Abstract
Objectives
We investigate the extent to which the electrocardiographic (ECG) properties of intact Scn5a+/− mice reproduce the corresponding clinical Brugada syndrome phenotype and use this model to investigate the role of conduction and repolarization abnormalities in the arrhythmogenic mechanism.
Methods and Results
The ECGs were obtained from anesthetized wild-type and Scn5a+/− mice, before and after administration of the known pro- and antiarrhythmic agents flecainide and quinidine. The ECG intervals were measured and their dispersions calculated. Scn5a+/− hearts showed ventricular arrhythmias, ST elevation, and conduction disorders including increased QT dispersion, accentuated by flecainide. Quinidine did not cause ventricular arrhythmias but exerted variable effects on ST segments and worsened conduction abnormalities.
Conclusions
The ECG features in an Scn5a+/− mouse establish it as a suitable model for Brugada syndrome and demonstrate abnormal conduction and repolarization phenomena. Altered QT dispersion, taken to indicate increased transmural repolarization gradients, may be useful in clinical risk stratification.
Introduction
The Brugada syndrome (BrS) is associated with increased incidences of polymorphic ventricular tachycardia (VT), exacerbated by flecainide but reduced by quinidine. Two major hypotheses have been suggested for this arrhythmogenicity. On the one hand, a depolarization defect might slow right ventricular (RV) action potential (AP) conduction,1 leading to a predisposition to reentrant arrhythmias. Alternatively, alterations in the time course of AP repolarization might result in a shortening of epicardial relative to endocardial AP durations (APDs), thereby increasing repolarization heterogeneities.2
These cellular mechanisms are potentially reflected in the electrocardiographic (ECG) features of BrS, of a persistent or transient ST elevation in the right precordial V1-V3 leads, often in combination with a pattern suggestive of right bundle branch block. This ECG pattern is central to the diagnosis of BrS. Flecainide and other class 1C drugs are often used to unmask the ST elevation,3 whereas there are early indications that quinidine can normalize the ST elevation seen in BrS.4
A significant proportion of BrS cases are associated with an inherited loss of Na+ channel function. This has prompted its recent experimental modeling using a genetic Scn5a+/− murine mutant, which shows a 50% reduction in sodium current in cellular studies and higher incidences of arrhythmogenesis compared to wild type (WT) when studied in Langendorff preparations.5 Recent studies have demonstrated both a conduction delay and increased repolarization gradients across the RV wall in the Scn5a+/− model.6 However, thus far, there have been few in vivo ECG studies in murine models whether for Scn5a+/− or in other primary electrophysiological disease. Yet, the ECG provides important measures of electrophysiological function, complementing studies of membrane currents at the cellular level and of AP waveforms in whole heart preparations. It has been the gold standard for determining effects of pharmaceutical compounds on cardiac electrophysiology. Furthermore, ECG recordings permit electrophysiological studies in whole animals with intact autonomic inputs to the heart.
Most available ECG studies have used the dog as a predictive preclinical species representing human electrophysiology, but BrS models are limited to pharmacological perfused wedge preparations. The recent extensive introduction of whole animal genetic murine models in studies of arrhythmic disease now requires the extension of ECG techniques to the mouse. Despite differences between murine and human ECG characteristics, particularly in QRS and T-wave morphology, ECG analysis is currently the only noninvasive method of studying cardiac electrophysiology in the intact mouse. Parameters such as the PR and QRS durations can give indications of conduction velocities within the heart, which may also provide indications of tendency toward reentrant arrhythmias. In addition, measurements such as the QT interval, representing the sum of ventricular depolarization and repolarization,7 may be used as a noninvasive surrogate for the APD and thereby help clarify the arrhythmogenic mechanism and aid clinical risk stratification.
The present experiments explore the extent to which ECG features in the genetic Scn5a+/− murine model reproduce the corresponding clinical phenotype, in particular in its arrhythmic features and characteristic ST elevation. We then use the murine model to explore for alterations in conduction velocity and repolarization times that might thereby be attributed to the Scn5a+/− mutation and compare these with features that occur in human BrS. A characterization of these may be useful in future studies assessing the effects of possible pharmacological interventions for BrS, for which the current mainstay of treatment is implanted cardioverter/defibrillator implantation.
