Abstract
In this study, six immunocompetent calves were experimentally infected with a noncytopathic strain of bovine viral diarrhea virus (BVDV), and the effects of the viral infection on parameters of the innate immune response of the host were analyzed. Clinical and virological data were compared with the temporal activation of the alpha/beta interferon-regulated Mx gene in white blood cells (WBC) and skin as well as the upregulation of the acute-phase serum proteins haptoglobin (Hp) and serum amyloid A (SAA). The viral strain used did provoke transient health impairment, namely, fever and leukopenia that were associated with viremia, viral shedding with nasal secretions, and antiviral seroconversion. Complete recovery was observed within 3 weeks. Elevated levels of SAA and Hp were apparent from days 4 to 13 and 8 to 11, respectively. In WBC, the levels of Mx mRNA and Mx protein were elevated from days 2 to 15. In the context of this study with BVDV, the level of Mx protein expression in WBC provided the most telling diagnostic window to monitor the host's ongoing innate immune response.
After tissue injury, trauma, or infection, the vertebrate host responds immediately with a variety of nonadaptive physiological changes, collectively known as the innate immune response. This response encompasses diverse countermeasures, such as the complement system, natural killer cells, the acute-phase response, and in the case of viral infections, the alpha/beta interferon (IFN-α/β) system (14). Initiation of the inflammatory and tissue-repairing cascade occurs primarily through recruitment of professional macrophages to the site of infection (6). Activated macrophages release a range of primary inflammatory mediators, the most important being interleukin 1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α). These factors prompt the release of a range of secondary cytokines and chemokines (e.g., IL-8, IL-12, and monocyte chemoattractant protein) from local stromal cells and resident tissue macrophages. The chemotactic activities of some of these molecules attract additional leukocytes to the inflammatory site, where they release further proinflammatory cytokines (6). One of the intensively studied systemic responses to an acute inflammatory stimulus is the alteration in the hepatic biosynthetic profile of acute-phase proteins (APPs), such as C-reactive protein (CRP), serum amyloid A (SAA), and haptoglobin (Hp) (17, 51). In veterinary medicine, there is growing interest in the possibility of using APPs as a marker of animal health or, alternatively, as an indicator of disease severity (12, 29). In cows, SAA and Hp were shown to be the most important APPs (12).
Viral infections commonly result in the induction, release, and remote action of type I IFNs, notably IFN-α, IFN-β, and IFN-ω (22, 35, 36, 49, 54) as well as the newly described IFN-τ (34, 40). Released IFN-α/β acts through specific receptors present on nucleated cells to transduce signals for the transcription of numerous genes, such as the genes encoding the double-stranded RNA (dsRNA)-dependent protein kinase (PKR), the 2′-5′-oligoadenylate synthetase (2′-5′-OAS), and the Mx proteins. PKR and 2′-5′-OAS are produced as inactive precursor molecules. Once activated by viral dsRNA intermediates, these effectors act in conjunction with RNase L to inhibit the host's cell transcription machinery and ultimately contribute to the induction of apoptosis of virus-infected cells. In ruminants, the IFN-α/β system is unique, encompassing, in addition to IFN-α/β/ω, the trophoblast-derived IFN-τ, which is a signal for maternal recognition of pregnancy (10, 16).
The Mx genes are specifically activated by IFN-α/β and by synthetic dsRNA, an established IFN-α/β inducer (2, 50, 60). Mx proteins are known to exert antiviral effects, especially towards RNA viruses, by targeting specific, yet poorly defined steps of the viral replication cycle (25, 28, 41, 42). Recent findings with La Crosse virus indicated an Mx protein-mediated sequestration of the viral nucleocapsid protein into perinuclear complexes (32).
Pestiviruses are members of the Pestivirus genus within the family Flaviviridae (57). This virus group comprises economically important animal pathogens, namely, classical swine fever virus, ovine border disease virus, and bovine viral diarrhea virus (BVDV). Pestiviruses are known for their ability to provoke transplacental infection, which may lead to fetal death, malformation, acute syndromes of the neonate, or immune tolerance and lifelong viral persistence, depending on the stage of gestation at which the infection takes place. Upon infection of immune competent animals, the severity of the clinical outcome ranges from subclinical or very mild in the majority of cases to acute fatal disease, depending on the virulence of the virus strain involved (26). BVDV is unusual due to the existence of two differing biotypes, namely, cytopathic (cpBVDV) and noncytopathic (ncpBVDV) virus types. Following transplacental infection during the first trimester of gestation, ncpBVDV is likely to provoke in the immunologically immature fetus a state of viral immunotolerance leading to lifelong virus persistence (52, 53). Persistently infected animals usually succumb to mucosal disease later in life either upon superinfection with or endogenous generation of an antigenically homologous cpBVDV (55, 57). While establishment of ncpBVDV persistence in the bovine fetus has been shown to correlate with a lack of IFN-α/β production (9), acute infection of the fetus with cpBVDV is paralleled by the production of endogenous IFN-α/β (9, 43).
