Abstract
Buruli ulcer disease (BUD) is an emerging disease caused by Mycobacterium ulcerans. In the present study we have characterized the serological reactivities of sera from volunteer case patients with laboratory-confirmed BUD and controls living in three different regions of Ghana where the disease is endemic to determine if serology may be useful for disease confirmation. Our results showed highly reactive immunoglobulin G (IgG) responses among patients with laboratory-confirmed disease, healthy control family members of the case patients, and sera from patients with tuberculosis from areas where BUD is not endemic. These responses were represented by reactivities to multiple protein bands found in the M. ulcerans culture filtrate (CF). In contrast, patient IgM antibody responses to the M. ulcerans CF (MUCF) proteins were more distinct than those of healthy family members living in the same village. A total of 84.8% (56 of 66) of the BUD patients exhibited strong IgM antibody responses against MUCF proteins (30, 43 and 70 to 80 kDa), whereas only 4.5% (3 of 66) of the family controls exhibited such responses. The sensitivity of the total IgM response for the patients was 84.8% (95% confidence interval [CI], 74.3 to 91.6%), and the specificity determined with sera from family controls was 95.5% (95% CI, 87.5 to 98.4%). These studies suggest that the IgM responses of patients with BUD will be helpful in the identification and production of the M. ulcerans recombinant antigens required for the development of a sensitive and specific serological assay for the confirmation of active BUD.
Buruli ulcer disease (BUD) is the most common mycobacterial disease after tuberculosis (TB) and leprosy among immunocompetent people in regions of Africa where BUD is endemic (34). The disease is characterized clinically by indolent, necrotizing skin ulcerations. Skin lesions progress over weeks to months from typically painless, subcutaneous nodules or plaques to large undermined ulcers, usually in the absence of systemic signs of illness. Adverse sequelae are common and include extensive scarring, flexion contractures, osteomyelitis, loss of limbs, and blindness. Over the past decade, there has been a considerable increase in the number of BUD cases in West Africa (15, 18, 19). In areas where the disease is endemic, BUD has replaced TB and leprosy as the most prevalent mycobacterial disease and affects up to 22% of the population in some communities (34). Although antibiotics have been shown to be effective against Mycobacterium ulcerans in vitro and in animal models of disease (5, 9), clinical trials have been inhibited by the absence of a good confirmatory assay, especially for the early stages of disease, when the possibility of clinical misclassification is highest. At present, the standard treatment strategy is limited to surgical excision, often followed by skin grafting. This intensive therapy and the need for long-term care create great economic burdens on affected communities (4).
Confirmation of BUD can be performed with tissues obtained directly from the excised skin or ulcer by combined laboratory methods such as Ziehl-Neelsen staining for acid-fast bacilli (AFB), bacterial culture for AFB, histopathology, and/or PCR (33). However, these tests may require advanced technical experience and are not always available; therefore, they are not routinely used for the case definition of BUD in developing countries where the disease is endemic. Consequently, the World Health Organization (WHO; Geneva, Switzerland) Global Buruli Ulcer Initiative challenged the research community to develop a simple and rapid diagnostic test that could be used to identify patients early during the course of infection (preferably at a preulcerative stage) so that the rate of detection of patients with BUD could be improved and preventive therapy and early treatment options could be fully implemented (31).
Because BUD is thought to mediate a selective suppression of human T-cell responses (21, 23), which results in a reduced delayed-type hypersensitivity reaction to M. ulcerans proteins in patients until late in the course of disease (10, 27), it has been thought that the detection of an immune response to infection and disease would not be diagnostic. Humoral immunity, however, may be useful for the diagnosis of disease, since serum samples from infected individuals from several geographically distinct regions where BUD is endemic have shown high antibody titers to M. ulcerans antigens (10, 12). In the study described in this report, we used Western blotting to characterize the immunoglobulin M (IgM) and IgG antibody responses of BUD patients to M. ulcerans proteins released into culture filtrates (CFs). Using serum samples obtained from patients with laboratory-confirmed BUD and matched healthy relatives from three different regions of Ghana where BUD is endemic, we now show that a distinct serological response is consistent with active BUD and that this specific response may be useful for the development of a serological test for BUD.
MATERIALS AND METHODS
Patients and study design.