Materials and methods
Mice aged 3 to 6 months were obtained from breeding pairs of heterozygote Scn5a+/− and WT inbred 129/sv mice, initially supplied by Harlan (UK). All procedures conformed to the UK Animals (Scientific Procedures) Act 1986. Mice were anesthetized intraperitoneally (IP) using Tribromoethanol (Avertin) (Sigma-Aldrich, Poole, UK) and custom-made chest and limb ECG leads attached. The anesthetic was chosen as it affects hemodynamics and electrophysiology to a lesser extent than other agents such as ketamine.8 A baseline ECG recording was obtained, and then either flecainide (20 mg/kg; Sigma-Aldrich) or quinidine (100 mg/kg; Sigma-Aldrich) was injected IP, and an ECG recording made for a 30-minute period. This flecainide dose has been used previously9 on both WT mice and mice with an SCN5A-1795insD mutation, in which it resulted in conduction abnormalities, whereas the quinidine dose compares with previous studies using 300 mg/kg IP in electrophysiological studies10 or 100 to 150 mg/kg in noncardiological studies.
The ECG signals were amplified and bandpass filtered between 15 and 1000 Hz using Neurolog amplifiers (Preamplifier: Model NL-100; AC amplifier: Model NL104) and filters (Model NL125/126, Digitimer, Welwyn Garden City, Herts, UK), and then digitized using a 1401plus interface (Cambridge Electronic Design, Cambridge, UK). Analysis of ECG waveforms was performed using Spike2 software (Cambridge Electronic Design).
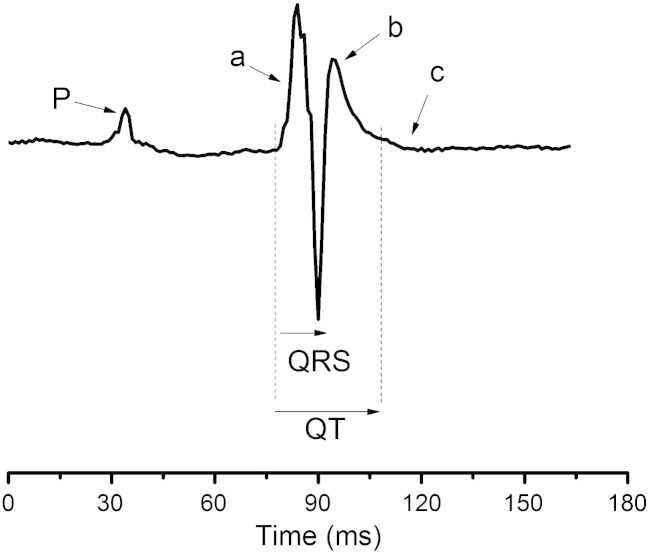
RR, PR, QRS, and QT intervals were measured from the ECG traces. The murine ECG differs from the human ECG in that the major deflection (a) representing the QRS is often followed by a secondary slower deflection (b) and sometimes a subtle third positive or negative wave (c), shown in Fig. 1. Some investigators designate b as the T wave and others c.11 We did not consistently find a c wave on our ECGs and therefore used the b wave to measure the QT interval, as shown. A heart rate correction factor was used, derived from Mitchell et al8 of QTc = QT/(RR/150)1/2, as the average RR interval in our mice was 150 milliseconds. Ten measurements of each ECG interval in both chest and limb leads were made for each mouse. Differences in time intervals between hearts were analyzed using Student t tests with a modified Bonferroni correction factor.12
Fig. 1.
Typical ECG complex from a WT heart before pharmacological manipulation. The murine ECG differs from the human ECG in that the major deflection (a) representing the QRS is followed by a secondary slower deflection (b), and sometimes, a subtle third positive or negative wave (c) follows. We did not consistently find a (c) wave on our ECGs and therefore used the (b) wave to measure the QT interval.
Results
Ten WT and 10 Scn5a+/− hearts were studied. In each group, 5 were subsequently exposed to flecainide and 5 to quinidine.
