Abstract
Balamuthia mandrillaris is one of the 4 amebas in fresh water and soil that cause diseases in humans. Granulomatous amebic encephalitis (GAE), caused by B. mandrillaris, is a rare but life-threatening condition. A 4-year-old, previously healthy, Thai girl presented with progressive headache and ataxia for over a month. Neuroimaging studies showed an infiltrative mass at the right cerebellar hemisphere mimicking a malignant cerebellar tumor. The pathological finding after total mass removal revealed severe necrotizing inflammation, with presence of scattered amebic trophozoites. Cerebrospinal fluid (CSF) obtained from lumbar puncture showed evidence of non-specific inflammation without identifiable organisms. A combination of pentamidine, sulfasalazine, fluconazole, and clarithromycin had been initiated promptly before PCR confirmed the diagnosis of Balamuthia amebic encephalitis (BAE). The patient showed initial improvement after the surgery and combined medical treatment, but gradually deteriorated and died of multiple organ failure within 46 days upon admission despite early diagnosis and treatment. In addition to the case, 10 survivors of BAE reported in the PubMed database were briefly reviewed in an attempt to identify the possible factors leading to survival of the patients diagnosed with this rare disease.
Keywords: Balamuthia mandrillaris, granulomatous amebic encephalitis, free-living ameba
INTRODUCTION
Encephalitis is an inflammatory process of the brain parenchyma associated with clinical evidence of brain dysfunction due to infectious, immune-mediated or primary inflammatory causes [1]. Of all identifiable infectious agents, virus and bacteria account for 90%, with ameba, though rare, also being capable of causing infectious encephalitis [2].
Amebas can be divided into 2 subgroups, parasitic and free-living ameba. Free-living amebas are widely distributed in nature, especially in fresh water and soil, but may occasionally invade and live as a parasite in the human tissue. Several species of Acanthamoeba, Balamuthia mandrillaris, Naegleria fowleri, and Sappinia pedata are pathogenic and opportunistic free-living amebas known to cause diseases in humans [3]. Since the first case of amebic encephalitis was reported in 1965 [4], free-living amebas have increasingly been recognized as a pathogen causing central nervous system infections in humans.
Pathogenic free-living amebas cause 2 distinct clinical forms of encephalitis; primary amebic meningoencephalitis (PAM) and granulomatous amebic encephalitis (GAE). PAM, caused by N. fowleri, is characterized by a fulminant course of disease with rapid progression to coma and death within a few days [5]. On the other hand, GAE, caused by Acanthamoeba spp. or B. mandrillaris, usually runs a subacute to chronic course, and eventually progresses to death [5].
Due to its rarity, difficulty in diagnosis and lack of proven effective treatment, only 10 patients with Balamuthia amebic encephalitis (BAE) were reported to have survived thus far [6-15]. The purpose of this report was to demonstrate the clinical presentation, investigation, and treatment outcome of a child with BAE, and to review the literature on cases with successful management. This report was informed and exempted by the Institutional Review Board, Faculty of Medicine, Chulalongkorn University, Thailand. The IRB also waived the written informed consent from the parent of the patient.
CASE DESCRIPTION
A previously healthy 4-year-old Thai female patient initially presented with 1-month history of subacute intermittent bi-temporal headache with projectile vomiting, in the absence of fever or diarrhea. Two weeks later, she developed unstable gait to the right side, and loss of distance approximation. There was no history of major illnesses, including sinusitis, pneumonia, chronic skin lesion, or any history of swimming in fresh water. She lived in Buriram province, the northeastern part of Thailand in rural settings, exposed to soil.
Physical examinations revealed an afebrile child with no meningeal signs. Her mental status was appropriate for her age. Abnormal neurological signs were head tilt to the right, jerky pursuit with nystagmus, hypometric succade, wide-based gait, and dysdiadochokinesia, which indicated right cerebellar hemispheric lesion. Computer tomography (CT) scan of the brain at a local hospital showed an ill-defined 4.4 cm isodensity mass without calcification in the posterior fossa displaying heterogeneous enhancement on post-contrast study and obstructive hydrocephalus. A referral was made with the diagnosis of a cerebellar tumor.
