Summary
Background
During cell division, chromosomes must clear the path of the cleavage furrow before the onset of cytokinesis. The abscission checkpoint in mammalian cells stabilizes the cleavage furrow in the presence of a chromatin obstruction. This provides time to resolve the obstruction before the cleavage furrow regresses or breaks the chromosomes, preventing aneuploidy or DNA damage. Two unanswered questions in the proposed mechanistic pathway of the abscission checkpoint concern factors involved in 1) resolving the obstructions, and 2) coordinating obstruction resolution with the delay in cytokinesis.
Results
We found that the 1-cell and 2-cell C. elegans embryos suppress furrow regression following depletion of essential chromosome segregation factors: topoisomerase IITOP-2, CENP-AHCP-3, cohesin, and to a lesser degree, condensin. Chromatin obstructions activated Aurora BAIR-2 at the spindle midzone, which is needed for the abscission checkpoint in other systems. Condensin I, but not condensin II, localizes to the spindle midzone in anaphase and to the midbody during normal cytokinesis. Interestingly, condensin I is enriched on chromatin bridges and near the midzone/midbody in an AIR-2 dependent manner. Disruption of AIR-2, the spindle midzone or condensin leads to cytokinesis failure in a chromatin-obstruction-dependent manner. Examination of the condensin-deficient embryos uncovered defects in both the resolution of the chromatin obstructions and the maintenance of the stable cleavage furrow.
Conclusions
We postulate that condensin I is recruited by Aurora BAIR-2 to aid in the resolution of chromatin obstructions and also helps generate a signal to maintain the delay in cytokinesis.
Introduction
During cell division, the completion of chromosome segregation prior to the onset of cytokinesis ensures the removal of DNA from the path of the cleavage furrow. Chromatin obstruction in the cleavage plane in human cells can lead to cleavage furrow regression resulting in tetraploid cells [1] and DNA damage [2]. The human 'abscission checkpoint' and the budding yeast 'NoCut' pathways sense chromatin obstructions in the cleavage plane and delay the completion of cytokinesis, presumably to allow more time for the obstruction to be resolved [1, 3].
Aurora B kinase is an essential part of the chromosome passenger complex and promotes multiple aspects of cell division (reviewed in [4, 5]). At anaphase, Aurora B localizes to the spindle midzone, a structure formed by antiparallel microtubules, where it promotes successful completion of cytokinesis. Importantly, Aurora B and the budding yeast Aurora homolog Ipl1 are critical for the transduction of the abscission checkpoint and NoCut pathway, respectively [1, 3]. Chromatin obstructions induce hyperactive Aurora B at the midbody and Ipl1 at the bud neck [1, 6]. Abscission is delayed when Aurora B promotes the phosphorylation of a component of the ESCRT machinery and MKLP1 in human cells [1, 2, 7]. In budding yeast, a stable intercellular canal correlates with increased levels of anillin homologs at the bud neck [3]. While the known downstream effectors delaying cytokinesis completion vary between human and budding yeast, the need for Aurora B/Ipl1 to initiate this response is conserved.
Effectors that promote resolution of chromatin obstruction in this response have not been identified. One candidate is the condensin complex that is highly conserved and essential for chromosome morphology and segregation (reviewed in [8]). Condensin normally aids in the resolution of sister chromatids to facilitate chromosome segregation at anaphase onset. Yeast condensin complexes also promote anaphase and telophase, by helping to resolve specific chromosomal regions and keeping the chromosomes resolved [9–12]. Curiously, a detectable fraction of condensin I complexes is observed at the spindle midzone in human cells and C. elegans embryos [13, 14]. Human chromosomes also continue to shorten at anaphase in a microtubule-dependent manner, but condensin depletion did not impair anaphase chromosome compaction [15]. Therefore it is unclear whether metazoan condensin promotes chromosome resolution at anaphase and why it localizes to the spindle midzone.
It is thought that cell cycle checkpoints are dampened in early embryonic divisions in many species [16–21] because these divisions occur rapidly [22]. These checkpoints typically minimize chromatin bridge formation and lagging anaphase chromosomes in other cell types, therefore we reasoned that the abscission checkpoint which helps to reduce the deleterious effects of chromatin obstruction during cytokinesis might be crucial in the early embryo. Whether the abscission checkpoint exists in vivo in an intact metazoan has not been addressed.
In this study, we show evidence that the cleavage furrow rarely regresses in the presence of chromatin obstructions in C. elegans embryos. Chromatin bridges induce activation of Aurora BAIR-2, which then recruits condensin I to the midzone and midbody. Loss of condensin impedes the resolution of chromatin obstructions and results in destabilized cleavage furrow in the presence of an obstruction. Therefore, our data suggest that a response similar to the abscission checkpoint also functions in the C. elegans embryo, and that condensin functions in obstruction resolution and the maintenance of the delay in the completion of cytokinesis.
Results
Chromatin obstructions rarely cause cytokinesis failure in early C. elegans embryos
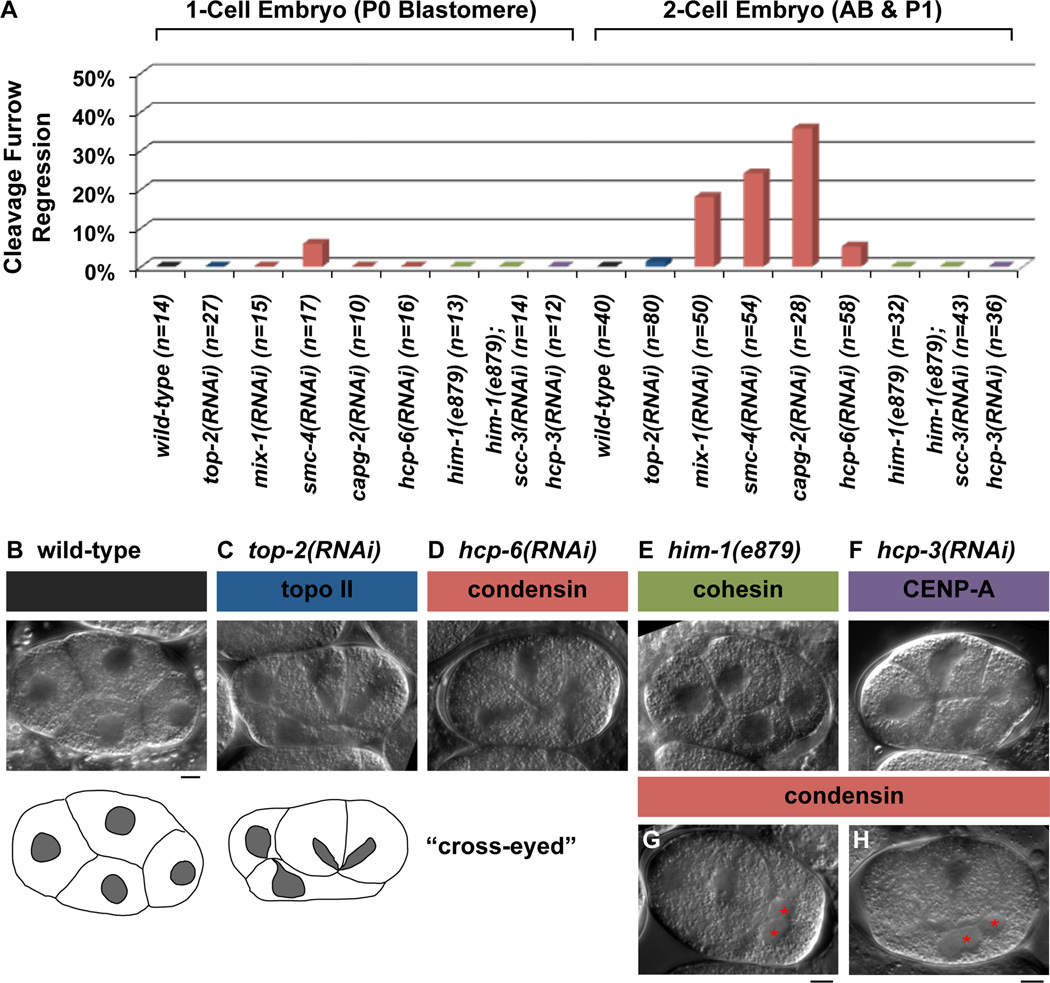
We used live time-lapse DIC microscopy to examine cytokinesis in the 1- and 2-cell embryos and addressed whether chromatin obstructions could trigger cytokinetic furrow regression. We monitored embryos until the onset of the third round of cell division to detect any late cytokinesis failures in the first and second round of divisions. Embryos were depleted of centromeric histone CENP-AHCP-3, topoisomerase IITOP-2, and subunits of two Structural Maintenance of Chromosomes (SMC) protein complexes, cohesin and condensin by RNAi (Figure 1). As expected, the RNAi depletion of genes essential for chromosome segregation produced the cross-eyed phenotype, where reformed nuclei are trapped close to the cleavage plane [23], indicating cytokinesis initiated despite the presence of chromatin obstructions (Figure 1, compare B to C–F). The cleavage furrow remained stable under these conditions and rarely regressed during the first division (Figure 1A, Table S1), consistent with previous observations [24]. We next examined smc-4 and top-2 RNAi treated embryos labeled to mark chromosomes and the plasma membrane with mCherry fluorescence. We confirmed that the cleavage furrow remains stable in the presence of chromatin obstructions (Figure S1), and the stability of the cleavage furrow is maintained under more potent RNAi depletion by injection (Table S1). The 2-cell embryo was also highly refractory to cytokinesis failure in the presence of chromatin obstructions but the frequency of furrow regression increased with more effective RNAi depletion of top-2 and CENP-Ahcp-3 (Figure 1A and Table S1). Interestingly, condensin depletion consistently had the highest frequency of furrow regression (up to 49%) in 2-cell embryos (Figure 1A and Table S1), resulting in binucleated AB and P1 blastomeres (Figure 1G and H, data not shown). However, the majority of cytokinesis in the 2-cell embryo did not fail despite chromosome segregation defects. Together, these findings indicate that the majority of cleavage furrows are stable in the presence of chromatin obstructions in the C. elegans embryo.
