Abstract
Low-grade salivary duct carcinoma (LG-SDC) is a rare neoplasm characterized by predominant intraductal growth, luminal ductal phenotype, bland microscopic features, and favorable clinical behavior with an appearance reminiscent of florid to atypical ductal hyperplasia to low grade intraductal breast carcinoma. LG-SDC is composed of multiple cysts, cribriform architecture with “Roman Bridges”, “pseudocribriform” proliferations with floppy fenestrations or irregular slits, micropapillae with epithelial tufts, fibrovascular cores, and solid areas. Most of the tumor cells are small to medium sized with pale eosinophilic cytoplasm, and round to oval nuclei, which may contain finely dispersed or dark condensed chromatin. Foci of intermediate to high grade atypia, and invasive carcinoma or micro-invasion have been reported in up to 23 % of cases. The neoplastic cells have a ductal phenotype with coexpression of keratins and S100 protein and are surrounded by a layer of myoepithelial cells in non-invasive cases. The main differential diagnosis of LG-SDC includes cystadenoma, cystadenocarcinoma, sclerosing polycystic adenosis, salivary duct carcinoma in situ/high-grade intraductal carcinoma, and papillary-cystic variant of acinic cell carcinoma. There is no published data supporting the continuous classification of LG-SDC as a variant of cystadenocarcinoma. Given that most LG-SDC are non-invasive neoplasms; the terms “cribriform cystadenocarcinoma” and LG-SDC should be replaced by “low-grade intraductal carcinoma” (LG-IDC) of salivary gland or “low-grade intraductal carcinoma with areas of invasive carcinoma” in those cases with evidence of invasive carcinoma.
Keywords: Low-grade salivary duct carcinoma, Low-grade intraductal carcinoma, Cribriform cystadenocarcinoma, Salivary duct carcinoma, Parotid gland, Salivary glands
Introduction
In 1996, Delgado et al. [1] reported 10 cases of a hitherto unknown salivary neoplasm characterized by predominant intraductal growth, luminal ductal phenotype, bland microscopic features, and favorable clinical behavior. The authors commented that the tumors “spanned an appearance reminiscent of florid to atypical ductal hyperplasia to low grade intraductal breast carcinoma” and proposed the term “low-grade salivary duct carcinoma” (LG-SDC). Although Delgado et al. [1] had documented the predominant intraductal growth pattern of LG-SDC, the 2005 World Health Organization Classification of Head and Neck Tumors adopted the term “low-grade cribriform cystadenocarcinoma” and regarded it as a variant of cystadenocarcinoma [2]. The re-labelling of LG-SDC as “low-grade cribriform cystadenocarcinoma” [2] created a yet to be resolved controversy in the taxonomy and classification of salivary gland neoplasms.
The main aims of this review article are to: (1) review the clinicopathologic features of reported cases of LG-SDC in the English literature; (2) investigate if the published data is useful in determining whether LG-SDC is an intraductal/non-invasive carcinoma rather than a cystadenocarcinoma; (3) explore the possible relation of LG-SDC to conventional salivary duct carcinoma. In addition, we will discuss the differential diagnosis between LG-SDC and two recently described benign salivary gland lesions; striated duct adenoma (SDA) and intercalated duct lesions (IDLs).
Clinical Features
LG-SDC is an exceedingly rare neoplasm with approximately 39 cases published in the English literature since 1996 [1, 3–13]. LG-SDC affects adults with a wide age spectrum (range 27–93; mean: 61.4; median: 62.5 years, respectively), and shows a female predilection (female/male ratio 1.5:1). Patients usually present with an asymptomatic and slow growing mass. No facial nerve paralysis has been recorded in any patient although one patient complained of paresthesia along the upper neck and ear [14]. Most LG-SDCs affect the parotid gland (84.6 %) with only a few arising in intraparotid lymph nodes (5.1 %), accessory parotid gland (2.6 %), submandibular gland (2.6 %), and minor salivary glands (5.1 %).
Pathologic Features
Macroscopically, LG-SDCs are well circumscribed, non-encapsulated and largely cystic lesions, containing serous to hemorrhagic fluid. Tumor size ranges up to 4 cm. At low-power magnification, LG-SDC exhibits multiple cysts of variable size, and smaller ducts (Fig. 1). The cysts and ducts display a diverse architecture with multiple patterns and a heterogenous luminal epithelial proliferation showing a ductal phenotype. The tumor patterns include cribriform architecture with “Roman Bridges” (Fig. 2) “pseudocribriform” proliferations with floppy fenestrations or irregular slits (Fig. 3) micropapillae with anastomosing and filigreed epithelial tufts (Fig. 4), fibrovascular cores, and solid areas. Mucinous secretion may be seen in the glandular lumens.
Fig. 1.
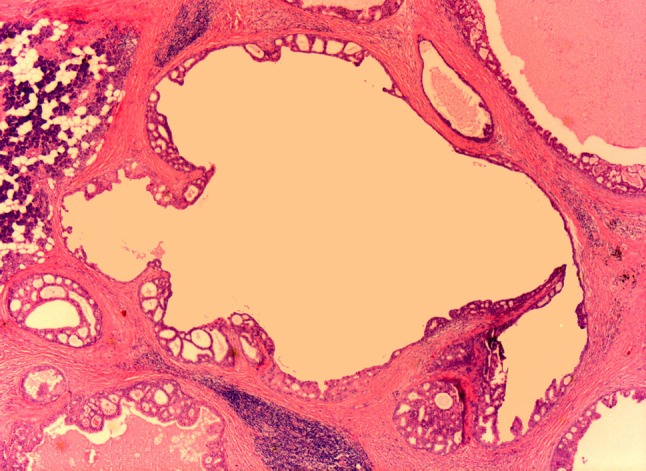
Low power appearance of LG-SDC showing cysts of various sizes and diameters with a large central cyst surrounded by smaller ones. Note the cribriform architecture with “Roman bridges” and epithelial tufts lining the cysts
Fig. 2.
