Abstract
Trichinosis is a parasitic zoonosis caused by the nematode Trichinella spiralis. Anthelmintics are used to eliminate intestinal adults as well as tissue-migrating and encysted larvae. This study aimed to investigate the effects of ivermectin and myrrh obtained from the aloe-gum resin of Commiphora molmol on experimental trichinosis. Ninety albino mice were orally infected with 300 T. spiralis larvae. Drugs were tested against adult worms at day 0 and day 5 and against encysted larvae on day 15 and day 35 post-infection (PI). Mature worms and encysted larvae were counted in addition to histopathological examination of muscle specimens. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total protein, albumin, globulin, urea, and creatinine values were estimated. Significant reductions in mean worm numbers were detected in ivermectin treated mice at day 0 and day 5 PI achieving efficacies of 98.5% and 80.0%, while efficacies of myrrh in treated mice were 80.7% and 51.5%, respectively. At days 15 and 35 post-infection, ivermectin induced significant reduction in encysted larval counts achieving efficacies of 76.5% and 54.0%, respectively, while myrrh efficacies were 76.6% and 35.0%, respectively. AST, ALT, urea, and creatinine levels were reduced, while total proteins were increased in response to both treatments compared to their values in the infected non-treated mice. Ivermectin use for controlling T. spiralis could be continued. Myrrh was effective and could be a promising drug against the Egyptian strains of T. spiralis with results nearly comparable to ivermectin.
Keywords: Trichinella spiralis, ivermectin, myrrh, mouse
INTRODUCTION
Trichinosis is a globally distributed foodborne helminthic infection [1]. In humans, Trichinella spiralis is the most prevailing and pathogenic species. However, 8 other species were reported worldwide [2]. In rats, T. spiralis larvae grow to the adult stage in about 4-5 days after infection. Thirteen days post-infection (PI), capsule formation in the diaphragm begins and is completed within week 5 PI [3]. Several anthelmintic drugs are used to eliminate intestinal stage adults ane tissue-migrating and encysted larvae. These are organophosphates, benzimidazoles, and diethylcarbamazine as well as certain immunomodulating and anti-mitotic agents [4]. However, it was reported that only benzimidazoles possess unequivocal efficacy at well-tolerated dosages [5]. Consequently, supplementary drugs that were proved to be successful against other nematodes are needed to be tested for management of trichinellosis [6].
Ivermectin and its related compounds are the most essential anthelmintics available nowadays [7]. It is a potent macrolytic lactone causing paralysis in nematodes and arthropods through influx of chloride ions across the cell membrane [8]. Several studies were carried out to report the anti-Trichinella activity of ivermectin [9-12]. However, they are relatively inadequate. Thus, more investigations are required to elucidate the effects. The curative prospective of medicinal plants is gradually increasing particularly because natural products are frequently proved to be less toxic, affordable, and free from adverse effects observed with synthetic drugs [13]. Myrrh is a natural drug obtained from the aloe-gum resin of Commiphora molmol [14]. In addition to its anti-bacterial and anti-fungal activities [15], it was recorded as a potent schistosomicidal, fasciolicidal, and molluscicidal agent [16-19]. In the available published literature on myrrh, studies to investigate its anti-Trichinella activity are scarce. Consequently, the present study aimed to show the myrrh effects on both intestinal and muscle phases of trichinosis in mice and to compare with ivermectin effects on the same infections.
MATERIALS AND METHODS
Experimental animals and parasite
One hundred Swiss albino mice (25-30 g) were obtained from Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt. Mice were laboratory bred for 6-8 weeks. They were parasite free. Ninety of them were infected orally with 300 larvae per mice, while the remaining 10 were kept as the non-infected non-treated control group. Trichinella spiralis larvae were obtained from infected pig muscles from Cairo Slaughter House (Cairo, Egypt).
Preparation of the inoculums
Infective larvae were recovered by digestion of muscles of infected pigs as follows: The material was immersed for 12 hr in a digestive fluid composed of 1,000 ml saline, 20 ml concentrated HCl and 20 g pepsin at 37℃ under continuous mixing with a mechanical stirrer. The suspension was centrifuged at 1,000 rpm for 2 min to sediment the larvae. The sediment was washed successively in saline (0.9% NaCl) and centrifuged [20]. Then, the sediment larvae were resuspended in 1.5% gelatin in saline to obtain a stable suspension. The required inoculum size for each number of larvae aimed at was adjusted after doing several counts using hemocytometer. Before infection, mice were starved for 12 hr after which they received the larvae by injection directly into the stomach, using a tuberculin syringe. The inoculums for each mouse were adjusted to contain about 300 larvae.