Figure 1.
Nomarski DIC time-lapse analysis of cell division in the 1-cell and 2-cell embryos. (A) Graph represents the percentages of P0 and AB/P1 cell divisions in wild-type and RNAi-treated embryos that exhibited a cytokinesis cleavage furrow regression defect in the presence of chromatin obstruction as monitored indirectly by the cross-eyed nuclei phenotype. The number of P0 or AB/P1 cell divisions (n) examined per condition is provided. (B–F) Representative still images of 4-cell embryos taken from Nomarski DIC time-lapse movies. Cartoon tracings show a wild-type embryo with each nucleus (grey) centered within the cell, compared with the top-2(RNAi) embryo which exhibited the defective cross-eyed nuclei morphology. (G–H) Nomarski DIC images of a condensin (G) mix-1 RNAi embryo and (H) smc-4 RNAi embryo after the P1 cell division had failed resulting in a binucleated cell (red asterisks). Scale bar, 5 µm.
Chromatin obstructions trigger hyperactivation of Aurora BAIR-2
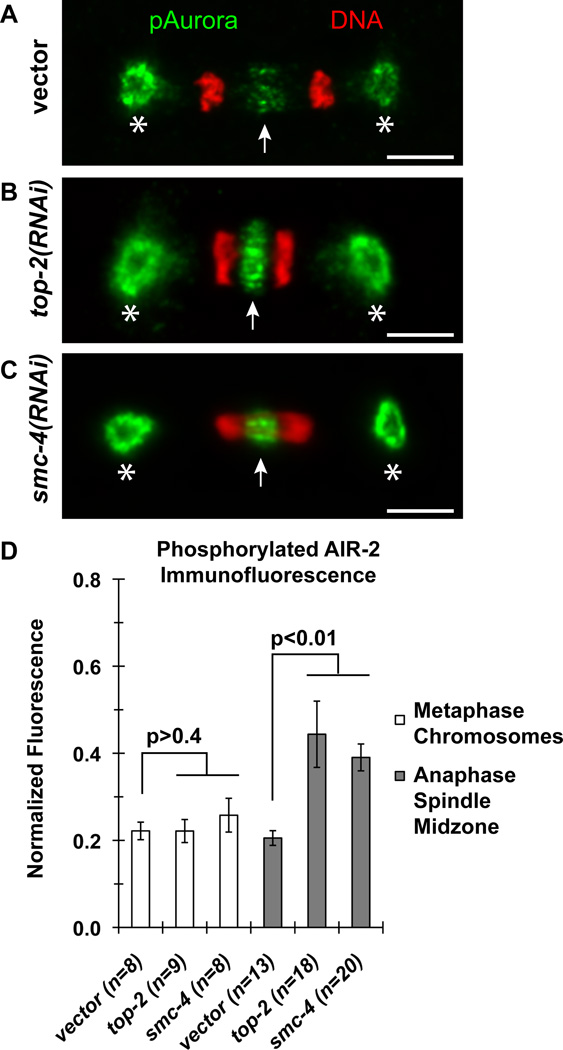
Activation of Aurora B at the spindle midzone and the midbody is a hallmark of the abscission checkpoint [1]. To determine if the C. elegans homolog of Aurora B, AIR-2, was similarly activated, we performed quantitative immunofluorescence microscopy using phosphorylation-specific antibodies that can detect activated AIR-2 [25]. In comparison to the vector RNAi control, depletion of top-2 and condensin smc-4 increased the level of phosphorylated AIR-2 staining at the spindle midzone (compare Figure 2A to B & C) by an average of 2-fold (Figure 2D). By contrast, the level of phosphorylated AIR-2 staining on metaphase chromosomes was unchanged (Figure 2D). After anaphase, phosphorylated AIR-2 staining persisted longer at the midbody when chromosome bridges are present (Figure S2A, B). However, immunostaining with antibodies that recognize all forms of AIR-2 detected no difference between vector RNAi, top-2 RNAi and smc-4 RNAi embryos (Figure S2C).
Figure 2.
Quantitative immunofluorescence microcopy of phosphorylated AIR-2 in 1-cell embryos. (A–C) Representative micrographs of phosphorylated Aurora kinase immunostaining recognizing phosphorylated AIR-1 at the centrosomes (asterisks) and phosphorylated AIR-2 at the spindle midzone (white arrows) of the 1-cell P0 blastomere division at anaphase. Phosphorylated AIR-1 and AIR-2 staining are shown in green and DAPI-DNA fluorescence shown in red. Scale bars, 5 µm. (D) Graph represents the average level of phosphorylated AIR-2 staining on metaphase chromosomes and at the anaphase spindle midzone normalized to the level of phosphorylated AIR-1 staining detected at the anterior spindle pole. The number of embryos (n) quantified per condition is provided. Error bars, SEM. The p-values are determined by two-tailed t-Tests.
We also examined AIR-2::GFP dynamics in live embryos. In control embryos, AIR-2::GFP moves from the metaphase plate to the central spindle and midbody (Figure S3A, and [25, 26]). AIR-2::GFP signal persists at the midbody into the next division when it apparently becomes internalized in the P1 daughter cell (Figure S3A and data not shown). In top-2 RNAi embryos, AIR-2::GFP was compressed at the spindle midzone (Figure S3C and Table S2), possibly due to the shorter spindle midzone in the presence of chromatin bridges which impede chromosome separation (see figure 4). AIR-2::GFP is also observed at the midbody in top-2 RNAi embryos until the time that the AB daughter cell enters mitosis, when AIR-2::GFP is recruited to all chromatin and is no longer focused only at the midbody (Figure S3C). Together these data indicate that the AIR-2 protein persists at the midbody throughout abscission and is activated in the presence of chromatin bridges.