Patients with BUD were enrolled in a case-control study carried out in three regions of Ghana where the disease is endemic: Upper Denkyira, Amansie West, and Asante Akim North. Case patients were included in the study if they met the WHO case definition for clinical BUD (33, 34). Controls from areas of endemicity included case patient family members (excluding persons with any history of BUD, TB, and leprosy) without signs of clinical disease were matched 1:1 with the case patients. The controls were not matched by age. The extent of the age mismatch was large at the household level but not at the village level, since uninfected individuals of the same age from the same village also served as controls. Samples from the family controls were not tested by the four confirmatory tests, as subjecting these apparently healthy individuals to such tests would have been unethical. We conducted this study under the assumption that all family controls were negative for active BUD and used the WHO case definition as the “gold standard” by which we compared the IgM responses. Since TB is also endemic in regions of Ghana where BUD is endemic, there was a need to define cross-reactive antibodies common in patients with Mycobacterium tuberculosis infection having a reduced possibility of exposure to M. ulcerans infection but living in the same environment as those persons infected or exposed to M. ulcerans. To discriminate against antigens cross-reactive for BUD and TB, 25 patients who were confirmed to have TB and who lived in regions of Ghana where BUD is not endemic were recruited into the study. Since the differential diagnosis for BUD includes onchocerciasis at the nodular stage of disease, 15 serum samples from patients with onchocerciasis from regions of Guatemala where BUD is not endemic were also acquired for this study from the Helminth Immunology Section, Laboratory of Parasitic Diseases, National Institutes of Health. Disease was confirmed in each of these patients by demonstration of the presence of Onchocerca parasites (microfilariae) in the skin.
A total of 66 serum samples from patients confirmed to have BUD and 66 serum samples from matched family controls were available for testing. Swabs of lesions and tissue samples of lesions and nodules were surgically obtained from BUD patients after they provided consent and were sent to the laboratory for culture, histopathology, testing for AFB, and PCR. Samples from all BUD patients studied were tested by the four diagnostic laboratory tests, and BUD was confirmed by at least two of these tests: the presence of AFB by Ziehl-Neelsen staining of material directly from swabs of lesions or tissue samples, culture of bacilli confirmed to be M. ulcerans on Lowenstein-Jensen or 7H11 agar medium, PCR for M. ulcerans-specific IS2404 DNA (28), or histopathology (14). It was previously shown (14, 28) that 83% of the patients who are AFB positive and histopathology positive for BUD were also PCR positive for M. ulcerans. BUD patients defined as having the nonulcerative stage of disease had no other evidence of the progressive ulcerative form of the disease, as defined by the presence of nodules, edema, plaques, or scars (33). BUD patients defined as having the active ulcerative stage of the disease had ulceration of the dermis; and many of these patients also had nodules, edema, plaques, or scars on other regions of their bodies. Serum samples were stored at 4°C and shipped to the serum bank at the Centers for Disease Control and Prevention (CDC) in Atlanta, Ga., and were maintained at −20°C until they were tested.
Serological analysis.
M. ulcerans CFs (MUCFs) were prepared as described previously (10). MUCF proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis by the method of Laemmli (17) on a 10 to 20% gradient on 10-well precast gels (Novex, San Diego, Calif.). The bands on the gels were visualized by silver staining (20), and staining was repeated routinely throughout the study period to reconfirm the integrity of the stored MUCF proteins. A single batch of an MUCF protein preparation was used throughout the study. All gels were run by using a MagicMark Western blot standard (Invitrogen Corporation, Carlsbad, Calif.) to ensure that the transferred proteins reactive to human antibodies could be compared directly to the molecular weight standard (the molecular weight standard is made up of recombinant Escherichia coli proteins that contain immunoglobulin-reactive regions to which primary and secondary antibodies react for direct detection of proteins on nitrocellulose membranes). Western blot analysis was carried out by standard protocols, as described previously (10). MUCF proteins fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis were transferred onto 0.45-μm-pore-size nitrocellulose membranes (Schleicher & Schuell, Inc., Keene, N.H.) and blocked overnight at 4°C with 1% blocking reagent (Roche Molecular Biochemicals, Indianapolis, Ind.). Nitrocellulose sheets were cut into strips for use for antibody detection. Sera from BUD patients were analyzed for antibodies to MUCF proteins by probing the nitrocellulose strips with serum samples at a 1:50 dilution. Sera from patients with TB and Onchocerca volvulus infection were tested at the same dilution. Bound antibody was detected with alkaline phosphatase-conjugated goat anti-human IgM antibody (Sigma Chemical Co., St. Louis, Mo.) or alkaline phosphatase-conjugated goat anti-human IgG (heavy and light chains) antibody (Pierce Biotechnology Inc., Rockford, Ill.) and the substrates nitroblue tetrazolium chloride-5-bromo-4-chloro-3-indolylphosphate, toluidine salt (Roche Molecular Biochemicals), according to the instructions of the manufacturer. All serum samples were tested in triplicate for the reproducibility and confirmation of the specific antibody reactivity to each M. ulcerans CF protein.
