Abstract
One of the major limitations of the use of adenoviruses as gene therapy vectors is the existence of preformed immunity in various populations. Recent studies have linked failure of adenoviral gene therapy trials to the presence of antiadenoviral neutralizing antibodies (NAb). Understanding the distribution and specificity of such antibodies will assist in the design of successful recombinant adenoviral gene therapies and vaccines. To assess the prevalence of NAb to adenovirus serotypes 5 and 35 (Ad5 and Ad35), we analyzed serum samples from adult immunocompetent individuals living in The Gambia, South Africa, and the United States by using a neutralization assay. Serum samples were incubated with A549 lung carcinoma cells and adenoviruses encoding enhanced green or yellow fluorescent proteins; results were analyzed by fluorescence microscopy and flow cytometry. Using this technique, we found a high prevalence of NAb against Ad5 in Gambian, South African, and U.S. subjects at both low and high titers. Conversely, all subjects displayed a low prevalence of NAb to Ad35; when present, anti-Ad35 NAb were seen at low titers. Because of the ability of adenoviruses to elicit systemic and mucosal immune responses, Ad35 with its low NAb prevalence appears to be an attractive candidate vector for gene therapy applications.
Natural adenoviral infection has a worldwide distribution. There are presently 51 serotypes of the virus (5, 34); most of these serotypes are pathogenic, but many have not been fully associated with specific human diseases. Adenoviruses are attractive for use as vectors because of their ability to infect both resting and dividing cells, their capacity to accommodate large transgenes, the low frequency of integration into the host genome, and the relative ease of production of recombinant virus in the laboratory (22). Other advantages include the ability of the virus to induce mucosal immunity as well as the feasibility of oral or intranasal administration (28). Because of these reasons, adenoviral vectors are important in the current search for effective vaccine platforms. In the area of airway and lung gene transfer, the vectors are of particular interest because they are able to infect a wide variety of nondividing cells, have a high affinity to airway epithelium, and exhibit excellent efficiency in gene transfer (21, 15). Similarly, the use of adenoviral gene transfer may benefit the treatment of chronic inflammatory diseases, such as rheumatoid arthritis (13), as well as malignancies (4).
A major hurdle that has prevented the effective application of these vectors is the host immune response, which may manifest as the induction of proinflammatory cytokines, a humoral antibody response that neutralizes the adenovirus, or a cellular immune response that targets and destroy cells expressing adenoviral antigens (2, 18, 19, 36). The first two immune responses prevent delivery of transgenes to target cells. The acute proinflammatory response is dose dependent (12) and may be prevented by using a low dose of the vector. The cellular immune response may be addressed if needed by deletion of the E2A and E4 genes of the adenoviral vector (1) or by reintroduction of immunosuppressive genes such as E3 (17). There remains, however, the challenge of circumventing the humoral immunity responsible for the generation of antibody against the adenoviral capsid, which can inhibit cell infection and transgene expression. There are two types of antibodies generated after adenoviral infection: nonspecific total antibodies (TAb) and serotype-specific neutralizing antibodies (NAb). TAb are generated against the adenoviral penton, fiber, core, and hexon (32). Detectable by enzyme-linked immunosorbent assay, TAb are present in individuals who have been exposed to adenovirus. NAb are generated against the adenoviral serotype responsible for the infection and are specific for fiber, penton, and hexon (31). The presence of NAb may have a more immediate impact on therapeutic efficacy and the ability to readminister the vector effectively (26). This role of NAb has been illustrated in a recent study of adenoviral gene transfer in the treatment of rheumatoid arthritis that demonstrated the failure of transgene transfer to synoviocytes as a result of preexisting NAb to the adenoviral vector (13).
Currently used gene transfer vectors based on adenovirus serotype 2 (Ad2) and Ad5 have serious limitations as vectors (27). Apart from having naturally infected more than 50% of the adult human population (9), the infectivity of these adenoviral group C vectors is dependent upon the coxsackievirus-adenovirus receptor, which is not present in all human cells (16, 35). Therefore, a search for other adenoviral serotypes that are independent of the coxsackievirus-adenovirus receptor, display high binding affinity, and have a low human infection rate (25) is necessary to ensure the successful application of adenoviral vector-based gene therapy and vaccine programs. Efforts to acquire adequate knowledge of the distribution and specificity of adenoviral NAb in target populations will ensure safety and enhance both primary and secondary expression of the transgene.