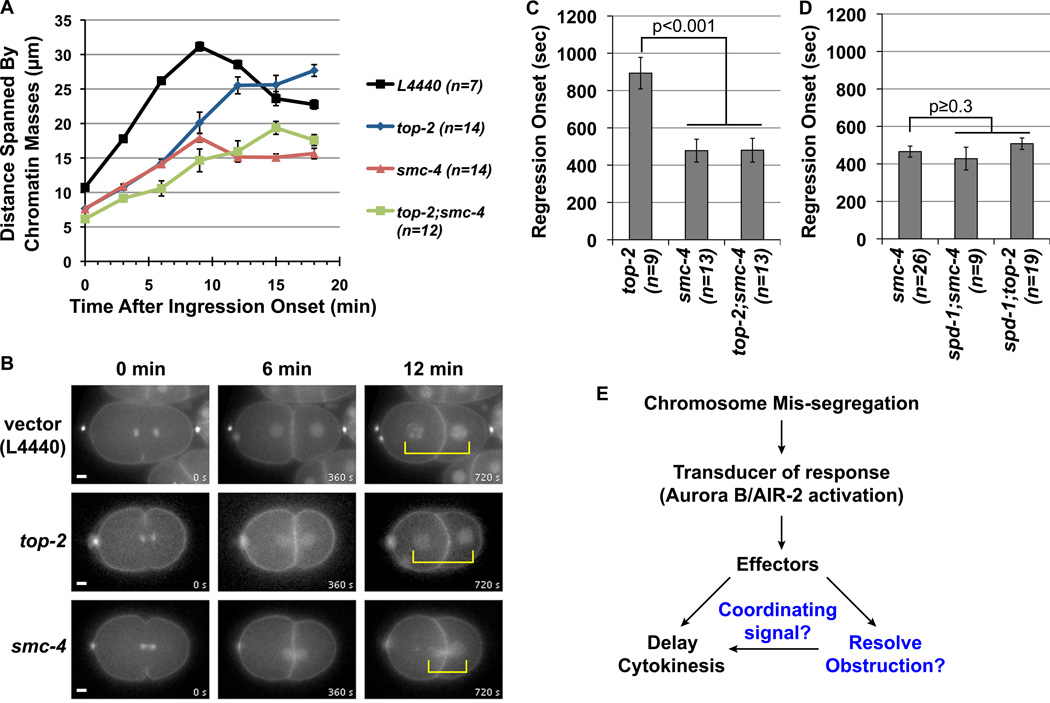
Figure 4.
Quantification of the extent of chromosome separation and the onset of the cleavage furrow regression in 1-cell P0 blastomeres. (A) Graph represents the average distance spanned by the separating chromosomes (y-axis) as a function of time after the onset of cleavage furrow ingression (x-axis). (B) Representative still images from the mCherry fluorescence time-lapse movies used for the quantification in (A). The movies are synchronized to the onset of ingression as t = 0 min. (C and D) Graphs represent the average time after the onset of ingression when the cleavage furrow began to regress. The p-values are determined by two-tailed t-Tests in comparisons to top-2(RNAi) and smc-4(RNAi) as indicated. For all graphs (A, C and D) the error bars represent SEM and n indicates the number of embryos examined for each test condition. (E) A schematic model for the response to chromosome mis-segregation mediated by AIR-2 activation to halt cytokinesis and facilitate obstruction resolution. The putative functions for condensin in aiding obstruction resolution and providing a signal to maintain the stable cleavage furrow are shown in blue.
Disruption of the spindle midzone destabilizes the cleavage furrow in the presence of chromatin obstructions
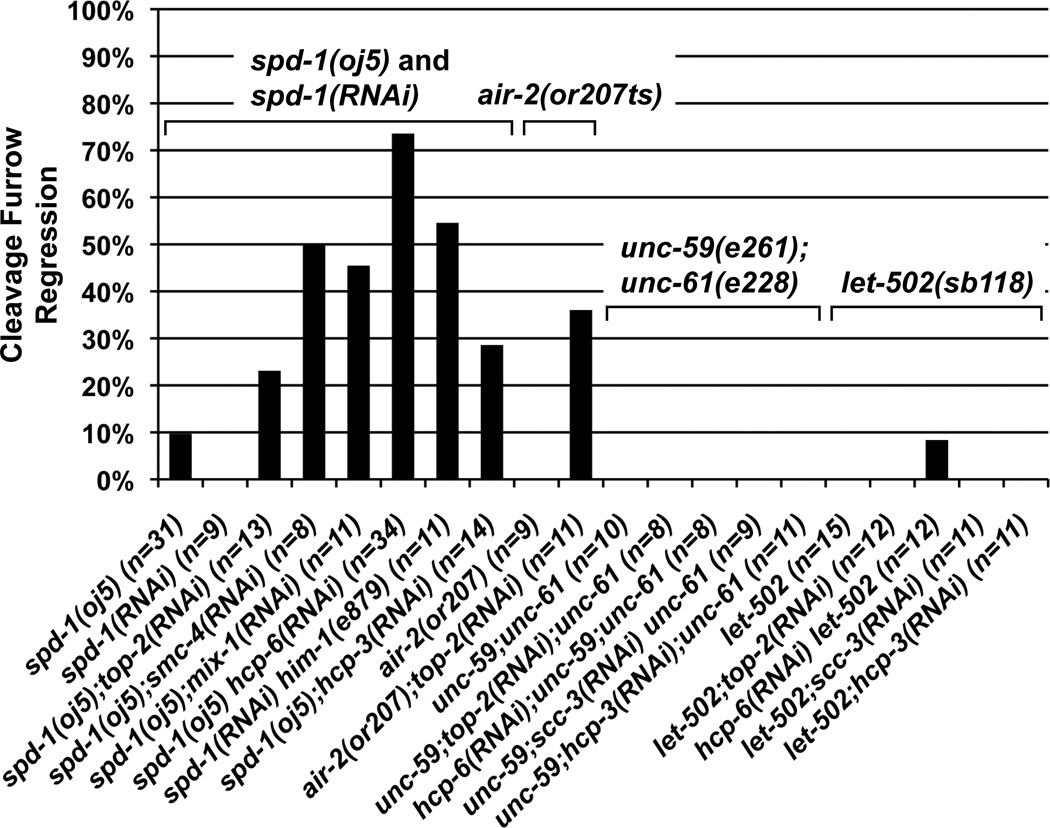
AIR-2 is required for normal chromosome segregation prior to its localization at the midzone [27–29], making it difficult to directly test its function during cytokinesis. To avoid segregation defects, we disrupted the spindle midzone by inactivating SPD-1 [30]. In C. elegans, the midzone is dispensable for cytokinesis in the 1-cell and 2-cell embryos (Figures 3 and S4G; [26, 30]). In spd-1(oj5) at 25°C and spd-1 RNAi embryos, AIR-2::GFP localization was significantly reduced at the middle of the spindle during anaphase and at the midbody (Figure S3B; [26]; and data not shown). As expected, AIR-2::GFP localization on mitotic chromosomes and AIR-2 function in chromosome segregation were not affected by loss of spd-1 (Figures S3B and S4A–F). Importantly, inactivation of spd-1 combined with chromosome segregation defects triggered significant increases in furrow regression in up to 74% of 1-cell embryos (Figure 3) and up to 92% failure in 2-cell embryos (Figure S4G). To directly test AIR-2 function during cytokinesis, we acutely shifted the temperature-sensitive air-2(or207) mutant after anaphase onset, previously shown to have fast inactivation kinetics [31]. Under this condition, we found 9/9 air-2(or207ts) embryos successfully completed cytokinesis without furrow regression (Figure 3). By contrast, chromatin bridges caused by top-2 RNAi resulted in furrow regression in 4/11 air-2(or207ts) embryos at the 1-cell stage (Figure 3). To test the possibility that any perturbation to cytokinesis will sensitize the embryo to cleavage furrow regression, we also examined chromosome segregation defects in embryos defective for the septin homologs unc-59 and unc-61 and the MRLC kinase let-502 [32–34]. These mutants are also largely dispensable for cytokinesis in early embryonic cell division, causing mild defects during furrow ingression [26, 33–35]. However, the cytokinesis cleavage furrows in the let-502 and septin defective backgrounds were stable in the presence of chromosome bridges (Figures 3 and S4G). These results, in combination with the hyperactivation of AIR-2 in the presence of chromatin obstructions, are consistent with AIR-2 acting at the spindle midzone and the midbody to prevent cleavage furrow regression. However, we cannot exclude the possibility that loss of other factors in the spd-1 and air-2 mutants also contribute to furrow regression when chromatin obstructions are present.