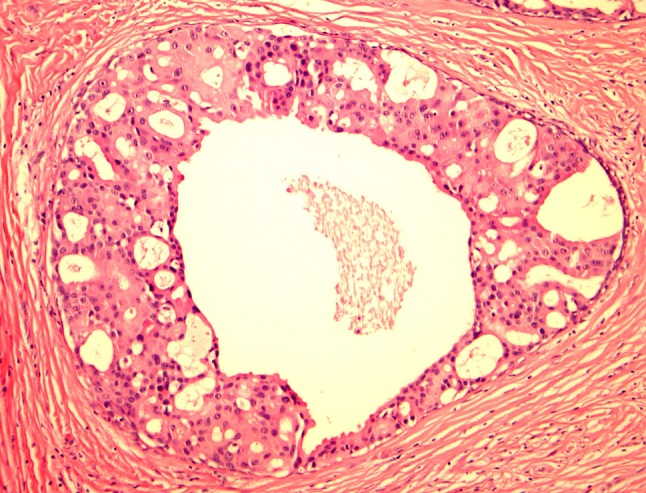
LG-SDC with round “rigid” cribriform, oval, and slit-like fenestrated spaces. The neoplastic cells are heterogeneous with elongated cells with small dark nuclei, and larger cells with moderate to abundant eosinophilic cytoplasm with round nuclei with visible nucleoli. Apical cytoplasmic blebs characteristic of apocrine metaplasia are readily seen. No distinctive myoepithelial cells are noted
Fig. 3.
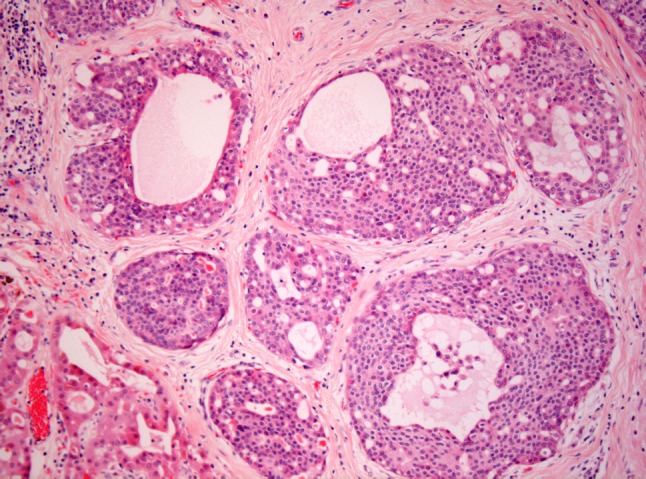
LG-SDC with “pseudocribriform” architecture. The ducts and lobules are occupied by a relatively monotonous ductal forming round spaces along with irregular fenestrated secondary lumina. Apocrine metaplasia can be observed in the lower left corner
Fig. 4.

LG-SDC with anastomosing and filigreed epithelial tufts. The tumor cells and exhibit dark nuclei or finely dispersed chromatin. Without immunohistochemical stains it is difficult to see a distinctive myoepithelial layer
At high-power magnification, LG-SDCs show bland but heterogeneous cytologic attributes (Figs. 2, 3). Most of the tumor cells are small to medium sized with indistinct cell borders, pale to eosinophilic cytoplasm, and round to oval nuclei, which may contain finely dispersed or dark condensed chromatin (Fig. 4). Elongated “streaming” cells are also seen (Fig. 5). Prominent nucleoli are absent in the majority of the cells but small eosinophilic nucleoli can be seen. Apocrine differentiation with apical snouts and cytoplasmic microvacuoles is occasionally present [1, 10, 14]. Some tumors contain fine lipofuscin-like, yellow to brown pigment within the intracytoplasmic vacuoles [1, 4]. Psammoma bodies and microcalcifications have been described in several cases [4, 14]. Secondary changes, such as hemorrhage, cholesterol clefts, and dystrophic calcification are relatively common. LG-SDC generally lacks cellular or nuclear pleomorphism, prominent nucleoli, significant mitotic activity and necrosis. However, transition to limited areas of high grade cytologic atypia (Fig. 6), including necrosis, has been illustrated in 5 cases (13 %) [1, 4, 12, 14]. Perineural and angiolymphatic invasion are absent.
Fig. 5.
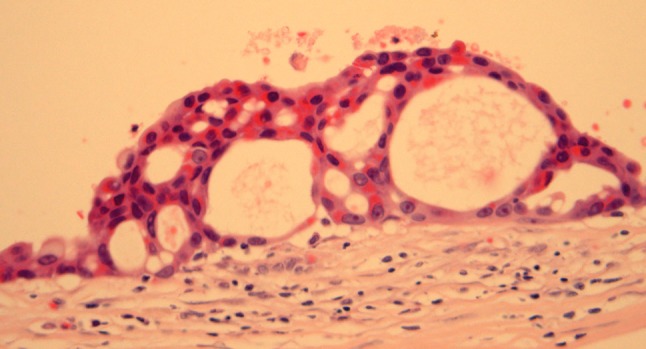
LG-SDC with streaming elongated cells with dark nuclei or nuclei with fine chromatin and small nuclei. The peripheral myoepithelial cells are barely visible
Fig. 6.
