Abstract
The BMP and Wnt/β-catenin signaling pathways cooperatively regulate osteoblast differentiation and bone formation. Although BMP signaling regulates gene expression of the Wnt pathway, much less is known about whether Wnt signaling modulates BMP expression in osteoblasts. Given the presence of putative Tcf/Lef response elements that bind β-catenin/TCF transcription complex in the BMP2 promoter, we hypothesized that the Wnt/β-catenin pathway stimulates BMP2 expression in osteogenic cells. In this study, we showed that Wnt/β-catenin signaling is active in various osteoblast or osteoblast precursor cell lines, including MC3T3-E1, 2T3, C2C12, and C3H10T1/2 cells. Furthermore, crosstalk between the BMP and Wnt pathways affected BMP signaling activity, osteoblast differentiation, and bone formation, suggesting Wnt signaling is an upstream regulator of BMP signaling. Activation of Wnt signaling by Wnt3a or overexpression of β-catenin/TCF4 both stimulated BMP2 transcription at promoter and mRNA levels. In contrast, transcription of BMP2 in osteogenic cells was decreased by either blocking the Wnt pathway with DKK1 and sFRP4, or inhibiting β-catenin/TCF4 activity with FWD1/β-TrCP, ICAT, or ΔTCF4. Using a site-directed mutagenesis approach, we confirmed that Wnt/β-catenin transactivation of BMP2 transcription is directly mediated through the Tcf/Lef response elements in the BMP2 promoter. These results, which demonstrate that the Wnt/β-catenin signaling pathway is an upstream activator of BMP2 expression in osteoblasts, provide novel insights into the nature of functional cross talk integrating the BMP and Wnt/β-catenin pathways in osteoblastic differentiation and maintenance of skeletal homeostasis.
Keywords: BMP, Wnt/β-catenin, Gene expression, Osteogenesis
Introduction
Bone morphogenetic protein 2 (BMP2) is a critical autocrine and paracrine growth factor that directs osteoblast differentiation and bone formation [1–4]. Signaling of BMP2 induces mesenchymal precursor cells to differentiate into mature osteoblasts by regulating signals that stimulate specific transcriptional programs required for bone formation [5,6]. Previous studies have shown that BMP2 expression is tightly associated with the status of osteoblast maturation [7,8], and is regulated in osteoblasts by other bone-related factors and signaling pathways including the TGF-β, hedgehog/Gli, PTH/CREB, estrogen receptor, NF-κB, PGE2, and microtubule signaling pathways [9–17]. Expression of BMP2 also is regulated by the BMP signaling pathway itself because BMP2 is an autoregulated protein [18–20]. These multiple mechanisms involved in regulation of BMP2 gene expression may provide potential therapeutic targets for skeletal diseases with bone loss. However, the major anabolic mechanisms, particularly at the transcriptional level, that control BMP2 expression for osteoblastic bone formation are still unknown.
Recent gain- and loss-of-function studies of LRP5/6, β-catenin, and many other molecules that participate in the Wnt signaling cascade identify the canonical Wnt signaling pathway as a crucial pathway in the skeleton controlling osteoblast differentiation and bone formation [21–28]. The evolutionarily conserved BMP and Wnt pathways are independent signaling mechanisms, by means of different ligands, receptors, and intracellular signal transducers. However, these two pathways control bone formation cooperatively. Activation of Wnt signaling induces differentiation of pluripotent mesenchymal cells into osteoblast progenitors that become osteoblasts, and maintains the precursor status of these osteoprogenitors. BMP signaling stimulates these cells to further differentiate into mature osteoblasts. After osteoprogenitors become osteoblasts, both pathways promote further differentiation, evidenced by increased alkaline phosphatase (ALP) activity and mineralization [29–32]. Functional integration of the BMP and Wnt signaling pathways mostly causes dependent and/or synergistic effects on osteoblast differentiation and bone formation [30,32,33].
Molecular studies have shown that functional communication between the BMP and Wnt pathways involves multiple mechanisms. Some extracellular proteins, such as sclerostin, CTGF, cerberus and sFRPs, are known to bind ligands/antagonists or/and receptors of both the BMP and Wnt pathways [34–40]. Intracellularly, Smads are found to form complexes with Wnt signaling molecules, such as Dishevelled-1, Axin, GSK3 and β-catenin. These complexes modulate phosphorylation and activity of Smads and β-catenin [41–46]. Perhaps the most compelling mechanism highlighting the cooperation between the BMP and Wnt pathways is the transcriptional regulation of their common target genes, which harbor both Smad and Tcf/Lef response elements (TREs). Smads can form a transcriptional complex with β-catenin/Tcf/Lef and co-activate transcription of many target genes through these binding elements in response to both BMP and Wnt signaling [47–52]. In our effort to identify potentially critical transcriptional mechanisms that control BMP2 expression in osteoblasts, we hypothesized that the Wnt signaling pathway is an upstream transactivator of BMP2 in osteoblasts. This takes into account that the BMP and Wnt signaling pathways tightly cooperate and regulate each other, and that multiple putative Tcf/Lef response elements (TREs) exist in the BMP2 promoter (vide infra). The purposes of the current study were to determine the Wnt/β-catenin signaling pathway activation effects on BMP2 gene expression and signaling in osteoblasts and to identify potential transcriptional mechanisms. Since both BMP and Wnt pathways are critical anabolic signaling pathways affecting bone formation, demonstration of Wnt regulation of BMP2 expression in osteoblasts will provide novel insights into the role of the functional communication between the BMP and Wnt signaling pathways that affects bone formation.
Material and methods
DNA constructs and recombinant proteins
The following expression plasmids were kindly provided by Dr. Bert Vogelstein (Johns Hopkins University School of Medicine, Baltimore, MD): pCI-neo-β-catenin for human β-catenin; pCI-neo-β-catenin(Δ45) for stabilized β-catenin form in which Ser45 is deleted; pCI-neo-β-catenin(S33Y) for stabilized β-catenin form in which Ser33 is replaced by Tyr33 [53]; pcDNA-Myc-TCF4 for human TCF4; pcDNA-Myc-ΔTCF4 for the dominant negative mutant form of TCF4 (ΔTCF4) that lacks amino acids 1–30; and the Wnt signaling reporter TOPFLASH (pGL3-OT) [54]. Murine inhibitor of β-catenin and TCF (ICAT) was expressed in a vector as pcDNA3.1-Flag-ICAT [55]. Expression plasmids pcDNA3-Flag-FWD1 for F-box/WD40-repeat protein 1 (FWD1), the mouse ortholog of β-transducin repeat-containing protein (β-TrCP), and pcDNA3-Flag-FWD1ΔF-dominant negative form of FWD1 in which the F-box is deleted [56] were gifts from Drs. Kei-ichi Nakayama and Hatakeyama (Kyushu University, Japan). Osteoblast-specific multiple-signaling reporter, 9×6-OC-Luc, was constructed by linking a sequence containing 9 copies of Tcf/Lef response elements (TRE), 9 copies of Smad binding elements (SBE), and 6 copies of Runx2 binding elements (OSE2) upstream of a basal mouse osteocalcin promoter and linked to a luciferase reporter in pGL3 vector (Promega, Madison, WI). The BMP2 promoter reporter − 2712/+165-Luc, made by linking mouse BMP2 promoter sequence − 2712/+165 to a cDNA for firefly luciferase in pGL3 vector, has been previously described [6,13,14,57]. The BMP signaling reporter, 12SBE-Luc that we previously described [13,17,57,58], was constructed by connecting 12 copies of BMP-specific SBEs upstream of a basal mouse osteocalcin promoter and firefly luciferase coding sequence in pGL3 vector. All recombinant murine proteins (Wnt3a, BMP2, noggin, DKK1, serum frizzled-related protein 4 [sFRP4], and a genetically engineered soluble form of the extracellular ligand-binding domain [amino acids 20–395] of Kremen-1 [sKremen]) were purchased commercially (R&D Systems, Minneapolis, MN).
Cell culture and transfection
Osteoblast and osteoblast precursor MC3T3-E1 and 2T3 cells [6,13,14,57] were cultured in α-minimal essential medium (α-MEM). Pluripotent mesenchymal C3H10T1/2 cells were cultured in RPMI medium 1640. Myoblastic C2C12 cells were cultured in Dulbecco's modified Eagle medium (DMEM). Cells were cultured at 37 °C in a humidified 5% CO2 incubator. The medium was supplemented with 10% fetal calf serum (FCS), 1% penicillin/streptomycin, and 1% l-glutamine. Cells at 70–80% confluence were transfected with luciferase reporters and/or expression plasmids using LipoFectamine Plus Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instruction.
Bone organ culture assay
Neonatal mouse calvarial cultures were performed as previously described [11,59,60]. Briefly, calvariae from 4-day old Institute for Cancer Research (ICR) Swiss pups were dissected and cut in half with the excised hemi-calvariae explanted on metal grids (on the surface) in 1 mL BGJ medium with Fitton-Jackson modification BGJ medium (Sigma) containing 0.1% BSA with glutamine. The bones were incubated at 37 °C in a 5% humidified incubator for 24 h, transferred to wells containing 1 mL of medium with test compounds, and then further incubated under the above conditions for 72 h. The bones were then removed, fixed in 10% buffered formalin for 24 h, decalcified in 14% EDTA overnight, and embedded in paraffin. Sections (7 µm thick) were then cut and stained with hematoxylin and eosin (H&E) to facilitate assessment of new bone formation and osteoblast proliferation (cellularity of sections) as detailed previously (60).
Western blot
Twenty-four hours after incubation of MC3T3-E1, 2T3, C3H10T1/2, and C2C12 cells with Wnt3a at 40 ng/mL or vehicle, or 36 h after transfection of C2C12 cells with the β-catenin expression vector, cells were lysed with RIPA cell lysate buffer. Cell lysates mixed with sample buffer were loaded on SDS-PAGE gels (Mini-PROTEIN II Ready gels, Bio-Rad, Hercules, CA). Proteins were transblotted onto a PVDF membrane (Bio-Rad) in a transblotting buffer (20 mM Tris, 150 mM glycine, 20% methanol, pH 8.0) at 4 °C and 100 V for 1 h. The membrane was blocked with 5% dry milk in TBS-T for 1 h at room temperature. Then the membrane was incubated with either a rabbit monoclonal anti-β-catenin antibody (Abcam, ab32572, Cambridge, MA) or a mouse monoclonal anti-β-actin antibody (Abcam, ab8226) in TBS-T for 2 h at room temperature. Incubation with horseradish peroxidase-conjugated anti-goat IgG antibody (Amersham Biosciences, Piscataway, NJ) was performed at room temperature for 1 h. After washes, immunoblots were detected using an enhanced chemiluminescence (ECL) system (Amersham Biosciences).