Figure 3.
Nomarski DIC time-lapse analysis of cell division. Graph represents the percentages of cell divisions exhibiting the cleavage furrow regression defect after chromosome mis-segregation were induced in the spd-1(RNAi) spd-1(oj5) mutant, the air-2(or207) mutant (acute inactivation), the unc-59(e261);unc-61(e228) mutant, and the let-502(sb118) mutant backgrounds in the 1-cell embryo. The number of cell divisions (n) examined per condition is indicated.
Depletion of condensin smc-4 leads to rapid onset of cytokinesis furrow regression
We next investigated the reason for the higher incidence of cytokinesis failure caused by loss of condensin smc-4 compared with top-2 in the second division. First, we examined chromosome segregation defects in late mitosis. In vector RNAi control embryo, the two sets of chromosomes continue to move apart after furrow ingression (Figure 4A and B). In both smc-4 and top-2 RNAi embryos, chromosomes remained in close proximity likely due to unresolved linkages between sister chromatids (Figure 4B). At six minutes after furrow ingression, average chromosome separation in smc-4 and top-2 RNAi embryos were significantly shorter compared to the vector RNAi control (Figure 4A). However by 12 minutes after ingression, chromosome separation in the top-2 RNAi embryos began to resemble vector RNAi, while chromosomes in the AB and P1 blastomeres in the smc-4 RNAi embryos remained closely linked together (Figure 4A and B). This indicates that the RNAi depletion of smc-4 had a more pronounced impairment of chromosome disjunction in late mitosis compared to top-2 RNAi.
To determine whether the more severe defects on chromosome disjunction due to smc-4 RNAi accounted for the increases in cytokinesis cleavage furrow regression, we examined whether the regression defect seen in the AB and P1 cell divisions always coincided with the presence of chromosome obstructions. We indeed observed a correlation between persistent obstruction and cytokinesis failure following top-2 and smc-4 RNAi, in cases where the ingressing furrow initially bisected separating chromosomes (Figure S5). These results support the model for a causal link between unresolved chromatin obstruction and cytokinesis failure in top-2 and smc-4 RNAi depleted embryos. Surprisingly, in some cases following smc-4 RNAi or top-2;smc-4 double RNAi, the cytokinetic cleavage furrow also regressed without bisecting the chromatin obstruction and appeared off-centered in the dividing cell (Figure S5B and C, yellow arrow). In these cases, the cleavage furrow regressed without any visible obstruction. This defect was not seen in the top-2 RNAi embryos (Figure S5A). This suggests that the loss of smc-4 may trigger additional defects other than obstruction resolution that contribute to furrow regression.
Interestingly, we noticed that the onset of furrow regression occurred significantly earlier in RNAi depleted embryos for smc-4 compared to top-2 (Figure 4C). Furrow regression occurred an average of 478 seconds after ingression in smc-4 RNAi embryos. By contrast in top-2 RNAi embryos, the cleavage furrow persisted significantly longer for an average of 893 seconds. The earlier regression onset phenotype for smc-4 RNAi also appeared in the top-2;smc-4 co-depleted embryos (Figure 4C). A predicted feature for the abscission checkpoint is that the delay in cytokinesis should be coordinated with obstruction resolution (Figure 4E). If condensin depletion is disrupting the maintenance of the stable cleavage furrow, then the timing of furrow regression onset in smc-4 RNAi embryos should resemble chromosome segregation defects in the spd-1(oj5) mutant background in which AIR-2 accumulation at the spindle midzone is compromised (Figure S3B). Consistent with this hypothesis, we found that top-2 and smc-4 depletions in the spd-1(oj5) background caused equally early furrow regression onset as smc-4 RNAi (Figure 4D). Moreover, smc-4 appeared to act after AIR-2 activation because the increased phosphorylation of AIR-2 at the spindle midzone was also found in the presence of chromatin obstructions in smc-4 RNAi embryos (Figure 2C and D). Collectively, these results support the idea that condensin smc-4 depletion affects cytokinesis not only by hindering chromosome disjunction, but that it could also disrupt the stability of the cleavage furrow (Figure 4E).
Condensin I localizes to the spindle midzone, midbody and chromatin obstruction in a manner dependent on AIR-2
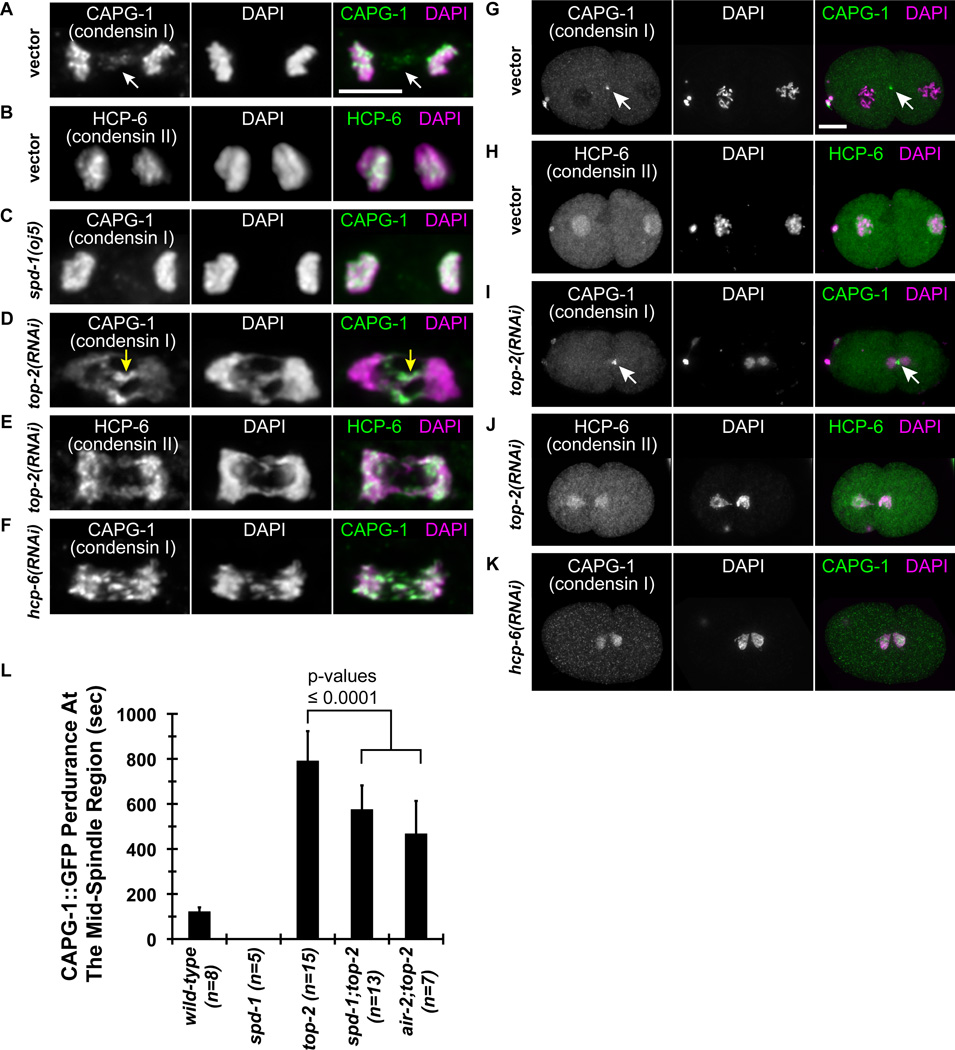
Given the observed effects of condensin depletion on cytokinesis, we next examined the localization of the condensin complexes after anaphase onset. Most metazoans have two paralogous condensin complexes, condensin I and condensin II, which share the same two SMC subunits but possess unique non-SMC subunits [8]. We previously found condensin I between separating chromosomes in anaphase [13], and this localization was confirmed in 17% (n=157) of wild-type anaphases by immunostaining to CAPG-1 (Figure 5A). At cytokinesis, the microtubules at the spindle midzone are constricted by the ingressing furrow becoming part of the midbody. Interestingly, we observed that CAPG-1 also localizes to the midbody, which to our knowledge is the first time this has been reported (Figure 5G). Unlike condensin I, HCP-6 (condensin II) staining was undetectable at the spindle midzone at anaphase (n=108; Figure 5B) or midbody (Figure 5H) in wild-type. Immunostaining to the SMC subunits of condensin, SMC-4 and MIX-1, were also observed at the midbody, suggesting that the condensin I complex is localized to the spindle midzone and midbody, and not just CAPG-1 (data not shown). The CAPG-1 immunostaining pattern was recapitulated in live embryos, in which there is a transient accumulation of CAPG-1::GFP between separating chromosomes in 56 of 58 anaphases examined (Figure S6A and G and Supplemental Movie 1), persisting for an average of 120 seconds in the wild-type 1-cell embryo (Figure 5L). CAPG-1::GFP also localizes to the midbody (Figures 5G and S6A). In summary, condensin I shows a reproducible localization to the spindle midzone and midbody in wild-type embryos, behaving similarly to Aurora B/AIR-2 and other chromosomal passenger proteins.
