Abstract
Normal piglets weaned onto soy- or egg-based diets generated antibody responses to fed protein. Concurrent infection with transmissible gastroenteritis virus (TGEV) did not affect the responses to dietary antigens at weaning, nor did it affect the subsequent development of tolerance. However, TGEV infection did enhance the primary immunoglobulin M (IgM) and IgG1, but not IgG2, antibody responses to injected soy in comparison to those of uninfected animals. Paradoxically, TGEV-infected animals showed an enhanced primary IgG1 antibody response to injected soy at 4 weeks of age, but they subsequently showed a reduced secondary response after an intraperitoneal challenge at 9 weeks of age in comparison to uninfected animals. The results suggest that an enteric virus, either used as a vaccine vector or present as a subclinical infection, may not have significant effects on the development of dietary allergies but may have effects both on the primary response and on the subsequent recall response to systemic antigens to which the animal is exposed concurrently with virus antigens.
In all individuals, small quantities of dietary proteins are absorbed intact across the intestinal mucosa (17, 29). Under normal circumstances, these absorbed proteins trigger immunological tolerance (so-called oral tolerance) rather than active immune responses (7, 8). The inappropriate induction of active immune responses to dietary antigens has been associated with food allergy, intolerance, and intestinal inflammation (4). The incidence of such allergic diseases appears to be increasing in the human infant population (5, 9, 28, 30). Several hypotheses have been proposed to account for the development of allergy in some individuals and for this increasing incidence. One possibility is that a viral infection occurring concomitantly with the induction phase of an immune response to a novel dietary antigen may result in the generation of a persistent allergy as a “bystander” effect (14, 24). In addition, viral infections have been implicated in the onset of other inappropriate responses, such as autoimmunity (19). Alternatively, there is increasing evidence that early life exposure to commensal and pathogenic organisms may protect humans against subsequent allergic disease (11, 21, 27).
The possibility that infections with enteric viruses may have long-term effects on other immune responses will also have implications for the future use of virus vectors for the mucosal delivery of vaccine antigens (10, 18, 22, 26). A coronavirus, transmissible gastroenteritis virus (TGEV), which targets the gut epithelium and would therefore be ideally suited to introduce antigens to this site, has been proposed as a vector for mucosal immunization in the pig and as a model for coronavirus vectors in other species (20, 23). It is therefore important to investigate the effect of a virus such as TGEV on immune responses to bystander antigens.
In previous studies, members of our laboratory demonstrated the reliable induction of primary immune responses to soy in piglets weaned onto soy protein at 3 weeks of age (3). Despite this strong primary response, these piglets subsequently generated systemic tolerance to soy antigens (2). This system, therefore, provides a model by which the effect of viral infections on primary responses to dietary antigens and subsequent tolerance can be studied. In this work, we compared primary and secondary responses to dietary (tolerogenic) and injected (priming) antigens with and without concomitant exposure to TGEV infection at the point of weaning or injection.
MATERIALS AND METHODS
Animals and diet.
All piglets used for this study were from six Large White/Landrace hybrid sows. Each group contained seven or eight animals, and the male/female ratios were 3:5 for groups 1 to 4 and 2:5 for groups 5 and 6. Animal housing and experimental procedures were all performed according to local ethical guidelines: all experiments were performed with a UK Home Office license and were approved by the University of Bristol Ethical Review group. Sows were fed soy- and egg-free diets for at least 1 month before parturition and were housed under specific-pathogen-free conditions under negative pressure provided by HEPA-filtered air to prevent the spread of infectious agents. Infected and uninfected groups were kept in separate air spaces. The weaning ovalbumin diet contained 10.5% egg-based protein, the soy-based diet contained 10.5% soy-derived protein, and the rest of the dietary protein was supplied by 5% cereal-based protein and 5.5% protein from bovine milk. Diets were produced by Parnutt Foods, Sleaford, Lincolnshire, United Kingdom.
TGEV infection.