Statistical analysis.
Immunoblot and epidemiological data were entered into Microsoft Excel worksheets for analysis. The relationship between the IgM antibody responses to M. ulcerans proteins of the case patients and the matched family controls and the other controls was assessed by use of a stratified conditional logistic regression analysis, which accounted for the matched nature of the data. Sensitivity estimates and 95% confidence intervals (CIs) were calculated for IgM antibody responses to MUCF proteins for patients with laboratory-confirmed BUD, and specificity estimates and 95% CIs were calculated for the healthy controls. All statistical tests were two sided and unadjusted for multiple comparisons. A P value ≤0.05 was considered statistically significant.
Ethical approval.
The study protocol was approved by the Institutional Review Boards of the Ghanaian Ministry of Health, Accra, Ghana; Groningen University Hospital, Groningen, The Netherlands; Emory University School of Medicine; and CDC. The protocol was also reviewed by WHO.
RESULTS
MUCF proteins were recognized on immunoblots by serum IgG antibodies from both BUD patients and their healthy family controls living in the same village (Fig. 1). Because a large number of similar MUCF proteins were reactive to IgG antibodies from both the BUD patients and the family controls in this study, it was not practical to determine the specific antigens associated with only the IgG antibody responses of the patients. However, the IgM antibody responses were primarily restricted to sera from patients confirmed to have BUD (Fig. 2). The sera of 84.8% (56 of 66) patients with confirmed BUD showed positive IgM antibody reactivities against one or more MUCF proteins, whereas the sera of only 4.5% (3 of 66) of healthy controls showed IgM reactivities (Table 1). By a matched analysis using stratified conditional logistic regression, the patients were significantly more likely than the family controls to demonstrate an IgM antibody response to MUCF (P < 0.001). None of the serum samples from the patients with TB or the patients with onchocerciasis from regions where BUD is not endemic were positive for an IgM antibody response against MUCF proteins, although all serum samples were positive for an IgG response to the MUCF proteins (data not shown). The sensitivity of the total IgM antibody response for BUD patients was 84.8% (95% CI, 74.3 to 91.6%), and the specificity was 95.5% (95% CI, 87.5 to 98.4%).
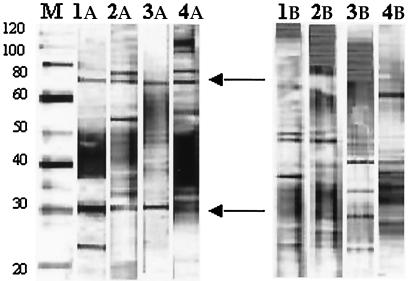
FIG. 1.
Reactivities of IgG antibodies against MUCF proteins on Western blots. Lane M, MagicMark molecular mass marker (Invitrogen), with molecular masses (in kilodaltons) given on the left; lanes 1A to 4A, representative sera from BUD patients with IgG antibody reactivities to different MUCF antigens; lanes 1B to 4B, representative sera from the corresponding patients' apparently healthy relative living in the same village. Common protein bands of 30 and 70 to 80 kDa recognized by both IgG and IgM, according to the molecular mass standard, are highlighted with arrows.
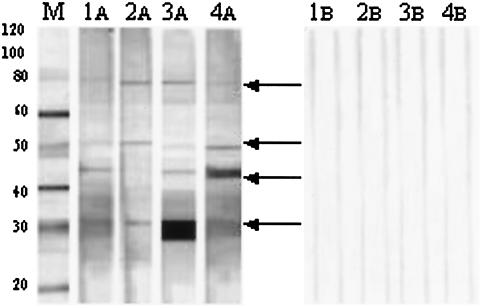
FIG. 2.