Drug formulation and dose
Ivermectin (Ivomec suspension; Merk, Sharp & Dohme AGVT Ltd, Oss, Holland) was administered orally at a dosage of 0.25 ml/mouse [21]. Myrrh (Mirazid suspension; Pharco Pharm. Co., Cairo, Egypt) was supplied in an emulsion form of 10% concentration and administered at a dose of 0.01 ml/mouse orally [22].
Study design
Groups of animals
Infected mice were classified into 3 groups; A, B, and C. Each group contained 30 mice. Group A and B received single oral doses of ivermectin and myrrh (0.25 ml and 0.01 ml per mouse, respectively). Group C was infected and left untreated as controls.
Dosage schedule
Each group of the treated animals was subdivided into 4 subgroups (I, II, III, and IV) each comprising 5 mice. Subgroups received the drugs on day 0, 5, 15, and 35 PI. Animals of groups I and II were sacrificed on day 7 PI to show the drug effects on the intestinal phase, while animals of groups III and IV were sacrificed on day 40 PI to detect the drug effects on the muscle phase. Infected untreated (control) group was subdivided into 2 subgroups I and II (10 mice each) and were sacrificed on day 7 and day 40 PI, respectively. The remaining 10 mice from each group were kept for 35 days PI. Blood samples were taken from them on day 5, 15, and 35 PI to explore the effects of infection and medications on serum biochemical molecules.
Parasitological methods
Adult worm detection in the intestines of mice
Adults were recovered from the intestine of mice by means of a method modified from Benham [23]. Briefly, the intestine was opened, washed, and then incubated in 10 ml saline at 37℃ for 2 hr to allow worms to leave the intestine to the container. Washing was done several times till the fluid become clear. Then, the fluid was collected and centrifuged at 1,500 rpm for 5 min. The supernatant was decanted and the sediment was reconstituted in a few drops of saline to be examined drop by drop under the dissecting microscope for counting the adults.
Counting the number of muscle larvae
The diaphragm of mice was carefully dissected in each mouse and examined using the trichinoscopy and the number of larvae per diaphragm was estimated.
Histopathological examinations
Muscle specimens taken from different groups were fixed in 10% formol-saline for 24 hr, washed in water for 12 hr, dehydrated in ascending grades of alcohols, cleared in xylene, and embedded in paraffin blocks which were sectioned at 10 µm thickness by microtome, then stained with hematoxylin and eosin [24].
Testing the drug effects
The anthelmintic effects of drugs were evaluated by calculating the mean number of living worms and larvae per mice. The efficacy of each drug was calculated according to the equation [25]: Efficacy (%)=A-B/A×100, where A=no. of worms or larvae extracted from control animals and B=no. of worms or larvae extracted from treated animals.
The drug effects on the serum biochemical parameters were determined as follows: The remaining 10 mice from each group were kept for 35 days PI. Collected sera were used to determine the values of liver and renal biomarkers, including the total protein, albumin, globulin, AST, ALT, urea, and creatinine levels [26].
Statistical analysis
Statistical analysis was performed using the SPSS 13.0 software package. A probability value of less than 0.05 was considered statistically significant.
Ethical consideration
The experimental animal studies were maintained under convenient conditions at the animal house in Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt. The research was approved from the Scientific Research Ethical Committee, Faculty of Medicine, Cairo University, Cairo, Egypt.
RESULTS
Drug effects on mature worms
The mean number of living T. spiralis worms per mice (w.p.m) and the efficacy in percentage of the tested anthelmintic drugs received on the same day of infection are shown in Table 1. The least mean adult count was found in group A which received ivermectin (0.7±0.7) and showed the most effective eradication of T. spiralis worms, with the drug efficacy of 98.5%, while the mean adult worm count was found to be 39.7±13.7 in group B which received myrrh with a satisfactory drug efficacy of 80.7%. The statistical analysis revealed a better eradication of adult worms achieved by ivermectin (P=0.0001). On day 5 PI, the mean adult worm count was 41.0±15.7 in group A which received ivermectin with the highest drug efficacy of 80% (P=0.001). Group B which received myrrh showed the mean adult count of 89.7±18.7 with an average efficacy of 51.5% (Table 1). The statistical analysis in relation to the day of drug administration revealed a better eradication of adults when it was administered on day 0 than day 5 in both groups A and B (P=0.001 and 0.01, respectively).
Table 1.