Piglets in three groups (groups 1, 3, and 5) were infected with a dose of 2 × 108 PFU of TGEV strain PUR46-MAD (1) at 2 days postweaning at the age of 29 days (Table 1). PUR46-MAD is an attenuated strain of TGEV that produces very mild or no enteritis and no mortality in conventional non-colostrum-deprived piglets. This strain grows to titers ranging between 102 and 103 PFU/g in the jejunum, ileum, and mesenteric lymph nodes and to titers between 105 and 107 PFU/g of tissue in the lung. The PUR46-MAD strain of TGEV is not found in feces in significant amounts. The susceptibility of piglets to infection by the PUR46-MAD strain of TGEV diminishes with age, being at a maximum during the first days of life.
TABLE 1.
Experimental protocol
| Piglet age (days) | Treatment | Protein source or adjuvant used for group
|
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| 27 | Weaning diet | Soy | Soy | Egg | Egg | Egg | Egg |
| i.p. injection | Soy | Soy | |||||
| 29 | Infection | TGEV | TGEV | TGEV | |||
| 54 | Dietary change | Fish | Fish | Fish | Fish | Fish | Fish |
| 68 | i.p. injection | Soy | Soy | Soy | Soy | Soy | Soy |
Experimental protocol.
Piglets from five sows were randomly allocated to six experimental groups, with each group containing seven or eight piglets. A summary of the experimental protocol is shown in Table 1. Piglets were weaned at 27 days of age onto a balanced diet in which the protein source was provided by soy (groups 1 and 2) or egg (groups 3 to 6). In addition, groups 5 and 6 were injected intraperitoneally (i.p.) with 2 mg of a soluble extract of soy, prepared as previously described (2, 3), plus 2 mg of Quil A (Superfos Biosector, Frederickssund, Denmark) in 2 ml of phosphate-buffered saline (PBS). Two days later, piglets in three groups (1, 3, and 5) were infected with a dose of 2 × 108 PFU of TGEV strain PUR46-MAD (1). The diets of all groups were changed to a fish-based diet at the age of 54 days. Piglets in all six groups were challenged by a final injection of 2 mg of soy in Quil A (age, 68 days). Blood samples were taken by venipuncture at ages 27 (weaning), 33, 40, 54, and 68 days.
Antibody responses to TGEV.
The presence of antibody to TGEV in serum was established as previously described (22a). Enzyme-linked immunosorbent assays (ELISAs) were performed with purified TGEV (0.2 μg per well) as the antigen, bound to 96-well microplates. Plate binding sites were saturated with 5% bovine serum albumin in PBS for 2 h at 37°C and then incubated with serial dilutions of the serum sample in 0.1% bovine serum albumin for 3 h at room temperature. Microplates were washed six times with 0.1% bovine serum albumin and 0.1% Tween 20 in PBS, and bound antibodies were detected by incubation with peroxidase-conjugated protein A diluted 1:2,000 in PBS with 0.1% bovine serum albumin. Color development was done with phenylenediamine dihydrochloride (Sigma FAST) as the peroxidase substrate for 15 min at room temperature. Reactions were stopped with 1.5 M H2SO4, and the absorbance was read at 492 nm. The ELISA titer was defined as the highest dilution of the serum giving a threefold increase over the background. The presence of anti-TGEV antibodies was considered confirmation that the TGEV infection had succeeded.
Antibody responses to soy and ovalbumin.
Antibodies against porcine immunoglobulin M (IgM), IgG1, and IgG2 specific for dietary and injected antigens were quantified by ELISA essentially as previously described (2). Briefly, ELISA plates (Labsystems) were coated with preoptimized concentrations of ovalbumin (5 μg/ml; grade V) (Sigma) and soy (5 μg/ml) overnight at 4°C. Plates were blocked with PBS-Tween plus 5% skimmed milk powder (Marvel) for 1 h, and trebling dilution series of test sera and reference sera, obtained from pigs that were hyperimmunized with ovalbumin and soy, respectively, were applied. Binding of antibodies was detected with monoclonal antibodies specific for porcine IgM (K52.1C3; Serotec), IgG1 (K139.3C8; Serotec), and IgG2 (K68.1G2; Serotec), all diluted at optimal dilutions of 1:50 in PBS-Tween, followed by 1:10,000 diluted goat anti-mouse IgG (Fc specific) conjugated to alkaline phosphatase (A1418; Sigma). Finally, pNPD (Sigma 104 phosphatase substrate) in carbonate buffer was added, and the optical density was read at the appropriate wavelength of 405 nm. The reference sera were assigned arbitrary antibody units of 1,000 (log 3) and were used to construct a standard curve. The quantities of antibody in test sera were determined by the interpolation of optical density values from all dilutions falling within the reference range. All antibody values were expressed relative to the standard.