Reactivities of IgM antibodies against MUCF proteins on Western blots. Lane M, MagicMark molecular mass marker (Invitrogen), with molecular masses (in kilodaltons) given on the left; lanes 1A to 4A, representative sera from BUD patients with IgM antibody reactivities to different M. ulcerans CF antigens; lanes 1B to 4B, representative sera from the corresponding patients' apparent healthy relative living in the same village. Specific protein bands of IgM antibody reactivity corresponding to 30, 43, 50, and 70 to 80 kDa in the region of the molecular mass standard are highlighted with arrows.
TABLE 1.
IgM and IgG antibody responses to MUCF proteins in with BUD and healthy family controls, TB patients without BUD from regions of Ghana where BUD is endemic, and onchocerciasis patients
| Clinical description | No. of individuals | No. (%) of individuals positive fora:
|
|
|---|---|---|---|
| IgM | IgG a | ||
| BUD patients | 66 | 56 (84.8) | 66 (100) |
| Healthy family controlsb | 66 | 3 (4.5) | 66 (100) |
| TB patients | 25 | 0 (0.0) | 25 (100) |
| Onchocerciasis patients | 15 | 0 (0.0) | 15 (100) |
A positive antibody response denotes the presence of any recognizable protein band from MUCF on Western blots.
Apparently healthy family controls living in the same village as the BUD patients.
Serum samples from BUD patients with positive IgM antibody reactivities recognized four distinct MUCF proteins, namely, proteins of 30, 43, 50, and 70 to 80 kDa (Fig. 2). The number of samples with IgM antibody reactivities for specific MUCF proteins was scored; and it was found that 72.7% (48 of 66) reacted with the 43-kDa region, 57.6% (38 of 66) reacted with the 30-kDa region, and 34.8% (23 of 66) reacted with the 50-kDa region (Table 2). Only 12.1% (8 of 66) of the serum samples from IgM-positive individuals recognized the high-molecular-mass protein of 70 to 80 kDa. In general, the sera from most BUD patients recognized more than one MUCF protein (Fig. 2), with only 25% (14 of 56) of the IgM-positive sera being reactive with only one antigen (data not shown).
TABLE 2.
IgM antibody response to specific M. ulcerans CF proteins in BUD patients and healthy family controls from three regions of Ghana where BUD is endemica
| Clinical descriptiona | No. (%) of serum samples recognizing CF protein ofb:
|
|||
|---|---|---|---|---|
| 30 kDa | 43 kDa | 50 kDa | 80 kDa | |
| BUD patients | 38 (57.6) | 48 (72.7) | 23 (34.8) | 8 (12.1) |
| Healthy family controls | 0 (0.0) | 2 (3.0) | 0 (0.0) | 3 (4.5) |
There were 66 individuals in each group.
Some serum samples recognized more than one M. ulcerans CF protein.
To determine if the discriminatory IgM antibody reactivity described here will be suitable for use in the diagnosis of all stages of active BUD, the reactivities were compared by stage of disease. The sera from a large percentage of the patients with preulcerative disease and no evidence of past disease or ulcers were reactive with the 43-kDa region of MUCF (85.7%; 18 of 21), while 66.7% (14 of 21) recognized the 30-kDa region (Table 3). Similarly, 68.6% (24 of 35) and 85.7% (30 of 35) of the serum samples from patients with the more progressive, ulcerative stage of disease recognized the 30- and 43-kDa MUCF proteins, respectively (Table 3).
TABLE 3.
IgM antibody response to specific MUCF proteins among BUD patients at different stages of disease progression
| Disease stagea | No. of individ- uals | No. of serum samples recognizing CF protein of:
|
|||
|---|---|---|---|---|---|
| 30 kDa | 43 kDa | 50 kDa | 80 kDa | ||
| Nonulcerative stage | 21 | 14 (66.7) | 18 (85.7) | 9 (42.9) | 5 (23.8) |
| Ulcerative stage | 35 | 24 (68.6) | 30 (85.7) | 14 (40) | 3 (8.6) |
Disease stage classification is based on the recommendations of the WHO Task Force on Buruli Ulcer and the International Conference on Buruli Ulcer Control and Research (see WHO Report on Global Buruli Ulcer Initiative, 2000), in which active form of disease is subdivided into ulcerative and nonulcerative stages. Of the 66 patients in this category, 56 were found to have clinical signs for only early active or ulcerative disease. Ten patients whose serum samples were not compared in the analysis summarized here had both early active and ulcerative forms of disease.