Alkaline phosphatase activity
C2C12 cells or primary calvarial cells seeded in 48-well plates were incubated with BMP2 at 100 ng/mL or Wnt3a at 40 ng/mL, in the presence or absence of noggin at 500 ng/mL or DKK1 at 100 ng/mL, in DMEM medium with 2.5% FCS for 24–48 h. After lysing in 0.05% Triton X-100 buffer, cell lysates were analyzed for ALP activity in 96-wells plates. Briefly, lysate (10 µL) was incubated with 90 µL of fresh AMP solution containing p-nitrophenyl phosphate substrate at 37 °C for 30–60 min, and then 0.5 N NaOH (100 µL) was added to stop the reaction. The plates were then read spectrophotometrically at 405 nm. ALP activity was determined using a p-nitrophenol standard curve and normalized to total cellular protein [6,13,14,57].
RT-PCR and quantitative real time PCR
Total RNAs prepared from C2C12, 2T3 or calvarial cells, treated with either Wnt-3a (20–80 ng/mL for 24 h) or transfected with β-catenin/TCF4 expression plasmids for 36 h, were extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA) and then reverse transcribed into cDNA using an iScript™ cDNA Synthesis Kit (Bio-Rad). The synthesized cDNAs were amplified by PCR for 35 cycles (94 °C for 1 min, 56 °C for 1 min, and 72 °C for 1 min) using a Taq PCR Master Mix Kit (Qiagen). The primers for mouse BMP2 were 5′-TGAGGATTAGCAGGTCTTTG and 5′-CACAACCATGTCCTGATAAT. Quantitative real time PCR of mouse BMP2, Col1a1 and Runx2 mRNAs was performed using a TaqMan® Environmental Master Mix kit 2.0 (Applied Biosystems, Carlsbad, CA) with the cDNA template and mouse TaqMan probes (BMP2 probe: Mm01340178_ml; Col1a1 probe: Mn02601853_g1; Runx2 probe: Mn00501578_m1, Applied Biosystems) on the 7300 real-time PCR system (Applied Biosystems). Mouse GAPDH mRNA, detected using a VIC-MGB probe (4352339E, Applied Biosystems), served as an endogenous control.
Luciferase reporter assay
C2C12 cells were plated in 24-well plates at 4 × 104 cells per well in DMEM with 10% FCS for 18–24 h before transfection. Cells were incubated for 4 h at 37 °C with 250 µL of Opti-MEM transfection solution containing LipofectAmine Plus Reagent (Invitrogen); 0.5 µg of reporter plasmids, including TOPFLASH, 9×6-OC-Luc, 12SBE-OC-Luc, and −2712/+165-Luc; 0.1 µg of pSV-β-Galactosidase (β-Gal) expression vector (Promega); and 0.05–0.2 µg of expression plasmids for proteins, including β-catenin, β-catenin(Δ45), β-catenin(S33Y), TCF4, ΔTCF4, ICAT, FWD1, and FWD1(ΔF). After 4 h of incubation, fresh DMEM medium (250 µL) containing 20% FCS was added. The cells were cultured in the presence or absence of BMP and Wnt ligands or antagonists, including BMP2, Wnt-3a, noggin, DKK1, and sFRP4, for 24–48 h. Cells were lysed with 100 µL of Reporter Lysis Buffer (Promega). Relative luciferase activities of cell lysates were measured using a luciferase assay kit (Promega) and normalized with β-Gal activity or Renilla activity [6,13,14,57].
Promoter mutagenesis
The putative TREs in the mouse BMP2 promoter were mutated by deleting core nucleotides within the TREs using GeneEditor™ in Vitro Site-Directed Mutagenesis System (Promega) following the manufacturer's protocol. Briefly, the BMP2 promoter sequence −2712/ + 165 was inserted into the mutagenesis vector pGEM®-11Zf(+). Denatured DNA templates were annealed with synthesized mutagenesis DNA oligonucleotides, 5′-TTCGGAGTTTCTTGCTGCTCCTTCCGC CTCC in which the nucleotides “TT” in the putative TRE “GCTTTGCT” at − 2269/ − 2263 bp of the promoter were deleted, or 5′-CTTCTGGTCTTTCT CGGTCTGCAAACTGGAAAGATCTGGT in which nucleotides “TTGCTTT” in the tandem TREs “TCTTTGCTTTGC” at − 1824/−1814 bp were deleted. After DNA ligation and transformation, mutated DNAs were verified by sequencing. The mutated BMP2 promoter sequences were recovered and inserted back into a pGL3 reporter vector. The luciferase activity of mutant reporters responding to β-catenin/TCF4 was measured as described above.
Calvarial cell culture
Calvarial osteoblastic cells were obtained from newborn mice (1–4 days). Briefly, calvarial bone tissues of pups were removed and subjected to multiple 15–25 min digestions in Hank's Buffered Salt Solution (HBSS) supplemented with 0.05% trypsin and 1.5 U/ml collagenase at 37 °C. Cells from the initial digestion were discarded, and cells from digestions 2–6 digestion were suspended in α-minimal essential media (α-MEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin and 1% l-glutamine. Cultured overnight, the cells were subcultured into the plates where they were treated the growth factors and their inhibitors. The osteoblast differentiation and BMP2 mRNA expression in these cells were determined as described above.
Results
Wnt/β-catenin signaling in osteoblasts
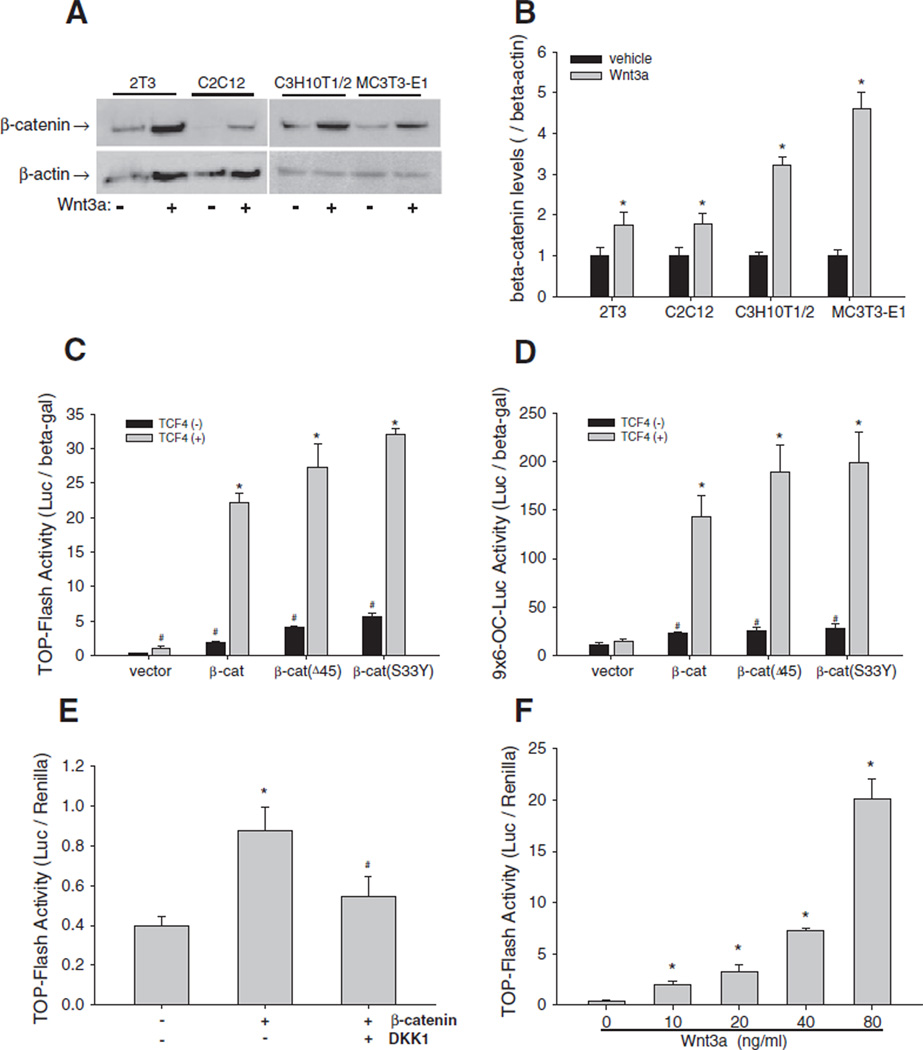
We performed a series of experiments to demonstrate that the canonical Wnt signaling pathway was intact and active in multiple murine C2C12 and C3H10T1/2 as well as osteoblast precursor 2T3 and MC3T3-E1 cell lines. C2C12 cells are primitive myoblastic cells that can undergo differentiation to preosteoblast-like cells, while C3H10T1/2 cells are pluripotent cells capable of differentiating along several lineages including osteogenic lineage. Immunoblotting revealed that incubation of these osteogenic cells with Wnt3a at 40 ng/mL for 24 h markedly increased intracellular levels of β-catenin (Fig. 1A). Quantitation of the Western blots showed that, compared with vehicle treatment, Wnt3a treatment significantly increased the β-catenin/β-actin (normalization control) ratio (Fig. 1B).
Fig. 1.
Wnt/β-catenin signaling in osteoblasts. (A and B) Effects of Wnt3a on β-catenin expression. 2T3, C2C12, C3H10T1/2, and MC3T3-E1 cells were treated with Wnt3a at 40 ng/mL for 24 h. β-catenin in cell lysates was detected by Western blot using an anti-β-catenin antibody. β-actin was used as control (A). The relative β-catenin levels were quantitated using Quantity One gel imaging system with normalization by β-actin (B). *: P<0.05 Wnt3a vs. vehicle (n=4). (C and D) Effects of β-catenin/TCF4 on Wnt reporter activity. C2C12 cells, which were transfected with TOPFLASH (C) or9×6-OC-Luc reporter (D), were transfected with expression vectors for wild-type and mutant β-catenin and TCF4at a dose of 0.2 µg DNA/well in 24-well plates for 36 h. Relative luciferase activity in the cell lysates was measured and normalized by β-galactosidase (β-gal) activity. #: P<0.05 β-catenin or TCF4 vs. vector; *: P<0 .01 β-catenin+TCF4 vs. β-catenin (n=6). (E) Effects of DKK1 on β-catenin activation of Wnt signaling activity. C2C12 cells carrying TOPFLASH reporter were incubated with DKK1 at 100 ng/mL for 36 h. Relative luciferase activity was measured with normalization by Renilla. *: P<0 .01 β-catenin vs. vector; #: P<0.01 β-catenin vs. β-catenin+DKK1 (n=6). (F) Effects of Wnt3a on Wnt signaling activity. C2C12 cells carrying TOPFLASH reporter were incubated with Wnt3a in a dose range of 10–80 ng/mL for 36 h. Relative luciferase activity was measured as described in (E). *: P<0 .01, Wnt3a vs. vehicle (n=6).
