Abstract
Background/Aims
Recurrence after hepatic resection is one of the most important factors impacting the prognosis and survival of patients with hepatocellular carcinoma (HCC). We identified prognostic factors affecting overall survival (OS) and disease-free survival (DFS) in patients with HCC after hepatic resection.
Methods
This study was of a retrospective cohort design, and 126 patients who underwent hepatic resection for HCC at Gachon University Gil Medical Center between January 2005 and December 2010 were enrolled. Various clinical, laboratory, and pathological data were evaluated to determine the prognostic factors affecting OS and DFS.
Results
Two- and 4-year OS and 2- and 4-year DFS were 78.1% and 65% and 51.1% and 26.6%, respectively. In a multivariate analysis, preoperative α-fetoprotein (> 400 ng/mL), tumor size (≥ 5 cm), multiple tumors (two or more nodules), presence of portal vein invasion, modified Union for International Cancer Control (UICC) stage III/IV, and Barcelona Clinic Liver Cancer (BCLC) stage B/C were independent prognostic factors affecting a shorter OS. In the multivariate analysis, presence of microvascular invasion, modified UICC stage III/IV, and BCLC stage B/C were independent prognostic factors for a shorter DFS.
Conclusions
The presence of vascular invasion was an independent poor prognostic factor for OS and DFS in patients with HCC after hepatic resection. Thus, close postoperative surveillance for early detection of recurrence and additional treatments are urgently needed in patients with vascular invasion after hepatic resection.
Keywords: Carcinoma, hepatocellular; Hepatic resection; Prognosis; Vascular invasion
INTRODUCTION
Since 2000, hepatoma has been the fifth most common cancer worldwide, comprising 5.6% of all cancers [1]. In Korea, an analysis of the incidence and prevalence of major cancers in 2008 suggested that hepatic neoplasm is the fifth most common cancer [2]. Hepatocellular carcinoma (HCC) comprises ~75% of all liver cancers in Korea [3]. The 5-year survival rate of HCC was 23.3% from 2004 to 2008, indicating a significantly poor prognosis compared to that of other cancers [2]. The prognosis of HCC is poor because it is an obstacle to active cancer therapy, as it causes earlier vascular invasion and is accompanied by rapid growth, chronic hepatitis, and liver cirrhosis. Furthermore, it is difficult to treat with curative therapy as it is often discovered at a more advanced stage due to the absence of specific symptoms unless a periodic inspection is conducted [4].
Treatments for HCC include hepatectomy, liver transplant, radiofrequency ablation (RFA), percutaneous ethanol injection, transarterial chemoembolization (TACE), anticancer therapy, and radiotherapy. The most likely curative therapy is liver transplantation; however, lack of donor livers, strict transplant criteria, and being dropped during the waiting period are major obstacles [5]. Therefore, hepatectomy is often conducted, and the recent mortality rate following hepatectomy has been reduced significantly due to preoperative examinations, development of surgical techniques, and improved management of patients, resulting in an increased long-term survival rate [6,7]. However, the 5-year reoccurrence rate of HCC after hepatectomy remains high at 77% to 100% [8].
HCC is staged on the basis of tumor size, number, and vascular invasion [9,10]. In the present study, these histopathological characteristics were evaluated to determine the prognostic factors affecting overall survival (OS) and disease-free survival (DFS) in patients with HCC after hepatectomy.
METHODS
Patient enrollment
A retrospective cohort study was conducted based on the individual medical records of 166 patients who underwent hepatectomy after being diagnosed with HCC at Gachon University Gil Medical Center from January 2005 to December 2010. The experimental protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Gachon University Gil Medical Center Human Research Committee.
Among the patients, 126 were finally selected with the exception of eight with ruptured a HCC, four with mixed HCC, two who died during the main operation, five with extrahepatic metastasis at the time of surgery, four with residual HCC after surgery, seven with no evidence of HCC in histopathological tissues due to preoperative treatment (RFA or TACE), one with no medical records, and nine who underwent liver transplantation. The cause of the existing liver disease, the presence of liver cirrhosis, the presence of cirrhosis complications, Child-Pugh classification, and preoperative HCC treatment method were investigated in all patients. Hepatitis B was defined as hepatitis B surface antigen (HBsAg) positive, hepatitis C as hepatitis C virus antibody (HCV Ab) positive, and liver cirrhosis was doubted radiologically as a result of laboratory inspection, so cases that underwent histopathological assessment were also included.
Laboratory findings
Preoperative serological tests, including alanine aminotransferase, total bilirubin, albumin, prothrombin time, hemoglobin, white blood cell count, platelets, sodium, creatinine, HBsAg, hepatitis B surface antibody, HCV Ab, α-fetoprotein (AFP), and indocyanine green retention at 15 minutes (ICG R15), were performed to evaluate residual liver function.
Radiological evaluation and HCC stage
Abdominal sonography, dynamic-contrast-enhanced computed tomography (CT), dynamic-contrast-enhanced magnetic resonance imaging (MRI), and hepatic antiography were performed before surgery, in which the modified Union for International Cancer Control (UICC) stage [9], and Barcelona Clinic Liver Cancer (BCLC) stage [10] were investigated.