Effects of ivermectin and myrrh on T. spiralis adult worms when administered on the same day (day 0) and on day 5 PI
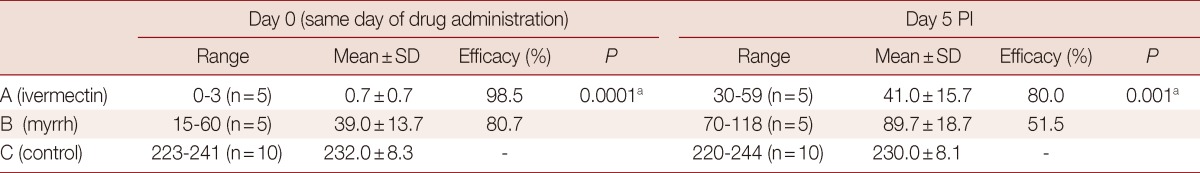
N, number of living mice.
aStatistically significant.
Effects on encysted larvae
Concerning the drug effects on the muscle phase, the larval count was estimated on day 40 PI for the 2 groups that received drugs on day 15 and 35 PI (Table 2). Among the mice which received the drugs on day 15 PI, the mean larval count was 1,119±30 in group A which received ivermectin with a satisfactory drug efficacy of 76.5%, whereas those which received myrrh, the mean larval count was 1,091±51 with a drug efficacy of 76.6%. Receiving the drugs on day 35 PI, the mean larval count of 2,336±1,786 was achieved with a drug efficacy of 54% in group A, whereas the mean larval count was 3,340±332 with a drug efficacy of 35% in group B. The statistical analysis revealed better eradication of larvae achieved by ivermectin (P=0.086). On comparing the drug effectiveness on the muscle phase in relation to the day of administration, statistically significant differences were found in groups A and B with a better eradication of larvae on day 15 than day 35 PI (P=0.008 and 0.076, respectively).
Table 2.
Effects of ivermectin and myrrh on T. spiralis larvae in muscles when administered on day 15 and day 35 PI
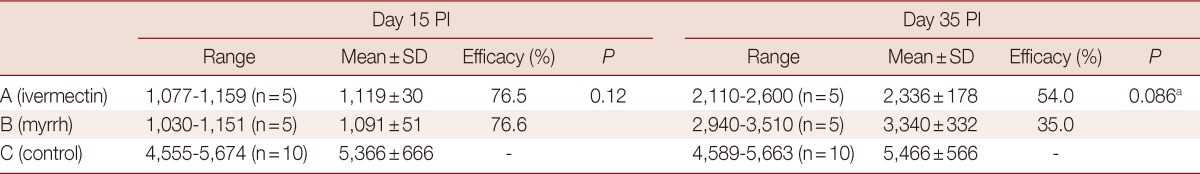
N, number of living mice.
aStatistically significant.
Histopathological examination
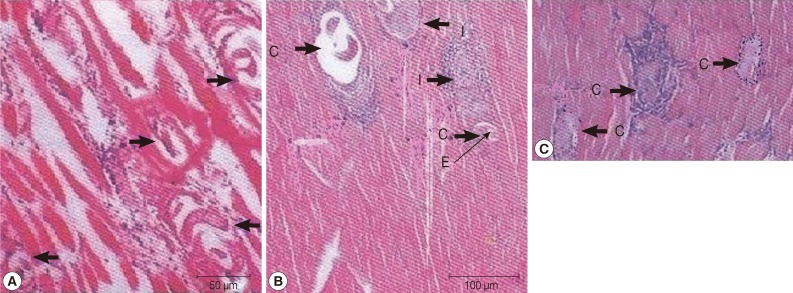
Fifteen days PI, group A which received ivermectin showed diffuse degenerative changes all over the muscle bundles, heavy inflammatory cellular infiltrations consisting of mainly histiocytes and lymphocytes. Encysted Trichinella larvae were diffusely present all over the muscle bundles (Fig. 1A). In group B which received myrrh, massive numbers of encysted larvae were detected all over the muscle bundles, in addition to diffuse mononuclear cellular infiltration affecting the bundles as well as the areas of encysted larvae. Larvae evidently showed degeneration and were replaced by amorphous material surrounded by inflammatory cells in a circumscribed round matter (Fig. 1B). On day 35 PI, both groups A and B showed massive numbers of encysted larvae surrounded by diffuse inflammatory cellular infiltration, with areas of coagulative necrosis, and many of cysts showed marked degenerative changes. However, these changes were more prominent in group B which received myrrh (Fig .1C).
Fig. 1.
(A) Hematoxylin-eosin staining of a muscle biopsy from group A , day 15 post-infection, showing multiple depositions of T. spiralis larval cysts (arrows) with mononuclear cell infiltrates surrounding the thickened capsule, areas of coagulative necrosis, and markedly degenerated muscle fibers. (B) Intersected T. spiralis larval cysts (C) within skeletal muscle tissue from group B, 15 days post-infection show degenerated cyst capsules surrounded by multiple cellular infiltrates (I) with replacement of one of the larvae by eosinophilic exudates (E). (C) Encysted T. spiralis larvae, 35 days post-infection surrounded by diffuse inflammatory cellular infiltration, with areas of coagulative necrosis and many of cysts (C) showed marked degenerative changes.