Next, we used luciferase reporters to determine the overexpression effects of β-catenin and TCF4 on signaling activity of the Wnt pathway. In C2C12 cells, transfection of wild-type β-catenin or its stabilized mutated forms, β-catenin(Δ45) and β-catenin(S33Y) substantially increased luciferase activity compared with the empty vector control in a Wnt-specific TOPFLASH reporter assay (Fig. 1C). Furthermore, co-transfection with TCF4 (a known co-activator of β-catenin) further synergistically increased the transcriptional activity of β-catenin and its mutant forms on TOPFLASH, compared with TCF4 empty vector (Fig. 1C, gray bars). Consistent with the results of the TOPFLASH assay, overexpression of the various forms of β-catenin and TCF4 in C2C12 cells significantly increased luciferase activity of the 9×6-OC-Luc reporter, which contains multiple copies of Tcf/Lef binding elements, compared with empty vector controls (Fig. 1D). Next, TOPFLASH-expressing C2C12 cells transfected with β-catenin were treated with DKK1, an antagonist of Wnt pathway. DKK1 attenuated the β-catenin-enhanced TOPFLASH activity (Fig. 1E). Further, treatment of C2C12 cells with Wnt3a over a wide dose range (10–80 ng/mL) markedly increased TOPFLASH activity in a dose-dependent manner (Fig. 1F). As Wnt signaling was substantially stimulated by Wnt3a at 40 ng/mL and 80 ng/mL, we used these doses of Wnt3a in subsequent experiments in this study. These results demonstrate that the Wnt signaling pathway is intact and active in osteoblasts.
Interaction between the BMP and Wnt signaling pathway in osteoblasts
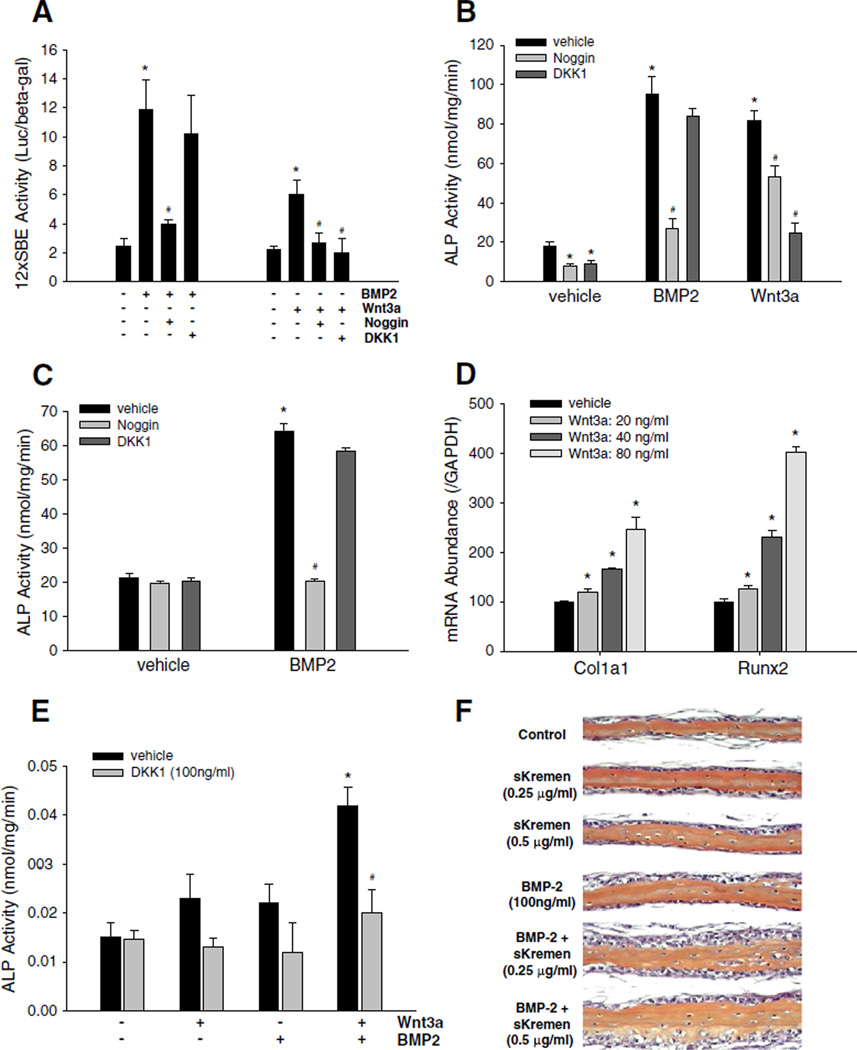
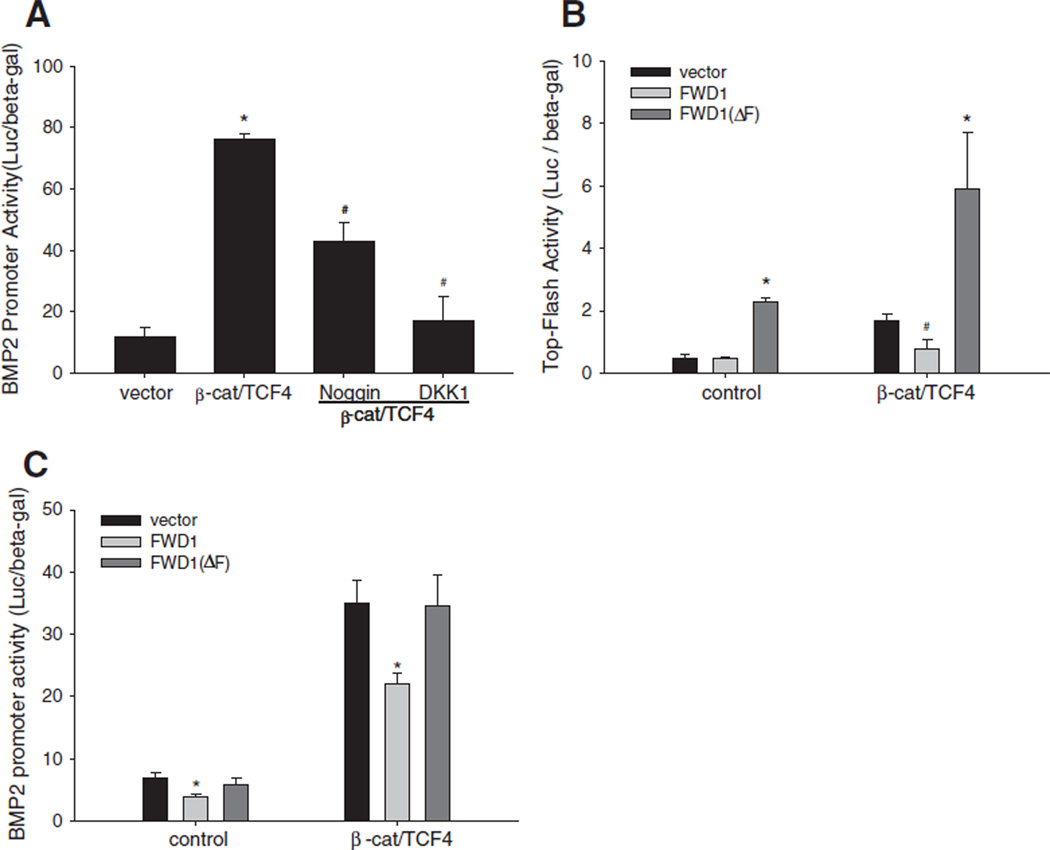
We next investigated the role of ligands and antagonists in cross-regulation of signaling activity of both BMP and Wnt pathways. First, we determined the effects of BMP and Wnt ligands on BMP signaling activity. C2C12 cells, transfected with a BMP/Smad-specific reporter construct 12SBE-Luc [13,17,57,58], were incubated with either BMP2 (100 ng/mL) or Wnt3a (40 ng/mL) for 36 h. As expected, BMP2 treatment increased reporter activity (Fig. 2A). However, Wnt3a also enhanced BMP/Smad reporter activity (Fig. 2A), indicating that activation of Wnt signaling cross-stimulates BMP signaling. Next, we determined the effects of BMP or Wnt antagonists on BMP reporter activity. As expected, treatment of C2C12 cells with the BMP antagonist noggin significantly attenuated BMP2-induced activation of the BMP reporter. However, incubation of the cells with the Wnt antagonist DKK1 did not block the stimulatory effect of BMP2 on reporter activity (Fig. 2A).
Fig. 2.
Interaction between Wnt and BMP signaling in osteoblasts. (A) Effects of BMP2 and Wnt3a and their antagonists on BMP signaling reporter activity. C2C12 cells, which were transfected with 12SBE-Luc reporter, were treated with BMP2 at 100 ng/mL, or Wnt3a at 40 ng/mL, in the presence or absence of noggin at 500 ng/mL or DKK1 at 100 ng/mL, for 36 h. Relative luciferase activity in the cell lysates was determined with β-gal normalization. *: P<0.01, BMP2 or Wnt3a vs. vehicle; #: P<0.01 noggin vs. BMP2, or noggin, DKK1 vs. Wnt3a (n=6). (B) Effects of BMP2 and Wnt3a and their antagonists on alkaline phosphatase (ALP) activity in C2C12 cells. C2C12 cells were treated with BMP2 or Wnt3a, with or without noggin or DKK1 as described above for 48 h. ALP activity in the cell lysates was determined using a Sigma ALP kit with normalization by total cell proteins. *: P<0.05 noggin or DKK1 or BMP2 or Wnt3a vs. vehicle; #: P<0.05, noggin vs. BMP2 or Wnt3a, or DKK1 vs. Wnt3a (n=6). (C) Effects of BMP2 on ALP activity of calvarial osteoblasts. Primary calvarial cells isolated from newborn mice were treated with BMP2 in the absence or presence of noggin or DKK1 at the doses as described above, for 48 h. ALP activity was quantitated as described above. *: P<0.01 BMP2 vs. vehicle;#: P<0.01 BMP2 vs. BMP2+noggin (n=6). (D) Effects of Wnt3a on Col1a1 and Runx2 expression in calvarial cells. The calvarial osteoblasts were incubated with Wnt3a at doses of 20, 40 and 80 ng/mL for 24 h. mRNA levels of Col1a1 and Runx2 were determined by real time PCR with GAPDH normalization. *: P<0 .01, Wnt3a vs. vehicle (n=6). (E) Effects of DKK1 on ALP activity induced by a combination of Wnt- and BMP2-induced signaling in neonatal calvariae. Hemi-calvariae were incubated ex vivo for 4 days with either Wnt3a (80 ng/ml) or BMP2 (100 ng/ml) or a combination of both in the presence or absence of DKK1. Conditioned media were harvested on day 4 and relative ALP levels determined. Data represent mean±SD (n≥3 calvariae/group). *: P<0.05, Wnt3a/BMP2 vs. Wnt3a or BMP2, or vehicle alone; #: P<0.05 Wnt3a/BMP2+DKK1 vs. Wnt3a/BMP2 (n≥3). (F) Soluble Kremen enhances BMP2-induced bone formation in neonatal mouse calvariae. Hemi-calvariae were treated with sKremen, BMP2 or a combination of both for 7 days with media and recombinant proteins completely replenished on day 4. Bones were processed for histology and stained with hematoxylin and eosin. Although soluble Kremen on its own did not have any effect, there was increased cellular proliferation and accumulation of mature osteoblasts adjacent to new bone in representative calvariae treated with BMP2 in the presence of sKremen compared with BMP2 alone. Representative calvariae from n≥3 calvariae/group are presented.