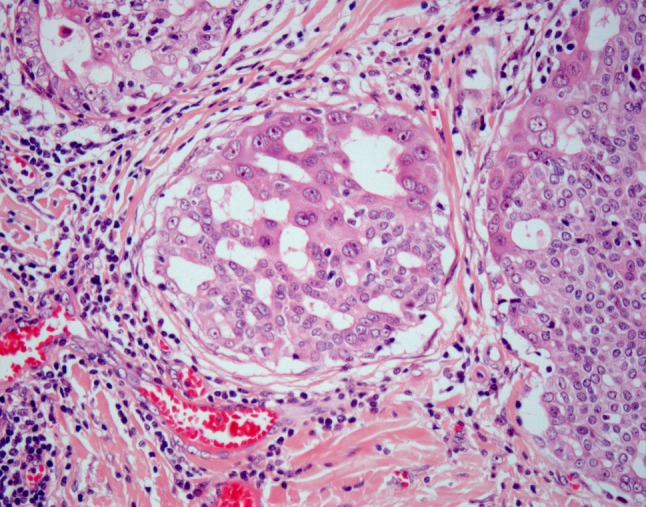
Cellular heterogeneity and cytologic progression in LG-SDC. Most of the neoplastic ductal cells are small with round nuclei containing fine chromatin; however, larger cells with vesicular chromatin and clearly visible nucleoli are also present
Invasive carcinoma or micro-invasion has been reported in 9 cases (23 %) of LG-SDC [1, 3–5, 10, 14] although the presence of invasion does not seem to adversely affect the patients’ outcome. In 6 of these 9 cases, the invasion was described as limited or focal, in 2 cases the invasive component was called “adenocarcinoma NOS” [10, 13], and in one case was an adenosquamous carcinoma [14]. The latter case had metastatic adenosquamous carcinoma in multiple periparotid and cervical lymph nodes.
Immunohistochemistry
Published immunohistochemistry reactions in LG-SDC are summarized in Table 1. The neoplastic cells have a distinctive ductal phenotype and are positive for AE1/AE3, CAM 5.2, cytokeratin 7 (CK7), cytokeratin 19 (CK19) and epithelial membrane antigen (EMA). High-molecular keratin (HMWK, CK34βE12) is reported as positive in both ductal and non-neoplastic myoepithelial neoplastic cells. Cytokeratin 20 (CK20) is negative in all reported cases. Smooth muscle actin (SMA), calponin, p63 and cytokeratin 14 (CK14) have clearly demonstrated a continuous layer of myoepithelial cells rimming the ducts and cyst spaces (Fig. 7). In tumors with areas of invasion, this myoepithelial layer appeared discontinuous [4]. Cytokeratin 5/6 (CK5/6) also highlighted myoepithelial cells in one additional case [6].
Table 1.
Immunohistochemical findings in LG-SDC
| Marker | No of cases positive expression | % Positive cases |
|---|---|---|
| SMA | 15/16, myoepithelium | 93.75 |
| Calponin | 13/13, myoepithelium | 100 |
| P63 | 8/8, myoepithelium | 100 |
| CK14 | 6/6, myoepithelium | 100 |
| AE1/AE3 | 6/6 | 100 |
| CK7 | 6/6 | 100 |
| CK19 | 3/3 | 100 |
| HMWK | 8/8 | 100 |
| CK 5/6 | 1/1 | 100 |
| EMA | 2/2 | 100 |
| CEAa | 6/7 | 85.71 |
| CK20 | 0/5 | 0 |
| S-100 | 22/26 | 84.61 |
| GCDFP-15 | 7/13 | 53.85 |
| ER | 1/7 | 14.29 |
| PR | 1/7 | 14.29 |
| AR | 5/8 | 62.50 |
SMA smooth muscle actin, CK keratin, HMWK high-molecular weight keratin, EMA epithelial membrane antigen, CEAa monoclonal carcinoembryonic antigen, GCDFP-15 gross cystic disease fluid protein-15, ER estrogen receptor, PR progesterone receptor, AR androgen receptor
Fig. 7.
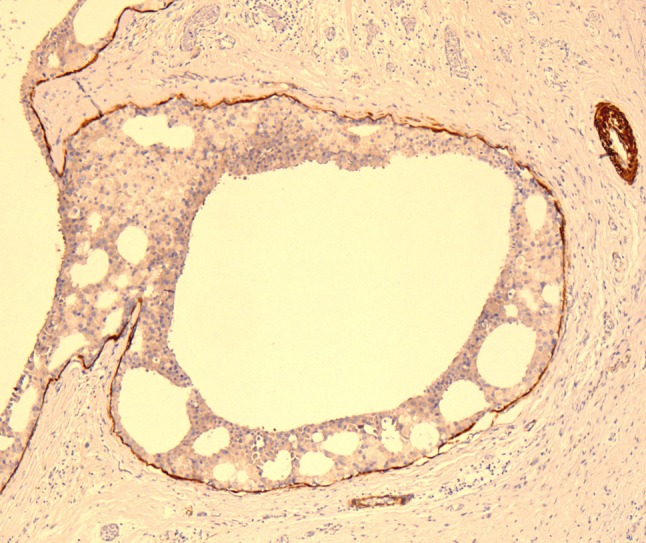
Calponin highlighting a thin myoepithelial cell layer at the periphery of ducts and cysts in LG-SDC
In normal salivary glands, S100 protein is only expressed by myoepithelial cells and intercalated duct cells. The ductal cells in LG-SDC show diffuse and strong nuclear and cytoplasmic immunoreactivity with S100 protein in most cases (21/25) (Fig. 8). In only three cases has S100 protein been reported as negative [7, 14]. Estrogen receptor (ER), progesterone receptor (PR) and HER-2/neu are usually absent [4, 6, 8]. Expression of androgen receptor (AR) has been noted in 5 of 8 cases [4, 5, 9, 14]. Occasionally, the luminal tumor cells have been positive for GCDFP-15, especially in areas with apocrine metaplasia [5, 9, 14].
