Abstract
Thrombospondin-related adhesive protein of Cryptosporidium 1 (TRAP-C1) belongs to a group of proteins that are also found in Toxoplasma gondii, Eimeria tenella, and Plasmodium species. TRAP-related proteins are needed for gliding motility, host-cell attachment, and invasion. The objective of this study was to characterize the antibody response to recombinant TRAP-C1 (rTRAP-C1) in healthy volunteers exposed to C. parvum and their association with clinical illness. A total of 31 healthy adult volunteers participated. Seven volunteers received the C. parvum TAMU isolate (inocula, 10 to 300 oocysts), and 24 volunteers received the C. parvum UCP isolate (500 to 105 oocysts). The total antibody (immunoglobulin M [IgM], IgG, and IgA) response to rTRAP C-1 was measured by enzyme-linked immunosorbent assays prior to and after exposure to Cryptosporidium parvum (days 0 to 45). Results of this study showed that individuals who were uninfected demonstrated higher reactivity at baseline compared to those who became infected. After challenge, increases in antibody reactivity were seen on days 30 and 45 compared to the results seen on days 0 to 5. The increases in antibody reactivity were statistically significant in subjects with diarrhea and with or without detectable oocysts compared to the results seen with those who were uninfected and asymptomatic. These findings suggest that increases in antibody reactivity to rTRAP-C1 occur after recent exposure to C. parvum.
Cryptosporidium parvum infects the intestinal mucosas of various mammals, including humans. This apicomplexan parasite can cause asymptomatic infection or result in self-limited diarrhea, sometimes accompanied by nausea, vomiting, abdominal cramping, and fever in healthy hosts (4). However, infection with Cryptosporidium species can also result in persistent, sometimes fatal, diarrheal disease and malnutrition, especially in those with an underlying immunodeficiency (1, 4, 22).
In other species of the phylum Apicomplexa, the micronemal proteins Etp100, thrombospondin-related adhesive protein (TRAP), and micronemal protein 2 (MIC2) identified in Eimeria tenella (26), Plasmodium falciparum (9, 23), and Toxoplasma gondii (15, 27), respectively, are characterized by similar structures and functions (12, 25). TRAP proteins are important in parasite attachment and invasion of host cells (3, 10, 14, 20, 25). Thrombospondin-1 stimulates focal adhesion disassembly through a sequence known as the Hep 1 peptide, which then mediates signaling through a receptor co-complex involving calreticulin and a low-density lipoprotein receptor-related protein (20, 21).
P. falciparum TRAP epitopes are recognized by both the humoral (2, 11) and cellular (13) immune systems and serve as potential candidates for vaccines (5, 7). Recently, the C. parvum gene encoding the thrombospondin-related adhesive protein of Cryptosporidium 1 (TRAP-C1) has been cloned and sequenced, and a fragment of the encoded polypeptide has been produced in Escherichia coli. Like other TRAP proteins, TRAP-C1 is characterized by the presence of the thrombospondin type I repeat, an amino acid sequence involved in the binding to sulfated glycoconjugates. TRAP-C1 has six such thrombospondin type I repeats and has been localized at the apical end of C. parvum sporozoites by immunolocalization, raising the possibility that this protein has adhesive properties similar to those described for other parasites. The objective of this study was to characterize the antibody response to recombinant TRAP-C1 (rTRAP-C1) in healthy volunteers exposed to C. parvum and their association with clinical illness.
MATERIALS AND METHODS
Human subjects, evaluation of stools, and definition of terms.