To explore the potential effects of the interaction between the two pathways on osteoblast differentiation, we examined the effects of BMP2 and Wnt3a in the presence or absence of their inhibitors on alkaline phosphatase (ALP) activity. Treatment with either BMP2 (100 ng/mL) or Wnt3a (40 ng/mL) for 48 h significantly increased ALP activity in C2C12 cells, but treatment with noggin or DKK1 decreased ALP activity in these cells (Fig. 2B). However, unlike noggin which substantially attenuated both BMP2-induced and Wnt-3a-induced ALP activity, DKK1 only attenuated Wnt3a-induced increase in ALP activity (Fig. 2B). These results indicate that Wnt signaling is likely an upstream activator of the BMP signaling pathway. We next confirmed the effects of Wnt3a and BMP2 on osteoblastic differentiation of primary calvarial cells. Incubation of primary calvarial cells with BMP2 increased ALP activity and this was blocked by concomitant treatment with noggin, but not DKK1 (Fig. 2C). Wnt3a over a dose range (20–80 ng/mL) also dose-dependently increased expression of osteoblast marker genes, including Col1a1 and Runx2, as assessed by real time PCR (Fig. 2D).
To further examine the cross talk between the Wnt and BMP2 signaling pathways, we next examined the effect of DKK1 on ALP activity in an ex vivo bone organ (neonatal calvaria) culture assay (which is predictive of osteoblast differentiation and new bone formation [11,59]). There was no significant increase in ALP in the presence of either Wnt3a or BMP2 alone. However, a combination of Wnt3a and BMP2 induced a significant increase in ALP. Although DKK1 on its own had no effect on basal ALP levels, it completely blocked the increase in ALP induced upon concurrent exposure of explanted hemi-calvariae to both Wnt3a and BMP2 (Fig. 2E). These results support the above in vitro data that strongly suggests the presence of cross talk between the Wnt-and BMP-signaling pathways. This indicates that BMP2-induced osteoblastic differentiation may be dependent, in part, on active Wnt signaling. To further explore this notion, we next examined the effect of sKremen on BMP2-induced new bone formation in the same neonatal calvarial assay. Available data in other systems suggest that DKK1 inhibits Wnt-induced signaling, in part, by forming a ternary complex with transmembrane receptors LRP5/6 and Kremen. This ternary complex is subsequently endocytosed; therefore, there are no ‘free’ LRP5/6 receptors available to transduce signals. As DKK1 is constitutively expressed and secreted in neonatal calvariae [61], we reasoned that by interfering with binding of Wnt3a to transmembrane LRP5/6 receptors, low levels of constitutively secreted Dkk1 dampen active Wnt signaling thereby attenuating osteoblastic response to BMP2. Therefore, sKremen, acting as a decoy to sequester DKK1 and block its biological activity, should “rescue” this attenuating effect of DKK1 and thereby augment the induction of new bone formation by exogenously applied BMP2. sKremen alone did not have any effect on new bone formation in this assay. Although as reported previously, BMP2 alone only had a marginal effect in this assay [11,59], more importantly, new bone formation (increased cellular proliferation and accumulation of mature osteoblasts adjacent to new bone) was markedly increased in bones treated with a combination of sKremen and BMP2 compared to BMP2 alone (Fig. 2F). Taken together, these data are consistent with the existence of a functional interplay between the Wnt and BMP pathways.
Effects of Wnt ligand and antagonists on BMP2 expression in osteoblasts
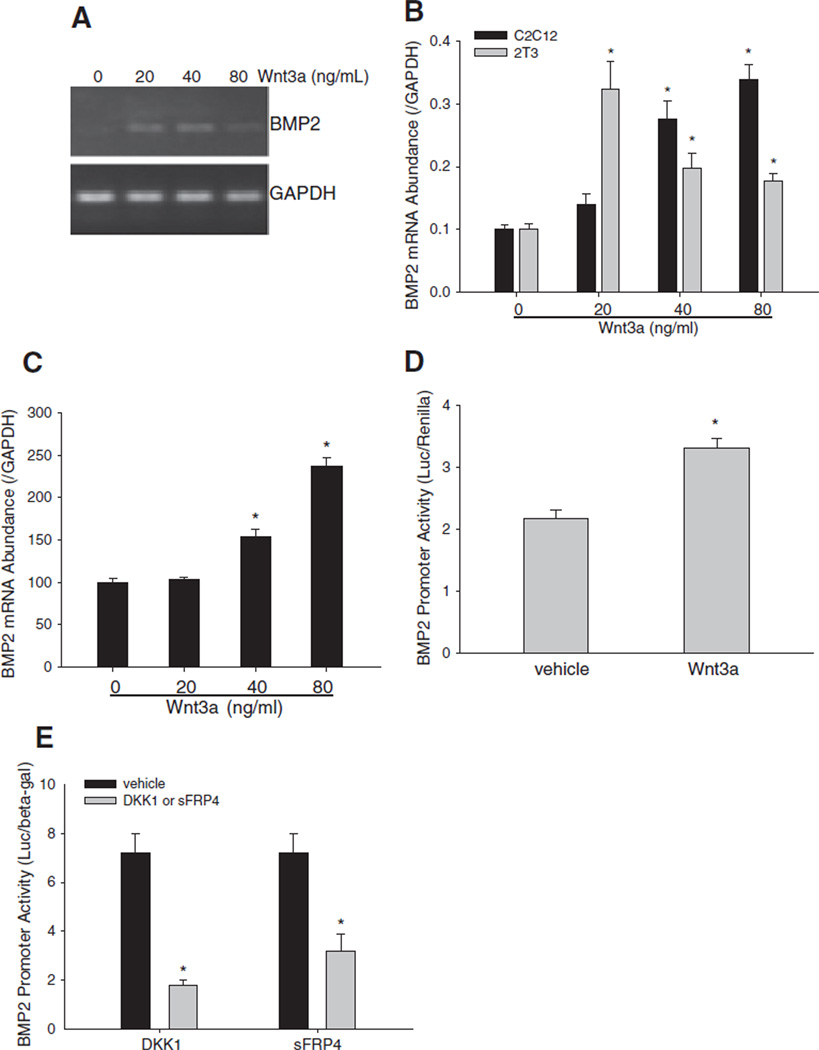
Wnt signaling regulates expression of BMP family members [62–64]. Sequence analysis showed that BMP2 (a prototype of the BMP family) promoter contains multiple putative binding elements for Tcf/Lef. Therefore, we hypothesized that Wnt/β-catenin signaling could function as a transcriptional stimulator of BMP2 expression in osteoblasts. This would provide a mechanism by which Wnt signaling activates BMP signaling. BMP2 mRNA levels were increased in osteoblast precursor C2C12 cells treated with Wnt3a (20–80 ng/mL) for 24 h, with a maximal effect at 40 ng/mL (Fig. 3A). Quantitative real time PCR further confirmed Wnt3a-enhancement of BMP2 expression in both C2C12 and 2T3 cells and identified a dose–response pattern of Wnt3a stimulation in C2C12 cells (Fig. 3B). We also examined the effects of Wnt3a on BMP2 mRNA expression in primary calvarial osteoblastic cells and found that Wnt3a dose-dependently increased BMP2 mRNA levels (Fig. 3C).
Fig. 3.
Effects of Wnt ligand and antagonists on BMP2 expression. (A and B) Effects of Wnt3a on BMP2 mRNA expression in C2C12 cells. (A) BMP2 PCR. C2C12 cells were treated with Wnt3a at a dose range from 20 to 80 ng/mL for 24 h. BMP2 mRNA levels in the cell lysates were determined by RT-PCR using mouse BMP2 primers, with GAPDH normalization. (B) Quantitative real time PCR of BMP2. C2C12 and 2T3 cells were treated with Wnt3a as described above. Relative BMP2 mRNA concentrations in the cell lysates were quantitated by real time PCR using TaqMan mouse BMP2 probe, and normalized by GAPDH. *: P<0.05 Wnt3a vs. vehicle (n=4). (C) Effects of Wnt3a on BMP2 mRNA expression in calvarial cells. Primary calvarial osteoblasts were incubated with Wnt3a at doses as described above. mRNA levels of BMP2 were determined as described above. *: P<0 .01 Wnt3a vs. vehicle (n=6). (D) Effects of Wnt3a on BMP2 promoter activity. C2C12 cells with BMP2 promoter reporter −2712/+165-Luc were treated with Wnt3a at 40 ng/mL for 36 h. Relative luciferase activity in the cell lysates was measured and normalized by Renilla activity. *: P<0.01, Wnt3a vs. vehicle (n=6). (E) Effects of Wnt antagonists on BMP2 promoter activity. C2C12 cells were transfected with BMP2 promoter reporter −2712/+165-Luc, and treated with DKK1 at 100 ng/mL or sFRP4 at 200 ng/mL for 36 h. Relative luciferase activity in the cell lysates was measured and normalized by β-gal activity. *: P<0.01 DKK1 or sFRP4 vs. vehicle (n=6).
Next, we tested the effects of Wnt signaling on BMP2 promoter activity. In C2C12 cells transfected with −2712/+165-Luc, a BMP2 promoter reporter that we have reported previously [6,11,14,16,17,57,59,65], Wnt3a treatment significantly enhanced BMP2 promoter activity, compared with vehicle control (Fig. 3D). We also determined the effects of DKK1 and sFRP4 (that competitively prevents Wnt ligands from binding to their receptors [66,67]) on BMP2 promoter activity. We found that incubation with either DKK1 or sFRP4 significantly reduced BMP2 promoter activity compared with vehicle control treatment (Fig. 3E). These results suggest that Wnt3a stimulates BMP2 transcription in osteoblasts.