To assess the prevalence of NAb to Ad5 and Ad35, we analyzed serum samples from adult immunocompetent individuals living in The Gambia, South Africa, and the United States by using a neutralization assay. The two populations in Africa were chosen because adenoviral vector application may be helpful in the development of preventive vaccines for the control of endemic African diseases, such as human immunodeficiency virus (HIV), Ebola hemorrhagic fever, and malaria. Serum samples from all subjects were incubated with A549 human lung carcinoma cells and adenoviruses encoding enhanced green or yellow fluorescent proteins; results were analyzed by fluorescence microscopy and flow cytometry. Although production of recombinant virus can be a lengthy process, the neutralization assay can be completed in 24 h. It is, therefore, a rapid and reproducible method of studying the distribution of NAb to adenoviral serotypes in the population.
MATERIALS AND METHODS
Study population.
Serum samples from 203 immunocompetent adult individuals were obtained for this study (age range, 17 to 68 years). All subject samples were HIV negative as determined by conventional enzyme-linked immunosorbent assay and/or PCR methods. Sources included the University of the Witwatersrand, Soweto, South Africa (53 samples), the Medical Research Council Banjul in The Gambia (50 samples) and, in the United States, the University of Pittsburgh (50 samples) and the Pittsburgh Central Blood Bank (50 samples). The samples from South Africa were from male and female volunteers. Pregnant women provided the samples from The Gambia. Half of the U.S. samples were from adult homosexual men, whereas the other half was from male and female blood donors. The samples were frozen at −86°C and banked in the various institutions after collection. Samples were shipped in dry ice to our facility in the Human Gene Therapy Center of The University of Pittsburgh. All the samples were thawed, aliquoted, and stored at −86°C until assayed. All samples in our study were subjected to identical treatment and storage conditions and were considered of equivalent quality. Approvals for the human subject research studies performed were obtained from the institutional review boards of the three institutions (University of Pittsburgh Exempt Protocol number 020192).
Cell culture.
A human lung carcinoma cell line, A549 (American Type Culture Collection), was cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 100 U of penicillin/ml, 100 mg of streptomycin/ml, and 2.5 mg of amphotericin B (Fungizone)/ml (all reagents from GIBCO BRL, Gaithersburg, Md.). Cells were cultured to confluence in 150- by 25-mm tissue culture dishes (Falcon; Becton Dickinson, San Jose, Calif.) at 37°C, trypsinized, washed with Hanks balanced buffer, resuspended with DMEM, and counted manually using the trypan blue exclusion method. For the neutralization assay, 105 cells were seeded into each microwell of a 96-well flat-bottom plate (Microtest; Becton Dickinson).
Construction of recombinant adenoviruses:.
Ad5-based vectors with E1/E3 deleted and expressing enhanced green fluorescent protein (EGFP) were constructed through Cre-lox recombination with reagents generously provided by S. Hardy (Somatix, Alameda, Calif.) (14). Briefly, a SalI-NotI fragment containing the EGFP gene from the plasmid pEGFP-N1 (Clontech, Palo Alto, Calif.) was inserted into the shuttle plasmid pAdlox and named pAdEGFP. Recombinant adenovirus with an E1/E3 substitution was generated by cotransfection of SfiI-digested pAdEGFP and ψ5 helper virus DNA into the adenoviral packaging cell line CRE8. Adenoviruses were propagated on CRE8 cells, purified by cesium chloride density gradient centrifugation, dialyzed, and stored at −70°C. An Ad35 vector with E3 deleted was generated by intermolecular homologous recombination of Ad35 genomic DNA with a plasmid containing enhanced yellow fluorescent protein (EFYP; plasmid pAd35EYFP) after cotransfection into HEK293 cells. pAd35EYFP contains 308 bp of E3 sequence (position 26888 to 27199), an expression cassette containing the EYFP gene under the control of a cytomegalovirus promoter, and the Ad35 sequence spanning position 30405 to the right inverted terminal repeat. This linearized plasmid was cotransfected into HEK293 cells with Ad35 genomic DNA that was pretreated with exonuclease III to remove the single strand of both 3′-terminal ends. The expression of EYFP was used to screen for the presence of recombinant virus using fluorescence microscopy. Adenoviruses were propagated on HEK293 cells (American Type Culture Collection), purified by cesium chloride density gradient centrifugation, dialyzed, and stored at −70°C.
Neutralization assay.
The neutralization assay was designed to quantitatively analyze the inhibition of adenoviral transduction by serotype-specific NAb present in diluted human serum samples. Ad5 with E1/E3 deleted and encoding EGFP (Ad5EGFP) and Ad35 with E3 deleted and bearing the gene for EYFP (Ad35EYFP) were used as the viruses. Recombinant adenoviruses were propagated in HEK293 cells, purified on cesium chloride density gradients, and dialyzed against storage buffer. Different cell lines were screened to determine the infection profile of the two viruses; the A549 cell line was chosen because it displayed similar infection profiles for both viruses.