Effects of drugs on serum biochemical parameters
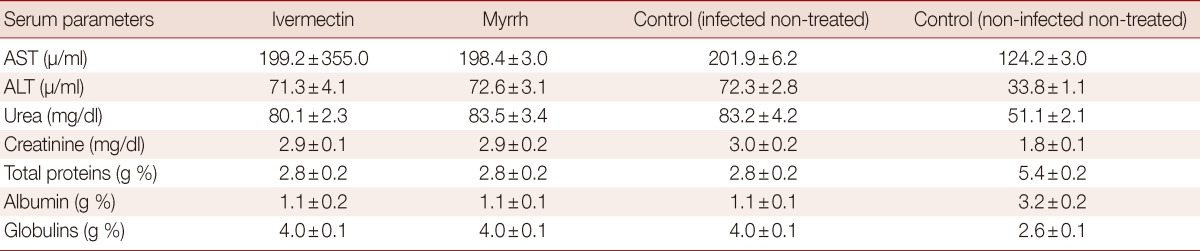
The effects of ivermectin and myrrh on the concentration of some serum biomoleculesare are shown in Tables 3 and 4. In the infected non-treated mice, significant increases in AST and ALT were revealed. Urea and creatinine levels also showed a highly significant increase throughout the experimental period. Mice infected with T. spiralis produced a significant decrease in the total protein, while the globulin levels were increased. Ivermectin and myrrh on day 5 and day 15 PI significantly reduced (P<0.05) the abnormal activities of AST, ALT, urea, and creatinine in infected mice. Furthermore, significant increases (P<0.05) were observed in total serum proteins and albumin concentrations in all the treatment groups when compared with the control group (Table 3). Until day 35 PI, ivermectin and myrrh did not significantly improve the altered parameters (Table 4).
Table 3.
Effects of ivermectin and myrrh on the concentrations of serum biochemical parameters on day 5 and day 15 PI
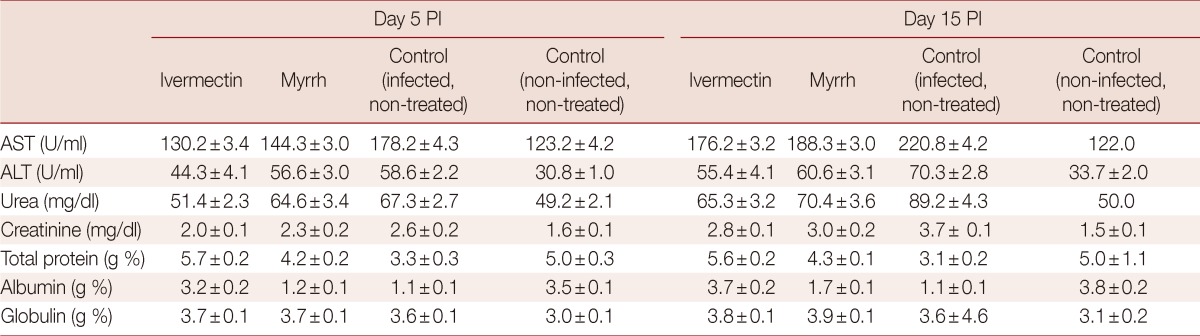
Table 4.
Effects of ivermectin and myrrh on serum biochemical parameters on day 35 PI
DISCUSSION
T. spiralis infection starts by invasion of host intestinal epithelium by the infective larvae that afterward create a collagen capsule within the striated muscles transforming the muscle cells into nurse cells [27]. Astonishingly, until now the mechanisms by which T. spiralis infective larvae distinguish, attack, creeps within the intestinal epithelium, and create their intramuscular niches are inadequately explained [28]. Efficacy studies reporting anti-Trichinella drug effects are insufficient; however, it had been reported that early administration of chemotherapy yielded effective results of trichinellosis therapy [29].