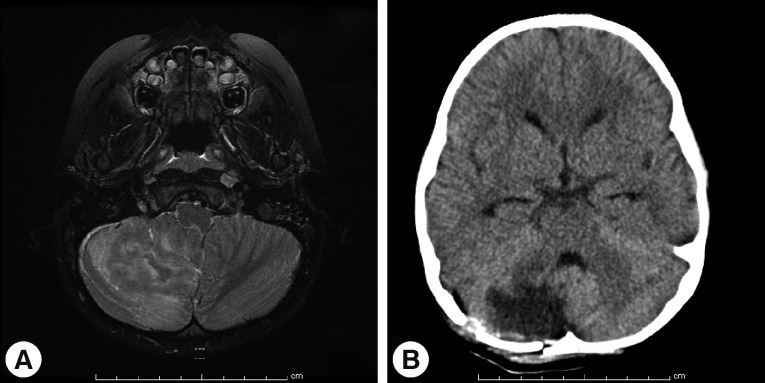
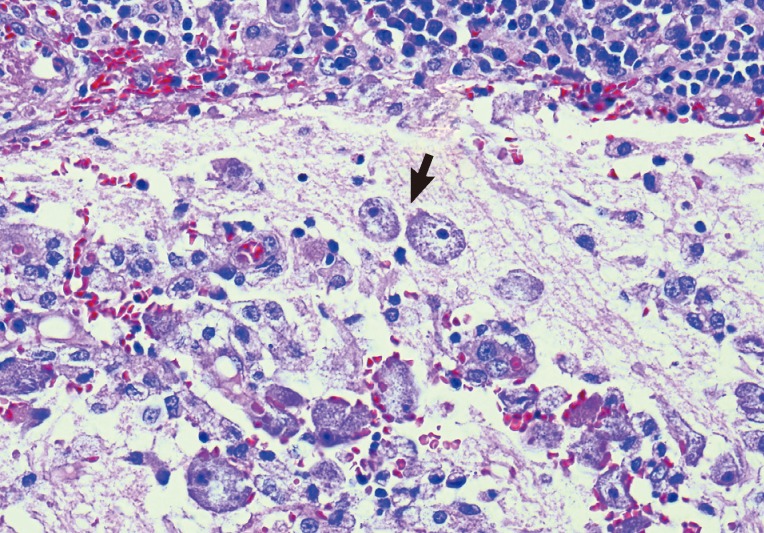
Magnetic resonance imaging (MRI) of the brain at King Chulalongkorn Memorial Hospital (Fig. 1) showed a 3.0×4.1×2.5 cm ill-defined infiltrating heterogeneously enhanced mass mainly occupying the right inferior cerebellar hemisphere with perilesional edema, causing right tonsillar herniation and mild obstructive hydrocephalus, highly suspicious for a malignant tumor such as medulloblastoma or high-grade glioma. Infectious cause, however, could not be excluded. Total excision of the mass was performed on day 7, and intraoperative finding revealed an infiltrative mass at the right cerebellum involving the hemisphere and tonsil with yellowish plaque and small cystic degeneration. The lesion was completely removed without any residual tumor detected by postoperative ultrasonography. The specimen was fixed in 10% neutral formalin, routinely processed, and embedded in paraffin wax. Pathological examination demonstrated severely inflamed cerebellar tissue, with presence of scattered amebic trophozoites (Fig. 2). The organisms tended to aggregate around blood vessels. No cyst form was observed. Dense lymphocyte infiltrates, aggregates of foamy histiocytes, few vague granulomata, and foci of tissue necrosis were found. The pathological diagnosis of amebic meningoencephalitis led to a prompt start of combined therapy of pentamidine (4 mg/kg/day), sulfasalazine (200 mg/kg/day), fluconazole (12 mg/kg/day), clarithromycin (14 mg/kg/day), and amphotericin B (1 mg/kg/day).
Fig. 1.
Pre- and postoperative imaging studies of the brain. (A) Axial T2-weighted MRI showing a 3.0×4.1×2.5 cm ill-defined infiltrating heterogeneous enhancing mass mainly occupying the right inferior cerebellar hemisphere with perilesional edema causing right tonsillar herniation and mild obstructive hydrocephalus. (B) Follow-up axial CT scan of the brain, 5 days post-operation, showing a hypodense lesion with perilesional vasogenic edema at the right cerebellar vermis and right cerebellar hemisphere, postoperative change.
Fig. 2.
Pathology of Balamuthia amebic encephalitis. Multiple amebic trophozoites in the cerebellar lesion are illustrated. The organisms tend to cling around a blood vessel (left side image). Note dense infiltrates of lymphocytes and plasma cells at top. H-E stain, ×400, Bar=50 µm.