In determining the optimal viral dose for the assay, we considered both the natural infectivity of the virus in A549 cells (in the absence of human serum) and the variability of infection in the presence of varying dilutions of serum containing Ad5-NAb and Ad35-NAb. A background level of greater than 50% but less than 100% cell infection was targeted, and the infectivity at viral particle/cell (Vp/c) ratios of 100, 1,000, 2,000, 4,000, and 5,000 was assessed.
Fourfold serum dilutions (1:8, 1:32, 1:128, and 1:512) were tested. In 96-well flat-bottom plates, 108 viral particles of adenovirus/well were combined with different serum dilutions and incubated for 1 h at 37°C. Subsequently, harvested A549 cells (105 cells/100 μl) were seeded into the wells of each assay plate and incubated for 24 h at 37°C.
Flow cytometric analysis.
After incubation, cells were inspected using fluorescence microscopy, harvested, washed, and resuspended in Hanks' solution. EGFP or EYFP expression was analyzed using a FACScan flow cytometer and CellQuest software (Becton Dickinson, Mountain View, Calif.). For each analysis, 1,000 events were collected. Results were subsequently statistically analyzed using Student's t test.
RESULTS
Optimization of conditions for the neutralization assay.
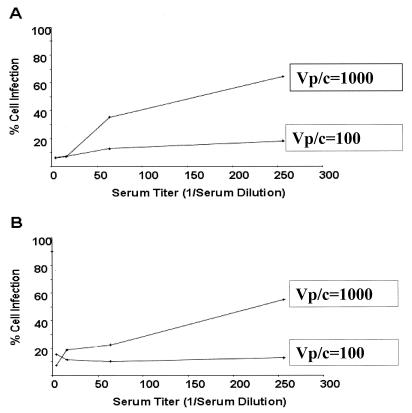
To determine the optimal conditions for adenoviral infection of the target A549 cells, infections with Ad5EGFP or Ad35EYFP were performed using different concentrations of viral particles in the absence or presence of various dilutions of serum samples containing NAb (Fig. 1). Poor cell infection using either Ad5EGFP or Ad35EYFP occurred at a Vp/c ratio of 100; 50% cell infection could not be achieved even at very low serum concentration. A dynamic curve of viral infection was obtained at Vp/c = 1,000 when either Ad5 or Ad35 was used; cell infection increased as serum NAb concentration decreased (Fig. 1) When a lower Vp/c ratio was used, the slope of the curve flattened out in the presence of samples with strong NAb to the adenoviral serotype. At higher Vp/c ratios, the slope of the curve increased dramatically; samples with lower-titer NAb were unable to prevent significant viral infection. Thus, to avoid a false-negative result that may occur because of viral saturation, a Vp/c ratio of 1,000 was chosen to perform the assay. NAb titer was determined as the reciprocal of the highest serum dilution that inhibited 50% cell infection compared to controls. In this assay, positivity at 8 and 32 was considered to be a low NAb titer, while neutralization at 128 and 512 was considered a high titer. Thus, all serum samples that did not inhibit >50% cell infection at 1:8 serum dilution were treated as NAb-negative samples. Each plate included a number of controls. Cells with recombinant adenoviruses (EGFP or EYFP) but no serum dilutions represented 100% fluorescence and, therefore, 0% inhibition of adenoviral transduction. A549 cells with no virus represented the background fluorescence and, thus, 100% neutralization of adenoviral infection. Other controls included previously identified highly negative and positive human samples (Table 1). Each test serum sample was assayed three or more times to confirm the consistency of the assay. All serum samples were treated identically.
FIG. 1.
Infection of A549 cells by recombinant adenoviruses at various Vp/c ratios in the presence of serum. (A) Infectivity profile of Ad5EGFP in the presence of diluted serum containing NAb indicates that a Vp/c ratio of 1,000 (or multiplicity of infection [MOI] of 10) yields optimal cell infection, with a clear inverse relationship between cell infection and serum NAb concentration. At Vp/c = 100 (or MOI = 1), the percentage of infected cells did not change with decreasing serum concentration (increasing titer), whereas at Vp/c = 1,000, cell infection increased with decreased serum concentration. Infection at higher viral doses suggested viral saturation (data not shown). (B) Infection of A549 cells with Ad35EYFP reveals a similar profile as with Ad5EGFP, with Vp/c = 1,000 as the optimal viral dose.