Figure 5.
Localization of condensin I but not condensin II specific subunits at the midzone and midbody. Immunofluorescence micrographs using antibodies to condensin I CAPG-1 and condensin II HCP-6 subunits in RNAi-treated embryos at anaphase in the P0 division (A-F, 5 µm scale bar) and at the midbody between the AB and P1 blastomeres (G-K, 10 µm scale bar). (L) The graph represents the average time duration that CAPG-1::GFP was detected at the mid-spindle region from the onset of anaphase. The number of embryos (n) examined per condition is indicated. Error bars, SD. The p-values are determined by two-tailed t-Tests.
To determine how condensin complexes might be involved in responding to chromatin obstructions during cytokinesis, we examined the localization of condensin I and condensin II after top-2 RNAi. CAPG-1 was highly enriched on chromatin bridges during anaphase (Figure 5D and Supplemental Movie 2) and persisted on chromatin obstructions in the midbody later than wild-type (Figure 5I and Figure S6C), similar to phosphorylated AIR-2. By contrast, HCP-6 immunostaining was not enriched on chromatin bridges at anaphase (Figure 5E) or in the midbody during late cytokinesis (Figure 5J), but instead had a uniform distribution on DNA. Interestingly, depletion of condensin II by hcp-6 RNAi reduced the enrichment of CAPG-1 immunostaining on chromatin bridges (Figure 5F) and the midbody (Figure 5K). CAPG-1::GFP did not properly accumulate on anaphase bridges and redistributed on all chromatin after hcp-6 RNAi (n = 12/14 one-cell embryos, Figure S6F), indicating the proper targeting of CAPG-1 after anaphase is dependent on condensin II. In contrast, AIR-2::GFP accumulated normally at the spindle midzone in anaphase and was observed on the midbody for an extended period of time in hcp-6 RNAi embryos, indicating that condensin I localization might be disrupted independent of the AIR-2 targeting mechanism (Figure S3E). CAPG-1::GFP localization near the spindle midzone and midbody regions was also stronger in top-2 RNAi embryos compared to wild-type (Figure S6A and C). CAPG-1::GFP persisted an average of ~800 seconds, which is approximately six times longer than wild-type (Figure 5L). At this stage the AB blastomere has entered prophase when CAPG-1::GFP redistributes onto all chromatin and is no longer focused at the midzone, similar to what is observed for AIR-2::GFP in top-2 RNAi embryos. Therefore, the increase and perdurance of CAPG-1 in spindle midzone and midbody regions in the presence of chromatin obstructions suggest that condensin I is actively recruited or maintained on the chromatin obstructions.
Given that AIR-2 has a role in the recruitment of condensin I to chromosomes in mitosis [13], we next tested whether the extended perdurance of CAPG-1::GFP near the spindle midzone and midbody in the top-2 RNAi embryos was dependent on AIR-2. We reduced AIR-2 activity at the spindle midzone region by depleting spd-1 in embryos that were co-depleted for top-2. Under these conditions, perdurance of AIR-2::GFP at the midbody was significantly reduced, and disappeared before cytokinesis failed (Figure S3D and Table S2). We also examined partial air-2 disruption in air-2;top-2 RNAi embryos, in which case we only analyzed embryos with the mildest air-2 phenotypes that were still capable of completing furrow ingression. In both cases, CAPG-1::GFP initially localized to chromatin obstructions at the center of the spindle, but the perdurance of CAPG-1::GFP was significantly less than top-2 RNAi control (Figure 5L and Figure S6C–E). Consistently, CAPG-1 levels were undetectable by immunofluorescence in fixed spd-1(oj5) mutant embryo anaphases (Figure 5C). Therefore, AIR-2 activity and the presence of bundled spindle midzone microtubules may collectively sustain condensin I accumulation at chromatin bridges and the midzone and midbody regions.
