Abstract
Vaccination against smallpox is again considered in order to face a possible bioterrorist threat, but the nature and the level of the immune response needed to protect a person from smallpox after vaccination are not totally understood. Therefore, simple, rapid, and accurate assays to evaluate the immune response to vaccinia virus need to be developed. Neutralization assays are usually considered good predictors of vaccine efficacy and more informative with regard to protection than binding assays. Currently, the presence of neutralizing antibodies to vaccinia virus is measured using a plaque reduction neutralization test, but this method is time-consuming and labor-intensive and has a subjective readout. Here, we describe an innovative neutralization assay based on a modified vaccinia virus Ankara (MVA) vector expressing the green fluorescent protein (MVA-gfp). This MVA-gfp neutralization assay is rapid and sensitive and has a high-throughput potential. Thus, it is suitable to monitor the immune response and eventually the efficacy of a large campaign of vaccination against smallpox and to study the vector-specific immune response in clinical trials that use genetically engineered vaccinia viruses. Most importantly, application of the highly attenuated MVA eliminates the safety concern in using the replication-competent vaccinia virus in the standard clinical laboratory.
Virus neutralization assays are useful tools to measure a reduction in titers of infectious virus mediated by antibodies. They serve as diagnostic tools and for basic research to monitor the humoral immune response to a virus. Conventional methods to measure anti-vaccinia virus neutralizing antibodies are usually performed using a plaque reduction neutralization test (PRNT) (7). Since a vaccinia virus-formed plaque represents a defined infectious unit of virus, the reduction in the number of plaques is directly related to the presence of a neutralizing activity.
The PRNT has been used to study the immune response to vaccinia virus, including modified vaccinia virus Ankara (MVA) as a vector (6, 14, 19), for studies on the structure of vaccinia virus particles (9) and to optimize the production of anti-vaccinia virus immune globulin (VIG) (17). However, the PRNT requires several days of assay time, is based on a visual readout, and uses the replication-competent vaccinia virus.
Here, we present a new neutralization assay that uses the highly attenuated replication-defective MVA carrying a green fluorescent protein reporter gene (MVA-gfp). This method uses the measurement of the green fluorescence of the Aequorea victoria gfp gene product in MVA-gfp-infected cells as a readout system. Samples, in this case blood specimens from a rabbit, mouse, or human, are incubated with standardized amounts of MVA-gfp, which then are added to target cells. After an overnight incubation, the expression of Gfp can be analyzed at a single-cell level by flow cytometry.
In this report, we established and tested our new strategy to measure anti-vaccinia virus neutralizing activity. The MVA-gfp neutralization assay was able to detect vaccinia virus-specific neutralizing antibodies in mice, rabbits, and humans. The sensitivity of our method was compared to those of two standard PRNTs using a VIG preparation. Finally, we demonstrated that the neutralization activity measured by our test was antibody mediated.
MATERIALS AND METHODS
Antibodies, mouse immunization, and human specimens.
Human anti-VIG preparation was obtained from the Statens Bakteriologiska Laboratorium (Stockholm, Sweden); the concentration of human immune globulins to vaccinia virus was equal to 160 mg/ml, and the preparation was stored as a liquid. The VIG preparation derives from healthy vaccinia virus-vaccinated Swedes from whom blood was taken regularly for this purpose during the period from 1960 to 1975. The rabbit anti-vaccinia virus antibody immunoglobulin G (IgG) fraction was from Firma Quartett (Berlin, Germany). The control rabbit anti-human immunodeficiency virus (anti-HIV) type 1 Nef serum was described previously (8). HLA-A*0201/Kb transgenic mice were vaccinated intraperitoneally twice with 108 PFU of MVA-hTyr (2), and 2 months after the second immunization blood was collected by cardiac aspiration. Plasma and sera from two MVA-nef-vaccinated subjects were collected during a phase I clinical trial in a cohort of chronically HIV-infected individuals (the study was approved by the German Bundesärztekammer) (1).
Cells and MVA viruses.
Human B-lymphoblastoid cell lines (B-LCL) were generated from peripheral blood mononuclear cells purified by standard Ficoll (Biochrom, Berlin, Germany) density centrifugation according to a previously described method (4). MVA-gfp was constructed as previously described (16). MVA-gfp preparations were obtained through amplification on chicken embryo fibroblast (CEF) cultures and purified using a standard methodology (13). Virus titers were determined by plaque assay (16) on confluent CEF monolayers grown in six-well tissue culture plates.