Histopathological examination
The largest tumor diameter was taken as the size of HCC, regardless of the number of tumors, and the number, microvascular invasion, portal vein invasion, infiltration of the tumor into the hepatic capsule, inclusion of margins, and Edmondson tissue grade were examined. Vascular invasion is divided into macrovascular and microvascular invasion, the former involving mostly large- to medium-sized vessels, which can be visually perceived, but the latter (mainly in small vessels such as portal vein branches in portal tracts, central veins in noncancerous liver tissue, and venous vessels in the tumor capsule and/or noncapsular fibrous septa) can be observed only by microscopy [11]. Gross tumor findings and hematoxylin-and-eosin-stained slides of HCC were reviewed by one pathologist.
Patient follow-up
A basic physical examination, liver function test, AFP, and dynamic contrast-enhanced CT were performed through outpatient consultations at intervals of 3 to 4 months after surgery. Recurrence was defined as HCC observed on dynamic contrast-enhanced CT, dynamic contrast-enhanced MRI, or hepatic angiography or if there was evidence after liver biopsy or resurgery.
Statistical analysis
The SPSS version 13.0 software (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Continuous variables are reported as means ± standard deviation and categorical variables are reported as frequencies (percentages). Five-year OS and 5-year DFS were measured by the Kaplan-Meier method. Cox proportional hazard model was used in the univariate and multivariate analyses of factors affecting OS and DFS. A p value < 0.05 was considered to indicate significance.
RESULTS
Baseline characteristics
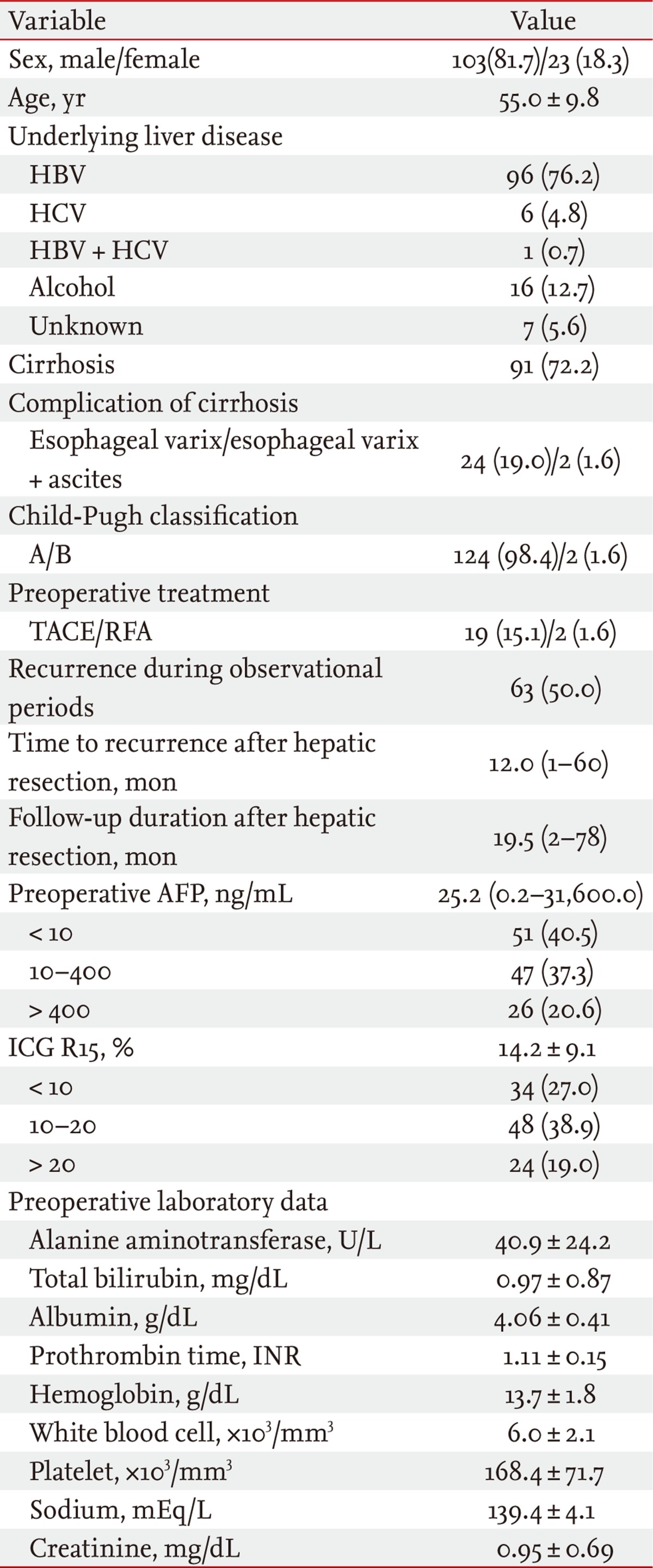
Of the 126 patients, 103 were male, and the average age at the time of hepatectomy was 55 years. Sixty-three (50.0%) patients developed recurrence after hepatectomy, and the time until recurrence after hepatectomy was a mean of 12 months, and the observation time was a mean of 19.5 months.