DISCUSSION
We have demonstrated for the first time that the humoral immune response during M. ulcerans infection may be useful for the confirmation of BUD. Because serum samples from infected individuals from several geographical areas (Africa and Australia) where BUD is endemic have been shown to have reproducibly high antibody responses to M. ulcerans antigens (10, 12), such a diagnostic test based on detection of a specific antibody may be globally important for the management of BUD. Serological tests based on humoral immunity have been shown to hold great potential for the diagnosis of related mycobacterial diseases, such as TB (13, 25, 26), and existing commercialized serological tests have used well-described recombinant antigens to detect IgG or other immunoglobulin classes in a dipstick or enzyme-linked immunosorbent assay format (for a review, see reference 22).
Shared antigens among the mycobacteria and other related bacteria could affect the specificity of a diagnostic test for mycobacterial disease based on detection of the humoral immune response (22). However, significant advances in multiantigen tests and characterization of nonprotein antigens may help to ameliorate the situation (25). Additionally, detection of an IgM antibody response has been attempted to discern more specific immune responses to mycobacterial antigens in patients with diagnoses of TB (13, 25) and leprosy (30). More recently, detection of IgM antibody reactive with Mycobacterium bovis BCG protein antigen A-60 in sera and pleural effusions was shown to enhance the sensitivity (77%) and specificity (94%) of identification of purified protein derivative-positive, BCG-vaccinated TB pleurisy patients (16) to distinguish them from patients with nontuberculous pleurisy; and serological confirmation of the multibacillary form of leprosy by detection of a specific IgM response to phenolic glycolipid I of Mycobacterium leprae has been shown (7). In the present study, we have shown that IgM antibody detection enables the identification of disease-specific antigens that may be useful for the development of a serological test for the diagnosis of BUD.
Although IgG-specific assays have traditionally been shown to have higher sensitivities than IgM-specific assays, most likely due to the lower titers of IgM that can occur during chronic mycobacterial diseases, our results show that the IgG antibodies could not be used to readily discern specific antigens between patients and family controls in the same villages where BUD is endemic. This repertoire of cross-reactive IgG antibodies to the MUCF antigens among patients and controls (Table 1) may be explained by the fact that the study population resides in a rural area and may be involved in activities that expose them to other environmental mycobacteria. Moreover, a high degree of conservation exists among the mycobacteria at the genomic level. For example, mycobacterial antigen 85 (Ag85A) from BCG is sufficiently conserved in M. ulcerans (84.1% amino acid sequence identity and 91% conserved residues) and allows cross-reactive protection from disease in animal models (29).
Only 4.5% (3 of 66) of the matched family controls had an IgM response against MUCF antigens. Early case detection based on clinical evaluation alone is difficult (3), so it is conceivable that these individuals may have had M. ulcerans infections and that the early cutaneous stages of disease were not detected by the attending physicians. Alternatively, it may be possible that these individuals had the disseminated form of M. ulcerans disease but did not present with the cutaneous form of the disease. In support of this speculation, Abalos and colleagues (1) reported on the increasingly frequent disseminated nonulcerative form of M. ulcerans infection in areas of endemicity. Portaels and colleagues (24) more recently noted that up to 50% of patients with BUD presenting to a hospital in Benin had nonulcerative lesions. The possibility that the matched family control individuals in our study had a noncutaneous form of disease therefore warrants additional studies to determine if a serological response may be useful in the diagnosis of these other forms of BUD, like that reported for extrapulmonary tuberculosis (32).
Previously, it was shown that sera from BUD patients have significant IgG antibody reactivities against two MUCF protein bands (approximately 36 or 38 and 70 kDa) that could be used to largely discriminate BUD patients and control individuals in an area of endemicity in Côte d'Ivoire (10). The case and control sera in that study had been collected in 1991 and were tested in 1999 and went through a series of freeze-thaw periods during their storage at CDC, possibly reducing their IgG titers. The control sera (from persons with no signs of disease) were also not matched to sera from patients with disease in the same village during their collection in Côte d'Ivoire. However, use of Western blot detection of specific proteins in the MUCF to discriminate the differences in the IgG responses of patients and unmatched control individuals was still required because 37% of the controls without disease (n = 27) had detectable IgG antibodies against any MUCF protein. Likewise, in this present study with fresh sera from Ghanaian case patients and controls, an IgG enzyme-linked immunosorbent assay of total MUCF was not able to discriminate between case patients and matched family controls (unpublished data). It was also more difficult to discriminate specific IgG responses between case patients and matched family controls by Western blotting due to the high titers of cross-reacting IgG antibodies in all individuals (Fig. 1; Table 1), with one exception. The IgG responses to the 70-kDa antigen by Western blotting were significantly different between sera from patients confirmed to have BUD and sera from patients with TB from an area of Ghana where BUD is not endemic (data not shown).