Anthelmintics are the chief drugs for treatment of trichinellosis. Glucocorticosteroids, protein, and electrolyte compensating preparations are supplementary administered groups [30]. Elimination of intestinal forms is significant for early and successful therapy which is the main target of anthelmintics applied during the first 3 days after infection [31]. Benzimidazole derivatives are the drugs most commonly used for treating human trichinellosis. Nevertheless; studies [32-33] reported that encysted larvae were not efficiently killed when these drugs were used experimentally in T. spiralis-infected mice. Despommier [34] explained this defective effect of albendazole on Trichinella late stages by the ability of the drug to induce a glucose depriving mechanism within the adult intestinal cells which become defective in absorbing nutrients and eventually, the adults depending on their own acquisition of glucose become starved. Yet, the encysted larvae depending on their energy supply on modulated muscle cells acting as nursing cells will not be affected. As a consequence of inadequate effectiveness of benzimidazole derivatives reported formerly against immature larval stages of Trichinella, ivermectin was used in the present study at a dose of 0.25 ml per mouse. Its administration on day 0 and 5 PI indicated that ivermectin was effective in removing the mature worms of T. spiralis, achieving efficacy of 98.5% and 80%, respectively, while efficacy of the drug against muscle larvae declined gradually as the time lags between the infection and the onset of treatment to be 76.5% and 54%, when given on days 15 and 35 PI, respectively.
Similar results were obtained by previous studies [9,11,12,35]. However, a bit different results were recorded by Song-Mingxin et al. [36] who found ivermectin less effective against the adult phase with a reduction rate ranging between 47.5% and 58.1% at a dose of 0.3 mg/kg, whereas it was effective against migrating and encysted larvae achieving reduction rates of 76.0-89.6% and 72.0-82.8%, respectively. Barton et al. [37] enlightened that the anthelmintic activity of ivermectin could be attributed to modulating GABA-gated chloride channels which are more accessible in nematodes than in vertebrates. El-Azzouni [11] implied that ivermectin has a direct effect on adults evidenced by topographic changes leading to degeneration and destruction of adults and consecutively reduction in the number of larvae. However, Ros-Moreno et al. [38] reported that the impact of ivermectin on the Trichinella surface is rather complex and diverse seeing that the role of γ-aminobutyric acid receptor of Trichinella as a target for ivermectin's remains indistinct. In the present study, when myrrh was given to Trichinella infected mice, it induced reduction of adult worm burden in addition to muscle larvae all over the study times achieving efficacies of 80.7, 51.5, 76.6, 35.0% when administered on day 0, 5, 15, and 35 PI, respectively.
During our search all through literature, myrrh effect on Trichinella has not yet been elucidated. In our study, at histopathological level, mice treated with both treatments showed diffuse degenerative changes, focal necrosis, inflammatory cellular infiltration and degeneration of encysted larvae that were replaced with amorphous material. However, more massive number of encysted larvae was noticed all over the muscle bundles when the treatments were given on day 35 PI than when given on day 15 PI, suggesting their higher effectiveness when given earlier.
The present findings were supported by those previously recorded by Kamel et al. [9] who noticed that degenerative changes in muscles were decreased while larval fragmentation was augmented when ivermectin was given on day 14 compared to less significant effects induced by ivermectin on day 35 PI. Similar results were reported by Soliman et al. [6] who stated that dormectin, ivermectin, and levamisole were unsuccessful in reduction of larval counts in the diaphragms of infected rats when injected at day 35 PI. These observations were conflicting with that recorded by El-Azzouni [11] who found that the ivermectin efficacy against encysted T. spiralis larvae was 73.5% when injected at week 6 PI. This discrepancy might be due to the differed medication schedules.
Our results revealed that T. spiralis infection significantly increased the activities of AST, ALT, levels of urea and creatinine in non-treated infected mice. These changes may be attributed to liver and kidney damages induced during migration of larvae as explained by Gamble et al. [26] who confirmed that elevation of AST and ALT is pointing to hepatic damage, whereas increased urea and creatinine is indicative of a kidney disease. Manifestly, in our study, the above-mentioned biochemical values remained elevated as the time passed between infections and starting treatments with ivermectin and myrrh. Yet, these values were still lower as compared to those of the control groups which might imply that both treatments might give the prospect to recovery of hepatic and renal cells towards normal after treatment. However, this consistently high levels of urea and creatinine despite the controlling effect of the drugs as compared to controls could be explained by reduced renal blood flow associated with higher serum urea concentration which may impair the secretory function of the kidney [39]. Thus, the superadded malfunction in the glomerular filtration results in retention of urea and creatinine, and this may be liable to augment their high serum levels in all treated groups.
Moreover, the increased amount of globulins in our experiment could be attributed to the compensatory mechanism to re-establish osmotic pressure which is reduced as a result of low albumin content by lost glomerular integrity in addition to elevated serum antibodies in response to the parasite or its metabolic products. Trivial effects of ivermectin and myrrh were noticed on the concentrations of serum biochemical parameters on day 35 PI compared to controls, validating the potential activity of the drugs used during earlier stages of infection. This is inconsistent with results obtained from a study by Arise and Malomo [40] who suggested that the repeated administration of ivermectin and/or albendazole may compromise the integral activity of hepatic and renal cells. Therefore, this controversy implies that despite its reported efficacy, ivermectin may possess the potential of adversely affecting liver and kidney functions.