Hepatitis B was the most common cause of existing liver disease, in 96 patients (76.2%), followed by 16 with alcoholic liver disease (12.7%), seven with unknown liver disease (5.6%), six with hepatitis C (4.8%), and one with a superinfection of hepatitis B and hepatitis C (0.7%). Liver cirrhosis was found in 91 patients (72.2%), of which 26 (20.6%) had esophageal varix or multiple complications of liver cirrhosis. A total of 124 patients (98.4%) were Child-Pugh class A; the remaining two (1.6%) were Child-Pugh class B. Nineteen patients (15.1%) received TACE as preoperative HCC treatment, and two (1.6%) received RFA.
The median preoperative serum AFP value was 25.2 ng/mL, and the mean preoperative ICG R15 value was 14.2%. The mean preoperative level of alanine aminotransferase was 40.9 U/L; total bilirubin, 0.97 mg/dL; albumin, 4.06 g/dL; prothrombin time (international normalized ratio), 1.11; hemoglobin, 13.7 g/dL; white blood cells, 6.0 × 103/mm3; blood platelets, 168.4 × 103/mm3; sodium, 139.4 mEq/L; and serum creatinine, 0.95 mg/dL (Table 1).
Table 1.
Baseline characteristics (n = 126)
Values are presented as number (%), mean ± SD, or median (range).
HBV, hepatitis B virus; HCV, hepatitis C virus; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; AFP, α-fetoprotein; ICG R15, indocyanine green retention at 15 minutes; INR, international normalized ratio.
Histopathological examination and preoperative HCC stage
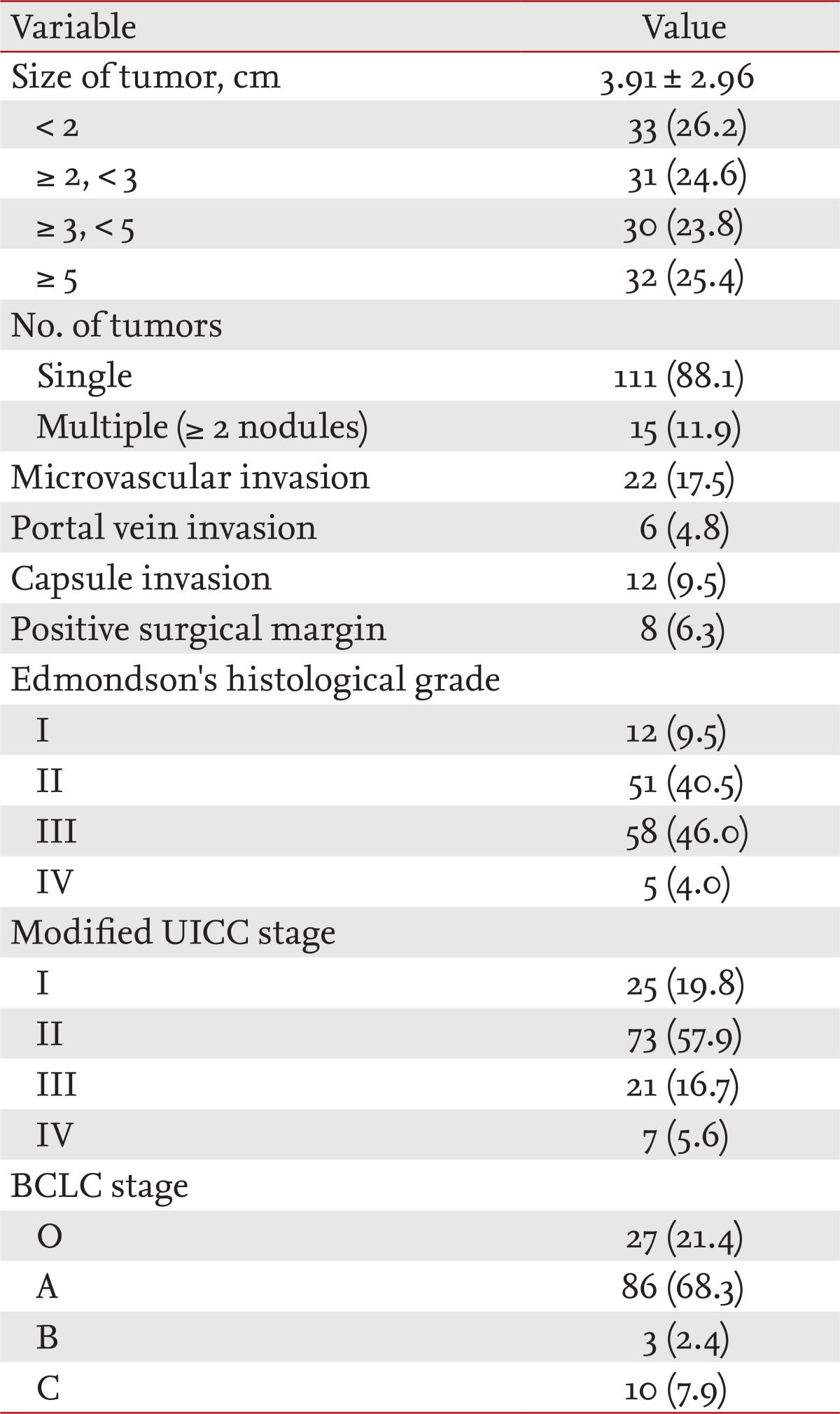
Average tumor size was 3.91 cm. A total of 111 patients (88.1%) had solitary carcinoma, and 15 (11.9%) had multiple tumors. In the pathological examination after hepatectomy, 22 (17.5%) had a microvascular invasion, six (4.8%) a portal vein invasion, 12 (9.5%) had infiltration of the tumor into the hepatic capsule, and eight (6.3%) had cancer cells on the margins. Twelve patients (9.5%) were assigned to Edmondson tissue grade I, 51 (40.2%) to grade II, 58 (46.0%) to grade III, and five (4.0%) to grade IV. Before hepatectomy, 25 patients (19.8%) were in modified UICC stage I, 73 (57.9%) were in stage II, 21 (16.7%) were in stage III, and seven (5.6%) were in stage IV. Twenty-seven patients (21.4%) were in BCLC stage O, 86 (68.3%) were in stage A, three (2.4%) were in stage B, and 10 (7.9%) were in stage C (Table 2).