Condensin I and II prevent cleavage furrow regression in top-2 RNAi 2-cell embryos
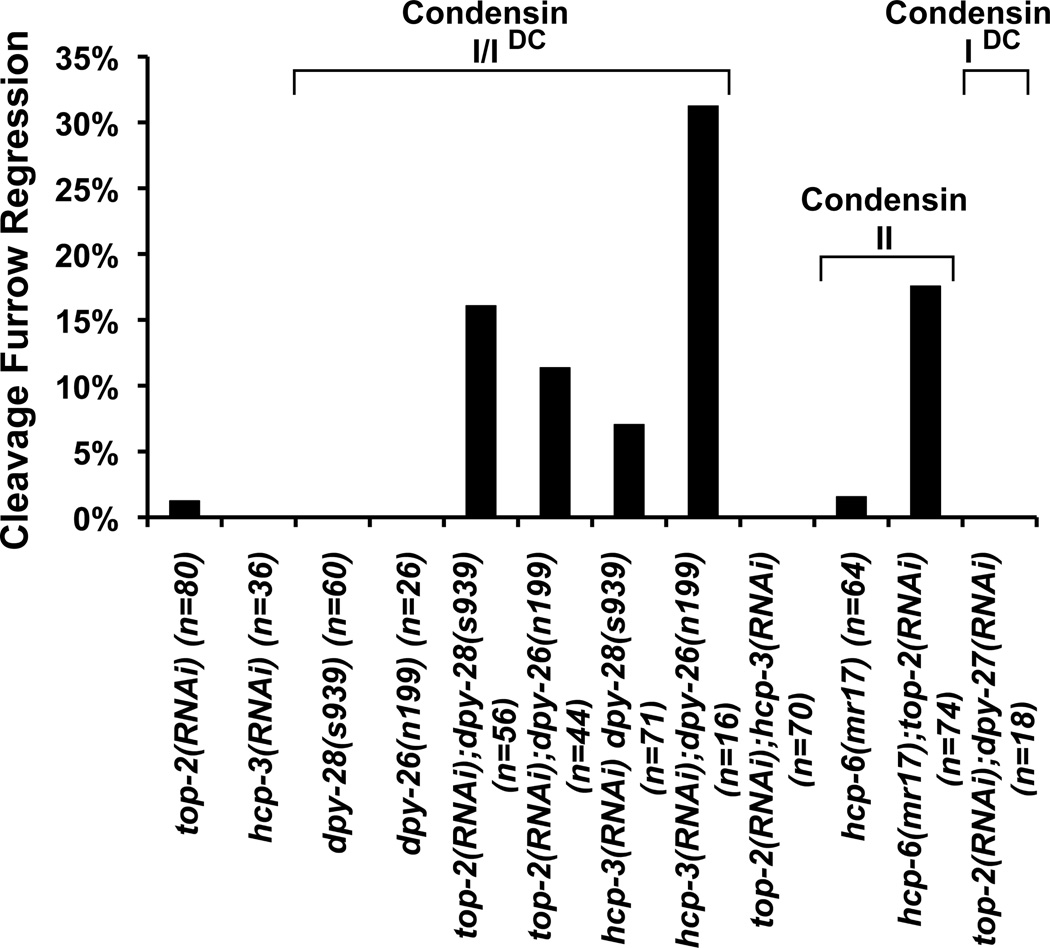
To test whether condensin I and II are necessary to prevent cleavage furrow regression, we induced chromosome segregation defects by top-2 RNAi in hypomorphic mutants of condensin I, dpy-28(s939) and dpy-26(n199), and of condensin II, hcp-6(mr17) [40–42]. We used milder RNAi conditions against top-2 that did not induce significant cytokinesis failure (1/80) in the 2-cell embryos, to measure any enhancement of the cytokinesis defect by the hypomorphic condensin mutants. The dpy-28(s939) and dpy-26(n199) mutations did not induce cytokinesis failure on their own (60 and 26 cell divisions examined, respectively), but are also known to have very mild chromosome segregation defects [36]. The hcp-6(mr17) mutant had only 1/64 cytokinesis failure. However combined with top-2 RNAi, the hypomorphic mutations in condensin I and condensin II synergistically enhanced the furrow regression defects (Figure 6). Similarly, depletion of CENP-Ahcp-3 enhanced cytokinesis failures in hypomorphic condensin mutants compared to no regression defects in the wild-type background (Figure 6). The more severe defect seen in hcp-3(RNAi);dpy-26(n199) embryos (n=16) compared to hcp-3(RNAi);dpy-28(s939) (n=71) may be due to the lower sample size in the dpy-26(n199) mutant background. By contrast, embryos co-depleted for top-2 and CENP-Ahcp-3 had no cytokinesis failure (70 cell divisions examined), indicating that combining chromosome segregation defective conditions do not necessarily enhance the cytokinesis failure rate (Figure 6). DPY-26 and DPY-28 are also present in the condensin-related complex (condensin IDC) that is crucial for X chromosome dosage compensation in C. elegans hermaphrodites. To rule out a role for this complex, we inactivated the condensin IDC-specific SMC-4 paralog, DPY-27 [37], in the top-2 RNAi background and found no significant cytokinesis defects (Figure 6). The requirement for condensin I is consistent with its wild-type localization to the spindle midzone and midbody regions and its enhanced accumulation on chromatin bridges in top-2 RNAi embryos. Since condensin II disruption reduces the enrichment of condensin I to the midzone and midbody (Figure 5F and K), the requirement for condensin II may be in part due to the disruption of condensin I. Collectively, these results indicate that both condensin I and condensin II are specifically needed to prevent furrow regression in the presence of chromatin bridges induced by top-2 RNAi.
Figure 6.
Condensin I and II are both required for robust cytokinesis in presence of chromatin obstruction. The graph represents the percentages of mutant and RNAi-treated embryos that exhibited a cytokinesis cleavage furrow regression defect in the AB and P1 blastomere divisions as monitored by Nomarski DIC time-lapse microscopy. The number of AB and P1 cell divisions (n) examined is provided for each test condition.
Discussion
Here, we examined whether the cleavage furrow is stabilized in the presence of chromatin obstructions in C. elegans embryos. We generated chromosome obstructions from deficiencies in topoisomerase IITOP-2, cohesin, condensin and CENP-AHCP-3, and found that the cleavage furrow rarely regresses. The presence of chromatin obstructions correlated with hyperactivation of Aurora BAIR-2 and the furrow was destabilized when Aurora BAIR-2 was acutely inactivated during cytokinesis, or when its localization to the midzone was disrupted in the spd-1 mutant background. Together these findings support the notion that a robust Aurora BAIR-2-mediated response to chromatin obstructions functions in C. elegans embryonic development.
We presented evidence that condensin I is enriched on chromatin obstructions specifically in the midbody in an Aurora BAIR-2-dependent manner. The RNAi knockdown of condensin SMC-4 hindered the resolution of the chromatin obstructions. The identification of condensin in this role fills a void in our understanding of how chromatin obstructions may be resolved during an abscission-checkpoint-like response. While factors that are required to resolve chromatin obstructions may vary depending on the nature of the obstruction (e.g. entangled DNA, incomplete DNA replication, merotelic attachments), we posit that condensin may fulfill a general requirement to keep the chromosomes apart once they are resolved. Collectively, our findings lend support to the model that Aurora B is the major transducer of the abscission checkpoint response capable of initiating both abscission regulation and obstruction resolution.
Condensin I is found at the spindle midzone and midbody in wild-type C. elegans embryos [13]. The precise role if any for condensin at the spindle midzone and midbody in cytokinesis remains to be defined. The fission yeast condensin complex was also found at the spindle midzone by Nakazawa et al. who speculated that condensin contributes to signaling for cytokinesis [12]. We also observed furrows that did not bisect the chromosomal mass before regressing when condensin was depleted, suggesting that the proper signaling necessary to position the furrow may be disrupted. In parallel to our findings, the knockout of condensin I in the chicken DT40 cell line also resulted in cytokinesis failures [38]. The observations that cytokinesis can fail in C. elegans smc-4 RNAi embryos and in condensin-I-knockout DT40 cells are congruent with the idea that condensin promotes stable cleavage furrows when chromosome segregation goes awry.
A mechanism that responds to chromatin obstructions at the cleavage plane could be particularly beneficial for C. elegans embryogenesis, in which cell divisions occur without cellular growth and the inter-spindle distances decrease with successive rounds of cell division. Ladouceur et al. have previously pointed out that chromosome compaction is likely adjusted to accommodate shorter inter-spindle distances in later rounds of C. elegans embryonic cell divisions [39], a phenomenon that has been confirmed in neurula stage Xenopus laevis embryos [40]. How this is accomplished in the embryo is unknown. Interestingly, budding yeast appears to regulate chromosome compaction to ensure the clearance of chromosomes from the cleavage plane [41]. The budding yeast Aurora homolog Ipl1 can direct hyper-condensation of artificially lengthened fusion chromosomes after anaphase onset, and the viability of budding yeast containing a fusion chromosome is reduced in hypomorphic condensin smc2 mutant [41]. Our findings in this study that C. elegans Aurora BAIR-2 is hyperactivated and is important for responding to chromatin obstruction at the spindle midzone in the first two rounds of embryonic division provide fodder for future study to address whether this mechanism acts constitutively in later rounds of embryonic cell divisions in C. elegans. The identification of C. elegans condensin I as a new player in an abscission-checkpoint-like response in embryos highlights the potential need for the abscission checkpoint in the context of development, and the need to explore condensin function at the interconnection between obstruction resolution and regulation of cytokinesis.