On the other hand, several studies reported that crude myrrh or extracts such as mirazid, have been used for the treatment of various health conditions with minor or no side effects [41]. In another study, serum chemistry of treated rabbits was within standard ranges following myrrh therapy, indicating that it was undisruptive to hepatic cells [42]. Moreover, a study on myrrh [43] supported its value in reduction of total leukocytes, eosinophil counts, and other hematologic disorders. This study proved that the elevated hepatic enzymes, bilirubin and high serum Fasciola-antibody titers were normalized and, strikingly, the clinical signs and symptoms attributed to fascioliasis were relieved.
From the current study, it is concluded that ivermectin gave the best results at dosage levels and formulations. In addition, myrrh was found to be effective against T. spiralis with results nearly comparable to that achieved by ivermectin. Accordingly, ivermectin and myrrh could have continued practical application in controlling T. spiralis. However, ivermectin resistance has become a major problem in agricultural parasites [44], notably in H. contortus, and may be emerging in human parasites [45] which implied the need for an alternative anthelmintic. This study established that myrrh could be a very promising drug in the treatment of the Egyptian strains of T. spiralis which could be persuading to evaluate the effectiveness of myrrh extract in the treatment of trichinosis in other countries where the infection is prevalent.
References
- 1.Murrell KD. Trichinellosis: now and forevermore? Parasite. 2001;8:S11–S13. doi: 10.1051/parasite/200108s2011. [DOI] [PubMed] [Google Scholar]
- 2.Hosking BC, Watson TG, Leathwick DM. Multigeneric resistance to oxfendazole by nematodes in cattle. Vet Rec. 1996;138:67–68. doi: 10.1136/vr.138.3.67. [DOI] [PubMed] [Google Scholar]
- 3.Teppema JS, Robinson JE, Ruitenberg EJ. Ultrastructural aspects of capsule formation in Trichinella spiralis infection in the rat. Parasitology. 1973;66:291–296. doi: 10.1017/s0031182000045224. [DOI] [PubMed] [Google Scholar]
- 4.Cabié A, Bouchaud O, Houzé S, Khuong MA, Ruggeri C, Ancelle T, Matheron S, Coulaud JP. Albendazole versus thiabendazole as therapy for trichinosis: A retrospective study. Clin Infect Dis. 1996;22:1033–1035. doi: 10.1093/clinids/22.6.1033. [DOI] [PubMed] [Google Scholar]
- 5.Campbell WC, Blair LS. Chemotherapy of Trichinella spiralis infections (a review) Exp Parasitol. 1974;35:304–334. doi: 10.1016/0014-4894(74)90037-x. [DOI] [PubMed] [Google Scholar]
- 6.Soliman GA, Taher ES, Mahmoud MA. Therapeutic efficacy of dormectin, ivermectin and levamisole against different stages of Trichinella spiralis in rats. Turkiye Parazitol Derg. 2011;35:86–91. doi: 10.5152/tpd.2011.22. [DOI] [PubMed] [Google Scholar]
- 7.McCavera S, Walsh TK, Wolstenholme AJ. Nematode ligand-gated chloride channels: an appraisal of their involvement in macrocyclic lactone resistance and prospects for developing molecular markers. Parasitology. 2007;134:1111–1121. doi: 10.1017/S0031182007000042. [DOI] [PubMed] [Google Scholar]
- 8.Chippaux JP, Boussinesg M, Prod'hon J. The use of ivermectin in control of onchocerciasis. Sante. 1995;5:149–158. [PubMed] [Google Scholar]
- 9.Kamel AM, El-Helal T, Hesham TM. Ivermectin and thiabendazole in experimental trichinosis; Trichinellosis: Proceedings of the eighth international conference on trichinellosis; Italy: 1993. p. 417. [Google Scholar]
- 10.Ramisz A, Balicka-Laurans M, Grupiniski T. Efficacy of Moxidectin on experimental infection of Trichinella spiralis in mice; Trichinellosis: Proceedings of the eighth international conference on trichinellosis; Italy: 1993. pp. 449–451. [Google Scholar]