Table 2.
Pathological characteristics and tumor stage (n = 126)
Values are presented as mean ± SD or number (%).
UICC, Union for International Cancer Control; BCLC, the Barcelona Clinic Liver Cancer.
Two- and 4-year survival and 2- and 4-year DFS after hepatectomy for HCC
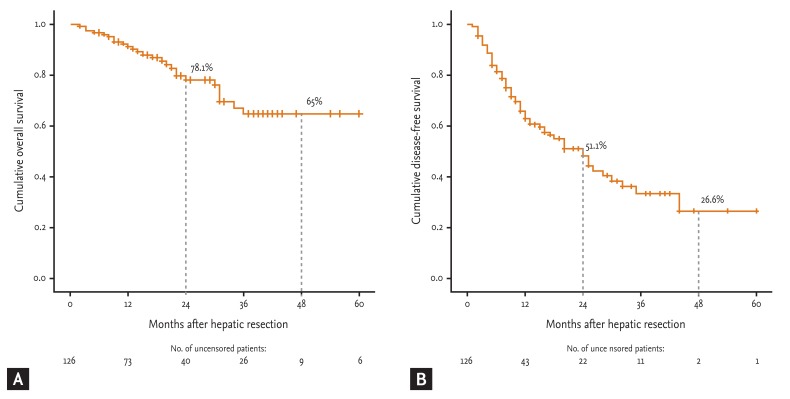
The 2- and 4-year OS and 2- and 4-year DFS were 78.1% and 65% and 51.1% and 26.6%, respectively, and the median 5-year OS and 5-year DFS were 63 and 24 months, respectively (Fig. 1).
Figure 1.
(A) Overall survival (OS) rate and (B) disease-free survival (DFS) rate for patients with hepatocellular carcinoma after hepatic resection. The OS and DFS rates were calculated by the Kaplan-Meier method.
Factors influencing survival rate after hepatectomy
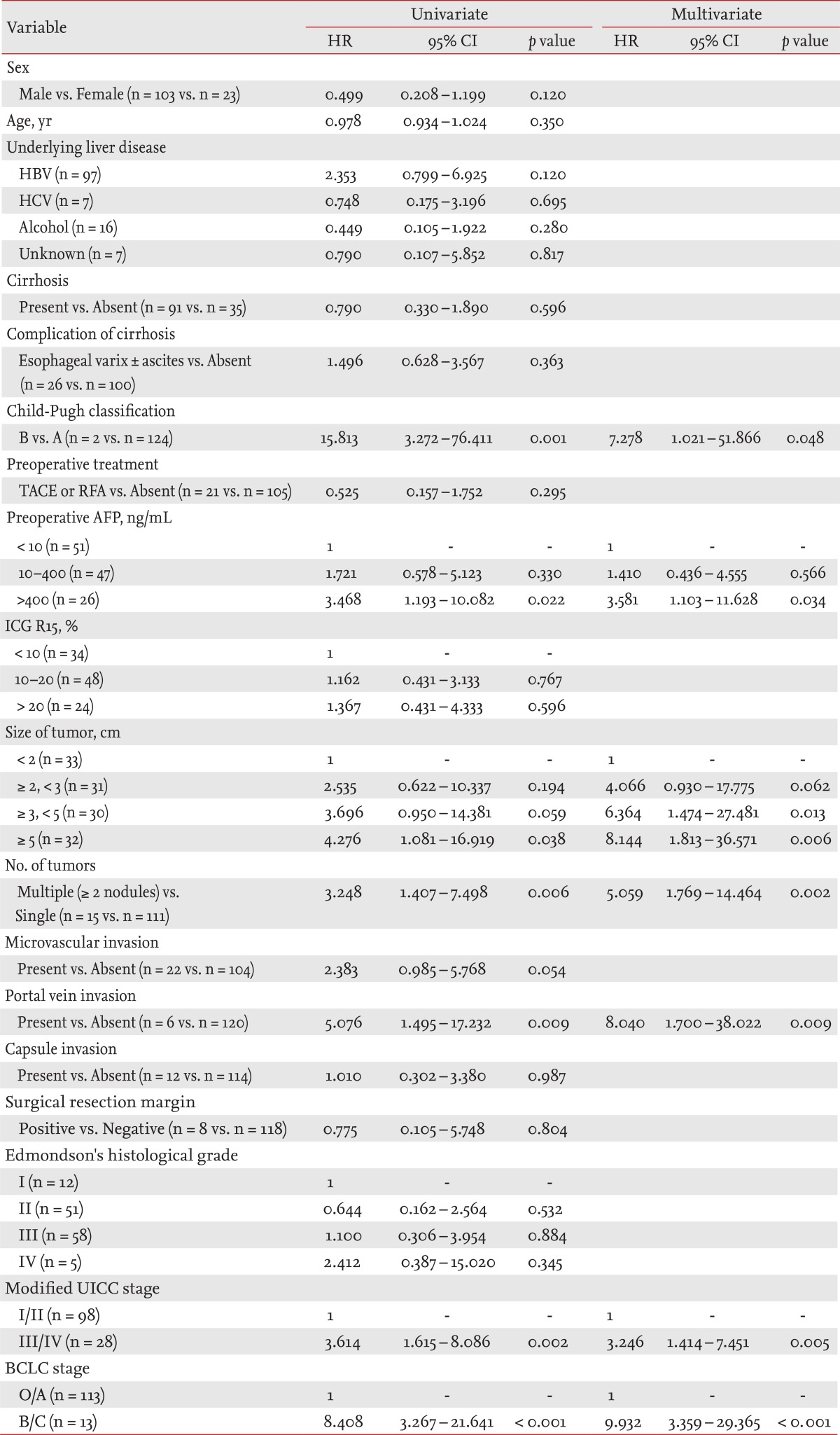
In the univariate analysis, survival rate was lower in patients with Child-Pugh class B (p = 0.001; hazard ratio [HR], 15.813; 95% confidence interval [Cl], 3.272 to 76.411), preoperative AFP > 400 ng/mL (p = 0.022; HR, 3.468; 95% CI, 1.193 to 10.082), tumor size ≥ 5 cm (p = 0.038; HR, 4.276; 95% CI, 1.081 to 16.919), more than two tumor nodules (p = 0.006; HR, 3.248; 95% CI, 1.407 to 7.498), portal vein invasion (p = 0.009; HR, 5.076; 95% CI, 1.495 to 17.232), modified UICC stage III/IV (p = 0.002; HR, 3.614; 95% CI, 1.615 to 8.086), and BCLC stage B/C (p < 0.001; HR, 8.408; 95% CI, 3.267 to 21.641). In the multivariate analysis performed with significant variables from the univariate analysis, Child-Pugh class B (p = 0.048; HR, 7.278; 95% CI, 1.021 to 51.866), preoperative AFP > 400 ng/mL (p = 0.034; HR, 3.581; 95% CI, 1.103 to 11.628), tumor size ≥ 5 cm (p = 0.006; HR, 8.144; 95% CI, 1.813 to 36.571), more than two tumor