TABLE 1.
Assessment of the neutralization assay using flow cytometry: results of controls in three different experiments
| Serotype and culture component(s) | % of A549 transduction in exp no.:
|
Mean | SD | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| AD5 | |||||
| A549 only | 6.82 | 6.71 | 5.44 | 6.32 | 0.77 |
| A549 + AD5EGFP | 94.7 | 91.14 | 91.70 | 92.51 | 1.91 |
| A549 + AD5EGFP + positive serum | 2.82 | 8.31 | 6.94 | 6.02 | 2.86 |
| A549 + A5EGFP + negative serum | 81.19 | 78.90 | 88.74 | 82.94 | 5.15 |
| AD35 | |||||
| A549 only | 6.64 | 6.62 | 1.92 | 5.06 | 2.72 |
| A549 + AD35EYFP | 95.54 | 97.10 | 97.67 | 96.77 | 1.10 |
| A549 + AD35EYFP + positive serum | 1.26 | 8.17 | 2.36 | 3.93 | 3.03 |
| A549 + AD35EYFP + negative serum | 85.29 | 83.24 | 83.19 | 83.90 | 0.98 |
Fluorescence microscopy and flow cytometry analysis.
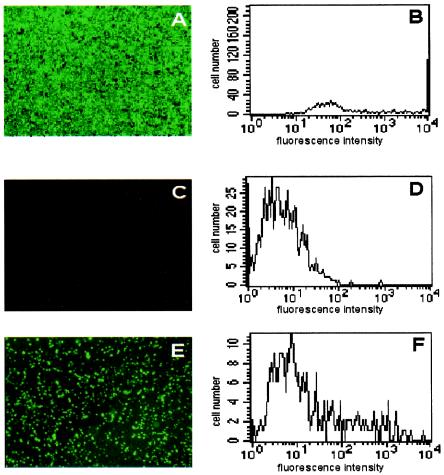
Using the optimized Vp/c ratio of 1,000, A549 cells incubated with recombinant adenoviruses in the presence of various dilutions of test serum samples for 24 h were assessed by fluorescence microscopy and flow cytometric analysis (Fig. 2). Total fluorescence detected in cells infected by Ad5EGFP or Ad35EYFP in the absence of diluted serum was considered to represent 0% inhibition of adenoviral transduction (Fig. 2A and B; Table 1). The background fluorescence detected in incubated cells with no virus represented 100% inhibition of adenoviral transduction (Fig. 2C and D; Table 1). Test serum samples were analyzed and compared to the controls (Fig. 2E and F).
FIG. 2.
Fluorescence microscopy and flow cytometry of A549 cells after neutralization assay. (A and B) A549 cells infected with virus Ad35EYFP in the absence of serum exhibit marked fluorescence, as seen in the micrograph (A) and the histogram (B). Similar results were seen in cells infected with Ad5EGFP. (C and D) A549 cells incubated with DMEM but not infected with virus display almost no background fluorescence in either the micrograph (C) or the histogram (D). (E and F) Representative fluorescence micrograph (E) and histogram (F) demonstrating A549 cells incubated with a test serum sample and virus.
Prevalence of adenoviral NAb in the study population.
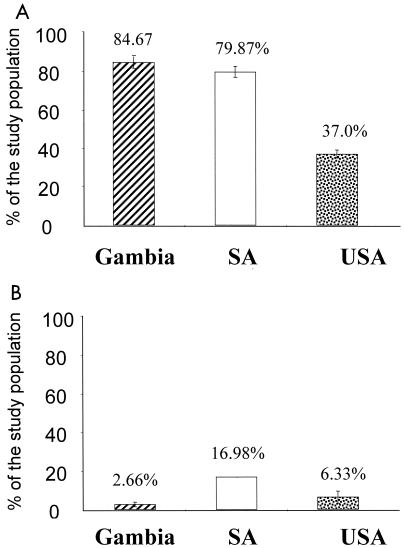
Using our developed neutralization assay, a high prevalence of Ad5-specific NAb was detected in both Gambian (84.67%; standard deviation [SD], 3.06%) and South African (79.87% [SD, 2.89%]) populations, with moderate prevalence in the U.S. population (37% [SD, 2.0%]); these results were seen at both low and high serum titers (Fig. 3A and Table 2). At low titers (8 and 32 together), The Gambia and South Africa groups had 47.33 and 56.60% prevalence, respectively, while the U.S. group had an average of 16.34% prevalence. At higher titers (128 and 512 together), the results in the three populations showed no marked difference; the Gambian and South African populations had 37.33 and 23.27% prevalence, respectively, while the U.S. group demonstrated 20.67% prevalence.