On postoperative day 5, cerebrospinal fluid (CSF) obtained from lumbar puncture showed lymphocytic pleocytosis (WBC 225 cell/mm3, mononuclear cell 100%, RBC 157 cell/mm3) with increased protein of 148 mg/dl and decreased glucose levels of 27 mg/dl (serum glucose level=126 mg/dl). No organisms were identified on gram and acid-fast bacillus stain and an Indian ink preparation. CSF culture for bacteria was negative. These findings were consistent with amebic meningoencephalitis.
In order to identify the specific causative agent, further molecular characterization was then performed, using the formalin-fixed brain tissue and fresh CSF sample as detailed below.
MATERIALS AND METHODS
Molecular diagnosis
The DNA was extracted from 1 mg of brain tissue samples and 100 µl (confirm needed) CSF. The standard organic method (phenol-chloroform extraction and alcohol precipitation) was applied for the DNA extraction. The resulting pellet was resuspended in a final volume of 30 µl of deionized water.
18S rRNA detection and sequencing
The gene encoding 18S rRNA was amplified by PCR using specific primer sets [16]. The reaction mixture consisted of 10 µl of 2.5X Mastermix (Eppendorf, Hamburg, Germany), 2 µl of template, 0.5 µM of each forward and reverse primer, and nuclease-free water to a final volume of 25 µl. Both first and second round amplifications were performed in an Eppendorf Thermocycler. The reactions comprised of initial denaturation at 94℃ for 3 min, followed by 40 cycles of denaturation at 94℃ for 30 sec, annealing at 55℃ for 45 sec and extension at 72℃ for 60 sec, and concluded by a final extension step at 72℃ for 7 min. The PCR products were subjected to 2% agarose gel electrophoresis (FMC Bioproducts Rockland, Maine, USA) and upon staining with ethidium bromide, visualized on a UV transilluminator. The band of the expected size was excised from the gel and purified using the agarose gel extraction mini kit (5PRIME, Hamburg, Germany) according to the manufacturer's specifications. The purified DNA was sequenced by First-BASE Laboratories SDNBHD (Selangor Darul Ehsan, Malaysia). The nucleotide sequences were analyzed by Basic Local Alignment Search Tool (BLAST) (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Direct sequencing of DNA extracted from both brain tissue and CSF showed 18S rDNA of B. mandrillaris after BLAST analysis.
Phylogenetic tree construction and analysis
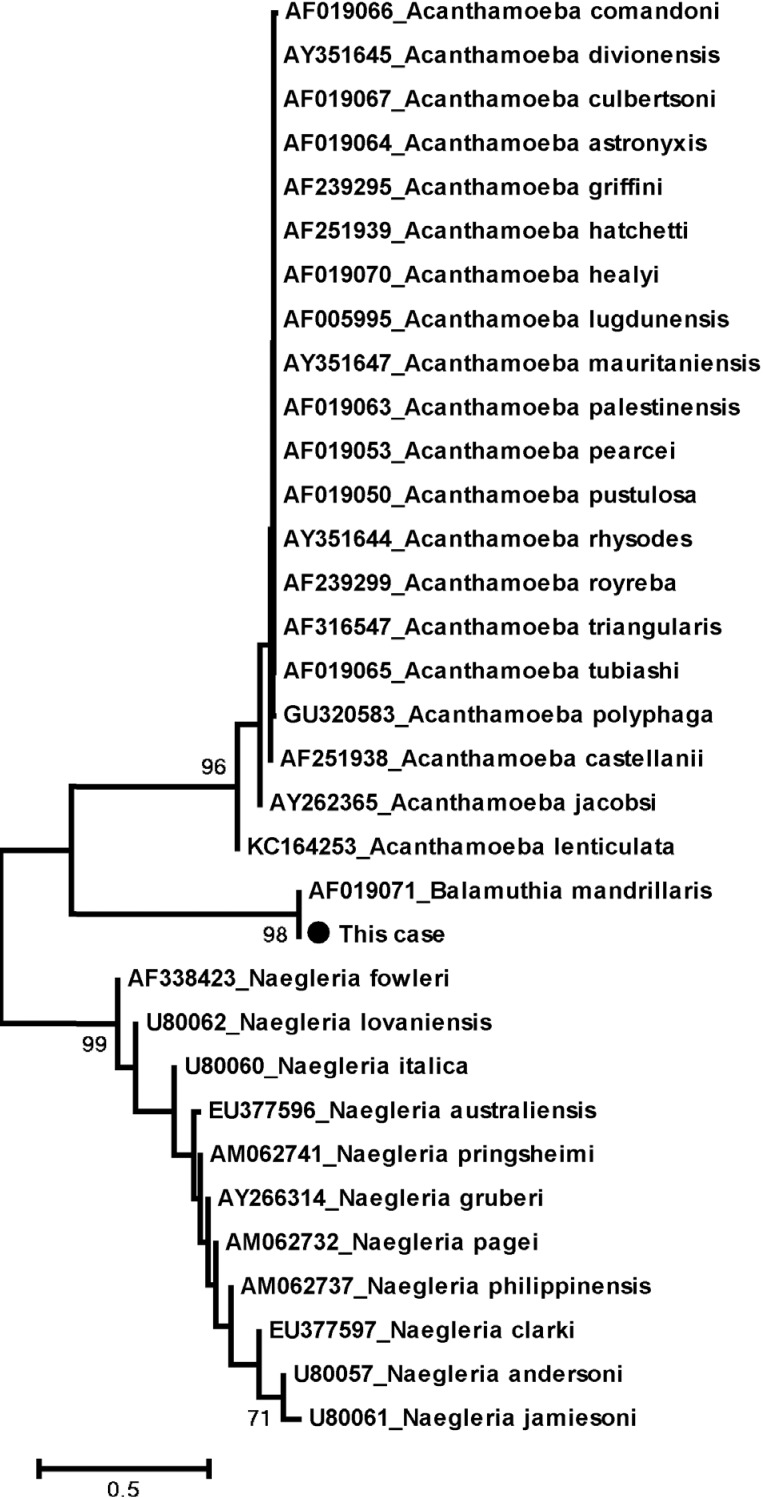
The Lasergene 6 Package (DNASTAR, Madison, Wisconsin, USA) was used to assemble the nucleotide sequences. The positive sequences were compared with the reference sequences (AF019071) available at GenBank. Alignment of the 18S ribosomal DNA sequences was performed by using Clustal W applying the BioEdit program (version 7.0.4.1) [17]. Phylogenetic trees were constructed for sequence analysis applying the neighbor-joining (NJ) method of the MEGA 4.0 program [18] and support tree topologies by boot-strapping applied with 1,000 replicates. According to Fig. 3, the phylogenetic tree also showed that this sequence is grouped with B. mandrillaris.