- 11.el-Azzouni MZ. Effect of ivermectin on experimental trichinosis. J Egypt Soc Parasitol. 1997;27:331–340. [PubMed] [Google Scholar]
- 12.Freedman DO, Zierdt WS, Lujan A, Nutman TB. The efficacy of ivermectin in the chemotherapy of gastrointestinal helminthiasis in humans. J Infect Dis. 1989;159:1151–1153. doi: 10.1093/infdis/159.6.1151. [DOI] [PubMed] [Google Scholar]
- 13.Abu El Ezz NM. Effects of Nigella sativa and Allium cepa oils on Trichinella spiralis in experimentally infected rats. J Egypt Soc Parasitol. 2005;35:511–523. [PubMed] [Google Scholar]
- 14.Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117–124. doi: 10.1016/s0378-8741(97)00137-2. [DOI] [PubMed] [Google Scholar]
- 15.Dolara P, Corte B, Ghelardini C, Pugliese AM, Cerbai E, Menichetti S, Lo Nostro A. Local anaesthetic and antifungal properties of sesquiterpenes from myrrh. Planta Med. 2000;66:356–358. doi: 10.1055/s-2000-8532. [DOI] [PubMed] [Google Scholar]
- 16.Massoud A, Salama O, Bennett I. Therapeutic efficacy of new schistosmicidal drug derived from myrrh in active intestinal schistosomiasis complicated with hepatosplenomegaly; Proceedings of International Congress of Parasitology; 1998. pp. 24–28. [Google Scholar]
- 17.El-Gohary Y, Massoud A, Kassem M, Abdo A, Hanno A, Salama O. Pilot study on a new fasciolicidal drug (Myrrh) Alex Med J. 1999;14:12–27. [Google Scholar]
- 18.Massoud AM, Fawzy SM, Salama OM. Laboratory studies on the molluscicidal and cercaricidal activities of Commiphora molmol. Egypt J Aquat Biol Fish. 2000;4:251–266. [Google Scholar]
- 19.Sheir Z, Nasr AA, Massoud A, Salama O, Badra GA, El-Shennawy H, Hassan N, Hammad SM. A safe, effective herbal anti-schistosomal therapy derived from myrrh. Am J Trop Med Hyg. 2001;65:700–704. doi: 10.4269/ajtmh.2001.65.700. [DOI] [PubMed] [Google Scholar]
- 20.Guenther S, Nöckler K, von Nickisch-Rosenegk M, Landgraf M, Ewers C, Wieler LH, Schierack P. Detection of T. spiralis, T. britovi and T. pseudospiralis in muscle tissue with real-time PCR. J Microbiol Methods. 2008;75:287–292. doi: 10.1016/j.mimet.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Coulaud JP, Lariviere M, Aziz MA, Gervais MC, Gaxotte P, Deluol AM, Cenac J. Ivermectin in onchocerciasis. Lancet. 1984;2:526–527. doi: 10.1016/s0140-6736(84)92608-4. [DOI] [PubMed] [Google Scholar]
- 22.Massoud A, Morsy TA, Haridy FM. Treatment of Egyptian dicrocoeliasis in man and animals with Mirazid. J Egypt Soc Parasitol. 2003;33:437–442. [PubMed] [Google Scholar]
- 23.Denham DA. Studies with methyridine and Trichinella spiralis. I. Effect upon the intestinal phase in mice. Exp Parasitol. 1965;17:10–14. doi: 10.1016/0014-4894(65)90003-2. [DOI] [PubMed] [Google Scholar]
- 24.Carleton MA, Drury GA, Willington EA, Cammeron H. Carleton,s histological technique. 4th ed. New York, Toronto, London: Oxford Univ Press; 1967. [Google Scholar]
- 25.Hosking BC, Watson TG, Leathwick DM. Multigeneric resistance to oxfendazole by nematodes in cattle. Vet Rec. 1996;138:67–68. doi: 10.1136/vr.138.3.67. [DOI] [PubMed] [Google Scholar]
- 26.Gamble HR, Wisnewski N, Wasson DL. Diagnosis of trichinellosis in swine by enzyme immunoassay, using a synthetic glycan antigen. Am J Vet Res. 1997;58:1417–1421. [PubMed] [Google Scholar]
- 27.Wu Z, Sofronic-Milosavljevic Lj, Nagano I, Takahashi Y. Trichinella spiralis: nurse cell formation with emphasis on analogy to muscle cell repair. Parasit Vectors. 2008;1:27. doi: 10.1186/1756-3305-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagliardo LF, McVay CS, Appleton JA. Molting, ecdysis, and reproduction of Trichinella spiralis are supported in-vitro by intestinal epithelial cells. Infect Immun. 2002;70:1853–1859. doi: 10.1128/IAI.70.4.1853-1859.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pozio E, Rinaldi L, Marucci G, Musella V, Galati F, Cringoli G, Boireau P, La Rosa G. Hosts and habitats of Trichinella spiralis and Trichinella britovi in Europe. Int J Parasitol. 2009;39:71–79. doi: 10.1016/j.ijpara.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Gottstein B, Pozio E, Nöckler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev. 2009;22:127–145. doi: 10.1128/CMR.00026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kociecka W. Trichinellosis: human disease, diagnosis and treatment. Vet Parasitol. 2000;93:365–383. doi: 10.1016/s0304-4017(00)00352-6. [DOI] [PubMed] [Google Scholar]