Fig. 8.

Diffuse expression of S100 protein in a LG-SDC with “pseudocribriform” architecture
Electron Microscopy
Ultrastructural examination of LG-SDC has been reported in only three cases [1]. Ductal cells displayed interdigitating cell membranes forming intercellular lumens, apical microvilli, and small numbers of organelles. Vacuolated ductal cells showed non-membrane bound empty spaces and lipid vacuoles while myoepithelial cells were characterized by a peripheral location and long cytoplasmic processes.
Molecular Pathology
Nothing is known about the genetic and molecular alterations of LG-SDC. No expression of p53, epidermal growth factor receptor (EGFR), or HER-2/neu has been found in the few cases investigated [6, 14].
Differential Diagnosis
The predominant multicystic architecture of LG-SDC raises a wide differential diagnosis (Table 2) which includes cystadenoma, cystadenocarcinoma, sclerosing polycystic adenosis, salivary duct carcinoma in situ/high-grade intraductal carcinoma, conventional salivary duct carcinoma, papillary-cystic variant of acinic cell carcinoma and mammary analog secretory carcinoma of salivary glands. In this review, we will focus our discussion to the differential diagnosis of LG-SDC with cystadenocarcinoma, salivary duct carcinoma in situ/high-grade intraductal carcinoma, and two rare and recently described benign lesions, ductal adenoma with striated duct differentiation [15] and intercalated duct hyperplasia/adenoma [16].
Table 2.
Low-grade salivary duct carcinoma
| Cystadenoma |
| Sclerosing polycystic adenosis |
| Cystadenocarcinoma |
| Salivary duct carcinoma in situ/high-grade intraductal carcinoma |
| Conventional salivary duct carcinoma |
| Acinic cell carcinoma, papillo-cystic variant |
| Mammary-analog secretory carcinoma of salivary glands |
Main differential diagnosis
Cystadenocarcinoma
Cystadenocarcinomas of salivary glands are a rare and morphologic heterogeneous group of neoplasms characterized by prominent cystic appearance-often with complex papillary architecture—infiltrative growth, and absence of myoepithelial cells [17–21]. Although most cystadenocarcinomas are low grade malignancies composed of small cuboidal cells with scanty cytoplasm displaying regular nuclei with inconspicuous nucleoli, and low mitotic activity [17, 20, 21], others exhibit large cuboidal or columnar cells with moderate to high nuclear atypia, prominent nucleoli, and frequent mitoses [17, 18, 22], and lastly, some are best classified as mucinous cystadenocarcinoma [22–24].
The re-labelling of LG-SDC as “low-grade cribriform cystadenocarcinoma” [2] created some confusion and controversy in terminology and obscured its relationship with cystadenocarcinoma. As originally described by Delgado et al. [1] and later confirmed by others [4], LG-SDC is an intraductal carcinoma with occasional limited invasion. Although both LG-SDC and cystadenocarcinomas share a cystic appearance, cystadenocarcinomas are clearly infiltrative neoplasms which lack cribriform architecture and non-neoplastic myoepithelial cells when stained with SMA, MSA, and other myoepithelial markers [17–20]. Given the relatively recent description of LG-SDC, we could speculate that some putative examples of low-grade “cystadenocarcinoma” in reality represent LG-SDC [17, 25].
Salivary Duct Carcinoma In-Situ/High-Grade Intraductal Carcinoma (HG-IDC)
Although not formally recognized in the 2005 edition of the WHO Classification of Head and Neck Tumors, there are rare but well documented cases of “high grade intraductal” carcinomas of salivary glands [26–28] or “salivary duct carcinoma in situ” [29]. High-grade intraductal carcinoma/salivary duct carcinoma in situ (HG-IDC) share with LG-SDC many features including partly cystic appearance, cribriform, solid, and micropapillary patterns, and neoplastic cells with ductal phenotype—including apocrine differentiation—surrounded by an attenuated layer of myoepithelial cells. The differences between LG-SDC and HG-IDC are nuclear grade and the presence of necrosis [27]. HG-IDC is composed of neoplastic ductal cells showing high nucleocytoplasmic ratio, large pleomorphic nuclei with prominent nucleoli, occasional to frequent mitoses, and foci of necrosis. Although the numbers of published cases of HG-IDC is quite small, the expression of S100 protein may help to separate these two lesions since HG-IDCs have been either negative or only partially positive for S100 protein [27, 29]. The segregation of intraductal carcinomas of salivary gland into low grade and high grade is not always straightforward since there are reports of tumors with “intermediate” grade or low grade cytologic features but containing foci of necrosis [12, 13, 27]. Patients affected experience a good prognosis with the majority alive without evidence of disease. We suggest grading these neoplasms similarly to breast carcinoma nuclear grading: nuclear grade 1 through 3.
Conventional Salivary Duct Carcinoma
Conventional salivary duct carcinoma (SDC) is one of the most aggressive malignancies affecting salivary glands. It occurs mostly in elderly patients, has a male predilection and usually present as a rapidly growing tumor in the parotid gland. Unlike LG-SDC, it is notorious for early distant metastases and high mortality [30]. SDC resembles high grade ductal carcinoma of breast, with both intraductal and widely invasive components. Comedonecrosis, perineural invasion and lymph-vascular tumor emboli are very common. By immunohistochemisty, the tumor cells are positive for AR and GCDFP-15 [31], and usually negative for S100 protein [30].