MVA-gfp neutralization assay.
Neutralizing activities were determined using B-LCL as target and the MVA-gfp vector (16). All blood samples were heat treated for 30 min at 56°C to inactivate complement. The assay was performed in a 96-well round-bottom tissue culture plate by adding 0.5 × 106 PFU of MVA-gfp to 40 μl of serial dilutions of plasma or serum in RPMI 1640 (Biochrom)-10% fetal calf serum. The incubation was carried out for 1 h at 37°C. Then, 0.5 × 106 B-LCL were added in 50 μl, and the incubation was carried out for two more hours under the same conditions. Cells were washed in media, transferred to a 96-well flat-bottom tissue culture plate, and kept overnight at 37°C. After being washed with phosphate-buffered saline (PBS), cells were fixed in 1% paraformaldehyde. The percentage of MVA-infected B-LCL was evaluated by measuring Gfp expression in a FACScalibur instrument (Becton Dickinson, Heidelberg, Germany). Intact cells were first discriminated from cellular debris on a side scatter-forward scatter dot plot (Fig. 1a), and the percentage of neutralization was defined as reduction in the number of Gfp-expressing cells (Fig. 1b) compared to the number of Gfp-expressing cells in untreated control wells (Fig. 1c). The percentage of neutralization was calculated as follows: (1 − [percentage of gfp-expressing cells/[percentage of Gfp-expressing cells in untreated controls]) × 100. Several dilutions were tested for each sample, and dose-response trend lines were calculated by fitting a variable slope sigmoidal equation (Hill equation) to calculate the inhibitory concentration that neutralizes 50% (IC50) of MVA-gfp infection using GraphPad (San Diego, Calif.) Prism software (version 4.00).
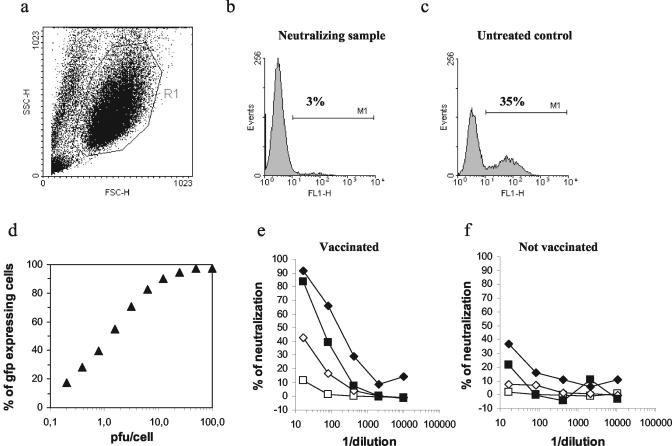
FIG. 1.
Set-up of the MVA-gfp neutralization assay. (a) Representative side scatter-forward scatter plot with a gate (R1) used to discriminate intact cells from cellular debris. (b and c) Representative histogram plots showing, respectively, a neutralizing sample and an untreated control sample. The percentages of Gfp-expressing cells are shown on each histogram plot. (d) B-LCL were infected with different numbers of PFU of MVA-gfp/cell, and the Gfp expression was measured after 14 h. (e and f) Different amounts of MVA-gfp were used in the neutralization assay (⧫ 0.5 PFU/cell; ▪, 1 PFU/cell; ◊ 2 PFU/cell; □, 6 PFU/cell), and the percentages of neutralization are shown for six serial dilutions. The same experiment was carried out with human plasma collected before (f) and 2 weeks after (e) the vaccination with MVA-nef.
Vaccinia virus Elstree plaque reduction assay.
The plaque reduction assay was performed by a slightly modified version of a standard method described previously (10). Briefly, a standard amount (70 PFU) of vaccinia virus (Elstree strain) was mixed with an equal volume of serial fourfold dilutions of VIG. Following 90 min of incubation at 37°C, 0.1 ml of the VIG-virus mixture was transferred, in triplicate, to a 48-well plate with confluent GMK cells. After 40 to 44 h of incubation at 37°C, cells were fixed and stained with 0.3% crystal violet-formalin solution and the number of plaques was counted.
MVA plaque reduction assay.
Virus-specific neutralizing antibodies were analyzed by a plaque reduction assay using recombinant MVA expressing the Escherichia coli lacZ reporter gene (MVA-LZ) (18). Twofold serial dilutions of sera were mixed with 200 PFU of MVA-LZ in a total volume of 200 μl of PBS and incubated for 2 h at 37°C. Afterwards, confluent CEF monolayers (grown on 24-well plates) were infected in duplicate with each dilution, and foci of virus- infected cells were visualized 48 h after inoculation by staining with 5-bromo-4-chloro-3-indolyl β-galactopyranoside substrate (X-Gal) (Roche Molecular Biochemicals, Mannheim, Germany) as described previously (3).