Fig. 3.
Phylogenetic tree analysis of Balamuthia mandrillaris.
Clinical course
An initial clinical response was noted for several days following surgical treatment and combined medical treatment. A follow-up CT scan (Fig. 1B) on postoperative day 5 showed no evidence of new lesions. However, the patient gradually deteriorated and eventually succumbed to multiple organ failure and brain death (declared on postoperative day 37), approximately 3 months after her first clinical symptoms.
DISCUSSION
B. mandrillaris, formerly known as leptomyxoid ameba (Leptomyxidae), was first identified in the brain of a pregnant mandrill baboon having died of encephalitis in 1986 [19]. In humans, it can cause diseases in both immunocompromised or immunocompetent individuals, especially young children and the elderly [20]. Recently, it has also been reported in solid organ transplant recipients [6,21]. To date, approximately 150 cases of BAE have been reported worldwide, mostly in the warm regions of the American continent, Asia, Australia, and Europe [22]. The first case of BAE in Thailand and Southeast Asia has been reported by Intalapaporn et al. [23]. The case involved a 23-year-old healthy man with prior history of motorcycle accident and upon falling into a swamp 6 months before, he developed a chronic nasal ulcer and had progressive central nervous system (CNS) deterioration leading to death in spite of receiving full empirical treatment. At autopsy, his brain section was sent for fluorescent immunohistochemical analysis to confirm B. mandrillaris infection. However, our case was the first Thai child with a premortal diagnosis of BAE.
Like other amebas, B. mandrillaris can be isolated from soil samples and fresh water [3]. B. mandrillaris can invade through skin wounds or be inhaled through nose or mouth, causing draining ulcers, chronic sinusitis or pneumonia before spreading hematogeneously to the CNS [20]. The skin lesion is typically a painless plaque or ulcer in the center of the face or extremity (especially the knee) [22]. Correct identification of the skin lesion is of great importance, allowing early diagnosis, weeks or months prior to CNS manifestation [3]. Nevertheless, most patients, including the present case, presented with neurological involvement from the start, without skin manifestation [20]. CNS symptoms consist of fever, personality changes, headache, focal seizures, nonspecific cranial nerve dysfunction, or localized motor deficit. Signs of increased intracranial pressure and alteration of consciousness may develop later as the disease progresses [24].