It was therefore important to discern to which MUCF antigens the sera from BUD patients and control individuals had IgM responses to determine likely candidate antigens for the detection of a specific immune response. The two IgM-reactive MUCF proteins (30 and 43 kDa) that were associated with both the preulcerative and the ulcerative stages of disease and that likewise discriminated BUD patients from TB patients and BUD patients from family controls were thus more easily detected than IgG-reactive proteins due to the elimination of the large amount of cross-reacting antibodies observed when IgG was detected in the same case patients and controls (Fig. 1 and 2). Of note, the sizes of these antigens may not be absolute, since posttranslational modifications and other tertiary structural properties of the proteins may contribute to variations in electrophoretic migratory patterns. Likewise, because of variations in native antigen expression by M. ulcerans (15) and potential batch-to-batch variabilities in the MUCFs, it will be necessary to identify the gene products of these MUCF antigens to further develop a recombinant antigen-based serological test. At present, we are exploiting the specificity obtained by detection of IgM in our banked serum specimens to screen an M. ulcerans genomic DNA expression library to identify the genes encoding the putative IgM-reactive 30-, 43-, and 70- to 80-kDa MUCF proteins.
The success of any serological test largely depends on the specificity, sensitivity, and positive predictive value of the antigen; and in most cases, an increase in specificity usually undermines the sensitivity. Chan and colleagues (8) noted that a test with a combination of several M. tuberculosis antigens was more efficient than a test with a single antigen. Bothamley (6) observed that no single antigen has 100% sensitivity for the serodiagnosis of TB and suggested the use of a combination of antigens to achieve high sensitivity and specificity. Because a high level of sensitivity of IgM reactivity to the two MUCF proteins was observed both for patients with confirmed preulcerative disease and for patients with confirmed ulcerative disease (Table 3) and because a high level of specificity was found between these patients and the matched family controls (Table 1), it is conceivable that a multiantigen serological test could be developed for the diagnosis of BUD in areas of endemicity. Such an assay is urgently needed for the identification of patients with the early stage of the disease and those exhibiting nonspecific clinical signs of infection. This would improve case detection and help define the true burden of BUD in countries where the disease is endemic. Such an assay would also facilitate the much-needed clinical trials by ensuring that suspect cases are appropriately classified prior to randomization. Early detection of patients with BUD followed by prompt treatment would definitely reduce the potential for serious sequelae (2), an important requirement for reduction of the disease burden in areas of endemicity.
Acknowledgments
This study was supported by grants from the USPHS (cooperative agreement U50/CCU416560) and the American Leprosy Mission (to C. H. King), the WHO BUD collaboration center at CDC (to D. A. Ashford and J. W. Tappero), and in part by a grant from the Buruli Ulcer Groningen Foundation (to T. S. van der Werf and W. T. A. van der Graaf). Ymkje Stienstra was the recipient of a WOTRO-NWO fellowship from the Dutch National Organization for Scientific Research.
We thank S. Etuaful, E. Klutse, and E. Quarshie for clinical support with patient care and the collection of tissue and serum samples and Thomas Nutman of NIH for providing the sera from patients with confirmed cases of onchocerciasis (which were collected with institutional review board approval). We also thank Kingsley Asiedu, director of the WHO Buruli Ulcer Initiative, for invaluable support, time, and suggestions and George Amofah, director of Public Health, MOH, Ghana, for support and expert advice that enabled the case-control study.