Incidence of ventricular arrhythmia
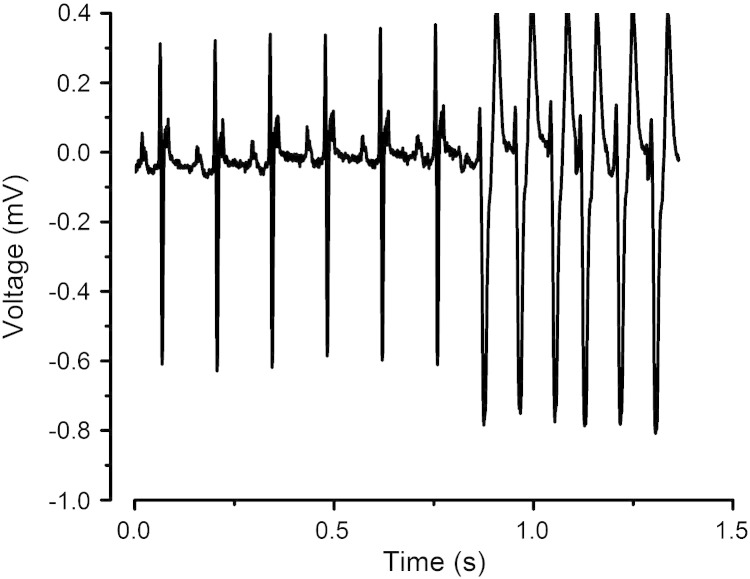
None of the WT hearts studied showed incidences of VT, whether before or after introduction of either flecainide or quinidine. None of the Scn5a+/− hearts showed incidences of VT before pharmacological intervention, although 1 Scn5a+/− heart demonstrated a bigeminal ECG pattern. However, the addition of flecainide then provoked VT in 2 Scn5a+/− hearts and nonsustained VT in a further heart (Fig. 2). Addition of quinidine did not lead to any ventricular arrhythmias.
Fig. 2.
Trace from a typical Scn5a+/− heart showing the onset of wide complex tachycardia following the addition of flecainide.
Presence of ST elevation
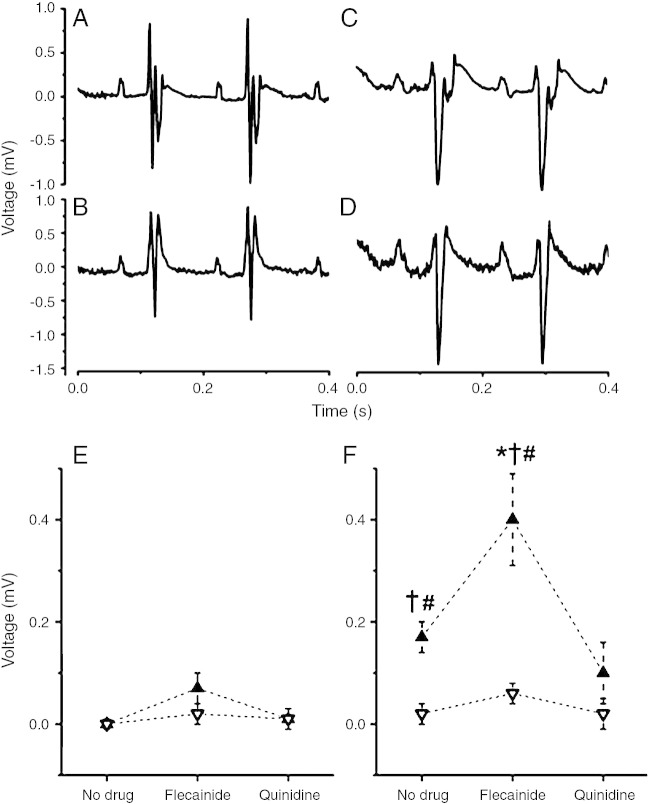
In WT hearts, there was no evidence of ECG ST elevation before pharmacological treatment. However, the addition of flecainide did cause some minor ST-segment elevation up to 0.09 ± 0.03 mV in the chest lead but not in the limb lead. Addition of quinidine did not affect the ST segment. Scn5a+/− hearts showed a degree of ST elevation in the chest lead, with a mean of 0.17 ± 0.03 mV. With the addition of flecainide, this significantly increased to a mean ST elevation of 0.40 ± 0.07 mV (Fig. 3). The ST elevation seen in untreated Scn5a+/− hearts was reduced to baseline by the addition of quinidine in 2 hearts but actually was slightly increased in 1 heart, from 0.16 to 0.22 mV.
Fig. 3.
A-D, Traces from chest leads (A, C) and limb leads (B, D) of a Scn5a+/− heart showing minor ST elevation before flecainide (A) and accentuated ST elevation after flecainide (C) in the chest leads but no appreciable ST elevation in the limb leads either before (B) or after (D) addition of flecainide. E and F, ST elevation measured from chest and limb leads for WT (E) and Scn5a+/− (F) hearts before and after the addition of flecainide or quinidine. Ten WT and ten Scn5a+/− hearts were used, with 5 of each exposed to each drug. Results from chest leads are denoted by solid symbols; results from limb leads are denoted by open symbols. †Results of t tests comparing WT and Scn5a+/− hearts giving values indicating significance. ⁎Results of t tests comparing values before and after drug in the same hearts giving values indicating significance. #Results of t tests comparing values between chest and limb leads in the same hearts giving values indicating significance.