Total IgG1 and IgG2.
The total immunoglobulin was quantified by competition ELISA. Plates were coated with a 0.5-μg/ml concentration of standard pig IgG (I-4381, batch 59H9001; Sigma) containing 39% IgG1 and 61% IgG2 in bicarbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.5 to 9.6). After the coating step, 50-μl samples of a dilution series of the standard pig IgG or of test sera in PBS-Tween were added, together with 50-μl aliquots of monoclonal antibodies specific for porcine IgG1 (K139.3C8; Serotec) and IgG2 (K68.1G2; Serotec) at pretitrated limiting dilutions of 1:300 for each. The remaining steps were the same as for the specific ELISA.
Statistical analysis.
ELISA data for the anti-TGEV antibody were not normally distributed and were analyzed by nonparametric Mann-Whitney tests (6). Log-transformed anti-soy and anti-ovalbumin data were normally distributed. The time course data were therefore analyzed by a three-way repeated value analysis of variance (31). The cutoff for statistical significance was set at the 0.05% level for the main effects and at the 0.01% level for interactions. In addition, the primary responses between days 27 and 33 and the recall responses between days 68 and 82 were analyzed by Student's unmatched two-tailed t test (6).
RESULTS
Concurrent infection with TGEV did not affect responses to either dietary antigen. However, exposure to TGEV did have a significant effect on the response to soy antigen given i.p. with an adjuvant: concurrently infected piglets developed transiently enhanced primary IgM and persistently enhanced IgG1 antisoy antibody responses.
TGEV infection.
The strain of TGEV used for this work was not observed to cause clinical signs, such as diarrhea, in any of the piglets. Table 2 shows the median antibody titers to TGEV for all six groups. In the infected groups, only 3 of 23 piglets (one piglet in group 1 and two piglets in group 3) failed to seroconvert, demonstrating successful infection. Conversely, only 1 piglet of 23 in the uninfected groups developed an antibody against TGEV, and this was transient and at a low level, probably reflecting a cross-reactive rather than specific antibody. Anti-TGEV antibody titers were significantly higher in the soy-weaned piglets (P < 0.01 for a comparison of group 1 and group 3).
TABLE 2.
Antibody titers against TGEV
| Piglet age (days) | Median titer of group against TGEV
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 27 | <20 | <20 | <20 | <20 | <20 | <20 |
| 33 | <20 | <20 | <20 | <20 | <20 | <20 |
| 40 | 1,500 | <20 | 500 | <20 | 1,500 | <20 |
| 54 | 2,500 | <20 | 500 | <20 | 500 | <20 |
| 68 | 2,500 | <20 | 500 | <20 | 500 | <20 |
| 84 | 2,500 | <20 | 500 | <20 | 500 | <20 |
Primary antibody responses to fed and injected antigens.
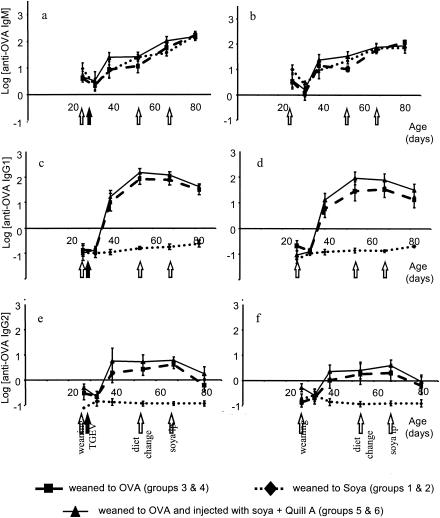
The IgM, IgG1, and IgG2 antibody responses to ovalbumin and soy are shown for the whole experimental period in Fig. 1 and 2. ELISA tests for specific ovalbumin antibody showed a rise in primary IgG1 and IgG2 antibody levels after weaning onto the egg-based diet for groups 3 to 6 (Fig. 1) compared to preweaning levels, in contrast to no such rise for groups 1 and 2, which were fed soy protein and no eggs. The first increases in IgG1 and IgG2 antibody were not yet apparent at day 33, which was 6 days postweaning, but we observed a large increase on day 40, or 13 days postweaning. At this point, the level of IgG1 anti-ovalbumin antibody had increased approximately 100-fold over preweaning levels. It increased further to a maximum of approximately 1,000-fold and remained elevated, even after the dietary protein source was changed to fish on day 54, until day 68, although it subsequently decreased slightly by day 84. The level of IgG2 antibody showed a smaller increase of about sevenfold over preweaning levels by day 40 and remained elevated until day 68 but decreased by day 84. IgM levels for all groups increased steadily, even for those without any exposure to ovalbumin, probably reflecting a general increase in nonspecific, low-affinity, high-avidity IgM rather than a specific IgM response to the egg diet. A comparison with the level of the reference hyperimmune serum, which had been assigned an arbitrary antibody level of 1,000 (log 3), showed that the mean IgG1 and IgM responses to the diet were about 10% of the levels in hyperimmune serum and the IgG2 response was about 1%.