nodules (p = 0.002; HR, 5.059; 95% CI, 1.769 to 14.464), portal vein invasion (p = 0.009; HR, 8.040; 95% CI, 1.700 to 38.022), modified UICC stage III/IV (p = 0.005; HR, 3.246; 95% CI, 1.414 to 7.451), and BCLC stage B/C (p < 0.001; HR, 9.932; 95% CI, 3.359 to 29.365) were independent factors associated with a lower survival rate (Table 3).
Table 3.
Factors affecting overall survival after hepatic resection in patients with hepatocellular carcinoma
Statistical analysis was carried out using the Cox proportional hazard model for the univariate and multivariate analyses of prognostic factors.
HR, hazard ratio; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; AFP, α-fetoprotein; ICG R15, indocyanine green retention at 15 minutes; UICC, Union for International Cancer Control; BCLC, the Barcelona Clinic Liver Cancer.
Factors influencing DFS after hepatectomy
In the univariate analysis of factors affecting DFS, Child-Pugh class B (p = 0.022; HR, 5.465; 95% CI, 1.279 to 23.345), more than two tumor nodules (p = 0.028; HR, 2.087; 95% CI, 1.081 to 4.030), microvascular invasion (p = 0.032; HR, 1.967; 95% CI, 1.059 to 3.656), portal vein invasion (p = 0.022; HR, 3.375; 95% CI, 1.196 to 9.525), modified UICC stage III/IV (p = 0.036; HR, 1.861; 95% CI, 1.042 to 3.321), and BCLC stage B/C (p < 0.001; HR, 5.248; 95% CI, 2.294 to 12.003) had a lower DFS. In the multivariate analysis performed with variables that were significant in the univariate analysis, microvascular invasion (p = 0.011; HR, 2.405; 95% CI, 1.228 to 4.711), modified UICC stage III/IV (p = 0.017; HR, 2.062; 95% CI, 1.138 to 3.736), and BCLC stage B/C (p < 0.001; HR, 5.488; 95% CI, 2.391 to 12.599) were independent factors associated with a lower DFS (Table 4).