Enzyme-linked immunosorbent assay (ELISA) to detect MVA binding antibodies.
Maxisorp plates (Nunc, Wiesbaden, Germany) were coated with sucrose gradient-purified MVA (at a protein concentration of 1 μg/ml) for 3 h at 37°C and overnight at 4°C. The plates were blocked with PBS containing 0.05% Tween 20 and 10% fetal calf serum for 60 min at 37°C. Serum samples were incubated for 60 min at 37°C, washed five times with PBS, and then incubated for 30 min with anti-mouse or anti-human alkaline phosphatase (diluted 1:1,000 in PBS). Following five washes, the plates were incubated with pNPP substrate (Sigma, Taufkirchen, Germany) at 37°C, and the optical density was measured after 20 min at a wavelength of 405 nm.
Antibody depletion.
Human serum (50 μl) was incubated with 250 μl of agarose beads coupled with affinity-isolated goat anti-human IgG, IgA, or IgM (Sigma) for 30 min at room temperature. After centrifugation the recovered plasma was inactivated for 30 min at 56°C and used in the MVA-gfp neutralization assay.
RESULTS
Dose response between MVA-gfp input and number of Gfp-expressing cells.
B-LCL were infected with different doses of MVA-gfp to determine the optimal concentration to be used in the neutralization assay. A typical sigmoid dose-response curve between MVA-gfp viral input and percentage of Gfp-expressing cells was observed in a logarithmic scale in a range between 100 and 0.2 PFU/cell (Fig. 1d). Next, we compared the neutralizing activities of two human plasma samples using different MVA-gfp inputs to evaluate the optimal multiplicity of infection (MOI) of virus to be used in our assay (Fig. 1e and f). The two plasma samples originated from the same subject and were collected before and after the administration of an MVA-nef vaccine. When the neutralization test was performed using a high MOI (6 PFU/cell), no significant neutralizing activity could be detected in the plasma collected after vaccination, whereas decreasing the MOI resulted in the detection of a significant neutralizing activity, with optimal detection when an MOI of 1 and 0.5 PFU/cell was used (Fig. 1e). No significant neutralizing activity at all PFU/cell ratios tested was detected using the plasma collected before MVA vaccination (Fig. 1f). According to these data, we decided to use an MVA-gfp input of 1 PFU/cell as a good compromise between the neutralization activity observed and the percentage of MVA-gfp-infected cells in untreated control wells.
The MVA-gfp neutralization assay is able to detect neutralizing antibodies in rabbit and mouse serum.
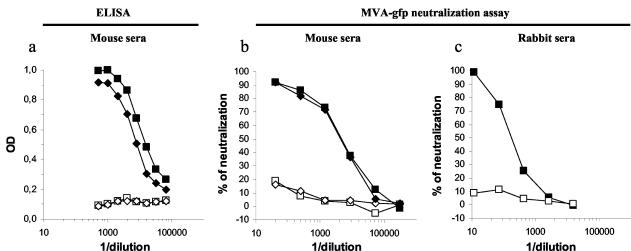
Two HLA-A*0201/Kb transgenic mice were vaccinated twice with a recombinant MVA-hTyr vector, and 2 months after the second vaccine administration sera were collected. As expected, using a standard ELISA we detected high levels of MVA-specific binding antibodies in the sera from the two vaccinated mice, while no antibodies were detected in two control sera (Fig. 2a). The same sera were analyzed using the MVA-gfp neutralization assay (Fig. 2b), and neutralizing activity was detected in sera from the two vaccinated mice (IC50s of 3,505 and 2,673) while the two control sera showed no significant neutralizing activity.
FIG. 2.
Neutralizing antibodies in mouse and rabbit specimens. (a) Sera from two MVA-vaccinated mice (⧫, ▪) and two naive mice (◊, □) were tested in ELISA for the presence of MVA-binding antibodies. (b) The same mouse sera were tested using the MVA-gfp neutralization assay. (c) An anti-vaccinia virus rabbit serum used for plaque-based titration assay was tested using the MVA-gfp neutralization assay (▪), together with an unrelated rabbit control serum (□). All three panels show the results of one representative experiment out of two.