Effects of overexpression of β-catenin on BMP2 expression in osteoblasts
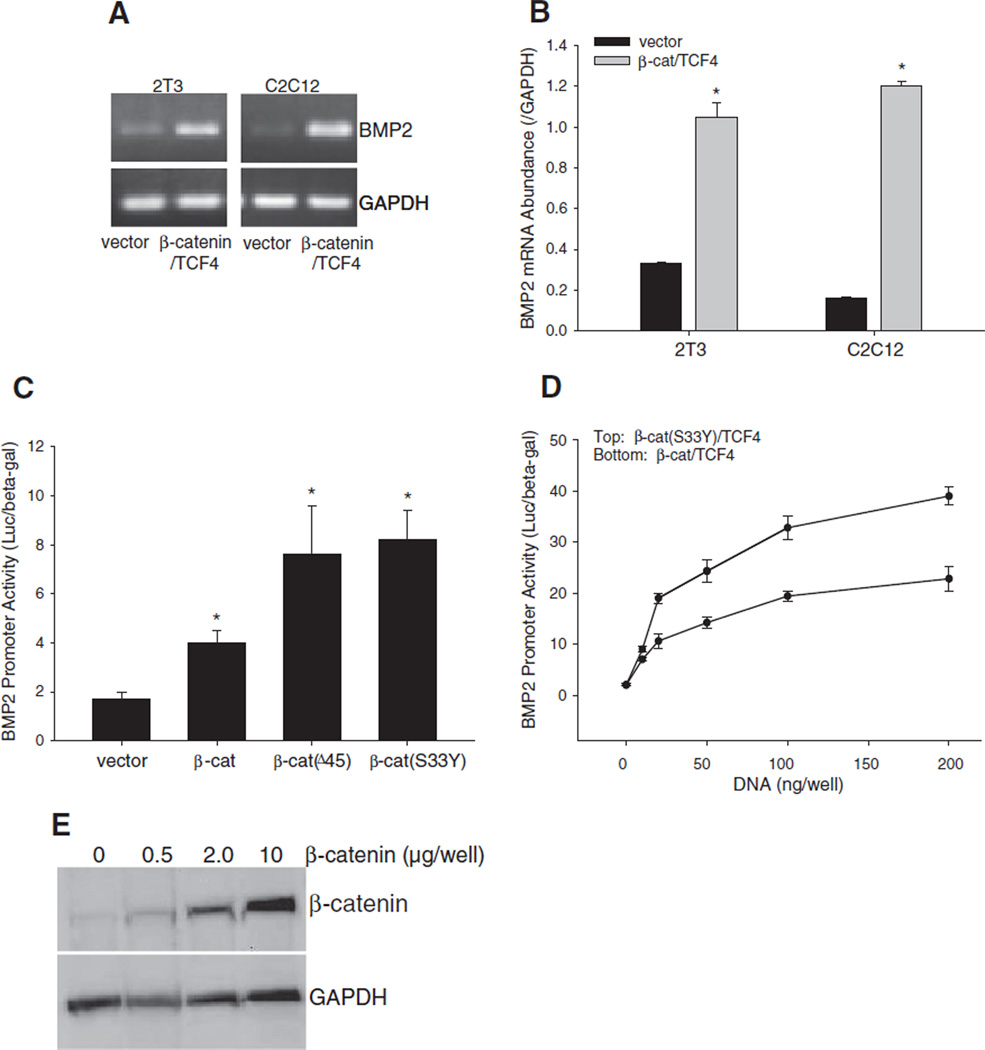
Next, we investigated whether modifying levels of β-catenin, a pivotal signaling molecule of the Wnt pathway, affects BMP2 expression. First, we determined the effects of overexpression of β-catenin. In both C2C12 and 2T3 cells, co-transfection of β-catenin with TCF4 markedly increased BMP2 mRNA concentrations, as evidenced by PCR assay with GAPDH normalization (Fig. 4A). Real time PCR further quantitatively confirmed the level of BMP2 mRNA in both cell lines was significantly elevated by β-catenin/TCF4 compared with vector controls (Fig. 4B). We also performed a BMP2 promoter reporter assay to determine the effects of β-catenin on BMP2 transcription and to compare the effects of different types of β-catenin. In C2C12 cells expressing the −2712/ +165-Luc reporter, the stable forms of β-catenin, β-catenin(Δ45) or β-catenin(S33Y) all exerted stronger stimulatory effects on BMP2 promoter activity than wild-type β-catenin (Fig. 4C). The stimulation extent of BMP2 promoter activity by either β-catenin/TCF4 or β-catenin(S33Y)/ TCF4 was dependent on the amount of DNA transfected (Fig. 4D). Together, these results suggest that β-catenin is a transcriptional activator of BMP2. We confirmed by immunoblotting that expression of exogenous β-catenin protein correlates dose-dependently with amount of β-catenin expression plasmid DNA transfected (Fig. 4E).
Fig. 4.
Effects of overexpression of β-catenin on BMP2 expression. (A and B) Effects of β-catenin/TCF4 on BMP2 mRNA expression. (A) BMP2 PCR. C2C12 and 2T3 cells were transfected with expression vectors for β-catenin and TCF4 at a dose of 0.5 µg DNA/well in 6-well plates for 24 h. BMP2 mRNA levels in the cell lysates were determined by RT-PCR using mouse BMP2 primers, with GAPDH normalization. (B) Quantitative real time PCR of BMP2. C2C12 and 2T3 cells were transfected with β-catenin/TCF4 as described above. BMP2 mRNA concentrations in the cell lysates were quantitated by real time PCR using TaqMan mouse BMP2 probe, and normalized to GAPDH. *: P<0.05 β-catenin/TCF4 vs. vector (n=4). (C and D) Effects of β-catenin/TCF4 on BMP2 promoter activity. C2C12 cells, which were transfected with −2712/+165-Luc reporter, were co-transfected with expression vectors for wild-type or mutant β-catenin at a dose of 0.2 µg DNA/well in 24-well plates (C) or co-transfected with β-catenin/TCF4 or β-catenin(S33Y)/TCF4 at a dose range of 0.05–0.2 µg DNA/well in 24-well plates (D) for 36 h. Relative luciferase activity in the cell lysates was measured and normalized by β-gal activity. *: P<0.05 β-catenin or its mutants vs. vector (n=6). (E) β-Catenin protein level correlates with amount of plasmid vector transfected. C2C12 cells in 6-well plates were transfected with β-catenin expression vector at varying doses (0.5, 2.0 and 10 µg DNA/well). After 36 h, cells were harvested and β-catenin protein levels in the respective cell lysates were determined by Western blot using anti-β-catenin antibody. GAPDH was used as an internal control.
Effects of inhibition of β-catenin activity on BMP2 expression in osteoblasts
Activation of Wnt signaling prevents β-catenin from ubiquitin-dependent proteolytic processing through the proteasome. Thus, we determined the effects on BMP2 expression of down-regulating β-catenin levels by several approaches, which include blocking transduction of signals via the Wnt cell surface receptor or overexpression of FWD1/β-TrCP (the E3 ligase that facilitates proteolytic degradation of β-catenin). In C2C12 cells transfected with the BMP2 promoter reporter construct, β-catenin/TCF4-enhanced BMP2 promoter activity was markedly decreased in the presence of DKK1, compared with vehicle control (Fig. 5A). Interestingly, noggin treatment also reduced BMP2 promoter activity induced by forced expression of β-catenin/TCF4 (Fig. 5A). We next evaluated the effects of FWD1 on Wnt signaling in osteoblasts by TOPFLASH assay. Although wild-type FWD1 had no effect on basal TOPFLASH activity, it reduced β-catenin/TCF4-induced TOPFLASH activity (Fig. 5B). In contrast, a dominant-negative form of FWD1 (FWD1ΔF) significantly increased both basal and β-catenin-induced activity of the Wnt signaling reporter (Fig. 5B). We also examined the effect of FWD1 and FWD1ΔF on BMP2 transcription. FWD1 significantly inhibited both basal and β-catenin/TCF4-stimulated BMP2 promoter activity (Fig. 5C). However, FWD1ΔF altered neither basal BMP2 promoter activity nor the β-catenin/TCF4-stimulated levels (Fig. 5C). Together with the findings from the overexpression studies above, it is likely that within the Wnt pathway, β-catenin functions as a pivotal signaling molecule regulating downstream BMP2 expression in osteoblasts.
Fig. 5.
Effects of down-regulation of β-catenin activity on BMP2 expression. (A) Effects of BMP or Wnt antagonists on β-catenin/TCF4 activation of BMP2 promoter activity. C2C12 cells were co-transfected with −2712/+165-Luc reporter and expression vectors for β-catenin and TCF4. Cells were treated with noggin at 500 ng/mL or DKK1 at 100 ng/mL for 36 h. Relative luciferase activity in the cell lysates was measured and normalized by β-gal activity. *: P<0.01 β-catenin/TCF4 vs. vector; #: P<0.05 noggin or DKK1 vs. vehicle (n=6). (B and C) Effects of FWD1/β-TrCP on Wnt signaling activity or BMP2 promoter activity. C2C12 cells were co-transfected with TOPFLASH (B) or −2712/+165-Luc reporter (C) and expression vectors for β-catenin and TCF4, and expression vectors for wild-type β-TrCP (FWD1) or mutant β-TrCP (FWD1ΔF) for 36 h. Relative luciferase activity in the cell lysates was measured and normalized by β-gal activity. In (B): *: P<0.01 FWD1ΔF vs. vector, or FWD1ΔF+β-catenin/TCF4 vs. vector+β-catenin/TCF4; #: P<0.05 FWD1+β-catenin/TCF4 vs. vector+β-catenin/TCF4. In (C): *: P<0.05 FWD1 vs. vector, or FWD1+β-catenin/TCF4 vs. vector+β-catenin/TCF4 (n=6).