- 32.Siriyasatien P, Yingyourd P, Nuchprayoon S. Effects of albendazole against early and late stages of Trichinella spiralis infection in mice. J Med Assoc Thai. 2003;86:S257–S262. [PubMed] [Google Scholar]
- 33.Casulli A, Morales MA, Gallinella B, Turchetto L, Pozio E. 2-Hydroxypropyl-beta-cyclodextrin improves the effectiveness of albendazole against encapsulated larvae of Trichinella spiralis in a murine model. J Antimicrob Chemother. 2006;58:886–890. doi: 10.1093/jac/dkl329. [DOI] [PubMed] [Google Scholar]
- 34.Despommier DD. Biology of Trichinella and trichinosis. In: Campell WC, editor. Trichinella and trichinosis. New York, USA: Plenum Press; 1983. pp. 42–75. [Google Scholar]
- 35.Omar M, El Assaly T, Amer A, El Enbawy M. Effects of synanthic and ivomec on with reference to their serological evaluation. Vet Med J. 1995;43:31–35. [Google Scholar]
- 36.Song-Mingxin L, Yixin Zhang X, Hui H, Tian Yang L. Efficacy of ivermectin and albendazole against the various phases of 4 Trichinella isolates in treating mice. Chin J Zoon. 2002;18:89–91. [Google Scholar]
- 37.Barton NJ, Mitchell PJ, Hooke FG, Reynolds J. The therapeutic efficacy and prophylactic activity of dormectin against Dictyocaulus vivparus in cattles. Aust Vet J. 1995;72:349–351. doi: 10.1111/j.1751-0813.1995.tb07540.x. [DOI] [PubMed] [Google Scholar]
- 38.Ros-Moreno RM, Moreno-Guzmán MJ, Jiménez-González A, Rodríguez-Caabeiro F. Interactions of ivermectin with gamma-aminobutyric acid receptors in Trichinella spiralis muscle larvae. Parasitol Res. 1999;85:320–323. doi: 10.1007/s004360050555. [DOI] [PubMed] [Google Scholar]
- 39.Whealton A, Watson AJ, Rock RC. Colorimetric Determination of serum urea concentration. In: Ashwood ER, Burtis CA, Tietz NW, editors. Tietz Textbook of Clinical Chemistry. 3rd ed. Philadelphia, USA: Saunders; 1994. pp. 1528–1531. [Google Scholar]
- 40.Arise RO, Malomo SO. Effects of ivermectin and albendazole on some liver and kidney function indices in rats. Afr J Biochem Res. 2009;3:190–197. [Google Scholar]
- 41.Kimura I, Yoshikawa M, Kobayashi S, Sugihara Y, Suzuki M, Oominami H, Murakami T, Matsuda H, Doiphode VV. New triterpenes, myrrhanol A and myrrhanone A, from guggul-gum resins, and their potent anti-inflammatory effect on adjuvant-induced air-pouch granuloma of mice. Bioorg Med Chem Lett. 2001;11:985–989. doi: 10.1016/s0960-894x(01)00111-1. [DOI] [PubMed] [Google Scholar]
- 42.Al-Mathal EM. Efficacy of Commiphora molmol against hepatic coccidiosis (Eimeria stiedae) in the domestic rabbit. J Food Agric Environ. 2010;8:1072–1080. [Google Scholar]
- 43.Massoud A, El Sisi S, Salama O, Massoud A. Preliminary study of therapeutic efficacy of a new fasciolicidal drug derived from Commiphora molmol (myrrh) Am J Trop Med Hyg. 2001;65:96–99. doi: 10.4269/ajtmh.2001.65.96. [DOI] [PubMed] [Google Scholar]
- 44.Wolstenholme AJ, Fairweather I, Prichard R, von Samson-Himmelstjerna G, Sangster NC. Drug resistance in veterinary helminthes. Trends Parasitol. 2004;20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Osei-Atweneboana MY, Eng JK, Boakye DA, Gyapong JO, Prichard RK. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: a two-phase epidemiological study. Lancet. 2007;369:2021–2029. doi: 10.1016/S0140-6736(07)60942-8. [DOI] [PubMed] [Google Scholar]