We next tested the neutralizing activity of a commercial anti-vaccinia virus rabbit serum routinely used in our laboratory to visualize vaccinia virus plaques. As a control we used a rabbit serum containing antibodies to the HIV regulatory protein Nef. No significant neutralizing activity was observed with the control serum, while the commercial anti-vaccinia virus serum was able to neutralize MVA-gfp with an IC50 of 189 (Fig. 2c).
Taken together, these experiments showed that anti-vaccinia virus neutralizing antibodies could be easily detected with our method.
The MVA-gfp neutralization assay is able to detect human vaccinia virus-specific immunoglobulins.
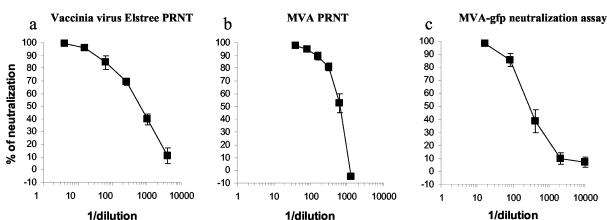
A major objective was to evaluate this test with human specimens and to compare our method with standard PRNTs. Thus, we tested the neutralizing activity in a preparation of VIGs using a conventional vaccinia virus strain, Elstree PRNT, an MVA PRNT, and the MVA-gfp neutralization assay. VIG is produced from blood of donors vaccinated with vaccinia virus and is the main efficient treatment for cases of progressive vaccinia following smallpox vaccination (5, 17). When the VIG preparation was analyzed by the vaccinia virus Elstree PRNT, we obtained an IC50 equal to 607 (Fig. 3a). Almost identical data were obtained in the PRNT performed with MVA (IC50 = 642) (Fig. 3b). Vaccinia virus neutralizing antibodies in the VIG preparation were also efficiently detected using the MVA-gfp neutralization assay, and we measured an IC50 of 284 (Fig. 3c). These results demonstrated that the MVA-gfp neutralization assay is able to detect human vaccinia virus neutralizing antibodies with a sensitivity comparable to that of conventional vaccinia Elstree and MVA PRNTs. Thus, like the conventional PRNTs, our assay should be able to detect antibody responses from vaccinated individuals, provided they are of sufficient magnitude.
FIG. 3.
A preparation of VIGs was tested in three parallel assays. (a) Neutralization activity of the VIG measured using the standard vaccinia virus Elstree PRNT. The average from six independent experiments is shown. (b) Neutralization activity of the VIG measured using an MVA PRNT. The average from two experiments is shown. (c) Neutralization activity of the VIG measured using the MVA-gfp neutralization assay. The average from three independent experiments is shown.
The neutralizing activity detected using the MVA-gfp neutralization assay is antibody mediated.
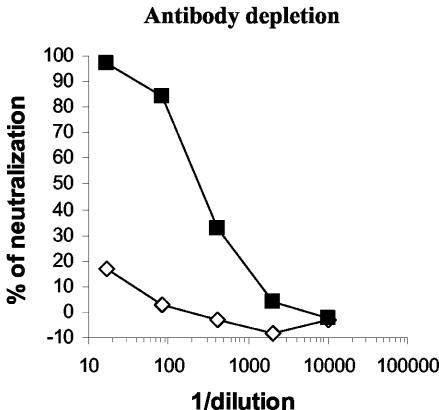
To demonstrate that the neutralizing activity observed in our assay was antibody mediated, we performed the MVA-gfp neutralization assay on immunoglobulin-depleted sera. A serum sample from a subject vaccinated three times with MVA-nef was depleted of specific IgG using anti-isotype antibodies coupled to agarose beads. As shown in Fig. 4, the neutralization activity of the serum was completely inhibited after depletion of IgG. Similar results were obtained using serum from another MVA-vaccinated subject (data not shown). We concluded that the observed neutralizing activity was exclusively antibody mediated. This experiment together with the results shown in Fig. 1e also demonstrated that the MVA-gfp neutralization assay is able to detect humoral immune responses elicited by the highly attenuated MVA in humans.
FIG. 4.
The neutralizing activity detected with the MVA-gfp assay is antibody mediated. A serum, from an individual vaccinated three times with MVA-nef was IgG depleted (◊) or mock depleted (▪) and then tested in the MVA-gfp neutralization assay. The results of one representative experiment out of three are shown.