FIG. 1.
Total antiovalbumin (OVA) Ig levels throughout the experimental period for all groups. (a, c, and e) Piglets infected with TGEV at 2 days postweaning. (b, d, and f) Piglets that were not infected. Results shown are means ± standard errors (n = 8).
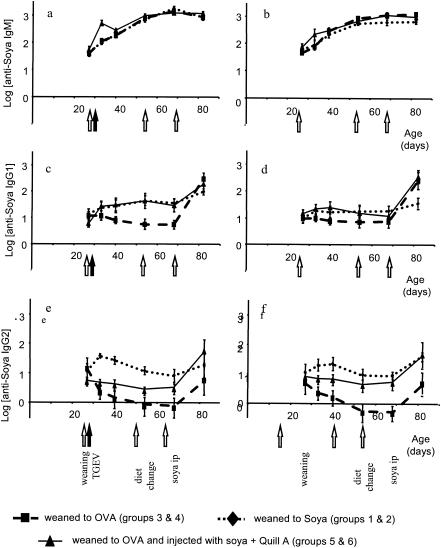
FIG. 2.
Total antisoy Ig levels throughout the experimental period for all groups. (a and b) IgM levels. (c and d) IgG1 levels. (e and f) IgG2 levels. Piglets were infected with TGEV at 2 days postweaning (a, c, and e) or were not infected (b, d, and f). Results shown are means ± standard errors (n = 8).
Similarly, weaning onto a diet containing soy resulted in antibody levels that were higher than those in the preweaning period and those for groups 3 and 4, which were not exposed to soy: there was an approximately twofold increase in both serum IgG1 and IgG2 antisoy antibody between days 27 and 40 (Fig. 2). As was the case for anti-ovalbumin antibody, antisoy IgM increased both in animals fed soy and in animals fed eggs (Fig. 2). The time course of the response to dietary soy was more rapid than the response to the egg-based diet and was already apparent at 6 days postweaning. A comparison with antibody levels found in the hyperimmune reference serum (log 3) showed that the responses to dietary soy were considerably lower, especially for IgG2. TGEV infection had no significant effect on primary responses to either dietary ovalbumin or soy antigens.
Like the response to dietary soy, the kinetics of the primary IgG response to injected soy were also rapid and apparent 6 days after the injection. This primary response to injected soy antigen in Quil A adjuvant resulted in an approximately threefold increase in serum IgG1 by day 40. However, the isotype pattern of the response to injected soy was different from the response to dietary antigen, in that the level of IgG2 antisoy antibody did not increase after immunization (approximately 0.8-fold change by day 40). The only evidence for an IgG2 antibody response to injected soy was a comparison of the injected groups 5 and 6 with soy-naïve piglets in groups 3 and 4, in which the level of IgG2 antisoy antibody declined rapidly (approximately 0.2-fold change over the same time period).