β-Catenin Transactivation of BMP2 expression through Tcf/Lef binding elements
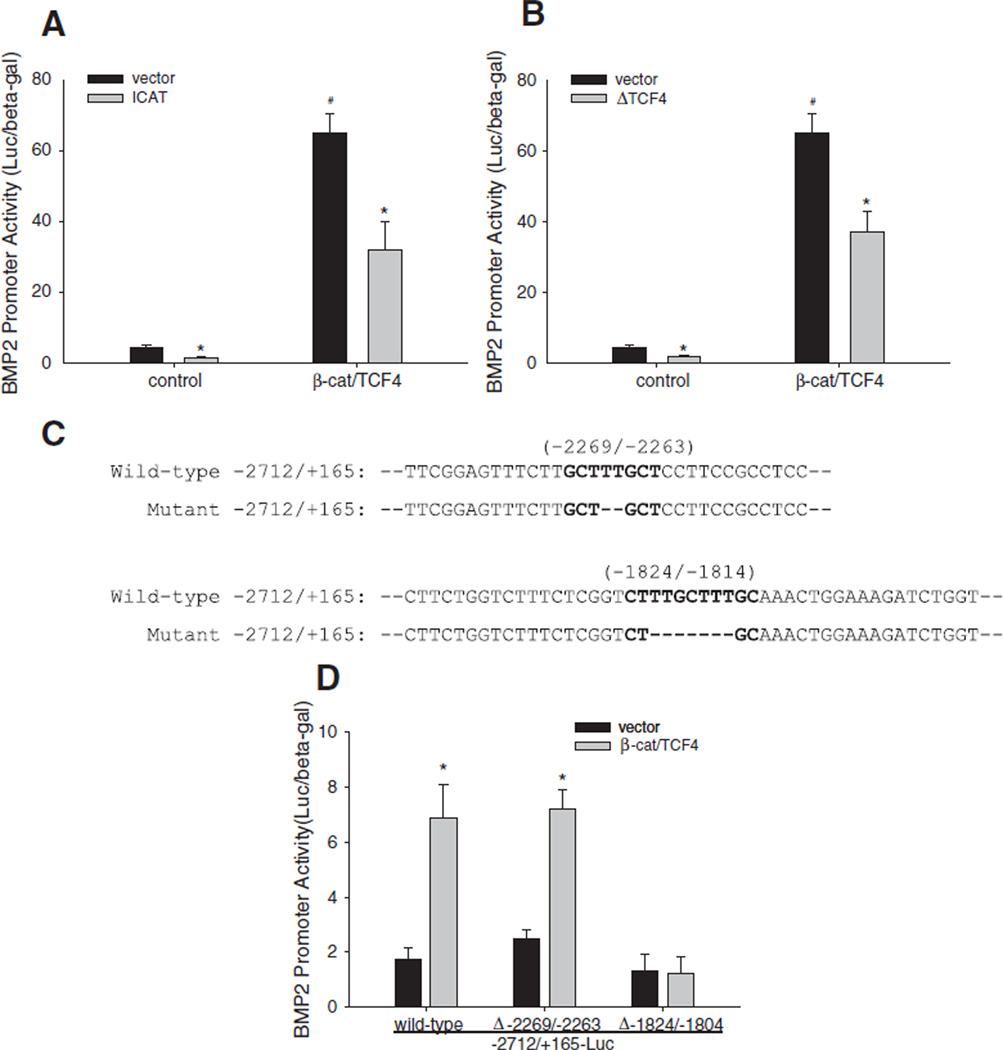
β-Catenin migrates into nuclei and forms a complex with transcriptional factor Tcf/Lef that transactivates target genes of the Wnt signaling pathway. To elucidate the functional interaction between β-catenin and Tcf/Lef in BMP2 gene activation, we used specific Wnt signaling inhibitors that inhibit formation of the β-catenin/Tcf/Lef complex, including (1) ICAT (an inhibitor of β-catenin and TCF that competitively blocks the interaction of β-catenin with Tcf/Lef [55,68,69]) and (2) a dominant negative mutant form of TCF4 (ΔTCF4) that prevents endogenous Tcf/Lef from binding with β-catenin and also blocks Tcf/Lef interaction with Tcf/Lef responsive elements (TRE) [70] in DNA. In C2C12 cells, forced expression of these inhibitors significantly reduced basal levels of BMP2 promoter activity (i.e. induced by endogenous β-catenin/Tcf/Lef complex) and also significantly attenuated the effect of exogenous β-catenin/TCF4 on BMP2 promoter activity (Fig. 6A and B). The β-catenin/Tcf/Lef transcription factor complex binds and initiates transcription through a TRE motif “C/T-CTTTG-A/T-A/T” in the promoter of target genes. By sequence analysis we identified three putative TREs in the BMP2 promoter that could potentially respond to β-catenin/Tcf/Lef. One TRE “CTTTGCT” was located between −2269/−2263 bp and other two tandem TREs “CTTTGCTTTGCA” were arrayed in the region of −1824/−1814 bp to −1814 bp (Fig. 6C). To evaluate the role of these putative TREs in β-catenin/TCF transactivation of BMP2 promoter, the TREs were mutated by deleting the core TRE sequences in the promoter using a site-directed mutagenesis approach (Fig. 6C) Their responsiveness to β-catenin/TCF4 was examined thereafter using the BMP promoter-luciferase reporter assay. Mutation of the binding site at −2269/−2263 did not alter the BMP promoter responsiveness to β-catenin/TCF (Fig. 6D). However, compared with wild-type BMP2 promoter reporter, the BMP2 promoter reporter where the tandem binding sites were deleted completely lost its responsiveness to stimulation by β-catenin/TCF4 (Fig. 6D). Together, these results suggest that the putative Tcf/Lef responsive elements at −1824/−1804 bp in the BMP2 promoter are functional binding sites for the β-catenin/Tcf/Lef transcription complex. This direct interaction between β-catenin/Tcf/Lef and these promotor specific binding sites contribute to transactivation of BMP2 expression in osteoblasts.
Fig. 6.
Transactivation of BMP2 promoter by Wnt/β-catenin signaling through Tcf/Lef response elements. (A and B) Effects of inhibitor of interaction of β-catenin and TCF4 on BMP2 promoter activity. C2C12 cells were co-transfected with −2712/+165-Luc reporter and expression vectors for β-catenin and TCF4, and expression vectors for ICAT (A) or ΔTCF4 (B) for 36 h. Relative luciferase activity in the cell lysates was measured and normalized by β-gal activity. *: P<0.05 ICAT or ΔTCF4 vs. vector; #: P<0.05 ICAT+β-catenin/TCF4 or ΔTCF4+β-catenin/ TCF4 vs. vector+β-catenin/TCF4 (n=6). (C) Mutagenesis of TREs in the BMP2 promoter. The core nucleotides (bold dashes) of putative TREs at −2269/−2263 and −1824/−1804 (bold) in the mouse BMP2 promoter reporter −2712/+165-Luc were deleted using synthesized mutagenesis DNA oligonucleotides. (D) Effects of mutated TREs on β-catenin/TCF4 activation of BMP2 promoter activity. C2C12 cells were co-transfected with−2712/+165-Luc reporter containing wild-type TREs or mutated TREs (Δ-2269/−2263, Δ-1824/−1804) with expression vectors for β-catenin and TCF4 for 36 h. Relative luciferase activity in the cell lysates was measured and normalized by β-gal activity.*:P<.01 β-catenin/TCF4 vs. vector (n=6).
Discussion
The BMP and Wnt signaling pathways tightly regulate each other. Both pathways play an important role in embryonic development and tumorigenesis. Activation of Wnt signaling induces expression of BMP family members including BMP2, BMP4, and BMP7 and increases expression of BMP target genes such as Msx and gremlin in the mesenchyme [62]. Similarly, in gastrointestinal cancer cells, β-catenin induces BMP2 expression [63]. Both pathways play a critical role in postnatal bone formation, as demonstrated by several genetically engineered mouse models during the past decade. However, the effects of Wnt signaling on BMP expression in osteoblasts, and the involved molecular mechanisms remain unclear. Given the existence of multiple putative TREs in the promoter of the BMP2 gene, this study was designed to test the central hypothesis that the Wnt pathway is an upstream activator of BMP2 expression in osteogenic cells.
β-Catenin is a pivotal signaling molecule of the Wnt pathway. Activation of canonical Wnt signaling with a Wnt ligand increased β-catenin protein levels in different osteoblastic cell lines. This increase could be a result of Wnt signaling preventing proteolytic processing of β-catenin (by the proteasome), resulting in β-catenin accumulation in the cytoplasm, as described in several cell types [56,71–75]. β-Catenin migrates from the cytoplasm into nuclei where it forms a complex with the co-activator Tcf/Lef and transactivates Wnt target genes [70,76,77]. Using a Wnt-specific reporter TOPFLASH [54], we showed that overexpression of β-catenin increased Wnt signaling activity which was synergistically increased by co-overexpression of TCF4. Moreover, forced expression of mutated forms of β-catenin, in which Ser45 was deleted [β-catenin(Δ45)] or Ser33 was replaced by Tyr33 [β-catenin(S33Y)] [53], produced more potent stimulatory effects on Wnt reporter than wild-type β-catenin. These mutants were previously found to protect β-catenin from proteolytic degradation. Therefore, the increased activity of these mutated β-catenin forms is most likely due to reduced degradation of β-catenin protein in the osteoblastic cells. However, this needs to further validated because of the uncertainty in the protein levels of the different forms post-transfection. In our previous effort to study the interaction between multiple anabolic signaling pathways in osteoblasts, we constructed an osteoblast-specific luciferase reporter, 9×6-OC-Luc. This reporter contains response elements for multiple signaling pathways, including TRE for the Wnt/β-catenin pathway. In the current study, we showed that β-catenin/TCF4 activated this reporter, which further demonstrated Wnt/β-catenin signaling in osteoblasts. However, this osteoblast-specific reporter also contains multiple copies of Smad response elements (SBE) and Runx2 binding elements (OSE2). Therefore it is possible that the stimulatory effect of β-catenin/TCF4 on the reporter could be mediated, at least in part, through these binding elements.
To clarify this, we utilized a BMP-specific reporter construct, 12SBE-OC-Luc [13,17,57,58]. We showed that, similar to BMP2, Wnt3a treatment also increased transcriptional activity of this BMP/ Smad reporter and this Wnt3a-induced response was blocked by noggin. This data strongly suggest that Wnt signaling acts as an upstream regulator of BMP signaling activity, and this function is dependent on BMP activity. Although DKK1 attenuated the effect of Wnt3a on the BMP/Smad reporter, it did not block BMP2-induced activation of the reporter. In addition, DKK1 also did not block BMP2-enhanced ALP activity in osteoblast cell lines and primary osteoblastic cells (Fig. 2B and C). We speculate that DKK1 did not block BMP2-activation of BMP signaling activity, despite the fact that it inhibits BMP2 expression in osteoblasts (as we convincingly demonstrated in this study), because it does not block the stimulatory effect of exogenously applied BMP2 ligand. Nonetheless, in ex vivo bone organ explant assays, sKremen synergistically enhanced the effect of BMP2 on osteoblast proliferation and new bone formation. This suggests that sKremen binding and sequestering DKK1 enhances LRP5/6 signaling and thereby concomitantly potentiates BMP2 signaling. Together, this data provides further evidence of complex interactions between the BMP and Wnt signaling pathways.