REFERENCES
- 1.Abalos, F. M., J. Aguiar, Sr., A. Guedenon, F. Portaels, and W. M. Meyers. 2000. Mycobacterium ulcerans infection (Buruli ulcer): a case report of the disseminated nonulcerative form. Ann. Diagn. Pathol. 4:386-390. [DOI] [PubMed] [Google Scholar]
- 2.Amofah, G., S. Asamoah, and C. Afram-Gyening. 1998. Effectiveness of excision of pre-ulcerative Buruli lesions in field situations in a rural district in Ghana. Trop. Doct. 28:81-83. [DOI] [PubMed] [Google Scholar]
- 3.Amofah, G., F. Bonsu, C. Tetteh, J. Okrah, K. Asamoa, K. Asiedu, and J. Addy. 2002. Buruli ulcer in Ghana: results of a national case search. Emerg. Infect. Dis. 8:167-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asiedu, K., and S. Etuaful. 1998. Socioeconomic implications of Buruli ulcer in Ghana: a three-year review. Am. J. Trop. Med. Hyg. 59:1015-1022. [DOI] [PubMed] [Google Scholar]
- 5.Bentoucha, A., J. Robert, H. Dega, N. Lounis, V. Jarlier, and J. Grosset. 2001. Activities of new macrolides and fluoroquinolones against Mycobacterium ulcerans infection in mice. Antimicrob. Agents Chemother. 45:3109-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bothamley, G. H. 1995. Serological diagnosis of tuberculosis. Eur. Respir. J. Suppl. 20:676S-688S. [PubMed] [Google Scholar]
- 7.Buhrer-Sekula, S., H. L. Smits, G. C. Gussenhoven, J. Van Leeuwen, S. Amador, T. Fujiwara, P. R. Klatser, and L. Oskam. 2003. Simple and fast lateral flow test for classification of leprosy patients and identification of contacts with high risk of developing leprosy. J. Clin. Microbiol. 41:1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, S. L., Z. Reggiardo, T. M. Daniel, D. J. Girling, and D. A. Mitchison. 1990. Serodiagnosis of tuberculosis using an ELISA with antigen 5 and a hemagglutination assay with glycolipid antigens. Results in patients with newly diagnosed pulmonary tuberculosis ranging in extent of disease from minimal to extensive. Am. Rev. Respir. Dis. 142:385-389. [DOI] [PubMed] [Google Scholar]
- 9.Dega, H., A. Bentoucha, J. Robert, V. Jarlier, and J. Grosset. 2002. Bactericidal activity of rifampin-amikacin against Mycobacterium ulcerans in mice. Antimicrob. Agents Chemother. 46:3193-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobos, K. M., E. A. Spotts, B. J. Marston, C. R. Horsburgh, Jr., and C. H. King. 2000. Serologic response to culture filtrate antigens of Mycobacterium ulcerans during Buruli ulcer disease. Emerg. Infect. Dis. 6:158-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gooding, T. M., P. D. Johnson, D. E. Campbell, J. A. Hayman, E. L. Hartland, A. S. Kemp, and R. M. Robins-Browne. 2001. Immune response to infection with Mycobacterium ulcerans. Infect. Immun. 69:1704-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gooding, T. M., P. D. Johnson, M. Smith, A. S. Kemp, and R. M. Robins-Browne. 2002. Cytokine profiles of patients infected with Mycobacterium ulcerans and unaffected household contacts. Infect. Immun. 70:5562-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grange, J. M., J. Gibson, E. Nassau, and T. Kardjito. 1980. Enzyme-linked immunosorbent assay (ELISA): a study of antibodies to Mycobacterium tuberculosis in the IgG, IgA and IgM classes in tuberculosis, sarcoidosis and Crohn's disease. Tubercle 61:145-152. [DOI] [PubMed] [Google Scholar]
- 14.Guarner, J., J. Bartlett, E. A. Whitney, P. L. Raghunathan, Y. Stienstra, K. Asamoa, S. Etuaful, E. Klutse, E. Quarshie, T. S. van der Werf, W. T. van der Graaf, C. H. King, and D. A. Ashford. 2003. Histopathologic features of Mycobacterium ulcerans infection. Emerg. Infect. Dis. 9:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King, C. H., D. A. Ashford, K. M. Dobos, E. A. Spotts-Whitney, P. L. Raghunathan, J. Guarner, and J. W. Tappero. 2001. Mycobacterium ulcerans infection and Buruli ulcer disease: emergence of a public health dilemma, p. 137-152. In W. M. Scheld, W. A. Craig, and J. M. Hughes (ed.), Emerging infections, vol. 5. ASM Press, Washington, D.C. [Google Scholar]