We thus demonstrate the occurrence of ventricular arrhythmias and chest lead ST elevation, particularly after exposure to a flecainide challenge, for the first time in intact anesthetized genetically modified animals used to model BrS. This reproduces the diagnostic clinical ECG features of the human condition and suggests that the murine Scn5a+/− mutant would be an appropriate model to explore for and identify those electrophysiological features that could be attributable to a genetic loss of Na+ channel function.
Presence of atrioventricular block
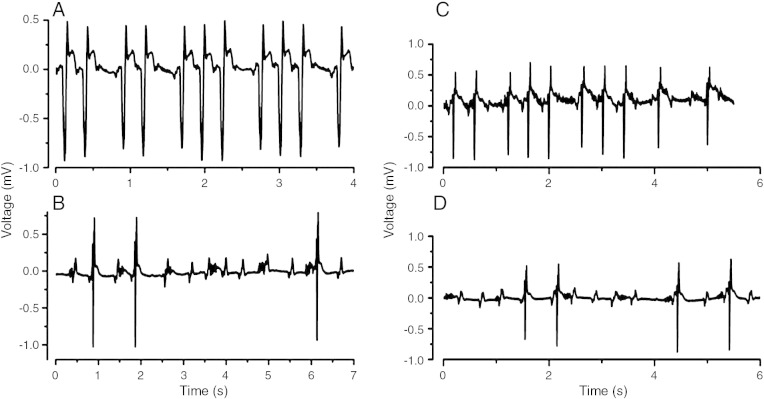
Untreated WT hearts showed no atrioventricular (AV) block. However, subsequent addition of flecainide and quinidine each caused 1 of 5 hearts to develop 2:1 heart block. Of 5 Scn5a+/− hearts, 2 showed short episodes of 2:1 AV block before exposure to flecainide. All 5 Scn5a+/− hearts exposed to flecainide showed various degrees of heart block, ranging from missed beats to persistent second-degree block, with 2:1 to 6:1 block, complete heart block, and sinus arrest (Fig. 4A, B). Interestingly, quinidine also produced heart block, with 1 heart showing 2:1 and 3:1 block, and another 3 showing block up to 5:1 (Fig. 4C, D).
Fig. 4.
Traces from Scn5a+/− hearts after flecainide challenge showing missed beats on a background of ST elevation (A) and high level AV block (B), and after introduction of quinidine showing missed beats (C) and high-level AV block (D).
ECG intervals
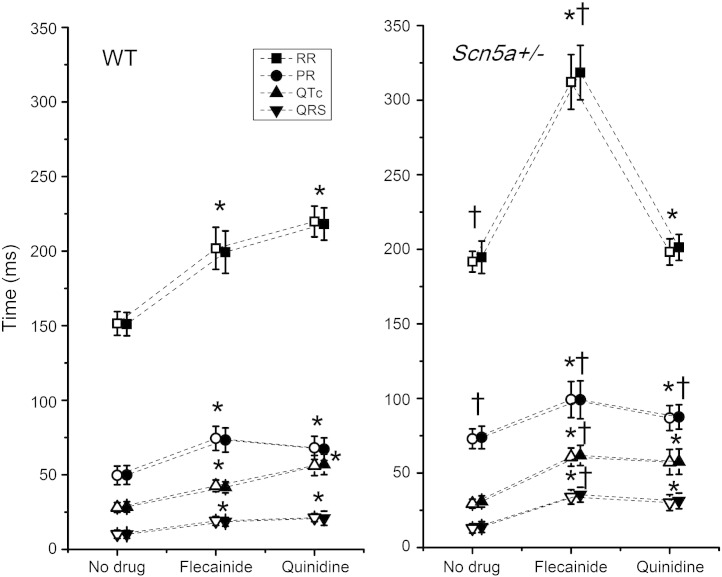
Untreated Scn5a+/− hearts showed increased RR and PR intervals but statistically indistinguishable QRS and QT intervals compared to WT. Both WT and Scn5a+/− mice showed widespread alterations in ECG intervals following the addition of either flecainide or quinidine. Both agents increased ECG RR, PR, QR, and QT intervals (Fig. 5), whether measured in the chest leads or the limb leads. However, flecainide exerted larger effects on these ECG intervals in Scn5a+/− than in WT. Consequently, when Scn5a+/− and WT were compared a second time in the presence of flecainide, the differences between the two reached significance for all intervals recorded. In contrast, quinidine increased the ECG intervals but did so to a similar degree in both the WT and Scn5a+/− hearts, and so the only difference between the two was then in the PR interval. All these effects on cardiac conduction were observed in both the chest lead and the limb lead of the ECG.