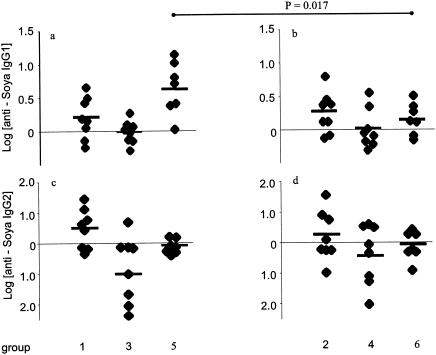
Although TGEV infection did not affect the primary responses to fed antigens, the IgG1 and IgM antibody responses to injected soy were significantly enhanced by TGEV infection. The mean log antisoy IgM on day 33, at 6 days postinjection, was 2.708 for the TGEV-infected group 5, compared to 2.047 for uninfected group 6 (Fig. 2a and b; P < 0.05). Figure 3 shows the log change in antisoy antibody levels for individual piglets between days 27 and 33 (the mean change in the antisoy IgG1 level for infected group 5 was 0.656 and that for uninfected group 6 was 0.174; P = 0.017).
FIG. 3.
Changes in antisoy IgG1 and IgG2 (Δ log) levels after a primary challenge (between days 27 and 33) in individual piglets. Significant differences (P values were obtained by Student's unmatched two-tailed t test) between groups are linked by horizontal lines.
Secondary antibody responses to i.p. challenge in tolerant and primed animals.
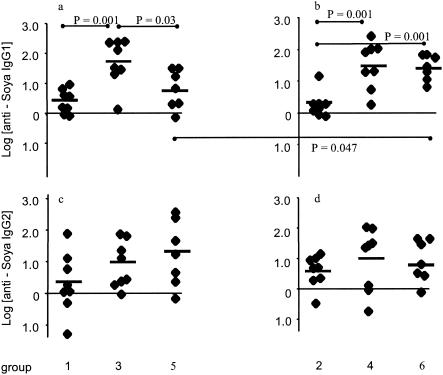
Figure 4 shows the log change in antisoy antibody levels between 68 and 82 days in response to an i.p. challenge at 68 days in individual piglets. Figure 4a and b show that feeding induced isotype-specific oral tolerance in both TGEV-infected and uninfected groups (P = 0.001), in that there were clearly decreased IgG1 responses to immunization in the animals that were previously fed on soy diets (groups 1 and 2) compared to the groups weaned onto an egg-based diet (groups 3 and 4). This induction of oral IgG1 tolerance was not affected by TGEV infection. In contrast to the effect of prior feeding on IgG1 responses to immunization, there was no significant effect of any of the treatments at weaning on IgG2 responses after i.p. challenge (Fig. 4c and d).
FIG. 4.
Changes in antisoy IgG1 and IgG2 levels between days 68 and 84 in individual piglets after an intramuscular challenge with soy plus Quil A. Significant differences (P values were obtained by Student's unmatched two-tailed t test) between groups are linked by horizontal lines.
In contrast, TGEV infection around the time of weaning did affect the secondary IgG1 response to soy in animals which were primed by an i.p. injection of soy at weaning (Fig. 4a and b). Only uninfected group 6 showed the expected increase in antibody level after the challenge, whereas the TGEV-infected group 5 did not (P = 0.047), despite the more vigorous primary response by this group.
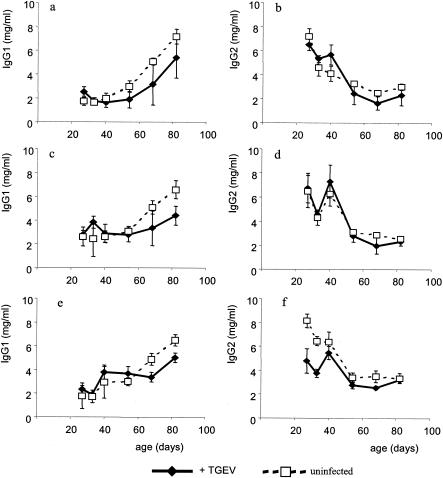
Total immunoglobulin.
Figure 5 shows the total IgG1 and IgG2 levels for all groups. The levels of total IgG1 rose from mean values of about 2 mg/ml at the point of weaning to values of 4 to 7 mg/ml at day 82 for all groups. There was a trend towards lower IgG1 levels in the infected groups during the later phase of the experiment; however, due to the high level of between-pig variation, these were not significant. In contrast to the rising IgG1 levels, IgG2 levels fell for all groups of piglets during the postweaning period, from a mean of 6.6 mg/ml to a mean of 2.8 mg/ml at day 82. There were no significant differences between infected and uninfected groups.
FIG. 5.