FIG. 3.
Prevalence of anti-Ad5 and anti-Ad35 NAb in the African and North American populations. (A) Anti-Ad5 NAb is present in The Gambian, South African, and U.S. populations at 84.67, 79.87, and 37.0% prevalence, respectively. (B) There is a very low prevalence of anti-Ad35 NAb in all three populations (<20%).
TABLE 2.
Prevalence of neutralizing antibody to the Ad5 or Ad35 serotype in 203 subjects
| Serotype and population | No. tested | Negative for Nab (% [SD]) | Positive for Nab (% [SD]) | Prevalence (% [SD]) at serum titer of:
|
|||
|---|---|---|---|---|---|---|---|
| 1:8 | 1:32 | 1:128 | 1:512 | ||||
| Ad5 | |||||||
| The Gambia | 50 | 15.33 (3.05) | 84.67 (3.06) | 11.33 (1.16) | 36.0 (4.0) | 33.33 (3.06) | 4 (2.82) |
| South Africa | 53 | 20.13 (2.89) | 79.87 (2.89) | 23.27 (1.09) | 33.33 (2.88) | 18.24 (1.09) | 5.03 (1.09) |
| United States | 100 | 63.0 (2.0) | 37.0 (2.0) | 6.67 (1.16) | 9.67 (3.51) | 15.67 (4.73) | 5.0 (1.0) |
| Ad35 | |||||||
| The Gambia | 50 | 97.33 (1.16) | 2.66 (1.16) | 2.66 (1.16) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| South Africa | 53 | 83.02 (0.0) | 16.98 (0.0) | 10.70 (2.88) | 6.28 (2.88) | 0 (0.0) | 0 (0.0) |
| United States | 100 | 93.67 (3.22) | 6.33 (3.22) | 5.33 (3.22) | 0.67 (0.58) | 0.33 (0.58) | 0 (0.0) |
In contrast, a low Ad35-specific NAb prevalence in all three different populations was discovered; only 2.66% (SD, 1.16) prevalence in the Gambian samples, 16.98% (SD, 0) in the South African samples, and 6.33% (SD, 3.22) in the U.S. samples were detected. These results were seen only at low titers (Fig. 3B and Table 2). Using Student's t test, the prevalence of NAb to Ad5 when compared to that for Ad35 in the study populations was highly statistically significant (P = 0.0001).
DISCUSSION
In this study, we analyzed serum samples from adult immunocompetent individuals living in The Gambia, South Africa, and the United States in order to assess the prevalence of NAb to Ad5 and Ad35. Using a neutralization assay, we demonstrated that NAb to Ad5 was found both at low and high titers in all populations. In contrast, only a low prevalence of NAb to Ad35 was seen on both continents, and it was found mostly at low titers.
Our findings are similar to other reports. In The Netherlands, Goossens et al. reported that 74% of the studied population exhibited Ad5-NAb and only 4% showed Ad35-NAb, also at low titers (13). Several studies were performed in the United States. Schulick et al. found a 57% prevalence of Ad5-NAb in an adult U.S. population (29). In another study, 7 (33.3%) out of 21 mesothelioma patients had NAb to Ad5 (26). Chen et al. found 46% prevalence of Ad5-NAb in prostate cancer patients and 60% prevalence in the normal healthy population (3). Thus, one can conclude that between 33 and 60% of the U.S. population have NAb to Ad5 as a result of natural infection.
While a high prevalence of NAb to group C adenoviruses (Ad2 and Ad5 included) was demonstrable in sera of 75 infants, none had NAb to Ad35 (6). In addition, a low prevalence of NAb to Ad35 was reported in previous assays of pooled gamma globulin (8). To our knowledge, there is no previously reported adenoviral NAb study in African populations.
Exposure to adenovirus, especially the major viral capsid proteins, usually results in the generation of immunoglobulin G and immunoglobulin A NAb, which attenuate cellular infection upon a second exposure (20, 37). In the case of Ad5, such circulating NAb have a significant impact on the efficacy and toxicity of systemically administered adenovirus (3). The use of the Ad5 vector has resulted in fatal toxicity (24) that was attributed to a high dose of vector (25). In addition, transgene transfer failure because of preexisting NAb was reported in rheumatoid arthritis patients treated with Ad5 (13). In another study, a lung cancer patient with high a Ad5-NAb titer experienced lower gene transfer (10).