Fig. 5.
ECG parameters from chest and limb leads measured as RR, PR, QRS, and QTc intervals for WT and Scn5a+/− hearts before and after the addition of flecainide or quinidine. Ten WT and ten Scn5a+/− hearts were used, with 5 of each exposed to each drug. Results from chest leads are denoted by solid symbols; results from limb leads are denoted by open symbols. †T tests comparing WT and Scn5a+/− hearts giving values indicating significance. *T tests comparing values before and after drug in the same hearts giving values indicating significance.
ECG dispersion
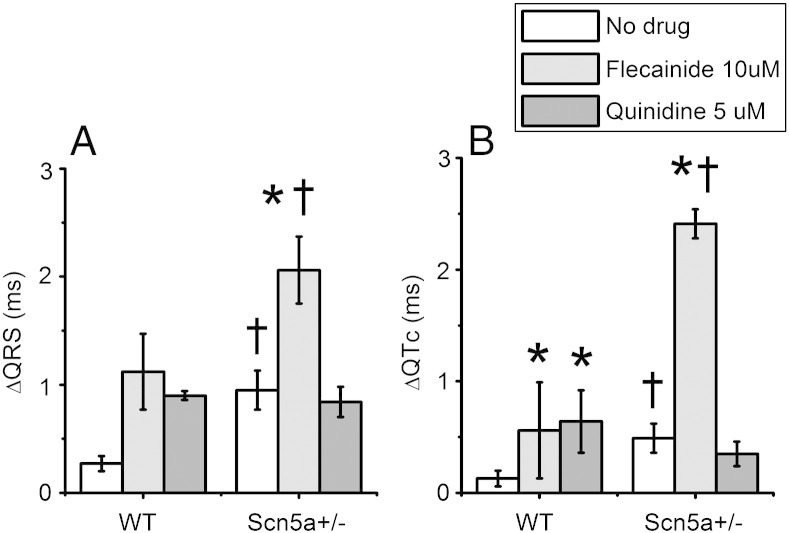
Scn5a+/− traces demonstrated increased dispersions in ECG intervals, attributable to Na+ channel loss. Dispersions of ECG intervals within each heart were explored by taking measurements from 2 ECG leads simultaneously—both from a chest lead and a limb lead (analogous to lead II), and the difference between the two in each heart calculated (Fig. 6).
Fig. 6.
QRS (A) and QTc (B) dispersion measured as the difference in time course in QRS or QTc intervals between chest leads and limb leads. Data are shown for WT and Scn5a+/− hearts before and after the addition of flecainide or quinidine. Ten WT and ten Scn5a+/− hearts were used, with 5 of each exposed to each drug. †T tests comparing WT and Scn5a+/− hearts giving values indicating significance. *T tests comparing values before and after drug in the same hearts with values indicating significance.
There was an increased difference between limb lead and chest lead in QRS and QTc intervals for Scn5a+/− hearts compared to WT hearts. Comparisons of the ECG dispersion in a given heart indicated that in WT hearts both flecainide and quinidine increased the dispersion to a significant level for the QTc intervals. In the Scn5a+/− hearts, flecainide increased the dispersion in both the QRS and QTc intervals. In contrast, quinidine did not increase the dispersion in either. Comparing WT and Scn5a+/− hearts after the addition of flecainide revealed a further difference in the QRS and QTc dispersions. However, the addition of quinidine served to reduce the difference between WT and Scn5a+/− hearts to statistically indistinguishable levels.
Discussion
BrS is characterized by increased incidences of ventricular arrhythmias exacerbated by flecainide but reduced by quinidine, and with an association of a significant proportion of cases with loss of function mutations involving the Na+ channel. The present experiments assessed the extent to which intact anesthetized heterozygotic Scn5a+/− mice replicate the ECG features of corresponding in vivo findings to allow them to be used as an experimental model for this condition. We then use this in vivo system to clarify ECG evidence for abnormalities of conduction and repolarization that are specifically attributable to loss of Na+ channel function.