We also demonstrated that activation of Wnt signaling by either treatment with Wnt3a or by overexpressing β-catenin/TCF4 strongly induced BMP2 transcription in osteoblasts. As we recognized, Wnt3a at a range of 20–80 ng/ml dose-dependently increased BMP2 mRNA expression in C2C12 cells, while it reached a peak at 20 ng/ml in 2T3 cells (Fig. 3B). A possible explanation for the difference in the response of the two cell lines is that 2T3 cells are less tolerant than C2C12 cells to exposure to exogenous Wnt3a, such that when Wnt3a reached higher concentrations it may initiate a negative feedback that attenuates Wnt action [78]. In contrast, inhibition of the Wnt pathway with Wnt antagonists (DKK1 and sFRP4) or by overexpressing E3 ubiquitin ligase (FWD1) reduced basal or Wnt/β-catenin-induced BMP2 expression in osteoblasts. Incubation with noggin also partially blocked the stimulatory effect of β-catenin/ TCF4 on BMP2 transcription. This cross-antagonistic effect of noggin could be attributed to the auto-regulatory property of BMP2 expression [8,18–20], i.e. β-catenin/TCF4 stimulates BMP2 expression which in turn further increases expression of BMP2 itself. In essence, noggin blocked this auto-induction, leading to partial inhibition of β-catenin/ TCF4 activation of BMP2 expression. FWD1/β-TrCP induces proteolytic degradation of β-catenin, thus blocking the Wnt-induced β-catenin-mediated signaling cascade [56,71–75]. As expected, we showed that overexpression of wild-type FWD1/β-TrCP reduced both basal and β-catenin-induced BMP2 transcription. This is due to its ability to induce degradation of both endogenous and exogenous β-catenin. However, in contrast with the stimulatory effects of the dominant-negative FWD1ΔF mutant in the TOPFLASH reporter (presumably by competitively inhibiting proteolytic processing of both endogenous and exogenous FWD1/β-TrCP), FWD1ΔF did not increase BMP2 promoter activity. Presently, it is unclear why the dominant-negative had no effect on BMP2 expression in osteoblasts and further studies are required to clarify this. Together, the above findings provide direct evidence that the Wnt/ β-catenin signaling pathway is a strong activator of BMP2 transcription in osteoblasts. Nevertheless, previous studies on Wnt regulation of BMP expression have shown a repressing function of Wnt on expression of the dpp gene, a Drosophila homologue of BMP2 [64], which suggested Wnt regulation of BMP2 expression could be a complex event.
Regulation of β-catenin activity also can occur at the transcriptional level within the nucleus. We showed that forced expression of either ICAT or ΔTCF4, both of which block physical interaction between β-catenin and TCF [55,68–70], antagonized both basal and β-catenin-enhanced BMP2 promoter activity. This suggests that formation of the β-catenin/TCF complex is required for Wnt activation of BMP2 transcription. TCF is known to bind to TREs, thus trans-activating target genes [47,49,77,79,80]. Our mutagenesis assay suggested that, among the three putative TREs in the mouse BMP2 promoter, two proximal tandem TREs (CTTTGCTTTGCA) are functional. However, it is yet to be determined if either or both together participate in β-catenin/TCF activation of the BMP2 gene. This will require further studies involving precise mutations of each tandem TRE.
Data from our published work and those from other laboratories have demonstrated that transcriptional regulation of BMP2 is complex, involving multiple signaling pathways [8,10,11,13,14,16,17,20], including the hedgehog (Hh)/Gli [11,13,14] and PTH/CREB pathways that we recently reported [17]. These pathways cross talk with the Wnt signaling pathway in controlling osteoblast differentiation and skeletal homeostasis. Therefore, it is likely that the Wnt pathway also cross talks with one or more of these other signaling pathways to modulate BMP2 expression in osteoblasts. The zinc finger transcription factors Gli2 and Gli3 mediate Hh signaling to target genes. We previously reported that Gli2 is a powerful enhancer of BMP2 transcription in osteoblasts [14], and a C-terminal truncated Gli3 acts as a transcriptional repressor (Gli3rep) [11]. We also found that Gli3rep markedly attenuated β-catenin/TCF4 activation of BMP2 transcription in osteoblasts (data not shown). This suggests another potential mechanism where BMP2 transcription is controlled by cross talk between the Hh and Wnt signaling pathways.
In summary, these findings provide compelling evidence for a mechanism by which the canonical Wnt pathway promotes the expression of the master osteogenic factor BMP2 in osteoblasts, through a direct transactivation by β-catenin/TCF. These studies strongly suggest that functional communication between the BMP and Wnt pathways play an important role in integrating the anabolic functions of both pathways in bone.
Acknowledgments
This manuscript is dedicated to the memory of the late Dr. Gregory Mundy for his support and constant encouragement of Drs. Ming Zhao and Babatunde Oyajobi over many years. We thank Alda Flores for performing the neonatal mouse calvarial assay and Ms. Elly Trepman for her help with editing the manuscript. The authors declare that there are no conflicts of interests.
Abbreviations
- ALP
alkaline phosphatase
- BGJ
medium, Biggers, Gwatkin, and Judah medium
- BMP
bone morphogenetic protein
- β-TrCP
β-transducin repeat-containing protein
- Col1a1
type I collagen 1a1
- CTGF
connective-tissue growth factor
- CREB
cAMP responsive element binding protein
- DKK1
Dickkopf-related protein 1
- FWD1
F-box/ WD40-repeat protein 1
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSK3
glycogen synthase kinase 3
- H&E
hematoxylin and eosin
- ICAT
murine inhibitor of β-catenin and transcription factor
- LRP5/6
low-density lipoprotein receptor-related protein 5/6
- Lef
lymphoid enhancer binding factor
- NF-κB
nuclear factor κB
- OSE2
Runx2 binding elements
- PGE2
prostaglandin E2
- PTH
parathyroid hormone
- RT-PCR
reverse transcription polymerase chain reaction
- SBE
Smad binding element
- sFRP4
secreted frizzled-related protein 4
- sKremen
soluble form of the extracellular ligand-binding domain (amino acids 20–395) of Kremen-1
- TCF (Tcf)
T cell specific factor
- TGF-β
transforming growth factor beta
- TRE
Tcf/Lef response elements or transcription regulatory elements
- Wnt
wingless integration site
References
- 1.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res. 1998:26–37. [PubMed] [Google Scholar]
- 3.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Harris MA, Rossini G, Dunstan CR, Dallas SL, Feng JQ, et al. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif Tissue Int. 1997;60:283–290. doi: 10.1007/s002239900230. [DOI] [PubMed] [Google Scholar]
- 5.Harris SE, Guo D, Harris MA, Krishnaswamy A, Lichtler A. Transcriptional regulation of BMP-2 activated genes in osteoblasts using gene expression microarray analysis: role of Dlx2 and Dlx5 transcription factors. Front Biosci. 2003;8:s1249–s1265. doi: 10.2741/1170. [DOI] [PubMed] [Google Scholar]
- 6.Zhao M, Harris SE, Horn D, Geng Z, Nishimura R, Mundy GR, et al. Bone morphogenetic protein receptor signaling is necessary for normal murine postnatal bone formation. J Cell Biol. 2002;157:1049–1060. doi: 10.1083/jcb.200109012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh-Choudhury N, Harris MA, Feng JQ, Mundy GR, Harris SE. Expression of the BMP 2 gene during bone cell differentiation. Crit Rev Eukaryot Gene Expr. 1994;4:345–355. doi: 10.1615/critreveukargeneexpr.v4.i2-3.30. [DOI] [PubMed] [Google Scholar]
- 8.Harris SE, Sabatini M, Harris MA, Feng JQ, Wozney J, Mundy GR. Expression of bone morphogenetic protein messenger RNA in prolonged cultures of fetal rat calvarial cells. J Bone Miner Res. 1994;9:389–394. doi: 10.1002/jbmr.5650090314. [DOI] [PubMed] [Google Scholar]
- 9.Arikawa T, Omura K, Morita I. Regulation of bone morphogenetic protein-2 expression by endogenous prostaglandin E2 in human mesenchymal stem cells. J Cell Physiol. 2004;200:400–406. doi: 10.1002/jcp.20031. [DOI] [PubMed] [Google Scholar]
- 10.Feng JQ, Xing L, Zhang JH, Zhao M, Horn D, Chan J, et al. NF-kappaB specifically activates BMP-2 gene expression in growth plate chondrocytes in vivo and in a chondrocyte cell line in vitro. J Biol Chem. 2003;278:29130–29135. doi: 10.1074/jbc.M212296200. [DOI] [PubMed] [Google Scholar]
- 11.Garrett IR, Chen D, Gutierrez G, Zhao M, Escobedo A, Rossini G, et al. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest. 2003;111:1771–1782. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris SE, Bonewald LF, Harris MA, Sabatini M, Dallas S, Feng JQ, et al. Effects of transforming growth factor beta on bone nodule formation and expression of bone morphogenetic protein 2, osteocalcin, osteopontin, alkaline phosphatase, and type I collagen mRNA in long-term cultures of fetal rat calvarial osteoblasts. J Bone Miner Res. 1994;9:855–863. doi: 10.1002/jbmr.5650090611. [DOI] [PubMed] [Google Scholar]
- 13.Zhao M, Ko SY, Liu JH, Chen D, Zhang J, Wang B, et al. Inhibition of microtubule assembly in osteoblasts stimulates bone morphogenetic protein 2 expression and bone formation through transcription factor Gli2. Mol Cell Biol. 2009;29:1291–1305. doi: 10.1128/MCB.01566-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao M, Qiao M, Harris SE, Chen D, Oyajobi BO, Mundy GR. The zinc finger transcription factor Gli2 mediates bone morphogenetic protein 2 expression in osteoblasts in response to hedgehog signaling. Mol Cell Biol. 2006;26:6197–6208. doi: 10.1128/MCB.02214-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou S, Turgeman G, Harris SE, Leitman DC, Komm BS, Bodine PV, et al. Estrogens activate bone morphogenetic protein-2 gene transcription in mouse mesenchymal stem cells. Mol Endocrinol. 2003;17:56–66. doi: 10.1210/me.2002-0210. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Zhang R, Ko SY, Oyajobi BO, Papasian CJ, Deng HW, et al. Microtubule assembly affects bone mass by regulating both osteoblast and osteoclast functions: stathmin deficiency produces an osteopenic phenotype in mice. J Bone Miner Res. 2011;26:2052–2067. doi: 10.1002/jbmr.419. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, Edwards JR, Ko SY, Dong S, Liu H, Oyajobi BO, et al. Transcriptional regulation of BMP2 expression by the PTH-CREB signaling pathway in osteoblasts. PLoS One. 2011;6:e20780. doi: 10.1371/journal.pone.0020780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng JQ, Chen D, Ghosh-Choudhury N, Esparza J, Mundy GR, Harris SE. Bone morphogenetic protein 2 transcripts in rapidly developing deer antler tissue contain an extended 5' non-coding region arising from a distal promoter. Biochim Biophys Acta. 1997;1350:47–52. doi: 10.1016/s0167-4781(96)00178-9. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh-Choudhury N, Abboud SL, Nishimura R, Celeste A, Mahimainathan L, Choudhury GG. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J Biol Chem. 2002;277:33361–33368. doi: 10.1074/jbc.M205053200. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh-Choudhury N, Choudhury GG, Harris MA, Wozney J, Mundy GR, Abboud SL, et al. Autoregulation of mouse BMP-2 gene transcription is directed by the proximal promoter element. Biochem Biophys Res Commun. 2001;286:101–108. doi: 10.1006/bbrc.2001.5351. [DOI] [PubMed] [Google Scholar]