Acinic Cell Carcinoma, Papillary Cystic Pattern
Acinic cell carcinoma, papillary cystic pattern should contain tumor cells with serous granules consistent with acinic cell differentiation. In difficult cases, a PAS stain can help to demonstrate the zymogen granules in the acinar cells. Immunohistochemically, acinic cell carcinoma is negative for S100 protein [32].
Mammary Analog Secretory Carcinoma
Mammary analog secretory carcinoma (MASC) resembles breast secretory carcinoma and as its breast counterpart, contains the t(12;15)(p13;q25) translocation, which leads to a ETV6-NTRK3 fusion gene [33]. MASC affects patients over a wide age range, occurs more commonly in males, and is found in both major and minor salivary glands [33–35]. MASC is well circumscribed but unencapsulated, and has microcystic, macrocystic, tubular and solid growth patterns. There are eosinophilic glassy secretions with occasionally empty bubbles in the cystic or tubular spaces. Papillary architecture with hobnailing of lining epithelial cells, and areas reminiscent with thyroid colloid are also reported [34]. The tumor cells have pink or vacuolated cytoplasm, vesicular nuclei and distinct nucleoli. The tumor is positive for mammaglobin, S100 protein, vimentin, CK19, CK8, CK18, MUC1, MUC4, HMWK and focally with GCDFP-15, while negative for p63 and calponin [33, 34].
Unlike the indolent LG-SDC, MASC is reported to have a 17.6 % rate of lymph node metastasis [35], and may sometimes behave aggressively [33].
Cystadenoma
Cystadenomas are uncommon neoplasms of salivary glands. They are well-circumscribed and composed of variable-sized cystic spaces. The lining epithelium also has a variable appearance, which can be flat, cuboidal or columnar and can have oncocytic, mucinous, epidermoid, and apocrine changes. There is no cellular atypia, solid growth, necrosis or mitoses. In a rare case, intraductal or intracystic epithelial proliferation with architecture resembling breast atypical ductal hyperplasia was described [36] but unlike LG-SDC, this case was reported to be negative for S100 protein and GCDFP-15, and showed only focal positivity for CK19 and HMWK.
Sclerosing Polycystic Adenosis
Sclerosing polycystic adenosis (SPA) is a mass lesion of salivary gland resembling breast fibrocystic change or sclerosing adenosis. Initially regarded as a reactive or inflammatory process, the demonstration of clonality by human androgen receptor (HUMARA) gene testing suggests that SPC might represent a neoplastic lesion [37]. SPA is well circumscribed and unencapsulated and is composed of a ductal and acinous proliferation in a hypocellular sclerotic background forming sclerotic nodules [38]. The ducts frequently show cystic changes and may have apocrine, mucinous, vacuolated and foamy appearance. Epithelial hyperplasia, dysplasia or even carcinoma in situ of the ductal epithelium has been reported. The lobules in SPA maintain a layer of myoepithelial cells demonstrated by SMA, calponin and S100 protein [38–40]. SPA develops local recurrence in around one-third of cases [39], but no metastasis or mortality has been reported.
Ductal Adenoma with Striated Duct Differentiation (DAS)
Ductal adenoma with striated duct differentiation is an extremely rare lesion characterized by a pure ductal component with striated duct differentiation lacking a continuous layer of myoepithelial cells [15]. Unlike LG-SDC, DASs are grossly solid and encapsulated. Microscopically, DASs are comprised of closely arranged ducts separated by a delicate intervening vasculature. Although cystic ducts are seen in DAS, these lesions lack the cribriform, pseudocribriform, or micropapillary architecture of LG-SDC, moreover they do not have a continuous myoepithelial layer when stained with SMA, smooth muscle myosin heavy chain (SMMHC), and calponin [15].
Intercalated Duct Lesions (IDLs)
Intercalated duct lesions are an uncommon group of small lesions displaying intercalated duct phenotype ranging from hyperplasia to adenoma [16]. Although some of the IDLs may form detectable masses, most often they are found in association with other salivary gland tumors, such as basal cell adenoma, epithelial-myoepithelial carcinoma, and basal cell adenocarcinoma. IDLs can be unifocal, multifocal or diffuse, and non-encapsulated or encapsulated. In contrast to the multicystic appearance of LG-SDC, IDLs are composed of small ducts with a mixture of bland ductal, acinic and mucus cells surrounded by a continuous layer of myoepithelial cells [16].
Treatment and Follow-Up
All patients with LG-SDC have undergone surgical resection of their tumors by superficial or total parotidectomy, submandibular resection or local wide excision. Five patients also had adjuvant radiotherapy. Follow-up and survival data indicate that the prognosis of LG-SDC is excellent; admittedly, most reported follow-up periods have been short (range 3 months to 19 years; median: 27 months; mean: 45.3 months). All but one case have neither tumor recurrences nor evidence of regional or distant metastases. The exception was an unusual case with LG-SDC associated with invasive adenosquamous carcinoma metastatic to multiple neck lymph nodes [14]. This patient remains well with no recurrent disease 91 months after surgery.
Discussion
LG-SDC is an exceedingly uncommon neoplasm of salivary glands. Since its initial description in 1996 [1], there have been approximately 39 reported cases in the English literature [3–13]. In the preceding paragraphs, we have summarized the clinical, light microscopic, and immunohistochemical features of cases which in our opinion, are well documented examples of LG-SDC. In their original description of LG-SDC, Delgado et al. [1] stated that “LG-SDC appears to be primarily an in situ (intraductal) process”. The architectural appearance, immunohistochemical findings, and ultrastructure strongly supported the authors’ conclusions; however, the adoption of the term “low-grade cribriform cystadenocarcinoma” [2] created a controversy that remains unresolved. Given this debate, we critically reviewed reported cases of LG-SDC with well documented IHC or EM findings. We found that in all 23 cases with appropriate description, a thin layer of non-neoplastic myoepithelial cells had been demonstrated by various combinations of p63, HMWK, CK14, SMA, MSA, and calponin or EM [1, 4–7, 9, 10, 14]. Using the criteria set forth by Cheuk, et al. [27] for the diagnosis of pure intraductal carcinoma of salivary gland we believe that there is compelling evidence to accept the original assertion by Delgado et al. [1], that LG-SDC is primarily an in situ, intraductal carcinoma and not a variant of cystadenocarcinoma.