Informed consent was obtained from all participating volunteers. This study was approved by The University of Texas-Houston Health Science Center Committee for the Protection of Human Subjects. Blood was collected on days 0 to 5, 30, and 45 postchallenge from healthy volunteers who participated in studies of Cryptosporidium infectivity as previously described (18). All volunteers were seronegative with respect to whole-C. parvum oocyst antigens, as determined by enzyme-linked immunosorbent assays (ELISA) prior to challenge. Seven volunteers received the C. parvum TAMU isolate (inocula containing from 10 to 300 oocysts), and 24 volunteers received the C. parvum UCP isolate (inocula containing from 500 to 105 oocysts). Sera were separated and stored at −80°C until use. Clinical information available included symptoms and characteristics of all stools passed for the first 2 weeks of the study and of two 24-h collections thereafter for a total of 6 weeks after challenge. Stools were examined for the presence of Cryptosporidium species by direct immunofluorescence assay (DFA) (6). Clinical and parasitologic data were categorized into the following groups. Volunteers with diarrhea included subjects who passed three unformed stools within an 8-h period, four or more unformed stools within a 24-h period, or stools with a total unformed weight of more than 200 g per 24-h period accompanied by at least two of the following gastrointestinal symptoms: abdominal pain and/or cramping, fecal urgency, excessive gas, tenesmus, nausea, or vomiting on at least one day during the duration of the study. Subjects were presumed infected when oocysts were detected in their stools by DFA or when they met the definition for diarrhea described above. Volunteers were presumed uninfected when they did not develop diarrhea as defined and stools were DFA negative.
Purification of rTRAP-C1.
Competent E. coli XL1-Blue MRF cells were transformed with a polyhistidine-tagged plasmid construct coding for the TRAP-C1 gene that expresses a fragment of TRAP-C1 from amino acid 295 to 491, as previously described (24). Protein expression was induced with the addition of 2 mM isopropyl-d-thiogalactopyranoside (IPTG) (Sigma, St. Louis, Mo.). Protein purification of rTRAP-C1 from the supernatant was performed by nickel affinity chromatography, as previously described (24). Protein concentration was determined by a bicinchoninic acid protein assay (Pierce, Rockford, Ill.) per the manufacturer's instructions.
ELISA for anti-rTRAP C1 antibodies.
Optimal concentrations of antigen and serum dilutions were determined by checkerboard analysis. Individual wells of Nunc flat 96-well immuno plates (Maxisorp Immuno Plates, Rochester, N.Y.) were coated with 0.2 μg of rTRAP-C1 in carbonate buffer (pH 9.5) overnight at 5°C. The plates were then washed six times with 0.2 M phosphate-buffered saline (PBS) containing 0.2% Tween 20 (PBST). Nonspecific binding sites were then blocked with 5% nonfat powdered milk (Sigma) in PBS and incubated for 1 h at 5°C. After a wash step with PBST performed as described above, serum diluted at a 1:4 ratio in 1% skim milk-PBS was added to each well in triplicate. After incubation for 1 h at 37°C and a wash step with PBST, polyclonal anti-human immunoglobulin A (IgA), IgG, and IgM raised in sheep and conjugated with horseradish peroxidase (Sigma) was used as the detecting antibody. After a final washing step with PBST, 3,3′,5,5′-tetramethylbenzidine with hydrogen peroxide (Pharmingen, San Diego, Calif.) was added and incubated at room temperature in the dark for 10 min. The reaction was stopped with the addition of 2.5 N sulfuric acid. The intensity of the color reaction was determined at 450 nm (MR5000; Dynatech, Chantilly, Va.). Optical density (OD) readings were recorded and stored. Serum samples from the same individual collected at different time points were assayed simultaneously on the same plate. Positive and negative controls consisting of serum samples with known high (OD, 0.600) and low (OD, 0.032) anti-rTRAP-C1 titers, respectively, were used for standardization between plates. To standardize plate-to-plate variability in the measurement of antibody response in the positive and negative controls, values for wells were required to be within 1 standard deviation of the mean value.
Statistical analysis.