DISCUSSION
Now that immunization with vaccinia virus is again being considered, the accurate measurement of antibody-mediated neutralization to vaccinia virus might be important to monitor the outcome of a vaccination campaign. Moreover, live-virus vector-based vaccination strategies are likely to increasingly use vaccinia virus-derived vectors, such as MVA, in future clinical trials.
The method described here is able to successfully monitor the neutralizing immune response to vaccinia virus elicited in different settings and offers several advantages compared to standard vaccinia virus PRNTs. The MVA-gfp neutralization assay allows the processing of several samples in the same experimental run and could be easily automated as all the procedures are performed in 96-well plates. Moreover, since MVA is a highly attenuated replication-deficient vaccinia virus vector (18) and is safe in immunocompromised individuals (1), it can also be handled by non-smallpox-vaccinated laboratory workers (12). These characteristics render our assay suitable for high-throughput applications and use in standard diagnostic laboratories. The standard vaccinia virus PRNT requires several rounds of replication before a visible plaque is formed, and the method is usually time-consuming, requiring 2 to 5 days according to the different protocols in use (7, 10, 16, 17). In contrast, the MVA-gfp neutralization assay requires less than 1 day to be performed since, after infection, Gfp fluorescence can be detected after 14 h. Moreover, our new method allows for autologous neutralization testing, since the target cell line can be chosen with the only restriction to support infection by MVA. Furthermore, MVA does not replicate in human B cells, allowing only a single round of infection in the target cell, hence more accurately measuring the number of viral particles able to enter the target cell. Finally, the use of a flow cytometry-based neutralization assay allows the precise counting of infected cells, making the assay quantitative and nonsubjective. Taken together, these considerations show net advantages of the MVA-gfp neutralization assay compared to the standard PRNTs.
The MVA-gfp neutralization assay uses an MOI of 1 PFU/cell, while the standard vaccinia virus Elstree and MVA PRNTs were performed with an MOI of 0.0007 and 0.0008 PFU/cell, respectively. Although low MOIs are usually associated with higher sensitivity in neutralization assays (see Fig. 1e and f), the VIG preparation tested in parallel in the three assays showed almost similar neutralizing activities, indicating that the sensitivity of our assay might be increased to be superior to that of conventional plaque reduction assay by lowering the MOI or by enhancing levels of expression of the gfp gene.
During vaccinia virus morphogenesis four different viral infectious forms are generated (15). Intracellular mature virions are made in cytoplasmic virus factories and move to the wrapping membranes of the trans-Golgi network to form the intracellular enveloped virus (IEV). Then, IEV particles fuse with the cellular membrane and form the cell-associated enveloped virus or the extracellular enveloped virus when particles are released from the cell. The relative contribution of the different forms of vaccinia virus to the observed neutralization is not addressed in this work. However, because of the extreme fragility of the outer membrane in extracellular enveloped virus, cell-associated enveloped virus, and IEV, intracellular mature virion is considered the predominant form in vaccinia virus stocks and the observed neutralizing activities are probably directed against this viral infectious form.
Recently, Manischewitz et al. described an interesting vaccinia virus neutralization assay based on the use of a β-galactosidase recombinant vaccinia virus (11). This novel assay is rapid and sensitive since it uses the enzymatic activity of the β-galactosidase as a readout system, yet it still requires the use of the replication-competent vaccinia virus and the use of a substrate to detect vaccinia virus-infected cells. The MVA-gfp neutralization assay adds to the advantages of using a reporter gene system, the safety of the highly attenuated MVA virus vector, and the ease of a flow-cytometry-based readout.
In summary, we estimate that our new MVA-gfp neutralization assay could be a good candidate method to eventually monitor anti-vaccinia virus immune responses in large-scale clinical trials. Moreover, the method could be used also in basic research since it gives a direct measurement of the neutralizing activity and has a quantitative and accurate readout.
Acknowledgments
We acknowledge Denise Boulanger for helpful suggestions and critical reading of the manuscript.