Due to the indolent clinical course, BAE is difficult to diagnose and mostly recognized postmortem. CSF usually reveals lymphocyte pleocytosis, with mild to severe elevation of protein levels (>1,000 mg/dl) and normal to low glucose levels which are nonspecific [3]. As B. mandrillaris trophozoites are rarely found in the CSF, a large number of patients were initially misdiagnosed for other organisms. CT scan and MRI typically show multifocally enhanced mass-like lesions with ring enhancement, edema, and hydrocephalus mimicking brain abscess, brain metastasis, or intracerebral hematoma [3]. A small number of solitary mass lesion was also reported [8-10,25,26] which mimicked a brain tumor with no obvious sign of encephalitis, similar to our case. Interestingly, a single mass in most cases situated in the anterior cranial fossa. To the best of our knowledge, this was the first case of BAE manifesting with a solitary cerebellar mass lesion.
Diagnosis of amebic encephalitis can be confirmed by microscopic identification of characteristic amebic trophozoites in the skin or brain tissue or in CSF. Skin and brain pathology typically show granulomatous inflammation formed by aggregation of foamy macrophages, multinucleated giant cells, and lymphocytes. Other findings may include presence of neutrophils, eosinophils, and small foci of vasculitis producing large areas of hemorrhage, infarction, and necrosis [22].
As B. mandrillaris is phylogenetically related to Acanthamoeba spp. (as shown in Fig. 3), it is not possible to morphologically distinguish between these 2 amebas. Moreover, GAE caused by Acanthamoeba spp. or B. mandrillaris are similar in terms of clinical course, neuroimaging, and pathological findings. Therefore, the diagnosis must be confirmed with indirect immunofluorescence (IIF) techniques, immunohistochemistry (IHC), or PCR [27,28]. Since IIF and IHC techniques for these amebas are readily available in only few specialized centers such as the Centers for Disease Control and Prevention (CDC; Atlanta, Georgia, USA), PCR technique, as performed on our patient, may be a more easily accessible tool leading to an earlier diagnosis and treatment of BAE before mortality.
Further to our own case, 10 patients who had survived BAE were searched for under "Balamuthia" in MEDLINE (PubMed). The 10 cases were summarized (Table 1) and reviewed in order to demonstrate common factors or antimicrobial agents that may contribute to a better chance of survival. The 10 survivors were distributed equally into 3 age groups, 4 children (<12 years), 3 middle-aged patients (13-60 years), and 3 elderly patients (>60 years). According to the data collected from all BAE cases, the proportion of elderly patients who survived appeared to be the highest, approximately 25% survival compared to less than 10% survival in the other groups. Thus, age might have contributed to the survival of the patients with BAE.
Table 1.