Total antigen-nonspecific immunoglobulin levels throughout the experimental period for all groups. (a and b) Piglets weaned onto a soy-based diet. (c and d) Piglets weaned onto an egg-based diet. (e and f) Piglets weaned onto an egg-based diet and injected with soy intramuscularly at weaning. IgG1 levels (a, c, and e) and IgG2 levels (b, d, and f) are shown. Results shown are means ± standard errors.
DISCUSSION
The two antigens used in this experiment were ovalbumin, an antigen which is not normally fed to sows, and soy, which is extremely likely to have been fed to the sows before the experimental period. This difference may be relevant, since unlike the case for humans, the placenta of the sow is not permeable to macromolecules, including antigens and immunoglobulins; however, the transfer of maternally derived antigens and antibodies of all isotypes can occur during the immediate postpartum period via the colostrum (25). Consistent with this, the decline in absolute levels of total IgG2, as well as specifically of antisoy IgG2, observed in piglets without any exposure to soy antigen after weaning suggests strongly that this may be in fact due to a decline in antibodies of maternal origin, either derived directly from milk or colostrum.
Importantly, responses to neither dietary antigen were affected by a concurrent infection with TGEV. This lack of effect included both the primary responses at weaning and the subsequent induction of oral tolerance. Thus, within the limits of the experimental design, our results suggest that TGEV is unlikely to cause inappropriate responses to dietary antigens when given as a vaccine vector or when occurring as a subclinical infection.
However, exposure to TGEV did have a significant effect on the response to soy antigen given i.p. with an adjuvant. Concurrently infected piglets developed transiently enhanced primary IgM and persistently enhanced IgG1 antisoy antibody responses. Paradoxically, these same piglets developed lower antibody responses upon a secondary challenge by i.p. injection than did the uninfected group. Three possibilities may account for this observation. First, the initial primary response in the presence of TGEV may have resulted in the preferential differentiation of high levels of effector cells (plasma cells and effector T cells) rather than the differentiation of memory T and B cells. Second, the response to the challenge may have been limited by the level of preexisting antibody; thus, the presence of more antibody in the infected piglets after primary immunization may have inhibited the secondary response (12, 15). Third, the secondary response may be limited to an absolute maximum: this might be suggested by the observation that final antibody levels in both groups were similar at the end of the experiments.
Therefore, the results suggest that a concomitant viral infection or the use of a viral vector to generate mucosal responses may act as a nonspecific adjuvant and enhance primary responses to systemic bystander antigens. Further, our results suggest that delayed effects on immune responses may also occur, as we observed a tendency towards lower total IgG1 levels in the later phase of the experimental period for infected animals. However, an interpretation of the mechanisms of these effects requires further investigation.
In addition to highlighting the impact of a TGEV infection at the point of weaning, this experiment revealed several other interesting effects. First, the decrease in IgG2 antisoy antibody after weaning for the group weaned onto an egg diet and not immunized and in total IgG2 for all groups suggests the decay of maternally derived (either via colostrum or milk) antisoy antibody. The more rapid kinetics of the response to fed soy than to fed ovalbumin may also suggest that the transfer of an antigen capable of priming the neonatal immune system had occurred before contact with a homologous antigen in the novel diet (25). Second, there was a difference in the ability of prior feeding to cause tolerance of IgG1 and IgG2 antibody responses after immunization. This effect could be attributable to a difference in Th1 or Th2 dependence of the two isotypes: several previous studies in mice have demonstrated an easier induction of Th1 tolerance by feeding (13). However, the Th dependence of IgG isotypes has not been demonstrated with pigs. Equally possible is the notion that IgG2 antibody responses to systemic challenge may be more variable between animals.
The two observations together might suggest that initial IgG1 and IgG2 responses occur to antigens fed to piglets at weaning but that the ability to produce diet-specific IgG1 antibody is subsequently downregulated as the pigs become tolerant, with the result that, as adults, IgG2 antibody to dietary antigen is the predominant isotype which is transferred to the next generation of piglets. This implies that the consequences of early life exposure to dietary antigens are extremely long lived and may affect the immune response of the next generation. The long-term persistence of maternally derived antibodies to dietary soy for over two generations has been observed for other species (16). The question of the effect of transfer of different levels of antifood antibody of different isotypes on the subsequent immune response of the offspring needs to be addressed. Future work could address this by using ovalbumin as a tolerogenic diet with or without the administration of antibody and/or antigen at weaning to investigate the effect of maternally derived antibody and/or antigen. In addition, a study using infection with a more virulent virus would also be interesting.