The Scn5a+/− mouse replicates the ECG features of BrS
These ECG studies demonstrated wide complex tachycardias in the Scn5a+/− mice, which confirmed previous studies describing VTs in Langendorff perfusion experiments.6 They then went on to demonstrate their accentuation by flecainide. This directly parallels its known arrhythmic effects in human VT. In contrast, quinidine exerted an antiarrhythmic effect in direct parallel with both its prevention of phase 2 reentry and of polymorphic VT in experimental canine pharmacological perfused wedge preparations2 and its therapeutic antiarrhythmic effects in human BrS.4
We then demonstrated for the first time that Scn5a+/− mice show an ST elevation specific to the chest lead, which is accentuated by flecainide. This directly correlates with the clinically observed ST elevation and its provocation by flecainide. Both these features are central to the ECG diagnosis of human BrS. The murine Scn5a+/− system thus provides an alternative to canine perfused wedge preparations in which ST elevation could only be replicated using multiple drug combinations rather than flecainide alone.13 Furthermore, the minor ST elevation in WT hearts with flecainide also replicates clinical reports of Brugada-type ECGs in normal patients treated with flecainide.14 Finally, the effect of quinidine on ST elevation is mirrored in studies either showing that quinidine can reverse the ST elevation4 or have a variable effect upon the ST interval15 in BrS.
The differences in morphology between the human and murine ECGs mean that the ST segment is not identical between the 2 species, and therefore, interpretations about ST-segment elevation must be made with care. It is possible that the ST elevation is a normal variant in the mice; however, the lack of ST elevation in any of the WT mice before drug administration would argue against this. The traces in the BrS mice with flecainide do appear to reproduce the coved appearance of a human BrS type 1 ECG. Furthermore, they resemble the ST elevation seen in murine ECGs after ischemia produced by occlusion of the left anterior descending artery.16
The Scn5a+/− mouse shows ECG evidence for conduction abnormalities directly attributable to loss of Na+ channel function
BrS has been associated not only with abnormalities in SCN5A but also mutations in several other genes. Our experiments explored for ECG properties in Scn5a+/− mice that might identify those features of BrS that might be identified with loss of Na+ channel function. These revealed abnormalities in conduction and repolarization.
The intact anesthetized Scn5a+/− mice showed features of second-degree AV block and increased PR intervals, consistent with the longer conduction latencies previously observed in Langendorff perfused Scn5a+/− hearts.6 These findings are directly translatable to clinical observations. Genetically heterogeneous groups of patients with BrS show varying findings of either similar17 or longer18 PR intervals and AV block; however, BrS patients with an identifiable SCN5A mutation have been reported generally to show longer PR intervals and more bradyarrhythmias19 than those without an identifiable SCN5A mutation. Furthermore, loss-of-function SCN5A mutations are associated with a heterogeneous range of conditions including Progressive Cardiac Conduction Disease and Sick Sinus Syndrome and not only with BrS. Together, these findings suggest that ECG features of conduction delay seen in BrS may be attributable specifically to the Na+ channel loss of function.
The conduction abnormalities in Scn5a+/− mouse are exacerbated by flecainide
Flecainide exacerbated the conduction delay in all mice, but particularly in the Scn5a+/−. This is consistent with its actions in pharmacological Na+ channel block when this is superimposed upon a preexisting reduction in Na+ channel function caused by Scn5a+/−. These findings directly correlate with clinical studies reporting that flecainide increases PR intervals and QRS durations both in control and BrS patients,17,20 with greater effects in the latter.
That quinidine also increased conduction abnormalities might suggest that theoretically it could worsen conduction in cases of BrS with an already diseased conduction system. However, in clinical trials, this has not been seen,21 and the effects shown here may be due to the relatively high doses of quinidine, in comparison to the low doses used clinically, where the effects of blocking K+ channels are likely to be much stronger that those blocking Na+ channels.
QT interval is not a direct correlate of APD
The clinical QT interval has been taken to be the sum of depolarization and repolarization time courses. The corresponding clinical findings vary, either reporting QTc intervals in patients with BrS within a reference range22 or that were significantly longer than controls.20 Several studies17,20 have shown that the QTc interval is prolonged with the introduction of flecainide both in BrS and control groups, but more so in patients with BrS.