Summary of demographic characteristics, clinical data, and treatment of the 10 survivors of Balamuthia amebic infection
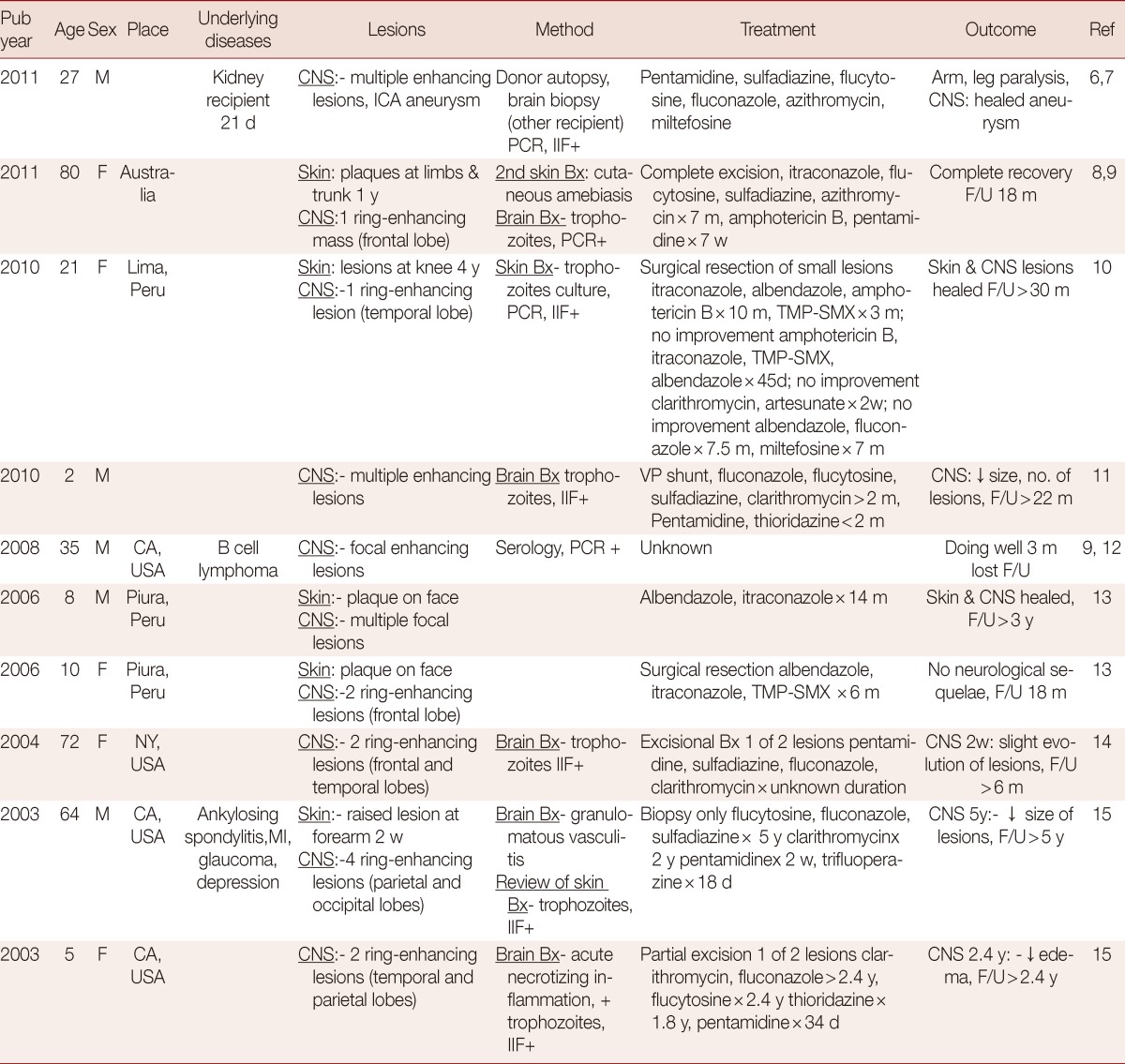
F, female; M, male; d, day; w, week; m, month; y, year; CNS, central nervous system; PCR, Polymerase chain reaction; Bx, biopsy, IIF, indirect immunofluorescence; ESRD, End stage renal disease; MI, myocardial infarction; ICA, internal carotid artery; VP, ventriculoperitoneal.
Concerning gender, male individuals were more susceptible to BAE, with male:female ratio of 2.5:1 [29], since they tend to be exposed to outdoor activities more than in females. However, male and female survivors were equal in number. Thus, the proportion of female survivors outnumbered that of males, with approximately 15% survival in females and 5% survival in males. Hence, female gender may play some role in increasing the chances of survival of these patients.
Skin lesions are the first clinical manifestation preceding neurological symptoms in approximately 1/4 of all patients with BAE. However, skin lesions appeared in up to 50% of the survivors. Therefore, skin manifestation may allow for early recognition and diagnosis of BAE leading to prompt treatment which potentially increase the patient's chances of survival.
In terms of treatment, apart from surgical approach [9], multi-drug regimens based on in vitro data and the clinical experience in successfully treated cases were also administered. The novel antimicrobial drug needs to have amebicidal activity, good penetration through the blood-brain barrier with minimal toxicity. Pentamidine, propamidine isethionates, polymyxin B, gramicidin S, and miltefosine were found to have strong inhibitory effects in vitro against B. mandrillaris [5,30,31]. Unfortunately, some of these amebicidal agents are of limited use because they are not suitable for parenteral use [30], except for miltefosine in 1 survivor and pentamidine in 4 survivors, including our patient.
Other antimicrobial agents with little or no in vitro effects included azithromycin, clarithromycin, the azole compounds, trimethoprim, sulfamethoxazole, and amphotericin B [30,31]. Of the 9 survivors with documented specific antimicrobial treatment regimens, almost all have shown similar treatment regimens consisting of azoles (fluconazole, itraconazole) in 8 of 9 survivors, macrolides (clarithromycin, azithromycin) in 7 of 9 survivors. In contrast with in vitro study results, azoles and macrolides may represent good choices as potential antimicrobial agents for treating BAE.
The optimal duration of drug therapy for BAE has not yet been ascertained due to the complete absence of any validated biochemical markers of response to treatment, but most survivors had been treated for a few months up to more than 5 years. However, long-term use of multiple drugs might be limited by some undesirable side effects, such as hyperglycemia due to pentamidine or hyponatremia due to thioridazine, resulting in discontinuation of medications [32].