- 21.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 22.Clement-Lacroix P, Ai M, Morvan F, Roman-Roman S, Vayssiere B, Belleville C, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102:17406–17411. doi: 10.1073/pnas.0505259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 25.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 27.Morvan F, Boulukos K, Clement-Lacroix P, Roman Roman S, Suc-Royer I, Vayssiere B, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21:934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 28.Ellwanger K, Saito H, Clement-Lacroix P, Maltry N, Niedermeyer J, Lee WK, et al. Targeted disruption of the Wnt regulator Kremen induces limb defects and high bone density. Mol Cell Biol. 2008;28:4875–4882. doi: 10.1128/MCB.00222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Bain G, Muller T, Wang X, Papkoff J. Activated beta-catenin induces osteoblast differentiation of C3H10T1/2 cells and participates in BMP2 mediated signal transduction. Biochem Biophys Res Commun. 2003;301:84–91. doi: 10.1016/s0006-291x(02)02951-0. [DOI] [PubMed] [Google Scholar]
- 31.Glass IIDA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18:1842–1853. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda T, Kokabu S, Ohte S, Sasanuma H, Kanomata K, Yoneyama K, et al. Canonical Wnts and BMPs cooperatively induce osteoblastic differentiation through a GSK3beta-dependent and beta-catenin-independent mechanism. Differentiation. 2010;80:46–52. doi: 10.1016/j.diff.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Kusu N, Laurikkala J, Imanishi M, Usui H, Konishi M, Miyake A, et al. Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J Biol Chem. 2003;278:24113–24117. doi: 10.1074/jbc.M301716200. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 36.Winkler DG, Sutherland MS, Ojala E, Turcott E, Geoghegan JC, Shpektor D, et al. Sclerostin inhibition of Wnt-3a-induced C3H10T1/2 cell differentiation is indirect and mediated by bone morphogenetic proteins. J Biol Chem. 2005;280:2498–2502. doi: 10.1074/jbc.M400524200. [DOI] [PubMed] [Google Scholar]
- 37.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development. 2004;131:2137–2147. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- 39.Misra K, Matise MP. A critical role for sFRP proteins in maintaining caudal neural tube closure in mice via inhibition of BMP signaling. Dev Biol. 2010;337:74–83. doi: 10.1016/j.ydbio.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, et al. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edlund S, Lee SY, Grimsby S, Zhang S, Aspenstrom P, Heldin CH, et al. Interaction between Smad7 and beta-catenin: importance for transforming growth factor beta-induced apoptosis. Mol Cell Biol. 2005;25:1475–1488. doi: 10.1128/MCB.25.4.1475-1488.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eivers E, Demagny H, Choi RH, De Robertis EM. Phosphorylation of Mad controls competition between wingless and BMP signaling. Sci Signal. 2011;4:ra68. doi: 10.1126/scisignal.2002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eivers E, Demagny H, De Robertis EM. Integration of BMP and Wnt signaling via vertebrate Smad1/5/8 and Drosophila Mad. Cytokine Growth Factor Rev. 2009;20:357–365. doi: 10.1016/j.cytogfr.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, et al. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z, Tang Y, Qiu T, Cao X, Clemens TL. A dishevelled-1/Smad1 interaction couples WNT and bone morphogenetic protein signaling pathways in uncommitted bone marrow stromal cells. J Biol Chem. 2006;281:17156–17163. doi: 10.1074/jbc.M513812200. [DOI] [PubMed] [Google Scholar]
- 46.Tang Y, Liu Z, Zhao L, Clemens TL, Cao X. Smad7 stabilizes beta-catenin binding to E-cadherin complex and promotes cell-cell adhesion. J Biol Chem. 2008;283:23956–23963. doi: 10.1074/jbc.M800351200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chakladar A, Dubeykovskiy A, Wojtukiewicz LJ, Pratap J, Lei S, Wang TC. Synergistic activation of the murine gastrin promoter by oncogenic Ras and beta-catenin involves SMAD recruitment. Biochem Biophys Res Commun. 2005;336:190–196. doi: 10.1016/j.bbrc.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 48.Hu MC, Rosenblum ND. Smad1, beta-catenin and Tcf4 associate in a molecular complex with the Myc promoter in dysplastic renal tissue and cooperate to control Myc transcription. Development. 2005;132:215–225. doi: 10.1242/dev.01573. [DOI] [PubMed] [Google Scholar]
- 49.Hussein SM, Duff EK, Sirard C. Smad4 and beta-catenin co-activators functionally interact with lymphoid-enhancing factor to regulate graded expression of Msx2. J Biol Chem. 2003;278:48805–48814. doi: 10.1074/jbc.M305472200. [DOI] [PubMed] [Google Scholar]
- 50.Labbe E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc Natl Acad Sci U S A. 2000;97:8358–8363. doi: 10.1073/pnas.150152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lei S, Dubeykovskiy A, Chakladar A, Wojtukiewicz L, Wang TC. The murine gastrin promoter is synergistically activated by transforming growth factor-beta/Smad and Wnt signaling pathways. J Biol Chem. 2004;279:42492–42502. doi: 10.1074/jbc.M404025200. [DOI] [PubMed] [Google Scholar]
- 52.Sakai D, Tanaka Y, Endo Y, Osumi N, Okamoto H, Wakamatsu Y. Regulation of Slug transcription in embryonic ectoderm by beta-catenin-Lef/Tcf and BMP-Smad signaling. Dev Growth Differ. 2005;47:471–482. doi: 10.1111/j.1440-169X.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- 53.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 54.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 55.Tago K, Nakamura T, Nishita M, Hyodo J, Nagai S, Murata Y, et al. Inhibition of Wnt signaling by ICAT, a novel beta-catenin-interacting protein. Genes Dev. 2000;14:1741–1749. [PMC free article] [PubMed] [Google Scholar]
- 56.Kitagawa M, Hatakeyama S, Shirane M, Matsumoto M, Ishida N, Hattori K, et al. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J. 1999;18:2401–2410. doi: 10.1093/emboj/18.9.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao M, Qiao M, Oyajobi BO, Mundy GR, Chen D. E3 ubiquitin ligase Smurf1 mediates core-binding factor alpha1/Runx2 degradation and plays a specific role in osteoblast differentiation. J Biol Chem. 2003;278:27939–27944. doi: 10.1074/jbc.M304132200. [DOI] [PubMed] [Google Scholar]
- 58.Li W, Yu B, Li M, Sun D, Hu Y, Zhao M, et al. NEMO-binding domain peptide promotes osteoblast differentiation impaired by tumor necrosis factor alpha. Biochem Biophys Res Commun. 2010;391:1228–1233. doi: 10.1016/j.bbrc.2009.12.048. [DOI] [PubMed] [Google Scholar]
- 59.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 60.Mohammad KS, Chirgwin JM, Guise TA. Assessing new bone formation in neonatal calvarial organ cultures. Methods Mol Biol. 2008;455:37–50. doi: 10.1007/978-1-59745-104-8_3. [DOI] [PubMed] [Google Scholar]
- 61.Clines GA, Mohammad KS, Bao Y, Stephens OW, Suva LJ, Shaughnessy JD, Jr, et al. Dickkopf homolog 1 mediates endothelin-1-stimulated new bone formation. Mol Endocrinol. 2007;21:486–498. doi: 10.1210/me.2006-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hill TP, Taketo MM, Birchmeier W, Hartmann C. Multiple roles of mesenchymal beta-catenin during murine limb patterning. Development. 2006;133:1219–1229. doi: 10.1242/dev.02298. [DOI] [PubMed] [Google Scholar]
- 63.Kim JS, Crooks H, Dracheva T, Nishanian TG, Singh B, Jen J, et al. Oncogenic beta-catenin is required for bone morphogenetic protein 4 expression in human cancer cells. Cancer Res. 2002;62:2744–2748. [PubMed] [Google Scholar]
- 64.Theisen H, Syed A, Nguyen BT, Lukacsovich T, Purcell J, Srivastava GP, et al. Wingless directly represses DPP morphogen expression via an armadillo/TCF/Brinker complex. PLoS One. 2007;2:e142. doi: 10.1371/journal.pone.0000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu H, Zhang R, Chen D, Oyajobi BO, Zhao M. Functional redundancy of type II BMP receptor and type IIB activin receptor in BMP2-induced osteoblast differentiation. J Cell Physiol. 2011;227:952–963. doi: 10.1002/jcp.22802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He B, Lee AY, Dadfarmay S, You L, Xu Z, Reguart N, et al. Secreted frizzled-related protein 4 is silenced by hypermethylation and induces apoptosis in beta-catenin-deficient human mesothelioma cells. Cancer Res. 2005;65:743–748. [PubMed] [Google Scholar]
- 67.Muley A, Majumder S, Kolluru GK, Parkinson S, Viola H, Hool L, et al. Secreted frizzled-related protein 4: an angiogenesis inhibitor. Am J Pathol. 2010;176:1505–1516. doi: 10.2353/ajpath.2010.090465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gottardi CJ, Gumbiner BM. Role for ICAT in beta-catenin-dependent nuclear signaling and cadherin functions. Am J Physiol Cell Physiol. 2004;286:C747–C756. doi: 10.1152/ajpcell.00433.2003. [DOI] [PubMed] [Google Scholar]
- 69.Kim YJ, Kim JT, Bae YC, Suh KT, Jung JS. ICAT participates in proliferation and osteogenic differentiation of human adipose tissue-derived mesenchymal stem cell. Life Sci. 2008;83:851–858. doi: 10.1016/j.lfs.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 70.Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, et al. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol. 2001;152:87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, et al. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol. 1999;9:207–210. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- 72.Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- 73.Latres E, Chiaur DS, Pagano M. The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene. 1999;18:849–854. doi: 10.1038/sj.onc.1202653. [DOI] [PubMed] [Google Scholar]
- 74.Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X. beta-Trcp couples beta-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci U S A. 1999;96:6273–6278. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marikawa Y, Elinson RP. beta-TrCP is a negative regulator of Wnt/beta-catenin signaling pathway and dorsal axis formation in Xenopus embryos. Mech Dev. 1998;77:75–80. doi: 10.1016/s0925-4773(98)00134-8. [DOI] [PubMed] [Google Scholar]
- 76.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 77.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 78.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Letamendia A, Labbe E, Attisano L. Transcriptional regulation by Smads: crosstalk between the TGF-beta and Wnt pathways. J Bone Joint Surg Am. 2001;83-A(Suppl. 1):S31–S39. [PubMed] [Google Scholar]
- 80.Theil T, Aydin S, Koch S, Grotewold L, Ruther U. Wnt and Bmp signalling cooperatively regulate graded Emx2 expression in the dorsal telencephalon. Development. 2002;129:3045–3054. doi: 10.1242/dev.129.13.3045. [DOI] [PubMed] [Google Scholar]