As is the case with in situ or intraductal carcinomas in other organs, LG-SDC may show progression to higher grade lesions and invasive carcinoma. Transition to focal areas of high grade cytologic atypia, including necrosis, has been described in 13 % of LG-SDC cases, [1, 4, 12, 14] whereas foci of focal invasion or associated invasive carcinoma were reported in 23 % of cases [1, 3–5, 10, 14]. The clinical impact of focal invasion or transition to high grade cytology is not completely clear, since the number of cases is still small and the median follow-up is only 27 months. However, it appears that the latter findings have no short term impact on local recurrences or the development of regional or distant metastases. A patient affected by a LG-SDC associated with an invasive adenosquamous carcinoma metastatic to multiple neck lymph nodes, remains well 91 months after completing treatment [14].
Is LG-SDC a precursor or a related lesion to conventional SDC? Currently there is no published data to satisfactorily answer this question. Delgado et al. [1] detailed many salient similarities and differences between LG-SDC and conventional SDC and concluded “that LG-SDC represents the low grade end of the spectrum of salivary duct neoplasms”. LG-SDC and conventional SDC are composed of neoplastic cells with ductal phenotype and varying degree of apocrine differentiation and AR expression [1, 6, 14, 31, 41] but exhibit significant clinicopathologic differences including the expression of S100 protein, with 84 % of LG-SDCs positive for S100 protein. In contrast, the data regarding S100 protein expression in conventional SDC is more controversial with Lewis et al. [30] reporting expression in only 4 % while Brandwein et al. [42] reported reaction in 100 % of cases. The presence of at least focal LG-SDC has not been described in any study of conventional SDC. In the most extensive study describing the presence of carcinoma in situ in conventional SDC, Di Palma et al. [43] reported the presence of intermediate or high-grade carcinoma in situ in 35 of 42 (83 %) SDCs with no cases containing a low-grade carcinoma in situ component. In another study investigating the presence of carcinoma in situ in 22 adenocarcinomas, NOS, Ihrler et al. [44] found no examples of LG-SDC.
The histologic resemblances between primary ductal neoplasms of the breast and salivary glands led to the recognition of conventional salivary duct carcinoma and LG-SDC [1, 45]. Is it time to accept the existence of primary in situ or intraductal carcinomas in salivary glands? Although the number of reported cases of LG-SDC, salivary duct carcinoma in situ, and intraductal carcinoma (HG-IDC) remains small, there is sufficient evidence to accept this group of neoplasms as bona fide intraductal carcinomas of salivary glands. Abandoning the labels “low-grade salivary duct carcinoma” and “salivary duct carcinoma in situ” and adopting the unifying terms of low-grade, intermediate-grade, and high-grade intraductal carcinomas has the advantage of avoiding reference to, and thereby potential confusion with, the more aggressive invasive salivary duct carcinoma and is more in keeping with the clinical behavior and biologic nature of these lesions. The criteria for diagnosis and grading of intraductal carcinomas of the salivary gland have been previously proposed by Cheuk et al. [27] and are summarized in Table 3.
Table 3.
Proposed criteria for the diagnosis of pure intraductal carcinoma of salivary glands
| Intraductal epithelial proliferation resembling conventional or apocrine atypical ductal hyperplasia or intraductal carcinoma of breast |
| Cribriform, “pseudocribriform”, micropapillary, solid, “comedo”, or clinging architecture |
| Low, intermediate, or high grade |
| Demonstration of non-neoplastic myoepithelial cells by immunohistochemistry (using an appropriate combination of calponin, SMA, MSA, p63, CK14, and others) |
| Exclusion of an invasive component by thorough sampling |
Modified from Cheuk et al. [27]
In conclusion, the so-called LG-SDC is a rare primary low-grade intraductal carcinoma of salivary gland with distinctive clinicopathological characteristics. There is no data supporting the continuous classification of LG-SDC as a variant of cystadenocarcinoma and given that most LG-SDC are non-invasive neoplasms; the terms “cribriform cystadenocarcinoma” and LG-SDC should be replaced by “low-grade intraductal carcinoma” (LG-IDC) of salivary gland or “low-grade intraductal carcinoma with areas of invasive carcinoma” in those cases with evidence of invasive carcinoma. Additional studies are needed to understand the relationship of LG-IDC with conventional SDC and adenocarcinoma, NOS.
References
- 1.Delgado R, Klimstra DS, Albores-Saavedra J. Low grade salivary duct carcinoma. A distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer. 1996;78:958–967. doi: 10.1002/(SICI)1097-0142(19960901)78:5<958::AID-CNCR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Brandwein-Gensler M, Gnepp DR. Low-grade cribriform cystadenocarcinoma. In: Barnes L, Eveson JW, Reichart P, et al editors. Pathology and Genetics Head and Neck Tumors. Lyon: IARC Press; 2005; 233.