The reduced Na+ current in BrS has been suggested to exaggerate the normally existing electrical heterogeneity within the ventricular wall and in particular accentuate the AP notch in the RV epicardium. Pitzalis et al20 have suggested that the greater the baseline AP notch, the longer the induced AP prolongation, which should be reflected in prolongation of the QT interval. However, other studies have related electrophysiological abnormalities in BrS with shortening of APDs in the RV epicardium.2,6 This contrasts with the normal or slightly prolonged QTc interval seen both clinically and in the present experiments.
It is possible that the QTc does not shorten in parallel with the APD in Scn5a+/− hearts owing to the selective nature of the AP shortening in the RV epicardium, with other ventricular regions less affected. The ECG represents a transthoracic interpretation of the electrical vector through the whole myocardium, and therefore, regions showing selective electrical changes are likely to be underrepresented. In contrast, increased transmural gradients of repolarization times may manifest as a prolonged QTc, as electrical repolarization would be recorded across a wider time frame. Danik et al11 found that the QTc interval was not correlated with the APD in murine studies.
QT dispersion as an indication of repolarization heterogeneity
Although there is disagreement to the extent that QT dispersion represents repolarization heterogeneities,23 there is evidence that it is useful in risk assessment in ischemia, chronic heart failure, and long QT syndrome. There remains controversy over the usefulness of QT dispersion as a marker of risk in BrS, with studies either supporting or refuting its value.24,25 Nevertheless, Na+ channel–blocking agents do appear to increase temporal QT dispersion in patients with BrS.20 We could not find clinical studies investigating the effect of quinidine on ECG dispersions in BrS. Our findings of heightened QT dispersion in Scn5a+/− hearts, further increased by flecainide but not by quinidine, directly parallel the respective pro- and antiarrhythmic effects of the 2 drugs experimentally and clinically. Furthermore, recent studies have implicated increased repolarization gradients across the RV wall in the arrhythmogenic mechanism in Scn5a+/− mice, which are increased by flecainide but reduced by quinidine.6
In conclusion, this article has extended analysis of BrS from limited pharmacological models in cellular or tissue preparations to a genetic Scn5a+/− model using the intact mouse. The model reproduces the clinical Brugada phenotype, lending weight to studies using it to investigate arrhythmogenic mechanisms. We attribute conduction abnormalities in BrS to a Na+ channel defect. Localized precordial ST elevation may reflect specific RV repolarization abnormalities. Finally, we show that the QT interval derived from the ECG is not a direct correlate of APD in a specific cardiac region but suggest that QT dispersion, as an indicator of increased transmural repolarization gradients, may be used in clinical risk assessment. Future studies measuring QT intervals simultaneously with monophasic action potentials could further investigate this.
Acknowledgments
This work was supported by the British Heart Foundation, the Medical Research Council, the Wellcome Trust, and the Biotechnology and Biological Research Council, UK. CAM was supported by a Medical Research Council Clinical Research Fellowship and a Sackler Studentship of the University of Cambridge School of Clinical Medicine. YZ was partially supported by the Chinese Nature Science Foundation (project numbers 30371571 and 30672209).
References
- 1.Meregalli P.G., Wilde A.A., Tan H.L. Pathophysiological mechanisms of Brugada syndrome: depolarization disorder, repolarization disorder, or more? Cardiovasc Res. 2005;67:367. doi: 10.1016/j.cardiores.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Yan G.X., Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100:1660. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 3.Brugada R., Brugada J., Antzelevitch C. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000;101:510. doi: 10.1161/01.cir.101.5.510. [DOI] [PubMed] [Google Scholar]
