zymogen granule poor intercalated duct cell predominant tumors as MASC based on demonstration of ETV6 translocation.
Acinic Cell Carcinoma
The most common tumor entity in salivary gland pathology that mimics MASC is AciCC. Classic AciCC is readily distinguishable from MASC even at the morphologic level, however zymogen granule poor AciCC shows a considerable morphologic overlap with MASC. The neoplastic cells of MASC resemble intercalated duct cells, and they have low grade nuclei with distinctive nuclear membranes and centrally located nucleoli. However, the large serous acinar cells with cytoplasmic PAS-positive zymogen-like granules, typical of AciCC, are completely absent from MASC. Moreover, AciCC is characterized by cytological and structural diversity, being composed of a mixture of serous acinar, intercalated duct-like, hobnail, vacuolated, clear, and non-specific glandular cells arranged in solid/lobular, microcystic, papillary-cystic, and follicular growth patterns [1]. In contrast, MASCs are structurally homogenous, uniformly composed of microcystic and slightly dilated glandular spaces with secretory material in lumina [6]. The major differential diagnostic feature of MASC is the absence of acinar cells. Other diagnostic criteria include a microcystic honeycombed pattern composed of small cysts often merging into larger cysts with resemblance to thyroid follicles, tubular spaces containing secretory material, and bland neoplastic cells with abundant eosinophilic cytoplasm which may on occasions appear foamy. Furthermore, MASCs display a characteristic immunohistochemical profile (S-100 protein+, mammaglobin+, vimentin+, and DOG1 absent). In equivocal cases, demonstration of the ETV6-NTRK3 translocation is diagnostic of MASC and is absent in conventional AciCC.
Cystadenocarcinoma/Adenocarcinoma NOS
Adenocarcinoma NOS is a poorly defined category of otherwise unclassifiable salivary gland carcinoma, and it usually represents a diagnosis of exclusion. Description of the MASC with a diagnostic ETV6-NTRK3 translocation allows a reclassification of certain cases of adenocarcinomas NOS as MASC.
Low Grade Cribriform Cystadenocarcinoma (Low Grade Salivary Duct Carcinoma)
Low grade cribriform cystadenocarcinoma (LGCC) must be considered in differential diagnosis of MASC as well. Although LGCC shares with MASC strong diffuse S-100 protein expression, LGCC shows a complete intact myoepithelial rim around tumor nests which is not a feature of MASC [11, 20]. However, ductal involvement consisting of a morphologically apparent connection of tumor to medium sized ducts in the major salivary glands and/or immunohistochemical evidence (p63) of a basal layer surrounding a portion of the tumor nests or cysts was noted in several cases of MASC [11]. This could be evidence of a ductal origin of MASC, which would further support a distinction from AciCC and mucoepidermoid carcinoma, or it could alternatively represent ductal extension of tumor akin to “cancerization of ducts” in the breast [11]. However, this ductal involvement with p63 positive basal cell layer is never as extensive as one sees in “low-grade cribriform cystadenocarcinoma” (LGCC).
Low Grade Mucoepidermoid Carcinoma
The immunohistochemical demonstration of high-molecular-weight cytokeratins (HMWK) and focal mucinous differentiation of MASC raises a differential diagnosis with mucoepidermoid carcinoma (MEC) [11, 20]. The distinction can be made by the lack of a cobblestone-like appearance with intercellular bridges, true squamoid areas or basal-like intermediate cells in MEC. In addition, MASC typically lacks p63 staining and shows diffuse S100 positivity in most cases, which would be distinctly unusual in MEC, as would papillary formations and hobnailing in the lining of the cysts. In our experience the HMWK positivity in MASC is less intense than is typically seen in MEC. Moreover, more than 50 % of MEC are characterized by a t(11; 19) translocation coding for a CRTC1-MAML2 fusion protein.