- 3.Chen KT. Cytology of salivary duct carcinoma. Diagn Cytopathol. 2000;22:132–135. doi: 10.1002/(SICI)1097-0339(200002)22:2<132::AID-DC17>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 4.Brandwein-Gensler M, Hille J, Wang BY, et al. Low-grade salivary duct carcinoma: description of 16 cases. Am J Surg Pathol. 2004;28:1040–1044. doi: 10.1097/01.pas.0000128662.66321.be. [DOI] [PubMed] [Google Scholar]
- 5.Kusafuka K, Itoh H, Sugiyama C, et al. Low-grade salivary duct carcinoma of the parotid gland: report of a case with immunohistochemical analysis. Med Mol Morphol. 2010;43:178–184. doi: 10.1007/s00795-009-0479-2. [DOI] [PubMed] [Google Scholar]
- 6.Laco J, Podhola M, Dolezalova H. Low-grade cribriform cystadenocarcinoma of the parotid gland: a neoplasm with favorable prognosis, distinct from salivary duct carcinoma. Int J Surg Pathol. 2010;18:369–373. doi: 10.1177/1066896910367649. [DOI] [PubMed] [Google Scholar]
- 7.Arai A, Taki M, Mimaki S, et al. Low-grade cribriform cystadenocarcinoma of the parotid gland: a case report. Auris Nasus Larynx. 2009;36:725–728. doi: 10.1016/j.anl.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Nakazawa T, Kondo T, Yuminomochi T, et al. Fine-needle aspiration biopsy of low-grade cribriform cystadenocarcinoma of the salivary gland. Diagn Cytopathol. 2011;39:218–222. doi: 10.1002/dc.21405. [DOI] [PubMed] [Google Scholar]
- 9.Weinreb I. Intraductal carcinoma of salivary gland (so-called low-grade cribriform cystadenocarcinoma) arising in an intra parotid lymph node. Head Neck Pathol. 2011;5:321–325. doi: 10.1007/s12105-011-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakatsuka S, Harada H, Fujiyama H, et al. An invasive adenocarcinoma of the accessory parotid gland: a rare example developing from a low-grade cribriform cystadenocarcinoma? Diagn Pathol. 2011;6:122. doi: 10.1186/1746-1596-6-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khurana KK, Pitman MB, Powers CN, et al. Diagnostic pitfalls of aspiration cytology of salivary duct carcinoma. Cancer. 1997;81:373–378. doi: 10.1002/(SICI)1097-0142(19971225)81:6<373::AID-CNCR12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Tatemoto Y, Ohno A, Osaki T. Low malignant intraductal carcinoma on the hard palate: a variant of salivary duct carcinoma? Eur J Cancer B Oral Oncol. 1996;32B:275–277. doi: 10.1016/0964-1955(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 13.Ide F, Mishima K, Saito I. Circumscribed salivary duct carcinoma of the palate: a non-threatening variant. Histopathology. 2004;45:89–91. doi: 10.1111/j.1365-2559.2004.01809.x. [DOI] [PubMed] [Google Scholar]
- 14.Weinreb I, Tabanda-Lichauco R, Van der KT, et al. Low-grade intraductal carcinoma of salivary gland: report of 3 cases with marked apocrine differentiation. Am J Surg Pathol. 2006;30:1014–1021. doi: 10.1097/00000478-200608000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Weinreb I, Simpson RH, Skalova A, et al. Ductal adenomas of salivary gland showing features of striated duct differentiation (‘striated duct adenoma’): a report of six cases. Histopathology. 2010;57:707–715. doi: 10.1111/j.1365-2559.2010.03682.x. [DOI] [PubMed] [Google Scholar]
- 16.Weinreb I, Seethala RR, Hunt JL, et al. Intercalated duct lesions of salivary gland: a morphologic spectrum from hyperplasia to adenoma. Am J Surg Pathol. 2009;33:1322–1329. doi: 10.1097/PAS.0b013e3181a55c15. [DOI] [PubMed] [Google Scholar]
- 17.Foss RD, Ellis GL, Auclair PL. Salivary gland cystadenocarcinomas. A clinicopathologic study of 57 cases. Am J Surg Pathol. 1996;20:1440–1447. doi: 10.1097/00000478-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Pollett A, Perez-Ordonez B, Jordan RCK, et al. High-grade papillary cystadenocarcinoma of the tongue. Histopathology. 1997;31:185–188. doi: 10.1046/j.1365-2559.1997.2270840.x. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi I, Kiyoshima T, Ozeki S, et al. Immunohistochemical and ultrastructural study of a papillary cystadenocarcinoma arising from the sublingual gland. J Oral Pathol Med. 1999;28:282–286. doi: 10.1111/j.1600-0714.1999.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 20.Aloudah NM, Raddaoui E, Aldhahri S, et al. Low-grade papillary cystadenocarcinoma of the parotid gland: presentation of a case with cytological, histopathological, and immunohistochemical features and pertinent literature review. Diagn Cytopathol. 2009;37:128–131. doi: 10.1002/dc.20971. [DOI] [PubMed] [Google Scholar]
- 21.Johnston NJ, Rose DS, Lutterloch MJ. Cystadenocarcinoma of salivary gland presenting as a cystic lesion in the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:201–204. doi: 10.1016/j.tripleo.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Kardos TB, Ferguson JW, McMillan MD. Mucus producing adenopapillary carcinoma of the oral cavity. Int J Oral Maxillofac Surg. 1992;21:160–162. doi: 10.1016/S0901-5027(05)80785-4. [DOI] [PubMed] [Google Scholar]
- 23.Notani K, Iizuka T, Yamazaki Y, et al. Mucinous adenocarcinoma of probable minor salivary gland origin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:738–740. doi: 10.1067/moe.2002.126698. [DOI] [PubMed] [Google Scholar]