Table 4.
Factors affecting disease-free survival after hepatic resection in patients with hepatocellular carcinoma
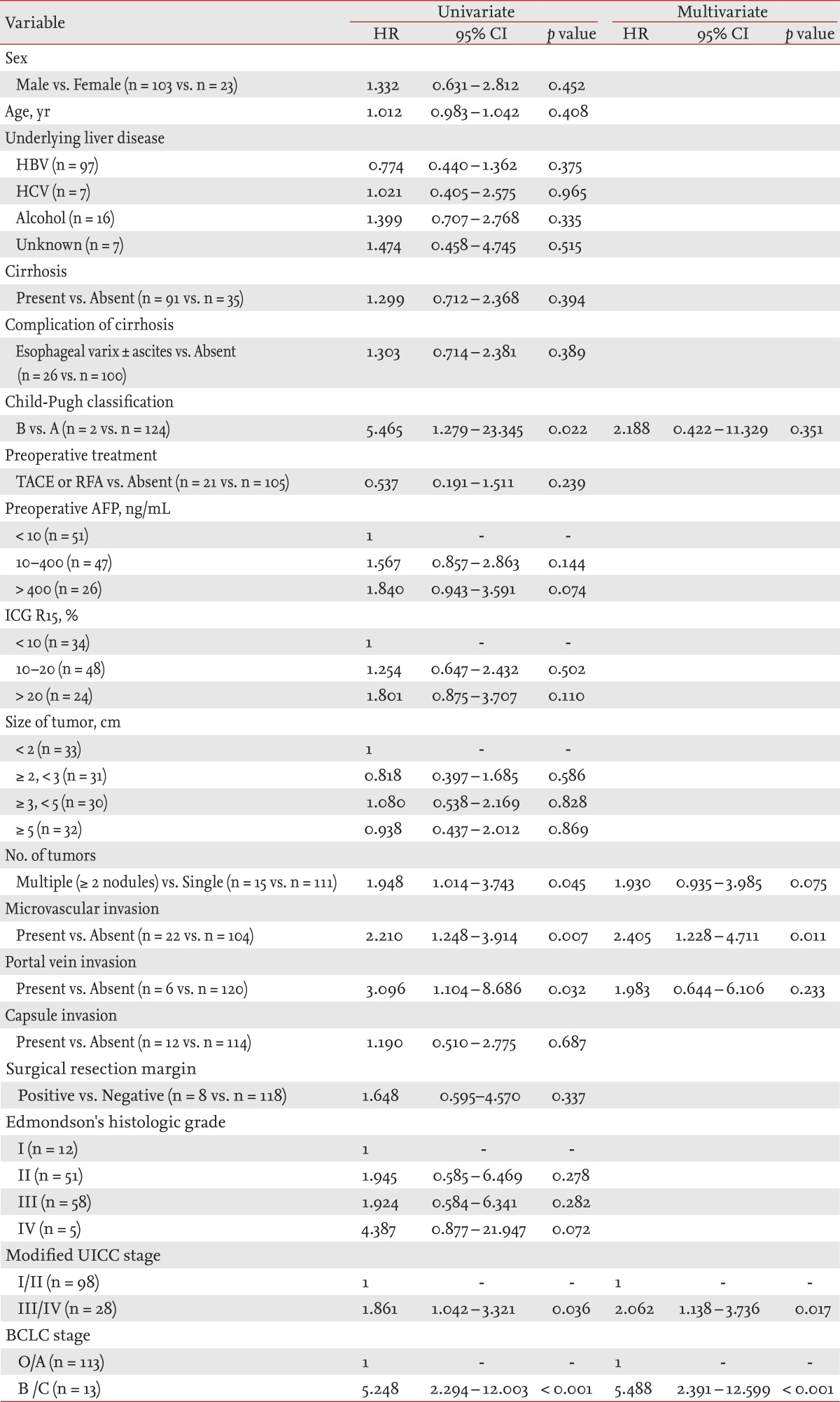
Statistical analysis was carried out using the Cox proportional hazard model for univariate and multivariate analyses of prognostic factors.
HR, hazard ratio; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; AFP, α-fetoprotein; ICG R15, indocyanine green retention at 15 minutes; UICC, Union for International Cancer Control; BCLC, the Barcelona Clinic Liver Cancer.
DISCUSSION
The high-risk group of HCC with liver cirrhosis has been actively selected, monitored, and evaluated; thus, HCC is diagnosed earlier and an increase in the long-term survival rate has been reported [12]. Additionally, the development of surgical techniques and improved patient care after surgery have led to a significant reduction in the death rate after hepatectomy, leading to a greater number of hepatectomies as curative treatment [6,7]. Previous studies reported that the 5-year survival rate after hepatectomy from HCC is 37% to 81.6% [6-8,13-17], and that the 5-year DFS is 15% to 46.6% [6-8,13-15,17]. However, more recent studies are reporting higher long-term OS rates. In the present study, the 5-year OS was 65%, and the 5-year DFS was 26.6%, suggesting that OS was high, but DFS was relatively low.