REFERENCES
- 1.Cosma, A., R. Nagaraj, S. Bühler, J. Hinkula, D. H. Busch, G. Sutter, F. D. Goebel, and V. Erfle. 2003. Therapeutic vaccination with MVA-HIV-1 nef elicits Nef-specific T-helper cell responses in chronically HIV-1 infected individuals. Vaccine 22:21-29. [DOI] [PubMed] [Google Scholar]
- 2.Drexler, I., E. Antunes, M. Schmitz, T. Wolfel, C. Huber, V. Erfle, P. Rieber, M. Theobald, and G. Sutter. 1999. Modified vaccinia virus Ankara for delivery of human tyrosinase as melanoma-associated antigen: induction of tyrosinase- and melanoma-specific human leukocyte antigen A*0201-restricted cytotoxic T cells in vitro and in vivo. Cancer Res. 59:4955-4963. [PubMed] [Google Scholar]
- 3.Drexler, I., K. Heller, B. Wahren, V. Erfle, and G. Sutter. 1998. Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J. Gen. Virol. 79:347-352. [DOI] [PubMed] [Google Scholar]
- 4.Geretti, A. M., M. E. Dings, C. A. van Els, C. A. van Baalen, F. J. Wijnholds, J. C. Borleffs, and A. D. Osterhaus. 1996. Human immunodeficiency virus type 1 (HIV-1)-and Epstein-Barr virus-specific cytotoxic T lymphocyte precursors exhibit different kinetics in HIV-1-infected persons. J. Infect. Dis. 174:34-45. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein, J. A., J. M. Neff, J. M. Lane, and J. P. Koplan. 1975. Smallpox vaccination reactions, prophylaxis, and therapy of complications. Pediatrics 55:342-347. [PubMed] [Google Scholar]
- 6.Hirsch, V. M., T. R. Fuerst, G. Sutter, M. W. Carroll, L. C. Yang, S. Goldstein, M. Piatak, Jr., W. R. Elkins, W. G. Alvord, D. C. Montefiori, B. Moss, and J. D. Lifson. 1996. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 70:3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz, J. B. 1987. The effect of the virus-serum incubation period upon vaccinia virus serum neutralization titers. J. Biol. Stand. 15:389-392. [DOI] [PubMed] [Google Scholar]
- 8.Kohleisen, B., M. Neumann, R. Herrmann, R. Brack-Werner, K. J. Krohn, V. Ovod, A. Ranki, and V. Erfle. 1992. Cellular localization of Nef expressed in persistently HIV-1-infected low-producer astrocytes. AIDS 6:1427-1436. [DOI] [PubMed] [Google Scholar]
- 9.Law, M., and G. L. Smith. 2001. Antibody neutralization of the extracellular enveloped form of vaccinia virus. Virology 280:132-142. [DOI] [PubMed] [Google Scholar]
- 10.Lennette, E. H., and N. J. Schmidt (ed.). 1979. Diagnostic procedures for viral, rickettsial and chlamydial infections, 5th ed., vol. 10, p. 257-308. American Public Health Association, Washington, D.C.
- 11.Manischewitz, J., L. R. King, N. A. Bleckwenn, J. Shiloach, R. Taffs, M. Merchlinsky, N. Eller, M. G. Mikolajczyk, D. J. Clanton, T. Monath, R. A. Weltzin, D. E. Scott, and H. Golding. 2003. Development of a novel vaccinia-neutralization assay based on reporter-gene expression. J. Infect. Dis. 188:440-448. [DOI] [PubMed] [Google Scholar]
- 12.Moss, B. 1996. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc. Natl. Acad. Sci. USA 93:11341-11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss, B., and P. L. Earl. 1991. Expression of proteins in mammalian cells using vaccinia viral vectors, p. 16.16.1-16.16.7. In F. M. Ausubel, R. Brent, R. Kingston, D. Moore, J. Seidman, J. Smith, and K. Struhl (ed.), Current protocols in molecular biology. Wiley, New York, N.Y.
- 14.Ramirez, J. C., M. M. Gherardi, D. Rodriguez, and M. Esteban. 2000. Attenuated modified vaccinia virus Ankara can be used as an immunizing agent under conditions of preexisting immunity to the vector. J. Virol. 74:7651-7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 16.Staib, C., I. Drexler, M. Ohlmann, S. Wintersperger, V. Erfle, and G. Sutter. 2000. Transient host range selection for genetic engineering of modified vaccinia virus Ankara. BioTechniques 28:1137-1148. [DOI] [PubMed] [Google Scholar]
- 17.Stienlauf, S., M. Shoresh, A. Solomon, T. Lublin-Tennenbaum, Y. Atsmon, Y. Meirovich, and E. Katz. 1999. Kinetics of formation of neutralizing antibodies against vaccinia virus following re-vaccination. Vaccine 17:201-204. [DOI] [PubMed] [Google Scholar]
- 18.Sutter, G., and B. Moss. 1992. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 89:10847-10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang, Z. Y., L. S. Wyatt, W. P. Kong, Z. Moodie, B. Moss, and G. J. Nabel. 2003. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J. Virol. 77:799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]