Acknowledgments
This work was supported by funds from the European Union under contract number Bio4-CT98-0239.
REFERENCES
- 1.Anton, I. M., S. Gonzalez, M. J. Bullido, M. Corsin, C. Risco, J. P. M. Langeveld, and L. Enjuanes. 1996. Cooperation between transmissible gastroenteritis coronavirus (TGEV) structural proteins in the in vitro induction of virus-specific antibodies. Virus Res. 46:111-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey, M., B. G. Miller, E. Telemo, C. R. Stokes, and F. J. Bourne. 1993. Specific immunological-unresponsiveness following active primary responses to proteins in the weaning diet of piglets. Int. Arch. Allergy Immunol. 101:266-271. [DOI] [PubMed] [Google Scholar]
- 3.Bailey, M., B. G. Miller, E. Telemo, C. R. Stokes, and F. J. Bourne. 1994. Altered immune-response to proteins fed after neonatal exposure of piglets to the antigen. Int. Arch. Allergy Immunol. 103:183-187. [DOI] [PubMed] [Google Scholar]
- 4.Baird, A. W., W. S. Barclay, B. L. Blazeryost, and A. W. Cuthbert. 1987. Affinity purified immunoglobulin-G transfers immediate hypersensitivity to guinea-pig colonic epithelium in vitro. Gastroenterology 92:635-642. [DOI] [PubMed] [Google Scholar]
- 5.Broadfield, E., T. M. McKeever, S. Scrivener, A. Venn, S. A. Lewis, and J. Britton. 2002. Increase in the prevalence of allergen skin sensitization in successive birth cohorts. J. Allergy Clin. Immunol. 109:969-974. [DOI] [PubMed] [Google Scholar]
- 6.Campbell, R. C. 1989. Statistics for biologists, 3rd ed. Cambridge University Press, New York, N.Y.
- 7.Challacombe, S. J., and C. Czerkinsky. 2002. Oral tolerance: probable mechanisms and possible therapeutic applications, p. 259-266. In J. Brostoff and S. J. Challacombe (ed.), Food allergy and intolerance, 2nd ed. Saunders, London, United Kingdom.
- 8.Challacombe, S. J., and T. B. Tomasi. 1980. Systemic tolerance and secretory immunity after oral immunization. J. Exp. Med. 152:1459-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charpin, D., and M. Gouitaa. 2001. Why is the prevalence of allergic diseases increasing? A critical assessment of some classical risk factors. Mediators Inflamm. 10:292-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, H. M. 2000. Recent advances in mucosal vaccine development. J. Controlled Release 67:117-128. [DOI] [PubMed] [Google Scholar]
- 11.Crane, J. 2002. Asthma and allergic diseases: is there a downside to cleanliness and can we exploit it? Eur. J. Clin. Nutr. 56:S39-S43. [DOI] [PubMed] [Google Scholar]
- 12.Galletti, R., P. Beauverger, and T. F. Wild. 1995. Passively administered antibody suppresses the induction of measles-virus antibodies by vaccinia-measles recombinant viruses. Vaccine 13:197-201. [DOI] [PubMed] [Google Scholar]
- 13.Hashiguchi, M., S. Hachimura, A. Ametani, and S. Kaminogawa. 2000. Th2 polarization enhanced by oral administration of higher doses of antigen. Cytotechnology 33:237-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hegele, R. G. 1999. Role of viruses in the onset of asthma and allergy: lessons from animal models. Clin. Exp. Allergy 29:78-81. [PubMed] [Google Scholar]
- 15.Jelonek, M. T., J. L. Maskrey, L. M. Cummins, Y. Arora, K. S. Steimer, M. White-Scharf, and M. A. Keller. 1998. HIV hyperimmune globulin or intravenous immune globulin inhibits response to an HIV vaccine. Biotechnol. Appl. Biochem. 27:89-95. [DOI] [PubMed] [Google Scholar]
- 16.Knippels, L. M. J., A. H. Penninks, and G. F. Houben. 1998. Continued expression of anti-soy protein antibodies in rats bred on a soy protein-free diet for one generation: the importance of dietary control in oral sensitization research. J. Allergy Clin. Immunol. 101:815-820. [DOI] [PubMed] [Google Scholar]