Data were analyzed with a commercial software program (Statview 5.0.1; SAS Institute, Cary, N.C.). Analysis of the relationship between baseline antibody response and infection was done with a t test. For pair-wise comparisons, sera collected on days 0 to 5 were considered baseline and those collected on days 30 or 45 were considered postchallenge. Increases in ELISA OD units for each of the outcome variables were analyzed by paired, two-tailed t tests. The comparison of OD units over time was performed with a Wilcoxon sign test. Fisher's exact test was used to analyze seroconversion in relationship to outcome. A volunteer was considered to have demonstrated seroconversion when his post challenge value was in excess of 2 standard deviations above the mean of baseline readings for the entire group. The level of significance was set at P values of < 0.05.
RESULTS
Antibody response to rTRAP-C1 after exposure.
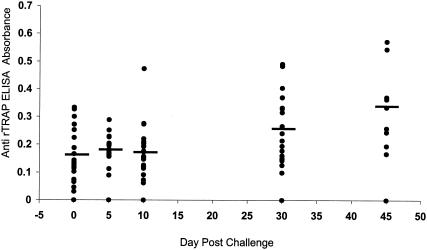
At entry into the study, all volunteers were identified as antibody negative to whole-C. parvum extracts by ELISA. A total of 31 volunteers were studied to detect the presence of antibodies to rTRAP-C1 by ELISA at two or more time points after exposure to Cryptosporidium oocysts. The time course of their antibody response as a group is shown in Fig. 1. Samples from 31 volunteers were available for testing on day 0 to 5, samples from 21 were available on day 10, samples from 19 were available on day 30, and samples from 9 were available on day 45. Statistically significant increases in antibody response from days 0 to 5 (mean ± standard deviation, 0.165 ± 0.08) and on days 30 (0.269 ± 0.121) and 45 (0.338 ± 0.140 [P < 0.05]) were observed.
FIG. 1.
Antibody (IgA, IgG, and IgM) response to rTRAP C-1 in volunteers exposed to C. parvum oocysts. Each point represents the mean of triplicate assays from serum samples collected on days 0 to 5, 10, 30, or 45. The horizontal bar represents the mean absorbance value for the group of volunteers.
Antibody response to rTRAP-C1 and clinical outcome.
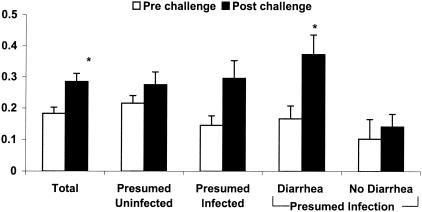
Prechallenge sera from 19 individuals were available for study. The mean baseline OD was significantly lower in volunteers who became infected than the baseline values for individuals who remained uninfected after challenge (0.150 ± 0.082 [n = 16] versus 0.233 ± 0.061 [n = 10], respectively [P < 0.05]). Table 1 shows paired values that include the prechallenge and postchallenge values available for the 19 subjects. Analysis of paired data for the 19 subjects as a group demonstrated a statistically significant increase in mean antibody response (from 0.183 ± 0.088 baseline to 0.285 ± 0.146 postchallenge [P < 0.05]) (Fig. 2). When stratified according to their clinical and parasitologic outcomes, 9 (47%) of 19 volunteers were presumed infected after exposure to oocysts and 10 were presumed uninfected. Among the nine subjects presumed infected, six (30%) had diarrhea meeting the definition and 3 were asymptomatic but had oocysts identified in their stools. Postchallenge sera from volunteers with presumed infection had mean OD readings that were higher than the results seen with the prechallenge sera (0. 146 ± 0.091 before challenge and 0.296 ± 0.170 after challenge [P < 0.01]). In comparison, sera from those presumed not infected did not demonstrate statistically significant increases in antibody response after challenge (0.216 ± 0.74 versus 0.275 ± 0.131). When members of the presumed-infected group were further divided according to symptoms, those with diarrhea (n = 6) showed a significant increase in mean OD (0.167 ± 0.099 at baseline and 0.373 ± 0.155 postchallenge [P < 0.01]); however, those without diarrhea (n = 3) did not (0.103 ± 0.062 versus 0.142 ± 0.040). Subgroup analysis of individuals with diarrhea showed that the increase in OD was statistically significant only for those individuals who had oocysts detectable by DFA (n = 4; 0.205 ± 0.102 before challenge and 0.446 ± 0.111 after challenge [P < 0.05]) compared to the results seen with those with diarrhea but without detectable oocysts (n = 2; 0.092 ± 0.037 and 0.228 ± 0.144).