Experimental Procedures
C. elegans growth, maintenance, and RNAi mediated knockdown
Strains used in this study are listed in Supplemental Materials. The air-2(or207) strain was maintained at <15°C by keeping plates on bubble wrap in an ice bucket (as described in [26]) and then shifted to room temperature for no more than 5 minutes during the mounting procedure before time-lapse imaging on embryos in the middle of furrow ingression was started at 25°C. All other temperature-sensitive strains were maintained at 15°C until L4 stage and then shifted to 25°C for 16 hours prior to imaging analysis. The remaining strains were maintained at 20°C as described [42]. For feeding RNAi, L4 stage hermaphrodites were plated on NGM plates supplemented with 1 mM IPTG and 25 µg/mL carbenicillin, and seeded with overnight cultures of HT115(DE3) E. coli containing the appropriate RNAi vector. This RNAi method was used for the Nomarski DIC time-lapse microscopy and condensin I and II immunostaining. We used log-phase E. coli seeded 1 day before the plates were used as described [43] for the mCherry fluorescence time-lapse microscopy and AIR-2 immunostaining experiments. Injection RNAi was performed with 1 mg/mL of double-stranded RNA as described [43]. Embryos were dissected from hermaphrodites after 1 to 2 days on feeding RNAi plates, or 1 day after injection. For partial air-2(RNAi) treatment, L4 hermaphrodites were first fed top-2(RNAi) for 24–32 hours and then transferred onto plates with both air-2 and top-2 RNAi feeding bacteria overnight for 13–18 hours. In all cases, we only analyzed images in which chromosome segregation defects are confirmed by the appearance of the cross-eyed phenotype in DIC imaging, or the formation of chromatin bridges in DAPI-stained DNA images and histone::mCherry timelapse movies.
Time-lapse microscopy and immunostaining
Nomarski DIC and mCherry fluorescence time-lapse microscopy and all fixed immuno- and DAPI-DNA fluorescence images were captured on an Olympus BX61 microscopy with a 60x PlanApochromat (NA 1.35) oil objective and a Hamamatsu ORCA ER camera. Live imaging by Nomarski DIC was performed as described [44] with approximately 20 1-µm z-series collected at 15-second intervals. To reduce phototoxicity for the mCherry imaging (Figure 4), the excitation light from an X-Cite UV light source (Leica) was filtered through an U25ND25 (Olympus) neutral density filter, and images were collected at 30-sec intervals with approximately 40 800-nm z-steps at each time point with the camera set at 4X binning and 49% of maximum digital gain. Live imaging of GFP::CAPG-1 embryos was captured by spinning disk laser confocal microscopy described in Supplemental Materials and [13]. Fixed images were captured at 200-nm and 250-nm z-steps and deconvolved using Huygens Essential version 4.2 (Scientific Volume Imaging). Images were processed using ImageJ and Photoshop CS2 software packages.
Peptide antibodies to CAPG-1 [36] and HCP-6 [45] and immunostaining were performed as described (protocol 21 in [46]). Immunostaining to phosphorylated Aurora (Cell Signaling, Danvers, MA) and AIR-2 was performed as described [25, 29] and phosphorylated Aurora immunofluorescence was quantified on z-series without deconvolution using the Measure Stack function in ImageJ 1.46. We manually selected an area of 8 µm x 8 µm around the spindle midzone and the anterior spindle pole. The fluorescence intensity from a blank region of the embryo of equal volume located away from the mitotic spindles and the midzone was subtracted from the midzone and the anterior pole measurements, and the ratio of spindle midzone:anterior pole fluorescence intensity was calculated as the normalized intensity value. For metaphase images, we selected an area of 6 µm x 6 µm around the metaphase chromosomes and the anterior spindle pole.
Supplementary Material
Highlights.
C. elegans embryo can prevent cleavage furrow regression in the presence of chromatin obstruction
Chromatin obstructions trigger activation of AIR-2 at the spindle midzone in embryos
Condensin I localizes to the spindle midzone and midbody in an AIR-2 dependent manner
Condensin loss compromises chromatin obstruction resolution and cleavage furrow stability
Acknowledgements
We thank Jill Schumacher for providing AIR-2 antibodies, Aaron Severson and Masanori Mishima for comments on the manuscript, and Stephanie Campbell, Netta Golenberg and Martha Snyder for assisting in strain construction. G.C. is supported by NIH grant GM079533, and R.C.C. is supported by NIH grant GM093173.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steigemann P, Wurzenberger C, Schmitz MH, Held M, Guizetti J, Maar S, Gerlich DW. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell. 2009;136:473–484. doi: 10.1016/j.cell.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Carlton JG, Caballe A, Agromayor M, Kloc M, Martin-Serrano J. ESCRT-III governs the Aurora B-mediated abscission checkpoint through CHMP4C. Science. 2012;336:220–225. doi: 10.1126/science.1217180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norden C, Mendoza M, Dobbelaere J, Kotwaliwale CV, Biggins S, Barral Y. The NoCut pathway links completion of cytokinesis to spindle midzone function to prevent chromosome breakage. Cell. 2006;125:85–98. doi: 10.1016/j.cell.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 4.Carmena M, Ruchaud S, Earnshaw WC. Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr. Opin. Cell Biol. 2009;21:796–805. doi: 10.1016/j.ceb.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat. Rev. Mol. Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 6.Mendoza M, Norden C, Durrer K, Rauter H, Uhlmann F, Barral Y. A mechanism for chromosome segregation sensing by the NoCut checkpoint. Nat. Cell Biol. 2009;11:477–483. doi: 10.1038/ncb1855. [DOI] [PubMed] [Google Scholar]
- 7.Capalbo L, Montembault E, Takeda T, Bassi ZI, Glover DM, D'Avino PP. The chromosomal passenger complex controls the function of endosomal sorting complex required for transport-III Snf7 proteins during cytokinesis. Open Biol. 2012;2:120070. doi: 10.1098/rsob.120070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirano T. Condensins: universal organizers of chromosomes with diverse functions. Genes Dev. 2012;26:1659–1678. doi: 10.1101/gad.194746.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Ambrosio C, Kelly G, Shirahige K, Uhlmann F. Condensin-dependent rDNA decatenation introduces a temporal pattern to chromosome segregation. Curr. Biol. 2008;18:1084–1089. doi: 10.1016/j.cub.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 10.D'Amours D, Stegmeier F, Amon A. Cdc14 and condensin control the dissolution of cohesin-independent chromosome linkages at repeated DNA. Cell. 2004;117:455–469. doi: 10.1016/s0092-8674(04)00413-1. [DOI] [PubMed] [Google Scholar]
- 11.Lavoie BD, Hogan E, Koshland D. In vivo requirements for rDNA chromosome condensation reveal two cell-cycle-regulated pathways for mitotic chromosome folding. Genes Dev. 2004;18:76–87. doi: 10.1101/gad.1150404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakazawa N, Mehrotra R, Ebe M, Yanagida M. Condensin phosphorylated by the Aurora-B-like kinase Ark1 is continuously required until telophase in a mode distinct from Top2. J. Cell Sci. 2011;124:1795–1807. doi: 10.1242/jcs.078733. [DOI] [PubMed] [Google Scholar]
- 13.Collette KS, Petty EL, Golenberg N, Bembenek JN, Csankovszki G. Different roles for Aurora B in condensin targeting during mitosis and meiosis. J. Cell Sci. 2011;124:3684–3694. doi: 10.1242/jcs.088336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerlich D, Hirota T, Koch B, Peters JM, Ellenberg J. Condensin I stabilizes chromosomes mechanically through a dynamic interaction in live cells. Curr. Biol. 2006;16:333–344. doi: 10.1016/j.cub.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 15.Mora-Bermudez F, Gerlich D, Ellenberg J. Maximal chromosome compaction occurs by axial shortening in anaphase and depends on Aurora kinase. Nat. Cell Biol. 2007;9:822–831. doi: 10.1038/ncb1606. [DOI] [PubMed] [Google Scholar]