We analyzed the factors influencing OS and DFS in patients who underwent hepatectomy for HCC by investigating patient age, gender, and cause of disease, clinical and laboratory data, HCC stage, and a postoperative pathological examination. In the multivariate analysis, patients with Child-Pugh class B, preoperative AFP > 400 ng/mL, HCC > 5 cm, multiple tumors, presence of portal vein invasion, modified UICC stage III/IV, and BCLC stage B/C showed a lower OS than those without those features, whereas patients with microvascular invasion, modified UICC stage III/IV, and BCLC stage B/C showed a lower DFS.
Calvet et al. [18] presented a prognostic model to calculate the relative risk of death with those factors related to survival rate in a multivariate analysis and included age, but the impact on long-term survival in several other studies remains controversial. In the present study, age and gender were not associated with OS or DFS. According to a recent data analysis, hepatitis B was the cause for HCC in 68% to 78% of cases, hepatitis C in 3% to 12%, and alcohol in 3% to 5% [19]. However, hepatitis B vaccination of newborns since 1995 is expected to reduce the occurrence of HCC caused by hepatitis B in the near future, which is supported by recent studies in Korea [20]. In our study, hepatitis B comprised 76.2% of the cases, alcoholic liver disease 12.7%, unknown liver disease 5.6%, hepatitis C 4.8%, and superinfection with hepatitis B and C 0.7% of cases. However, the cause of the existing liver disease was not associated with OS or DFS after hepatectomy. We found that 72.2% of all patients had liver cirrhosis, but that liver cirrhosis was not associated with OS or DFS. This was probably because most patients who underwent hepatectomy were Child-Pugh class A; thus, patients with good liver function were selected.
The Child-Pugh classification has long been used as a preoperative inspection to evaluate the safety of hepatectomy, but it is insufficient. Several institutions in Korea are using the ICG R15, presented by Japan, as a residual liver function test before surgery [21]. Child-Pugh class A patients comprised 98.4% of our sample, and the survival and DFS rates were significantly lower compared to patients with Child-Pugh class B in the univariate analysis and it was also an independent prognostic factor reducing survival rate in the multivariate analysis. Laboratory parameters used to calculate the Child-Pugh classification score and ICG R15-used to evaluate the presence of liver cirrhosis complications and preoperative residual liver function-were not associated with OS or DFS.
The 2009 Hepatocellular Carcinoma Medical Guidelines reduced the serum AFP criterion for diagnosing HCC from 400 to 200 ng/mL [22]; however, we still do not have a classification of increased serum AFP levels in relation to survival rate after hepatectomy. In this study, the preoperative serum AFP was categorized into < 10, 10 to 400, and > 400 ng/mL. Survival was lower for patients with serum AFP > 400 ng/mL, and this was an independent prognostic factor.
Vitale et al. [23] reported that BCLC stage reflected the prognosis better than other staging systems when they analyzed 3-year survival rates in 225 subjects who underwent surgery for HCC. In the present study, modified UICC stage III/IV and BCLC stage B/C were independent poor prognostic factors affecting OS and DFS, and both reflected the prognosis relatively well. Seven patients with modified UICC stage IV A were T4N0M0, three patients with BCLC stage B had multiple carcinomas with the largest size > 3 cm, and 10 patients with BCLC stage C were all suspected to have portal vein invasion preoperatively (six individuals had right portal vein and four left portal vein), which were pathologically proven after surgery.
Since the report by Okuda et al. [24] on the characteristics of small HCC, much effort has focused on discovery of small HCCs, and many researchers have reported that the prognosis of small HCCs is good [25,26]. Chen et al. [17] found that the overall prognosis is well reflected by tumor size when performing hepatectomy for HCCs < 5 cm, but that the 5-year OS and DFS are best predicted by 3-cm criterion. In the present study, the OS in patients with HCCs ≥ 5 cm was poor, but it was not associated with DFS. The number of HCCs has been reported as a significant prognostic factor in most studies and is considered an important prognostic factor in many other HCC stages [9,10]. In our study, 15 patients with multiple tumors had two tumors. OS and DFS were significantly lower for cases of multiple tumors in the univariate analysis, and the presence of multiple tumors was an important prognostic factor for a lower survival rate in the multivariate analysis.
Vascular invasion in patients with HCC is frequently portal vein invasion, suggesting the possibility of movement of cancer cells through blood vessels. A study of patients who underwent a liver transplant for HCC reported that vascular incidence and histopathological differentiation are prognostic factors and that histopathological differentiation and size of the carcinoma are predictive of vascular invasion [27]. In the present study, portal vein invasion was found in six patients, OS and DFS were significantly lower in the univariate analysis, and portal vein invasion was an independent prognostic factor for a lower OS rate in the multivariate analysis. Lim et al. [28] reported that microvascular invasion is superior predictive factor for the recurrence and survival rates than the Milan criteria. Furthermore, microvascular invasion after surgery reflected the prognosis well compared to the Milan criteria before surgery in a long-term prospective study of 454 patients with hepatectomy from HCC [28]. In the present study, microvascular invasion was an independent prognostic factor associated with DFS in the multivariate analysis.