TABLE 1.
Antibody (IgA, IgG, and IGM) response to rTRAP C-1 in 19 volunteers challenged with C. parvum oocystsa
| Outcome variable | No. of volunteers (n) | Prechallenge (OD ± SD) | Postchallenge (OD ± SD) | P |
|---|---|---|---|---|
| Presumed infected | 9 | 0.146 ± 0.091 | 0.296 ± 0.170 | 0.01 |
| Diarrhea | 6 | 0.167 ± 0.099 | 0.373 ± 0.155 | 0.03 |
| DFA+ | 4 | 0.205 ± 0.102 | 0.446 ± 0.111 | 0.03 |
| DFA− | 2 | 0.092 ± 0.037 | 0.228 ± 0.144 | NSb |
| No diarrhea | 3 | 0.103 ± 0.062 | 0.142 ± 0.040 | NS |
| Uninfected | 10 | 0.216 ± 0.074 | 0.275 ± 0.131 | NS |
| DFA+ | 7 | 0.161 ± 0.097 | 0.315 ± 0.182 | 0.03 |
| DFA− | 12 | 0.195 ± 0.084 | 0.267 ± 0.127 | 0.04 |
| Total | 19 | 0.183 ± 0.088 | 0.285 ± 0.146 | 0.003 |
Volunteers are categorized by clinical and/or parasitological outcomes.
NS, not significant.
FIG. 2.
Antibody (IgA, IgG, and IgM) response to rTRAP C-1 in 19 volunteers before (empty bars) and after (filled bars) exposure to C. parvum oocysts. Volunteer results are categorized by clinical outcome and expressed as the mean ± standard error for each group. Definitions of the various groups can be found in the text. *, P < 0.05.
When oocyst excretion was considered the outcome variable, statistically significant increases in reactivity were noted for those in whose samples oocysts could be identified by DFA (n = 7; 0.161 ± 0.097 before challenge and 0.315 ± 0.182 after challenge [P < 0.05]) compared to the results seen with those in whose samples oocysts could not be identified (0.195 ± 0.184 before challenge and 0.267 ± 0.127 after challenge [P < 0.05]). Seroconversion was observed for 3 (75%) of 4 individuals in the diarrhea and DFA+ group, whereas only 3 (20%) of 15 subjects without diarrhea or oocysts seroconverted (P = 0.07).
DISCUSSION
Although serological studies performed using whole-C. parvum extracts (8, 16, 19) have identified specific proteins or groups of proteins that correlate with illness and/or protection, the assays were not standardized and the functions of the proteins recognized by immune serum are not known. This study examined the antibody response to an isolated C. parvum protein that may be important in host cell invasion and thus could constitute a virulence marker. The results of this study demonstrated that healthy individuals develop antibody responses to the rTRAP-C1 polypeptide following Cryptosporidium infection.
In related parasites, TRAP is important in sporozoite motility and invasion (10, 17, 25) and is a target for the host immune response (7, 13). In this study, we investigated the humoral antibody response to a recombinant fragment of TRAP-C1 that contains thrombospondin type I repeats in healthy adults exposed to C. parvum. The similarity of TRAP C-1 to its counterparts in Plasmodium, Toxoplasma, and Eimeria species suggests that this protein might play a crucial role in C. parvum invasion of host enterocytes and elicit protective immune responses. In this study, there was a temporary increase in antibody response to Cryptosporidium infection; however, we cannot exclude the possibility of the presence of cross-reactivity from disease-state sera for those subjects infected with other related parasites as mentioned above.