- 4.Alings M., Dekker L., Sadee A. Quinidine induced electrocardiographic normalization in two patients with Brugada syndrome. Pacing Clin Electrophysiol. 2001;24(9 Pt 1):1420. doi: 10.1046/j.1460-9592.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- 5.Papadatos G.A., Wallerstein P.M., Head C.E. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci U S A. 2002;99:6210. doi: 10.1073/pnas.082121299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin C.A., Zhang Y., Grace A.A. Increased right ventricular repolarization gradients promote arrhythmogenesis in a murine model of Brugada syndrome. J Cardiovasc Electrophysiol. 2010;1 doi: 10.1111/j.1540-8167.2010.01767.x. [Electronic publication ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butrous G. The QT interval and other representation of ventricular repolarization. Curr Opin Cardiol. 1987;2:43. [Google Scholar]
- 8.Mitchell G.F., Jeron A., Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol. 1998;274(3 Pt 2):H747. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- 9.Remme C.A., Verkerk A.O., Nuyens D. Overlap syndrome of cardiac sodium channel disease in mice carrying the equivalent mutation of human SCN5A-1795insD. Circulation. 2006;114:2584. doi: 10.1161/CIRCULATIONAHA.106.653949. [DOI] [PubMed] [Google Scholar]
- 10.Berul C.I., Aronovitz M.J., Wang P.J. In vivo cardiac electrophysiology studies in the mouse. Circulation. 1996;94:2641. doi: 10.1161/01.cir.94.10.2641. [DOI] [PubMed] [Google Scholar]
- 11.Danik S., Cabo C., Chiello C. Correlation of repolarization of ventricular monophasic action potential with ECG in the murine heart. Am J Physiol Heart Circ Physiol. 2002;283:H372. doi: 10.1152/ajpheart.01091.2001. [DOI] [PubMed] [Google Scholar]
- 12.Jaccard J., Wan C.K. Sage Publications; Thousand Oaks (CA): 1996. LISREL approaches to interaction effects in mulitple regression. [Google Scholar]
- 13.Fish J.M., Antzelevitch C. Role of sodium and calcium channel block in unmasking the Brugada syndrome. Heart Rhythm. 2004;1:210. doi: 10.1016/j.hrthm.2004.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura W., Segawa K., Ito H. Class IC antiarrhythmic drugs, flecainide and pilsicainide, produce ST segment elevation simulating inferior myocardial ischemia. J Cardiovasc Electrophysiol. 1998;9:855. doi: 10.1111/j.1540-8167.1998.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe H., Chinushi M., Washizuka T. Variable electrocardiographic effects of short-term quinidine sulfate administration in Brugada syndrome. Pacing Clin Electrophysiol. 2005;28:372. doi: 10.1111/j.1540-8159.2005.09494.x. [DOI] [PubMed] [Google Scholar]
- 16.Li R.A., Leppo M., Miki T. Molecular basis of electrocardiographic ST-segment elevation. Circ Res. 2000;87:837. doi: 10.1161/01.res.87.10.837. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu W., Antzelevitch C., Suyama K. Effect of sodium channel blockers on ST segment, QRS duration, and corrected QT interval in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2000;11:1320. doi: 10.1046/j.1540-8167.2000.01320.x. [DOI] [PubMed] [Google Scholar]
- 18.Tukkie R., Sogaard P., Vleugels J. Delay in right ventricular activation contributes to Brugada syndrome. Circulation. 2004;109:1272. doi: 10.1161/01.CIR.0000118467.53182.D1. [DOI] [PubMed] [Google Scholar]
- 19.Makiyama T., Akao M., Tsuji K. High risk for bradyarrhythmic complications in patients with Brugada syndrome caused by SCN5A gene mutations. J Am Coll Cardiol. 2005;46:2100. doi: 10.1016/j.jacc.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 20.Pitzalis M.V., Anaclerio M., Iacoviello M. QT-interval prolongation in right precordial leads: an additional electrocardiographic hallmark of Brugada syndrome. J Am Coll Cardiol. 2003;42:1632. doi: 10.1016/j.jacc.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Viskin S., Wilde A.A., Tan H.L. Empiric quinidine therapy for asymptomatic Brugada syndrome: time for a prospective registry. Heart Rhythm. 2009;6:401. doi: 10.1016/j.hrthm.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alings M., Wilde A. “Brugada” syndrome: clinical data and suggested pathophysiological mechanism. Circulation. 1999;99:666. doi: 10.1161/01.cir.99.5.666. [DOI] [PubMed] [Google Scholar]
- 23.Burnes J.E., Ghanem R.N., Waldo A.L. Imaging dispersion of myocardial repolarization, I: comparison of body-surface and epicardial measures. Circulation. 2001;104:1299. doi: 10.1161/hc3601.094276. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda T., Sakurada H., Sakabe K. Assessment of noninvasive markers in identifying patients at risk in the Brugada syndrome: insight into risk stratification. J Am Coll Cardiol. 2001;37:1628. doi: 10.1016/s0735-1097(01)01197-4. [DOI] [PubMed] [Google Scholar]
- 25.Fauchier L., Pierre B., Robin I. 13. Brugada syndrome, LQTS, and ARVC: 13.5 abnormal QT dynamics and nocturnal temporal QT dispersion in Brugada syndrome. Europace. 2005;7:S21. [Google Scholar]