- 16.Brauchle M, Baumer K, Gonczy P. Differential activation of the DNA replication checkpoint contributes to asynchrony of cell division in C. elegans embryos. Curr. Biol. 2003;13:819–827. doi: 10.1016/s0960-9822(03)00295-1. [DOI] [PubMed] [Google Scholar]
- 17.Conn CW, Lewellyn AL, Maller JL. The DNA damage checkpoint in embryonic cell cycles is dependent on the DNA-to-cytoplasmic ratio. Dev. Cell. 2004;7:275–281. doi: 10.1016/j.devcel.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Dasso M, Newport JW. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus . Cell. 1990;61:811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- 19.Encalada SE, Willis J, Lyczak R, Bowerman B. A spindle checkpoint functions during mitosis in the early Caenorhabditis elegans embryo. Mol. Biol. Cell. 2005;16:1056–1070. doi: 10.1091/mbc.E04-08-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sibon OC, Stevenson VA, Theurkauf WE. DNA-replication checkpoint control at the Drosophila midblastula transition. Nature. 1997;388:93–97. doi: 10.1038/40439. [DOI] [PubMed] [Google Scholar]
- 21.Wei Y, Multi S, Yang CR, Ma J, Zhang QH, Wang ZB, Li M, Wei L, Ge ZJ, Zhang CH, et al. Spindle assembly checkpoint regulates mitotic cell cycle progression during preimplantation embryo development. PLoS One. 2011;6:e21557. doi: 10.1371/journal.pone.0021557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Farrell PH, Stumpff J, Su TT. Embryonic cleavage cycles: how is a mouse like a fly. Curr. Biol. 2004;14:R35–R45. doi: 10.1016/j.cub.2003.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonnichsen B, Koski LB, Walsh A, Marschall P, Neumann B, Brehm M, Alleaume AM, Artelt J, Bettencourt P, Cassin E, et al. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans . Nature. 2005;434:462–469. doi: 10.1038/nature03353. [DOI] [PubMed] [Google Scholar]
- 24.Bembenek JN, White JG, Zheng Y. A role for separase in the regulation of RAB-11-positive vesicles at the cleavage furrow and midbody. Curr. Biol. 2010;20:259–264. doi: 10.1016/j.cub.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heallen TR, Adams HP, Furuta T, Verbrugghe KJ, Schumacher JM. An Afg2/Spaf-related Cdc48-like AAA ATPase regulates the stability and activity of the C. elegans Aurora B kinase AIR-2. Dev. Cell. 2008;15:603–616. doi: 10.1016/j.devcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewellyn L, Carvalho A, Desai A, Maddox AS, Oegema K. The chromosomal passenger complex and centralspindlin independently contribute to contractile ring assembly. J. Cell Biol. 2011;193:155–169. doi: 10.1083/jcb.201008138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagstrom KA, Holmes VF, Cozzarelli NR, Meyer BJ. C. elegans condensin promotes mitotic chromosome architecture, centromere organization, and sister chromatid segregation during mitosis and meiosis. Genes Dev. 2002;16:729–742. doi: 10.1101/gad.968302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaitna S, Pasierbek P, Jantsch M, Loidl J, Glotzer M. The aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous Chromosomes during meiosis. Curr. Biol. 2002;12:798–812. doi: 10.1016/s0960-9822(02)00820-5. [DOI] [PubMed] [Google Scholar]
- 29.Schumacher JM, Golden A, Donovan PJ. AIR-2: An Aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos. J. Cell Biol. 1998;143:1635–1646. doi: 10.1083/jcb.143.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verbrugghe KJ, White JG. SPD-1 is required for the formation of the spindle midzone but is not essential for the completion of cytokinesis in C. elegans embryos. Curr. Biol. 2004;14:1755–1760. doi: 10.1016/j.cub.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 31.Severson AF, Hamill DR, Carter JC, Schumacher J, Bowerman B. The aurora-related kinase AIR-2 recruits ZEN-4/CeMKLP1 to the mitotic spindle at metaphase and is required for cytokinesis. Curr. Biol. 2000;10:1162–1171. doi: 10.1016/s0960-9822(00)00715-6. [DOI] [PubMed] [Google Scholar]
- 32.Diogon M, Wissler F, Quintin S, Nagamatsu Y, Sookhareea S, Landmann F, Hutter H, Vitale N, Labouesse M. The RhoGAP RGA-2 and LET-502/ROCK achieve a balance of actomyosin-dependent forces in C. elegans epidermis to control morphogenesis. Development. 2007;134:2469–2479. doi: 10.1242/dev.005074. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen TQ, Sawa H, Okano H, White JG. The C. elegans septin genes, unc-59 and unc-61, are required for normal postembryonic cytokineses and morphogenesis but have no essential function in embryogenesis. J. Cell. Sci. 2000;113:3825–3837. doi: 10.1242/jcs.113.21.3825. [DOI] [PubMed] [Google Scholar]
- 34.Piekny AJ, Mains PE. Rho-binding kinase (LET-502) and myosin phosphatase (MEL-11) regulate cytokinesis in the early Caenorhabditis elegans embryo. J. Cell Sci. 2002;115:2271–2282. doi: 10.1242/jcs.115.11.2271. [DOI] [PubMed] [Google Scholar]
- 35.Maddox AS, Lewellyn L, Desai A, Oegema K. Anillin and the septins promote asymmetric ingression of the cytokinetic furrow. Dev. Cell. 2007;12:827–835. doi: 10.1016/j.devcel.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Csankovszki G, Collette K, Spahl K, Carey J, Snyder M, Petty E, Patel U, Tabuchi T, Liu H, McLeod I, et al. Three distinct condensin complexes control C. elegans chromosome dynamics. Curr. Biol. 2009;19:9–19. doi: 10.1016/j.cub.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chuang PT, Albertson DG, Meyer BJ. DPY-27:a chromosome condensation protein homolog that regulates C. elegans dosage compensation through association with the X chromosome. Cell. 1994;79:459–474. doi: 10.1016/0092-8674(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 38.Green LC, Kalitsis P, Chang TM, Cipetic M, Kim JH, Marshall O, Turnbull L, Whitchurch CB, Vagnarelli P, Samejima K, et al. Contrasting roles of condensin I and condensin II in mitotic chromosome formation. J. Cell Sci. 2012;125:1591–1604. doi: 10.1242/jcs.097790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ladouceur AM, Ranjan R, Maddox PS. Cell size: chromosomes get slapped by a midzone ruler. Curr. Biol. 2011;21:R388–T390. doi: 10.1016/j.cub.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Kieserman EK, Heald R. Mitotic chromosome size scaling in Xenopus . Cell Cycle. 2011;10:3863–3870. doi: 10.4161/cc.10.22.17975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neurohr G, Naegeli A, Titos I, Theler D, Greber B, Diez J, Gabaldon T, Mendoza M, Barral Y. A midzone-based ruler adjusts chromosome compaction to anaphase spindle length. Science. 2011;332:465–468. doi: 10.1126/science.1201578. [DOI] [PubMed] [Google Scholar]
- 42.Brenner S. The genetics of Caenorhabditis elegans . Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahringer J. WormBook. The C. elegans Research Community, WormBook; 2006. Reverse genetics. http://www.wormbook.org. [Google Scholar]
- 44.Verbrugghe KJ, Chan RC. Imaging C. elegans Embryos using an Epifluorescent Microscope and Open Source Software. J. Vis. Exp. 2011;49:e2625. doi: 10.3791/2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan RC, Severson AF, Meyer BJ. Condensin restructures chromosomes in preparation for meiotic divisions. J. Cell Biol. 2004;167:613–625. doi: 10.1083/jcb.200408061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaham S, editor. WormBook. The C. elegans Research Community, WormBook; Methods in cell biology. http://www.wormbook.org. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.