Our results showed that volunteers demonstrated increases in anti-TRAP-C1 response as a function of time after exposure and that the degree of antibody response correlated with the characteristics of clinical illness and oocyst shedding. Subgroup analysis demonstrated that increases in antibody response were most notable for those volunteers who had infection intensities in the range detectable by immunofluorescence assays. Among the individuals who had infections, volunteers with diarrhea had greater increases in antibody response than those who did not have diarrhea. Furthermore, subjects with both diarrhea and detectable oocysts exhibited the highest relative increase in antibody response. Individuals who had both diarrhea and detectable oocysts showed a trend toward a higher seroconversion rate than those who did not have diarrhea. Of interest, when oocyst excretion was used as an outcome variable (regardless of clinical outcome) increases were noted for subjects in whose samples oocysts could not be identified as well as for those with detectable oocysts. This further supports our previous observation that Cryptosporidium-related diarrhea can occur in the absence of detectable oocysts by DFA (19). That is, the occurrence of diarrhea is not always correlated with the number of oocysts shed.
The overall antibody response to infection increased from days 0 to 5 to day 30 and day 45. The delay in antibody detection after exposure likely reflects the time needed for initial replication of the parasite and development of a detectable antibody concentration.
Although we have been able to demonstrate significant antibody responses to TRAP-C1, our work has several limitations. First, participants in the study were preselected on the basis of negative serology results with respect to total oocyst antigens. Therefore, this study did not address relationships between preexisting anti-TRAP-C1 antibodies and protection (although subjects with lesser reactivity at baseline were more susceptible to infection). Second, not all volunteers were exposed to the same C. parvum isolate (TAMU and UCP) or inoculum size (since sera used in this study were drawn from volunteers who participated in previous challenges designed to determine infective doses of different isolates) (6). Whether infection with specific isolates elicits distinct antibody reactivity results is unknown. A separate statistical analysis (data not shown) failed to identify an effect from the different isolates or inocula used. Rather, the antibody reactivity correlates more with infection (proven or presumed). Both isolates (TAMU and UCP) used in this study, as well as the isolate used to prepare rTRAP-C1, belong to the same genotype, genotype 2, which can infect both human and animals. The genetic homogeneity may explain the lack of differences between the isolates. It would be of interest to know whether antibody response to rTRAP-C1 would differ in those individuals infected with genotype 1 oocysts. Third, all antibody isotypes were studied simultaneously. Studies that evaluate the individual isotypes and fecal IgA response to TRAP-C1 are in progress. Fourth, conclusions from this study are based on a small number of subjects. Nevertheless, the data presented here suggest that healthy adults convalescing from Cryptosporidium infection develop antibody responses to TRAP-C1, a protein that most likely is involved in enterocyte cell invasion. Although the antibody response was modest in this study, we believe that the current serological assays for Cryptosporidium infection are not very sensitive. However, refinement of an ELISA-based TRAP-C1 assay such as the one described herein may be of use in determining susceptibility to infection and/or recent exposure.
Acknowledgments
This work was supported by grants from The U.S. Food and Drug Administration (FD-U-001621-01), U.S. Environmental Protection Agency (CR-819814 and CR-824759), National Institutes of Health (R01 AI 41735-01 and RR-02558), and the British Biological Research Council.