Currently, patients recently diagnosed with this disease are being treated according to the reported successful regimens; however, case fatality of this disease is still high. Due to the limited number of survivors reported, the prognostic factors and effective treatment regimens have so far remained elusive and require more detailed further research.
BAE is a rare but extremely life-threatening disease. Although it has become more recognizable among physicians, more case reports and additional research will still be required for more information and a better insight into this disease. A high index of suspicion of clinicians, immunostaining or molecular techniques to promote early diagnosis, multidisciplinary team approach, and prompt initiation of antimicrobial therapy are key factors which might improve survival of the patients.
ACKNOWLEDGMENTS
This work was supported by the Higher Education Research Promotion and National Research University Project of Thailand (HR1155A-55), Thailand Research Fund (DPG5480002), Office of the Commission on Higher Education, Center of Excellence in Clinical Virology, Chulalongkorn University, CU Centenary Academic Development Project and King Chulalongkorn Memorial Hospital. We would like to express our gratitude to the entire staff of the Gastroenterology unit, Department of Medicine, Liver Transplant Unit, Center of Excellence in Clinical Virology, Faculty of Medicine, Chulalongkorn University and Hospital, Thai Red Cross Society, Thailand.
References
- 1.Falchek SJ. Encephalitis in the pediatric population. Pediatr Rev. 2012;33:122–133. doi: 10.1542/pir.33-3-122. [DOI] [PubMed] [Google Scholar]
- 2.Glaser CA, Honarmand S, Anderson LJ, Schnurr DP, Forghani B, Cossen CK, Schuster FL, Christie LJ, Tureen JH. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006;43:1565–1577. doi: 10.1086/509330. [DOI] [PubMed] [Google Scholar]
- 3.Visvesvara GS, Moura H, Shuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 4.Fowler M, Carter RF. Acute pyogenic meningitis probably due to Acanthamoeba sp: a preliminary report. Br Med J. 1965;2:740–742. doi: 10.1136/bmj.2.5464.734-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visvesvara GS. Free-living amebae as opportunistic agents of human disease. J Neuroparasitology. 2010;1 doi: 10.4303/jnp/N100802. [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Balamuthia mandrillaris transmitted through organ transplantation-Mississippi, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1165–1170. [PubMed] [Google Scholar]
- 7.Orozco LD, Khan MA, Fratkin JD, Hanigan WC. Asymptomatic aneurysm of the cavernous and supraclinoid internal carotid artery in a patient with Balamuthia mandrillaris encephalitis. J Clin Neurosci. 2011;18:1118–1120. doi: 10.1016/j.jocn.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 8.Botterill E, Yip G. A rare survivor of Balamuthia granulomatous encephalitis. Clin Neurol Neurosurg. 2011;113:499–502. doi: 10.1016/j.clineuro.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Doyle JS, Campbell E, Fuller A, Spelman DW, Cameron R, Malham G, Gin D, Lewin SR. Balamuthia mandrillaris brain abscess successfully treated with complete surgical excision and prolonged combination antimicrobial therapy. J Neurosurg. 2011;114:458–462. doi: 10.3171/2010.10.JNS10677. [DOI] [PubMed] [Google Scholar]
- 10.Martínez DY, Seas C, Bravo F, Legua P, Ramos C, Cabello AM, Gotuzzo E. Successful treatment of Balamuthia mandrillaris amoebic infection with extensive neurological and cutaneous involvement. Clin Infect Dis. 2010;51:e7–e11. doi: 10.1086/653609. [DOI] [PubMed] [Google Scholar]
- 11.Cary LC, Maul E, Potter C, Wong P, Nelson PT, Given C, 2nd, Robertson W., Jr Balamuthia mandrillaris meningoencephalitis: survival of a pediatric patient. Pediatrics. 2010;125:e699–e703. doi: 10.1542/peds.2009-1797. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Balamuthia amebic encephalitis-California, 1999-2007. MMWR Morb Mortal Wkly Rep. 2008;57:768–771. [PubMed] [Google Scholar]
- 13.Seas RC, Bravo PF. Amebic granulomatosis encephalitis due to Balamuthia mandrillaris: a fatal disease increasingly recognized in Latin America. Rev Chilena Infectol. 2006;23:197–199. doi: 10.4067/s0716-10182006000300001. [DOI] [PubMed] [Google Scholar]