The limitations of this study should be mentioned. It was of a retrospective design, based on the medical records from a single tertiary care hospital, so sample selection was likely biased; therefore, a large-scale prospective study is necessary.
Vascular invasion was an independent factor associated with OS and DFS in patients who underwent hepatic resection for HCC. Close postoperative follow-up and additional treatments are required to detect early recurrence in patients with confirmed postoperative vascular invasion.
KEY MESSAGE
1. The presence of microvascular invasion is an independent poor prognostic factor for overall survival and tumor recurrence in patients with hepatocellular carcinoma after hepatic resection.
2. Close postoperative surveillance for early detection of recurrence is needed in patients with vascular invasion after hepatic resection.
Footnotes
No potential conflict of interest relevant to this article is reported.
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Ministry of Heath and Welfare. 2008 Annual Report of Cancer Statistics in Korea. Gwacheon: Ministry of Heath and Welfare; 2010. [Google Scholar]
- 3.Ministry of Heath and Welfare. Cancer Incidence in Korea 1999-2001. Gwacheon: Ministry of Heath and Welfare; 2005. [Google Scholar]
- 4.Park JW, Kim CM. Epidemiology of hepatocellular carcinoma in Korea. Korean J Hepatol. 2005;11:303–310. [PubMed] [Google Scholar]
- 5.Sharma P, Balan V, Hernandez JL, et al. Liver transplantation for hepatocellular carcinoma: the MELD impact. Liver Transpl. 2004;10:36–41. doi: 10.1002/lt.20012. [DOI] [PubMed] [Google Scholar]
- 6.Lee KU, Koh YT, Kim KH, et al. Prognostic factors of hepatocellular carcinoma after curative hepatic resection. Korean J Hepatobiliary Pancreat Surg. 1997;1:41–58. [Google Scholar]
- 7.Poon RT, Fan ST, Lo CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minagawa M, Makuuchi M, Takayama T, Kokudo N. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003;238:703–710. doi: 10.1097/01.sla.0000094549.11754.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueno S, Tanabe G, Nuruki K, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res. 2002;24:395–403. doi: 10.1016/s1386-6346(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 10.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 11.Gouw AS, Balabaud C, Kusano H, Todo S, Ichida T, Kojiro M. Markers for microvascular invasion in hepatocellular carcinoma: where do we stand? Liver Transpl. 2011;17(Suppl 2):S72–S80. doi: 10.1002/lt.22368. [DOI] [PubMed] [Google Scholar]
- 12.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 13.Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330–339. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Choi SB, Lee JG, et al. Prognostic factors and 10-year survival in patients with hepatocellular carcinoma after curative hepatectomy. J Gastrointest Surg. 2011;15:598–607. doi: 10.1007/s11605-011-1452-7. [DOI] [PubMed] [Google Scholar]
- 15.Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127(5 Suppl 1):S248–S260. doi: 10.1053/j.gastro.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 16.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–799. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YL, Ko CJ, Chien SY, et al. Tumor size as a prognostic factor in resected small hepatocellular carcinoma: a controversy revisited. J Gastroenterol Hepatol. 2011;26:851–857. doi: 10.1111/j.1440-1746.2010.06595.x. [DOI] [PubMed] [Google Scholar]
- 18.Calvet X, Bruix J, Gines P, et al. Prognostic factors of hepatocellular carcinoma in the west: a multivariate analysis in 206 patients. Hepatology. 1990;12(4 Pt 1):753–760. doi: 10.1002/hep.1840120422. [DOI] [PubMed] [Google Scholar]
- 19.Song IH, Kim KS. Current status of liver diseases in Korea: hepatocellular carcinoma. Korean J Hepatol. 2009;15(Suppl 6):S50–S59. doi: 10.3350/kjhep.2009.15.S6.S50. [DOI] [PubMed] [Google Scholar]
- 20.Oh KC, Park SH, Park JC, et al. Is the prevalence of cryptogenic hepatocellular carcinoma increasing in Korea? Korean J Gastroenterol. 2005;45:45–51. [PubMed] [Google Scholar]
- 21.Makuuchi M, Sano K. The surgical approach to HCC: our progress and results in Japan. Liver Transpl. 2004;10:S46–S52. doi: 10.1002/lt.20044. [DOI] [PubMed] [Google Scholar]
- 22.Korean Liver Cancer Study Group; National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 23.Vitale A, Saracino E, Boccagni P, et al. Validation of the BCLC prognostic system in surgical hepatocellular cancer patients. Transplant Proc. 2009;41:1260–1263. doi: 10.1016/j.transproceed.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 24.Okuda K, Nakashima T, Obata H, Kubo Y. Clinicopathological studies of minute hepatocellular carcinoma: analysis of 20 cases, including 4 with hepatic resection. Gastroenterology. 1977;73:109–115. [PubMed] [Google Scholar]
- 25.Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245:909–922. doi: 10.1097/01.sla.0000254368.65878.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–1536. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 27.Jonas S, Bechstein WO, Steinmuller T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 28.Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108–113. doi: 10.1097/SLA.0b013e31821ad884. [DOI] [PubMed] [Google Scholar]