- 24.Yakirevich E, Sabo E, Klorin G, et al. Primary mucin-producing tumors of the salivary glands: a clinicopathological and morphometric study. Histopathology. 2010;57:395–409. doi: 10.1111/j.1365-2559.2010.03639.x. [DOI] [PubMed] [Google Scholar]
- 25.Danford M, Eveson JW, Flood TR. Papillary cystadenocarcinoma of the sublingual gland presenting as a ranula. Br J Oral Maxillofac Surg. 1992;30:270–272. doi: 10.1016/0266-4356(92)90274-M. [DOI] [PubMed] [Google Scholar]
- 26.Anderson C, Muller R, Piorkowski R, et al. Intraductal carcinoma of major salivary gland. Cancer. 1992;69:609–614. doi: 10.1002/1097-0142(19920201)69:3<609::AID-CNCR2820690302>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Cheuk W, Miliauskas JR, Chan JK. Intraductal carcinoma of the oral cavity: a case report and a reappraisal of the concept of pure ductal carcinoma in situ in salivary duct carcinoma. Am J Surg Pathol. 2004;28:266–270. doi: 10.1097/00000478-200402000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Chen KT. Intraductal carcinoma of the minor salivary gland. J Laryngol Otol. 1983;97:189–191. doi: 10.1017/S002221510009397X. [DOI] [PubMed] [Google Scholar]
- 29.Simpson RH, Desai S, Di Palma S. Salivary duct carcinoma in situ of the parotid gland. Histopathology. 2008;53:416–425. doi: 10.1111/j.1365-2559.2008.03135.x. [DOI] [PubMed] [Google Scholar]
- 30.Lewis JE, McKinney BC, Weiland LH, et al. Salivary duct carcinoma. Clinicopathologic and immunohistochemical review of 26 cases. Cancer. 1996;77:223–230. doi: 10.1002/(SICI)1097-0142(19960115)77:2<223::AID-CNCR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 31.Kapadia SB, Barnes L. Expression of androgen receptor, gross cystic disease fluid protein, and CD44 in salivary duct carcinoma. Mod Pathol. 1998;11:1033–1038. [PubMed] [Google Scholar]
- 32.Chiosea SI, Griffith C, Assaad A, Seethala RR. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol. 2012;36:343–350. doi: 10.1097/PAS.0b013e318242a5b0. [DOI] [PubMed] [Google Scholar]
- 33.Skalova A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 34.Connor A, Perez-Ordonez B, Shago M, Skalova A, Weinreb I. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol. 2012;36:27–34. doi: 10.1097/PAS.0b013e318231542a. [DOI] [PubMed] [Google Scholar]
- 35.Chiosea SI, Griffith C, Assaad A, Seethala RR. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology. 2012;61:387–394. doi: 10.1111/j.1365-2559.2012.04232.x. [DOI] [PubMed] [Google Scholar]
- 36.Fahim L, Weinreb I, Alexander C, Perez Ordonez B. Epithelial proliferation in small ducts of salivary cystadenoma resembling atypical ductal hyperplasia of breast. Head Neck Pathol. 2008;2:213–217. doi: 10.1007/s12105-008-0061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skalova A, Gnepp DR, Simpson RH, Lewis JE, Janssen D, Sima R, et al. Clonal nature of sclerosing polycystic adenosis of salivary glands demonstrated by using the polymorphism of the human androgen receptor (HUMARA) locus as a marker. Am J Surg Pathol. 2006;30:939–944. doi: 10.1097/00000478-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Smith BC, Ellis GL, Slater LJ, Foss RD. Sclerosing polycystic adenosis of major salivary glands. A clinicopathologic analysis of nine cases. Am J Surg Pathol. 1996;20:161–170. doi: 10.1097/00000478-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Gnepp DR. Sclerosing polycystic adenosis of the salivary gland: a lesion that may be associated with dysplasia and carcinoma in situ. Adv Anat Pathol. 2003;10:218–222. doi: 10.1097/00125480-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Gnepp DR, Wang LJ, Brandwein-Gensler M, Slootweg P, Gill M, Hille J. Sclerosing polycystic adenosis of the salivary gland: a report of 16 cases. Am J Surg Pathol. 2006;30:154–164. doi: 10.1097/01.pas.0000186394.64840.1d. [DOI] [PubMed] [Google Scholar]
- 41.Hui KK, Batsakis JG, Luna MA, et al. Salivary duct adenocarcinoma: a high grade malignancy. J Laryngol Otol. 1986;100:105–114. doi: 10.1017/S0022215100098807. [DOI] [PubMed] [Google Scholar]
- 42.Brandwein MS, Jagirdar J, Patil J, et al. Salivary duct carcinoma (cribriform salivary carcinoma of excretory ducts). A clinicopathologic and immunohistochemical study of 12 cases. Cancer. 1990;65:2307–2314. doi: 10.1002/1097-0142(19900515)65:10<2307::AID-CNCR2820651024>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 43.Di Palma S, Simpson RH, Marchio C, et al. Salivary duct carcinomas can be classified into luminal androgen receptor-positive, HER2 and basal-like phenotypes. Histopathology. 2012;61:629–643. doi: 10.1111/j.1365-2559.2012.04252.x. [DOI] [PubMed] [Google Scholar]
- 44.Ihrler S, Sendelhofert A, Weiler C, et al. Preinvasive intraductal neoplasia in salivary adenocarcinoma, not otherwise specified. Virchows Arch. 2006;449:159–163. doi: 10.1007/s00428-006-0208-3. [DOI] [PubMed] [Google Scholar]
- 45.Kleinsasser O, Klein HJ. Hubner G [Salivary duct carcinoma. A group of salivary gland tumors analogous to mammary duct carcinoma] Arch Klin Exp Ohren Nasen Kehlkopfheilkd. 1968;192:100–105. doi: 10.1007/BF00301495. [DOI] [PubMed] [Google Scholar]