Because of the limitations of currently used adenoviral vectors (Ad2 and Ad5) and the need to improve transgene expression, a search is required for alternative serotypes that are less prevalent in the human population. Ad35 appears to possess qualities of the ideal adenoviral vector. It has a high binding affinity to human epithelial cells (25). Recently, it was reported to transduce dendritic cells, smooth muscle cells, synoviocytes (33), and most human cells (30) more efficiently than Ad5. In addition, Ad35 infection is uncommon in immunocompetent individuals (8). Our data and that of others (13) demonstrate the low prevalence of Ad35-specific NAb in immunocompetent individuals from several continents, suggesting the low incidence of human infection caused by this adenovirus serotype worldwide. Given the low or absent titer of preexisting NAb in the general population, minimal doses of an Ad35 vector may be used for gene therapy and vaccine applications, thereby reducing toxicity (25) without compromising efficacy. In addition, our investigators have recently generated a replication-incompetent Ad35-based vector with E1/E3 deleted in our facility (11). By using this vector, the desirable qualities of Ad35 will ensure successful delivery and expression of the transgenes without compromising the safety of the recipients. It may be possible, therefore, to achieve the elusive therapeutic goals with Ad35 vectors.
There are some important limitations of our study. The sample size, which was somewhat limited because of the difficulty involved in obtaining serum samples from the studied populations, will need to be increased. Secondly, we studied only populations in Africa and North America. Although similar results were obtained in a European study, it is premature to make global generalizations. It will be interesting to evaluate similar data from Australia, South America, and Asia.
Continued investigation will be important in considering adenoviral vectors as preventive vaccine platforms for HIV and other infectious diseases because of the global prevalence of adenoviral NAb. Based on our findings and those of others, the use of Ad5 as a vector in the preparation of HIV or other vaccines may require caution in African, North American, and European populations. The results of our study demonstrate the importance of screening target populations for adenoviral NAb to specific serotypes before embarking on large-scale adenoviral vector gene therapy or vaccine programs.
This study focused on the determination of preexisting NAb to the candidate vector Ad35 and the commonly used vector Ad5 in three target populations. Given the low prevalence of anti-Ad35 NAb, use of the vector may be suitable for circumventing the humoral response to primary immunization in gene therapy or vaccine applications. Use of the same vector for a second immunization or boost may not be of any therapeutic benefit because of the potential generation of NAb (29). Based on previous studies (20), using another vector (serotype) with low preexisting NAb in the population is preferable. Identification of other serotypes with advantages such as those of Ad35 merits further exploration.
Our study did not address the T-cell response to virally infected cells. Cross-reactivity among different adenoviral serotypes arising from recognition by the cellular immune system of adenoviral epitopes expressed by infected cells has been reported (23). In addition, Flomemberg et al. demonstrated T-cell proliferation in response to Ad35 in individuals without serologic evidence of Ad35 infection (7).
In conclusion, our findings provide a broader picture of the prevalence of NAb to the commonly used Ad5 vector and propose a novel candidate vector based on serotype Ad35. Our discovery of low Ad35-specific NAb in the studied populations supports the feasibility of using Ad35 in future gene therapy and vaccine programs.
Acknowledgments
This work was supported by National Institutes of Health grants AI52806-01 and U01HL66949 (A.G.).
We thank Sarge-Njie Ramou and Baldeh Ignatius of the Medical Research Council of The Gambia and the staff of the Infectious Disease unit of the University of Witwatersrand, Soweto, South Africa, for their roles in providing the serum samples to our institution.