Conclusions
In conclusion, it is likely that MASC is more common than currently recognized. The identification of ETV6-NTRK3 fusion in MASC adds yet another gene fusion to the growing list of chimeric genes of diagnostic importance for salivary gland carcinomas. Previous studies have shown that mucoepidermoid carcinomas are characterized by CRTC1/CRTC3-MAML2 or EWSR1-POU5F1 fusions [24, 25] and adenoid cystic carcinomas by MYB-NFIB fusions [26]. In addition, Antonescu et al. [27] recently identified a consistent EWSR1-ATF1 fusion in hyalinizing clear cell carcinoma of minor salivary glands. It is likely that other histological subtypes of salivary gland carcinomas may also be characterized by tumor-specific gene fusions. The use of molecular diagnostic techniques has become increasingly frequent in modern surgical pathology and helps to identify previously unrecognized tumor entities. Furthermore, molecular targets may provide new avenues for therapeutic management of patients and/or be useful in predicting prognosis.
References
- 1.Barnes L, Eveson J, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. World Health Organization classification of tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 2.Gnepp DR, Henley JD, Simpson RHW, Eveson J. Salivary and Lacrimal glands. In: Gnepp DR, editors. Diagnostic surgical pathology of the head and neck. 2nd ed. Philadelphia: Saunders Elsevier, pp. 413–562.
- 3.Hunt JL. An update on molecular diagnostics of squamous and salivary gland tumors of head and neck. Arch Pathol Lab Med. 2011;135:602–609. doi: 10.5858/2010-0655-RAIR.1. [DOI] [PubMed] [Google Scholar]
- 4.McCord C, Weinreb I, Perez-Ordonez B. Progress in salivary gland pathology: new entities and selected molecular features. Diagnostic Histopathol. 2012;18(6):253–260. doi: 10.1016/j.mpdhp.2012.03.005. [DOI] [Google Scholar]
- 5.Skalova A, Vanecek T, Simpson RHW, Michal M. Molecular advances in salivary gland pathology and their practical application. Diagnostic Histopathol. 2012;18(9):388–396. doi: 10.1016/j.mpdhp.2012.08.002. [DOI] [Google Scholar]
- 6.Skalova A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, Starek I, Geierova M, Simpson RHW, Passador-Santos F, Ryska A, Leivo I, Kinkor Z, Michal M. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 7.Tognon C, Knezevich SR, Huntsman D, Roskelley CD, Melnyk N, Mathers JA, Becker L, Carneiro F, MacPherson N, Horsman D, Poremba C, Sorensen PHB. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–376. doi: 10.1016/S1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 8.Fehr A, Loning T, Stenman G. Mammary analogue secretory carcinoma of the salivary glands with ETV6-NTRK3 gene fusion. Letter to Editor. Am J Surg Pathol 2011; 35: 1600–1602. [DOI] [PubMed]
- 9.Griffith C, Seethala R, Chiosea SI. Mammary analogue secretory carcinoma: a new twist to the diagnostic dilemma of zymogen granule poor acinic cell carcinoma. Virchows Arch. 2011;459:117–118. doi: 10.1007/s00428-011-1098-6. [DOI] [PubMed] [Google Scholar]
- 10.Lei Y, Chiosea SI. Re-evaluating historic cohort of salivary acinic cell carcinoma with new diagnostic tools. Head and Neck Pathol. 2012;6:166–170. doi: 10.1007/s12105-011-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor A, Perez-Ordoñez B, Shago M, Skálová A, Weinreb I. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol. 2012;36:27–34. doi: 10.1097/PAS.0b013e318231542a. [DOI] [PubMed] [Google Scholar]
- 12.Chiosea SI, Griffith C, Assad A, Seethala RR. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol. 2012;36:343–350. doi: 10.1097/PAS.0b013e318242a5b0. [DOI] [PubMed] [Google Scholar]