- 14.Jung SJ, Schelper RL, Visesvara GS, Chang HT. Balamuthia mandrillaris meningoencephalitis in an immunocompetent patient: an unusual clinical course and a favorable outcome. Arch Pathol Lab Med. 2004;128:466–468. doi: 10.5858/2004-128-466-BMMIAI. [DOI] [PubMed] [Google Scholar]
- 15.Deetz TR, Sawyer MH, Billman G, Schuster FL, Visvesvara GS. Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clin Infect Dis. 2003;37:1304–1312. doi: 10.1086/379020. [DOI] [PubMed] [Google Scholar]
- 16.Qvarnstrom Y, Visvesvara GS, Sriram R, da Silva AJ. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J Clin Microbiol. 2006;44:3589–3595. doi: 10.1128/JCM.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999;41:95–98. [Google Scholar]
- 18.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 19.Visvesvara GS, Martinez AJ, Schuster FL, Leitch GJ, Wallace SV, Sawyer TK, Anderson M. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol. 1990;28:2750–2756. doi: 10.1128/jcm.28.12.2750-2756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz JH. The public health threat from Balamuthia mandrillaris in the southern United States. J La State Med Soc. 2011;163:197–204. [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Notes from the field: transplant-transmitted Balamuthia mandrillaris-Arizona, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:1182. [PubMed] [Google Scholar]
- 22.Bravo FG, Alvarez PJ, Gotuzzo E. Balamuthia mandrillaris infection of the skin and central nervous system: an emerging disease of concern to many specialties in medicine. Curr Opin Infect Dis. 2011;24:112–117. doi: 10.1097/QCO.0b013e3283428d1e. [DOI] [PubMed] [Google Scholar]
- 23.Intalapaporn P, Suankratay C, Shuangshoti S, Phantumchinda K, Keelawat S, Wilde H. Balamuthia mandrillaris meningoencephalitis: the first case in Southeast Asia. Am J Trop Med Hyg. 2004;70:666–669. [PubMed] [Google Scholar]
- 24.Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol. 2004;34:1001–1027. doi: 10.1016/j.ijpara.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh PS, Ghosh D, Loddenkemper T, Prayson RA, Tekautz T, Sriram CS, Danziger-Isakov L. Necrotizing granulomatous meningoencephalitis due to Balamuthia in an immunocompetent child. Neurology. 2011;77:801–802. doi: 10.1212/WNL.0b013e31822b0100. [DOI] [PubMed] [Google Scholar]
- 26.Tavares M, Correia da Costa JM, Carpenter SS, Santos LA, Afonso C, Aguiar A, Pereira J, Cardoso AI, Schuster FL, Yagi S, Sriram R, Visvesvara GS. Diagnosis of first case of Balamuthia amoebic encephalitis in Portugal by immunofluorescence and PCR. J Clin Microbiol. 2006;44:2660–2663. doi: 10.1128/JCM.00479-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuster FL, Yagi S, Gavali S, Michelson D, Raghavan R, Blomquist I, Glastonbury C, Bollen AW, Scharnhorst D, Reed SL, Kuriyama S, Visvesvara GS, Glaser CA. Under the radar: Balamuthia amebic encephalitis. Clin Infect Dis. 2009;48:879–887. doi: 10.1086/597260. [DOI] [PubMed] [Google Scholar]
- 28.Guarner J, Bartlett J, Shieh WJ, Paddock CD, Visvesvara GS, Zaki SR. Histopathologic spectrum and immunohistochemical diagnosis of amebic meningoencephalitis. Mod Pathol. 2007;20:1230–1237. doi: 10.1038/modpathol.3800973. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqui R, Khan NA. Balamuthia amoebic encephalitis: an emerging disease with fatal consequences. Microb Pathog. 2008;44:89–97. doi: 10.1016/j.micpath.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Schuster FL, Visvesvara Gs. Axenic growth and drug sensitivity studies of Balamuthia mandrillaris, an agent of amebic meningoencephalitis in humans and other animals. J Clin Microbiol. 1996;34:385–388. doi: 10.1128/jcm.34.2.385-388.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuster FL, Guglielmo BJ, Visvesvara GS. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J Eukaryot Microbiol. 2006;53:121–126. doi: 10.1111/j.1550-7408.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 32.Schuster FL, Visvesvara GS. Opportunistic amoebae: challenges in prophylaxis and treatment. Drug Resist Updat. 2004;7:41–51. doi: 10.1016/j.drup.2004.01.002. [DOI] [PubMed] [Google Scholar]