REFERENCES
- 1.Armentano, D., J. Zabner, C. Sacks, C. C. Sookdeo, M. P. Smith, J. A. St. George, S. C. Wadsworth, A. E. Smith, and R. J. Gregory. 1997. Effect of the E4 region on the persistence of transgene expression from adenovirus vectors. J. Virol. 71:2408-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brody, S. L., M. Metzger, C. Danel, M. A. Rosenfeld, and R. G. Crystal. 1994. Acute responses of non-human primates to airway delivery of an adenovirus vector containing the human cystic fibrosis transmembrane conductance regulator cDNA. Hum. Gene Ther. 5:821-836. [DOI] [PubMed] [Google Scholar]
- 3.Chen, Y., D.-C. Yu, D. Charlton, and D. R. Henderson. 2000. Pre-existing adenovirus antibody inhibits systemic toxicity and anti-tumour activity of CN706 in the nude mouse LNCaP xenograft model: implications and proposal for human therapy. Hum. Gene Ther. 11:1553-1567. [DOI] [PubMed] [Google Scholar]
- 4.Cichon, G., H. H. Boeckh-Herwig Schmidt, E. Wehnes, T. Muller, P. Pring-Akerblom, and R. Burger. 2001. Complement activation by recombinant adenoviruses. Gene Ther. 8:1794-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Jong, J. C., A. G. Wermenbol, M. W. Verweij-Uijterwaal, K. V. Slaterus, G. J. van Doornum, S. H. Khoo, and J. C. Hierholzer. 1999. Adenovirus from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J. Clin. Microbiol. 37:3940-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatz, W., and R. Wigand. 1985. Infection rate with adenovirus 1 to 39 in infants and adults. Immun. Infekt. 13:108-112. [PubMed] [Google Scholar]
- 7.Flomemberg, P., V. Piaskowski, R. L. Truitt, and J. T. Casper. 1995. Characterization of human proliferative T cell responses to adenovirus. J. Infect. Dis. 171:1090-1096. [DOI] [PubMed] [Google Scholar]
- 8.Flomemberg, P. R., M. Chen, G. Munk, and M. S. Horwitz. 1987. Molecular epidemiology of adenoviral type 35 infections in immunocompromised hosts. J. Infect. Dis. 155:1127-1134. [DOI] [PubMed] [Google Scholar]
- 9.Foy, H. M., and J. T. Grayston. 1976. Adenoviruses, p. 53-69. In A. S. Evans (ed.), Viral infections of humans: epidemiology and control. Plenum, New York, N.Y.
- 10.Gahery-Segard, H., V. Molinnier-Frankel, C. Leboulaire, P. Saulinier, P. Opolon, R. Lengagne, E. Gautier, A. Lecesne, L. Zitvogel, A. Venet, C. Schatz, M. Courtney, T. Lechevalier, T. Tursz, J.-G. Guillet, and F. Farace. 1997. Phase 1 trial of recombinant adenovirus gene transfer in lung cancer: longitudinal study of the immune responses to transgene and viral products. J. Clin. Investig. 100:2218-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao, W., P. D. Robbins, and A. Gambotto. 2003. Human adenovirus type 35: nucleotide sequence and vector development. Gene Ther. 10:1941-1949. [DOI] [PubMed] [Google Scholar]
- 12.Gilgenkrantz, H., D. Duboc, V. Julliard, D. Couton, A. Pavirani, J. G. Guilet, P. Briand, and A. Kahn. 1995. Transient expression of genes transferred in vivo into heart using first generation adenoviral vectors: role of the immune response. Hum. Gene Ther. 6:1265-1274. [DOI] [PubMed] [Google Scholar]
- 13.Goossens, P. H., R. Vogels, E. Piteterman, M. J. Havenga, A. Bout, F. C. Breedveld, D. Valerio, and T. W. Huizinga. 2001. The influence of synovial fluid on adenovirus-mediated gene transfer to the synovial tissue. Arthritis Rheum. 44:48-52. [DOI] [PubMed] [Google Scholar]
- 14.Hardy, S., M. Kitamura, T. Harris-Stansil, Y. Dai, and M. L. Phipps. 1997. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 71:1842-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hitt, M. M., and J. Gauldie. 2000. Gene vectors for cytokine expression in vivo. Curr. Pharm. Design 6:613-632. [DOI] [PubMed] [Google Scholar]
- 16.Huang, S., T. Kamata, Y. Takada, Z. M. Ruggeri, and G. R. Nemerow. 1996. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J. Virol. 70:4502-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilan, Y., G. Droguett, N. Roy Chowdury, Y. Li, K. Sengupta, N. R. Thummala, A. Davidson, J. Roy Chowdury, and M. S. Horwitz. 1997. Insertion of the adenoviral E3 region into a recombinant viral vector prevents antiviral humoral and cellular immune responses and permits long-term gene expression. Proc. Natl. Acad. Sci. USA 94:2587-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jooss, K., L. A. Turka, and J. M. Wilson. 1998. Blunting of immune responses to adenoviral vectors in mouse liver and lung with CTLA4Ig. Gene Ther. 5:309-319. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan, J. M., J. A. St. George, S. E. Pennington, L. D. Keyes, R. P. Johnson, S. C. Wadsworth, and A. E. Smith. 1996. Humoral and cellular immune responses of non-human primates to long term repeated lung exposure to Ad2/CFTR-2. Gene Ther. 3:117-1127. [PubMed] [Google Scholar]