- 13.Chiosea SI, Griffith C, Assad A, Seethala RR. Clinicopathological characterization of mammary analogue carcinoma of salivary glands. Histopathology. 2012;61:387–394. doi: 10.1111/j.1365-2559.2012.04232.x. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz S, Zenk J, Muller M, Ettl T, Wunsch PH, Hartmann A, Agaimy A. The many faces of acinic cell carcinomas of the salivary glands: a study of 40 cases relating histological and immunohistological subtypes to clinical parameters and prognosis. Histopathology. 2012;61:395–408. doi: 10.1111/j.1365-2559.2012.04233.x. [DOI] [PubMed] [Google Scholar]
- 15.Rastatter JC, Jatana KR, Jennings LJ, Melin-Aldana H. Mammary analogue secretory carcinoma of parotid gland in a pediatric patient. Otolaryngol Head Neck Surg. 2012;146:514–515. doi: 10.1177/0194599811419044. [DOI] [PubMed] [Google Scholar]
- 16.Petersson F, Lian D, Chau YP, Yan B. Mammary analogue secretory carcinoma: the first submandibular case reported including findings on fine needle aspiration cytology. Head Neck Pathol. 2012;6:135–139. doi: 10.1007/s12105-011-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito S, Ishida E, Skalova A, Matsuura K, Kumamoto H, Sato I. Short communication: a case of mammary analogue secretory carcinoma of parotid gland. Pathol Internat. 2012;62:149–152. doi: 10.1111/j.1440-1827.2011.02759.x. [DOI] [PubMed] [Google Scholar]
- 18.Levine P, Fried K, Krevitt LD, Wang B, Wenig BM. Aspiration biopsy of mammary analogue secretory carcinoma of accessory parotid gland: another diagnostic dilemma in matrix-containing tumors of the salivary glands. Diagn Cytopathol 2012. [DOI] [PubMed]
- 19.Kratochvil FJ, Stewart JCB, Moore SR. Mammary analog secretory carcinoma of salivary glands: a report of 2 cases in the lip. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:630–635. doi: 10.1016/j.oooo.2012.07.480. [DOI] [PubMed] [Google Scholar]
- 20.Laco J, Švajdler M, Jr, Andrejs J, Hrubala D, Hácová M, Vaněček T, Skálová A, Ryška A. Mammary analogue secretory carcinoma of salivary glands: a report of 2 cases with expression of basal/myoepithelial markers (calponin, CD10 and p63 protein). Pathol Res Pract 2013- in press. [DOI] [PubMed]
- 21.Chenevert J, Duvvuri U, Chiosea S, Dacic S, Cieply K, Kim J, Shiwarski D, Seethala RR. DOG1: a novel marker of salivary acinar and intercalated duct differentiation. Mod Pathol. 2012;25:919–929. doi: 10.1038/modpathol.2012.57. [DOI] [PubMed] [Google Scholar]
- 22.Griffith CC, Stelow EB, Saqi A, Khalbuss WE, Schneider F, Chiosea SI, Seethala R. The cytological features of mammary analogue secretory carcinoma. A series of 6 molecularly confirmed cases. Cancer Cytopathol. 2013;121:234–241. doi: 10.1002/cncy.21249. [DOI] [PubMed] [Google Scholar]
- 23.Tognon CE, Somasiti AM, Evdokimova VE, et al. ETV6-NTRK3-mediated breast epithelial cell transformation is blocked by targeting the IGF1R signaling pathway. Cancer Res. 2011;71:1060–1070. doi: 10.1158/0008-5472.CAN-10-3096. [DOI] [PubMed] [Google Scholar]
- 24.Behboudi A, Enlund F, Winnes M, Andrén Y, Nordkvist A, Leivo I, Flaberg E, Szekely L, Mäkitie A, Grenman R, Mark J, Stenman G. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1-MAML2 fusion oncogene. Genes Chromosomes Cancer. 2006;45:470–481. doi: 10.1002/gcc.20306. [DOI] [PubMed] [Google Scholar]
- 25.Moller E, Stenman G, Mandahl N, Hamberg H, Molne L, van den Oord JJ, Brosjo O, Mertens F, Panagopoukos I. POU5FI, encoding a key regulator of stem cell pluripotency, is fused to EWSRI in hidradenoma of the skin and mucoepidermoid carcinoma of the salivary glands. J Pathol. 2008;215:78–86. doi: 10.1002/path.2327. [DOI] [PubMed] [Google Scholar]
- 26.Persson M, Andrén Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci USA. 2009;106:18740–18744. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonescu C, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC, Perez-Ordonez B, Have C, Asa SL, Leong IT, Bradley G, Klieb H, Weinreb I. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50(7):559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]