REFERENCES
- 1.Arenas-Pinto, A., G. Certad, G. Ferrara, J. Castro, M. A. Bello, and L. T. Nunez. 2003. Association between parasitic intestinal infections and acute or chronic diarrhoea in HIV-infected patients in Caracas, Venezuela. Int. J. STD AIDS 14:487-492. [DOI] [PubMed] [Google Scholar]
- 2.Bharadwaj, A., P. Sharma, S. K. Joshi, B. Singh, and V. S. Chauhan. 1998. Induction of protective immune responses by immunization with linear multiepitope peptides based on conserved sequences from Plasmodium falciparum antigens. Infect. Immun. 66:3232-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke, L. E., F. M. Tomley, M. H. Wisher, I. J. Foulds, and M. E. Boursnell. 1990. Regions of an Eimeria tenella antigen contain sequences which are conserved in circumsporozoite proteins from Plasmodium spp. and which are related to the thrombospondin gene family. Mol. Biochem. Parasitol. 41:269-279. [DOI] [PubMed] [Google Scholar]
- 4.Current, W. L., and L. S. Garcia. 1991. Cryptosporidiosis. Clin. Lab. Med. 11:873-897. [PubMed] [Google Scholar]
- 5.Dolo, A., D. Modiano, O. Doumbo, A. Bosman, T. Sidibe, M. M. Keita, S. Naitza, K. J. Robson, and A. Crisanti. 1999. Thrombospondin related adhesive protein (TRAP), a potential malaria vaccine candidate. Parassitologia 41:425-428. [PubMed] [Google Scholar]
- 6.DuPont, H. L., C. L. Chappell, C. R. Sterling, P. C. Okhuysen, J. B. Rose, and W. Jakubowski. 1995. The infectivity of Cryptosporidium parvum in healthy volunteers. N. Engl. J. Med. 332:855-859. [DOI] [PubMed] [Google Scholar]
- 7.Flanagan, K. L., T. Mwangi, M. Plebanski, K. Odhiambo, A. Ross, E. Sheu, M. Kortok, B. Lowe, K. Marsh, and A. V. Hill. 2003. Ex vivo interferon-gamma immune response to thrombospondin-related adhesive protein in coastal Kenyans: longevity and risk of Plasmodium falciparum infection. Am. J. Trop. Med. Hyg. 68:421-430. [PubMed] [Google Scholar]
- 8.Frost, F. J., A. A. de la Cruz, D. M. Moss, M. Curry, and R. L. Calderon. 1998. Comparisons of ELISA and Western blot assays for detection of Cryptosporidium antibody. Epidemiol. Infect. 121:205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilberger, T. W., J. K. Thompson, M. B. Reed, R. T. Good, and A. F. Cowman. 2003. The cytoplasmic domain of the Plasmodium falciparum ligand EBA-175 is essential for invasion but not protein trafficking. J. Cell Biol. 162:317-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jewett, T. J., and L. D. Sibley. 2003. Aldolase forms a bridge between cell surface adhesins and the actin cytoskeleton in apicomplexan parasites. Mol. Cell 11:885-894. [DOI] [PubMed] [Google Scholar]
- 11.John, C. C., J. S. Zickafoose, P. O. Sumba, C. L. King, and J. W. Kazura. 2003. Antibodies to the Plasmodium falciparum antigens circumsporozoite protein, thrombospondin-related adhesive protein, and liver-stage antigen 1 vary by ages of subjects and by season in a highland area of Kenya. Infect. Immun. 71:4320-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kappe, S., T. Bruderer, S. Gantt, H. Fujioka, V. Nussenzweig, and R. Menard. 1999. Conservation of a gliding motility and cell invasion machinery in apicomplexan parasites. J. Cell Biol. 147:937-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lalvani, A., N. Hurt, M. Aidoo, P. Kibatala, M. Tanner, and A. V. Hill. 1996. Cytotoxic T lymphocytes to Plasmodium falciparum epitopes in an area of intense and perennial transmission in Tanzania. Eur. J. Immunol. 26:773-779. [DOI] [PubMed] [Google Scholar]