- 16.Kunter, E., C. K. Ilvan, A. Isitmangil, T. Turken, O. Okutan, Z. Kartaloglu, and S. Cavuslu. 2003. The value of pleural fluid anti-A60 IgM in BCG-vaccinated tuberculous pleurisy patients. Clin. Microbiol. Infect. 9:212-220. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 18.Marston, B. J., M. O. Diallo, C. R. Horsburgh, Jr., I. Diomande, M. Z. Saki, J. M. Kanga, G. Patrice, H. B. Lipman, S. M. Ostroff, and R. C. Good. 1995. Emergence of Buruli ulcer disease in the Daloa region of Côte d'Ivoire. Am. J. Trop. Med. Hyg. 52:219-224. [DOI] [PubMed] [Google Scholar]
- 19.Meyers, W. M., N. Tignokpa, G. B. Priuli, and F. Portaels. 1996. Mycobacterium ulcerans infection (Buruli ulcer): first reported patients in Togo. Br. J. Dermatol. 134:1116-1121. [PubMed] [Google Scholar]
- 20.Morrissey, J. H. 1981. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal. Biochem. 117:307-310. [DOI] [PubMed] [Google Scholar]
- 21.Pahlevan, A. A., D. J. Wright, C. Andrews, K. M. George, P. L. Small, and B. M. Foxwell. 1999. The inhibitory action of Mycobacterium ulcerans soluble factor on monocyte/T cell cytokine production and NF-kappa B function. J. Immunol. 163:3928-3935. [PubMed] [Google Scholar]
- 22.Perkins, M. D. 2000. New diagnostic tools for tuberculosis. Int. J. Tuberc. Lung Dis. 4:S182-S188. [PubMed] [Google Scholar]
- 23.Pimsler, M., T. A. Sponsler, and W. M. Meyers. 1988. Immunosuppressive properties of the soluble toxin from Mycobacterium ulcerans. J. Infect. Dis. 157:577-580. [DOI] [PubMed] [Google Scholar]
- 24.Portaels, F., J. Aguiar, M. Debacker, C. Steunou, C. Zinsou, A. Guedenon, and W. M. Meyers. 2002. Prophylactic effect of Mycobacterium bovis BCG vaccination against osteomyelitis in children with Mycobacterium ulcerans disease (Buruli ulcer). Clin. Diagn. Lab. Immunol. 9:1389-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pottumarthy, S., V. C. Wells, and A. J. Morris. 2000. A comparison of seven tests for serological diagnosis of tuberculosis. J. Clin. Microbiol. 38:2227-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raja, A., U. D. Ranganathan, R. Bethunaickan, and V. Dharmalingam. 2001. Serologic response to a secreted and a cytosolic antigen of Mycobacterium tuberculosis in childhood tuberculosis. Pediatr. Infect. Dis. J. 20:1161-1164. [DOI] [PubMed] [Google Scholar]
- 27.Stanford, J. L., W. D. Revill, W. J. Gunthorpe, and J. M. Grange. 1975. The production and preliminary investigation of Burulin, a new skin test reagent for Mycobacterium ulcerans infection. J. Hyg. (London) 74:7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stienstra, Y., T. S. van der Werf, J. Guarner, P. L. Raghunathan, E. A. Spotts Whitney, W. T. van der Graaf, K. Asamoa, J. W. Tappero, D. A. Ashford, and C. H. King. 2003. Analysis of an IS2404-based nested PCR for diagnosis of Buruli ulcer disease in regions of Ghana where the disease is endemic. J. Clin. Microbiol. 41:794-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanghe, A., J. Content, J. P. Van Vooren, F. Portaels, and K. Huygen. 2001. Protective efficacy of a DNA vaccine encoding antigen 85A from Mycobacterium bovis BCG against Buruli ulcer. Infect. Immun. 69:5403-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torrella, A., R. L. Solis, N. Rodriguez, Y. Medina, M. Pita, I. Perez, and N. Licourt. 1994. Ultra micro ELISA to the detection of IgM antibodies in Mycobacterium leprae using dry blood samples. Rev. Inst. Med. Trop. Sao Paulo 36:131-138. [PubMed] [Google Scholar]
- 31.van der Werf, T. S., W. T. van der Graaf, J. W. Tappero, and K. Asiedu. 1999. Mycobacterium ulcerans infection. Lancet 354:1013-1018. [DOI] [PubMed] [Google Scholar]
- 32.Wilkins, E. G., and J. Ivanyi. 1990. Potential value of serology for diagnosis of extrapulmonary tuberculosis. Lancet 336:641-644. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. 2001. Buruli ulcer: diagnosis of Mycobacterium ulcerans disease. World Health Organization, Geneva, Switzerland.
- 34.World Health Organization. 2000. Buruli ulcer: Mycobacterium ulcerans infection. World Health Organization, Geneva, Switzerland.