- 20.Kass-Eisler, A., L. Leinwand, J. Gall, B. Bloom, and E. Falck-Pedersen. 1996. Circumventing the immune response to adenovirus-mediated gene therapy. Gene Ther. 3:154-162. [PubMed] [Google Scholar]
- 21.Kay, M. A., D. Liu, and P. M. Hoogerbugge. 1997. Gene therapy. Proc. Natl. Acad. Sci. USA 94:12744-12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozarsky, K., and J. M. Wilson. 1993. Gene therapy: adenovirus vectors. Curr. Opin. Genet. Dev. 3:499-503. [DOI] [PubMed] [Google Scholar]
- 23.Mack, C. A., W. R. Song, H. Carpenter, T. J. Wickham, I. Kovesdi, B. G. Harvey, C. J. Magovern, O. W. Isom, T. Rosengart, E. Falck-Pedersen, N. R. Hackett, R. G. Crystal, and A. Mastrangeli. 1997. Circumvection of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum. Gene Ther. 8:99-109. [DOI] [PubMed] [Google Scholar]
- 24.Marshall, E. 1999. Gene therapy death prompts review of adenovirus vector. Science 286:2244-2245. [DOI] [PubMed] [Google Scholar]
- 25.Mei, Y.-F., K. Lindman, and G. Wadel. 2002. Human adenoviruses of subgenera B, C and E with various tropisms differ in both binding to and replication in the epithelial A549 and 293 cells. Virology 295:30-43. [DOI] [PubMed] [Google Scholar]
- 26.Molnar-Kimber, K. L., D. H. Sterman, M. Chang, E. H. Kang, M. ElBash, M. Lannuti, A. Elshami, K. Gelfand, J. M. Wilson, L. R. Kaiser, and S. M. Albelda. 1998. Impact of preexisting and induced humoral and cellular immune responses in an adenovirus-based gene therapy; phase 1 clinical trial for localized mesothelioma. Hum. Gene Ther. 9:2121-2133. [DOI] [PubMed] [Google Scholar]
- 27.Pickles, R. J., D. McCarthy, H. Matsui, P. J. Hart, S. Randell, and R. Boucher. 1998. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J. Virol. 72:6014-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnell, M. J. 2001. Viral vectors as potential HIV-1 vaccines. FEMS Microbiol. Lett. 200:123-129. [DOI] [PubMed] [Google Scholar]
- 29.Schulick, A. H., G. Vassalli, P. F. Dunn, G. Dong, J. J. Rade, C. Zamarron, and D. A. Dichek. 1997. Established immunity precludes adenovirus-mediated gene transfer in rat carotid arteries. Potential for immunosuppression and vector engineering to overcome barriers of immunity. J. Clin. Investig. 99:209-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seshidhar Reddy, P., S. Ganesh, M. P. Limbach, T. Brann, A. Pinkstaff, M. Kaloss, M. Kaleko, and S. Connelly. 2003. Development of adenovirus serotype 35 as a gene transfer vector. Virology 311:384-393. [DOI] [PubMed] [Google Scholar]
- 31.Toogood, C., J. Crompton, and R. Hay. 1992. Antipeptide antisera define neutralizing epitopes on the adenovirus hexon. J. Gen. Virol. 73:1429-1435. [DOI] [PubMed] [Google Scholar]
- 32.Tursz, T., A. Le Cesne, P. Baklerou, E. Gautier, P. Opolon, C. Schatz, A. Pavirini, M. Courtney, D. Lamy, T. Ragot, P. Saulnier, A. Andremont, R. Monier, M. Perrcaudt, and T. Le Chevalier. 1996. A study of a recombinant adenovirus-mediated gene transfer in lung cancer patients. J. Natl. Cancer Inst. 88:1857-1863. [DOI] [PubMed] [Google Scholar]
- 33.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M. P. de Bethune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Cecchini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of pre-existing adenovirus immunity J. Virol. 77:8263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wadell, G., A. Allard, and J. C. Hierholzer. 1999. Adenovirus, p. 970-982. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 35.Walters, R. W., T. Grunst, J. M. Bergelson, R. W. Finberg, M. J. Welsh, and J. Zabner. 1999. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J. Biol. Chem. 274:10219-10226. [DOI] [PubMed] [Google Scholar]
- 36.Yang, Y., G. Trinchieri, and J. M. Wilmson. 1995. Recombinant IL-12 prevents formation of blocking IgA antibodies to recombinant adenovirus and allows repeated gene therapy to mouse lung. Nat. Med. 1:890-893. [DOI] [PubMed] [Google Scholar]
- 37.Yang, Y., Q. Li, H. C. Ertl, and J. M. Wilson. 1995. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses J. Virol. 69:2004-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]