- 14.Lovett, J. L., D. K. Howe, and L. D. Sibley. 2000. Molecular characterization of a thrombospondin-related anonymous protein homologue in Neospora caninum. Mol. Biochem. Parasitol. 107:33-43. [DOI] [PubMed] [Google Scholar]
- 15.Meissner, M., M. Reiss, N. Viebig, V. B. Carruthers, C. Toursel, S. Tomavo, J. W. Ajioka, and D. Soldati. 2002. A family of transmembrane microneme proteins of Toxoplasma gondii contain EGF-like domains and function as escorters. J. Cell Sci. 115:563-574. [DOI] [PubMed] [Google Scholar]
- 16.Moss, D. M., C. L. Chappell, P. C. Okhuysen, H. L. DuPont, M. J. Arrowood, A. W. Hightower, and P. J. Lammie. 1998. The antibody response to 27-, 17-, and 15-kDa Cryptosporidium antigens following experimental infection in humans. J. Infect. Dis. 178:827-833. [DOI] [PubMed] [Google Scholar]
- 17.Nussenzweig, I., and I. Menard. 2000. Analysis of a malaria sporozoite protein family required for gliding motility and cell invasion. Trends Microbiol. 8:94-97; discussion, 96-97. [DOI] [PubMed] [Google Scholar]
- 18.Okhuysen, P. C., C. L. Chappell, J. Crabb, L. M. Valdez, E. T. Douglass, and H. L. DuPont. 1998. Prophylactic effect of bovine anti-Cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clin. Infect. Dis. 26:1324-1329. [DOI] [PubMed] [Google Scholar]
- 19.Okhuysen, P. C., C. L. Chappell, C. R. Sterling, W. Jakubowski, and H. L. DuPont. 1998. Susceptibility and serologic response of healthy adults to reinfection with Cryptosporidium parvum. Infect. Immun. 66:441-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orr, A. W., C. A. Elzie, D. F. Kucik, and J. E. Murphy-Ullrich. 2003. Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration. J. Cell Sci. 116:2917-2927. [DOI] [PubMed] [Google Scholar]
- 21.Orr, A. W., C. E. Pedraza, M. A. Pallero, C. A. Elzie, S. Goicoechea, D. K. Strickland, and J. E. Murphy-Ullrich. 2003. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J. Cell Biol. 161:1179-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen, C. 1992. Cryptosporidiosis in patients infected with the human immunodeficiency virus. Clin. Infect. Dis. 15:903-909. [DOI] [PubMed] [Google Scholar]
- 23.Robson, K. J., J. R. Hall, M. W. Jennings, T. J. Harris, K. Marsh, C. I. Newbold, V. E. Tate, and D. J. Weatherall. 1988. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature 335:79-82. [DOI] [PubMed] [Google Scholar]
- 24.Spano, F., L. Putignani, S. Naitza, C. Puri, S. Wright, and A. Crisanti. 1998. Molecular cloning and expression analysis of a Cryptosporidium parvum gene encoding a new member of the thrombospondin family. Mol. Biochem. Parasitol. 92:147-162. [DOI] [PubMed] [Google Scholar]
- 25.Sultan, A. A., V. Thathy, U. Frevert, K. J. Robson, A. Crisanti, V. Nussenzweig, R. S. Nussenzweig, and R. Menard. 1997. TRAP is necessary for gliding motility and infectivity of plasmodium sporozoites. Cell 90:511-522. [DOI] [PubMed] [Google Scholar]
- 26.Tomley, F. M., L. E. Clarke, U. Kawazoe, R. Dijkema, and J. J. Kok. 1991. Sequence of the gene encoding an immunodominant microneme protein of Eimeria tenella. Mol. Biochem. Parasitol. 49:277-288. [DOI] [PubMed] [Google Scholar]
- 27.Wan, K. L., V. B. Carruthers, L. D. Sibley, and J. W. Ajioka. 1997. Molecular characterisation of an expressed sequence tag locus of Toxoplasma gondii encoding the micronemal protein MIC2. Mol. Biochem. Parasitol. 84:203-214. [DOI] [PubMed] [Google Scholar]