- 17.Lippard, V., O. Schloss, and P. Johnson. 1936. Immune reactions induced in infants by intestinal absorption of incompletely digested cows milk protein. Am. J. Dis. Health 51:562-574. [Google Scholar]
- 18.Morrow, C. D., M. J. Novak, D. C. Ansardi, D. C. Porter, and Z. Moldoveanu. 1999. Recombinant viruses as vectors for mucosal immunity. Curr. Top. Microbiol. Immunol. 236:255-273. [DOI] [PubMed] [Google Scholar]
- 19.Olson, J. K., J. L. Croxford, and S. D. Miller. 2001. Virus-induced autoimmunity: potential role of viruses in initiation, perpetuation, and progression of T-cell-mediated autoimmune disease. Vir. Immunol. 14:227-250. [DOI] [PubMed] [Google Scholar]
- 20.Ortego, J., D. Escors, H. Laude, and L. Enjuanes. 2002. Generation of a replication-competent, propagation-deficient virus vector based on the transmissible gastroenteritis coronavirus genome. J. Virol. 76:11518-11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pack, C. D., A. E. Cestra, B. Min, K. L. Legge, L. Q. Li, J. C. Caprio-Young, J. J. Bell, R. K. Gregg, and H. Zaghouani. 2001. Neonatal exposure to antigen primes the immune system to develop responses in various lymphoid organs and promotes bystander regulation of diverse T cell specificities. J. Immunol. 167:4187-4195. [DOI] [PubMed] [Google Scholar]
- 22.Ryan, E. J., L. M. Daly, and K. H. G. Mills. 2001. Immunomodulators and delivery systems for vaccination by mucosal routes. Trends Biotechnol. 19:293-304. [DOI] [PubMed] [Google Scholar]
- 22a.Smerdou, C., I. M. Auton, J. Plana, R. Curtiss, and L. Enjuanes. 1996. A continuous epitope from transmissible gastroenteritis virus S protein fused to E. coli heat-labile toxin B subunit expressed by attenuated Salmonella induces serum and secretory immunity. Virus Res. 41:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sola, I., S. Alonso, S. Zuniga, M. Balasch, J. Plana-Duran, and L. Enjuanes. 2003. Engineering the transmissible gastroenteritis virus genome as an expression vector inducing lactogenic immunity. J. Virol. 77:4357-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strobel, S. 2001. Immunity induced after a feed of antigen during early life: oral tolerance v. sensitisation. Proc. Nutr. Soc. 60:437-442. [DOI] [PubMed] [Google Scholar]
- 25.Telemo, E., M. Bailey, B. G. Miller, C. R. Stokes, and F. J. Bourne. 1991. Dietary antigen handling by mother and offspring. Scand. J. Immunol. 34:689-696. [DOI] [PubMed] [Google Scholar]
- 26.van Ginkel, F. W., H. H. Nguyen, and J. R. McGhee. 2000. Vaccines for mucosal immunity to combat emerging infectious diseases. Emerg. Infect. Dis. 6:123-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Mutius, E. 2001. Infection: friend or foe in the development of atopy and asthma? The epidemiological evidence. Eur. Respir. J. 18:872-881. [DOI] [PubMed] [Google Scholar]
- 28.von Mutius, E. 2002. Worldwide asthma epidemic. Immunol. Allergy Clin. N. Am. 22:701-712. [Google Scholar]
- 29.Wilson, A. D., C. R. Stokes, and F. J. Bourne. 1989. Effect of age on absorption and immune-responses to weaning or introduction of novel dietary antigens in pigs. Res. Vet. Sci. 46:180-186. [PubMed] [Google Scholar]
- 30.Wuthrich, B. 1989. Epidemiology of the allergic diseases—are they really on the increase. Int. Arch. Allergy Appl. Immunol. 90:3-10. [DOI] [PubMed] [Google Scholar]
- 31.Zar, J. H. 1998. Biostatistical analysis, 4th ed. Prentice Hall, Upper Saddle River, N.J.