These in vivo observations are supported by in vitro findings showing that ncpBVDV isolates do not induce IFN-α/β (1, 11, 39, 48) and block the induction of IFN-α/β by other activators, such as cpBVDV, Semliki Forest virus, and dsRNA (47, 48). A recent in vitro study indicated impairment of several signal transducers of the IFN-β gene by ncpBVDV, with interferon regulatory factor 3 being responsible for the permanent inhibition of IFN-α/β induction (5). On the basis of these in vivo and in vitro observations, it was concluded that the inability of cpBVDV to establish persistent infection in the bovine fetus was related to its IFN-α/β-inducing capacity (1, 9, 48). However, a recent report showed that ncpBVDV does induce IFN-α/β in 1-month-old calves (8). This suggests that the ability of ncpBVDV to induce immunotolerance is dependent not only on the viral biotype but also on the developmental stage of both the host's innate and adaptive immune system.
In this study and using immunocompetent calves, we analyzed the effects of a transient ncpBVDV infection on IFN-α/β-regulated Mx gene expression and the acute-phase response by determining the levels of SAA and Hp in serum. Detection of Mx gene products in white blood cells (WBC) was chosen as a marker for IFN-α/β upregulation rather than measuring IFN itself. Here we show that detection of IFN-α/β-induced Mx protein in blood samples or skin biopsy specimens may be a useful tool to obtain information about the health status of virally infected animals.
MATERIALS AND METHODS
Animals and virus.
Six conventionally reared, outbred, colostrum-fed fattening calves of different breeds (two males, four females; 3 to 6 months of age), obtained from the faculty-owned cattle herd, were held in an isolated experimental station, fed reconstituted milk powder, and allowed free access to water, hay, and straw. The veterinary office of the Canton of Zurich approved all experimental procedures. Upon arrival of the calves at the experimental station, BVDV antigen could not be detected in the blood samples of any of the animals, and they were all shown to be seronegative for BVDV-specific antibodies. During the 4-week adaptation period, two blood and skin biopsy specimens were taken on preinoculation days −18 and −8. On day 0, the animals were inoculated orally with 5 × 107 of the ncpBVDV strain 110/39, grown on embryonic bovine turbinate cells. This virus was isolated in 1995 from a 22-month-old, persistently infected Swiss cow that showed no disease symptoms (P. Schaller, unpublished data). Starting on the day of infection (day 0) through day 13 postinfection, the following samples were taken daily: (i) anticoagulated jugular blood samples for hematological examinations and isolation of WBC, (ii) sera for detection of antiviral antibodies and APP, (iii) skin biopsy specimens for immunohistochemistry, and (iv) nasal swabs for virus isolation. Between days 15 and 24, similar samples were taken on five occasions. Skin biopsy specimens were taken from the shoulder area after local anesthesia with lidocaine and using a commercially available punching device that provides 6-mm-diameter skin samples (Stiefel Laboratories, Winterthur, Switzerland). The skin biopsy specimens were either immediately snap-frozen or fixed in 4% buffered formaldehyde solution for immunohistochemical evaluation.
Clinical and hematological studies.
The animals were examined daily with respect to general condition, body temperature, respiratory distress (nasal discharge, coughing), and diarrhea. The WBC count was determined using an automated procedure (CELL-DYN 3500; Abbott Laboratories, Abbott Park, Ill.).
Virological examinations.
Virus isolation from nasal swabs and WBC was performed in cultured bovine embryonic turbinate cells as described elsewhere (4, 58, 59). Serum antibodies were measured by two different methods. First, an indirect enzyme-linked immunosorbent assay (ELISA) system targeting the nonstructural protein NS3 was performed essentially as described previously (7). Second, conventional neutralization tests were performed with the homologous virus strain. The presence of ncpBVDV in the inoculated cell cultures was recorded by immunohistochemistry (48). Immunohistochemical testing for BVDV antigens in skin cryostat sections was performed with a panel of BVDV-specific monoclonal antibodies (MAbs) (58, 59).
Determination of the levels of APPs in serum.
Serum samples were stored at −20°C until the end of the sampling period. SAA and Hp levels in serum were evaluated using commercially available test kits (PHASE SAA and PHASE Hp; Tridelta Development Ltd., Greystones, Ireland) and following the manufacturer's instructions. PHASE SAA is a solid-phase sandwich ELISA, and PHASE Hp measures the peroxidase activity of hemoglobin which, in the presence of binding Hp, is preserved upon lowering the pH value. In the case of Hp, the sera were additionally evaluated with a MAb-based capture ELISA (20, 27). The cutoff for positive samples was defined as the background optical density plus twice the standard deviation for each assay.
Detection of Mx mRNA and Mx protein in WBC and skin biopsy specimens.
Expression of Mx mRNA and Mx protein in WBC was determined as described previously (38). Briefly, anticoagulated blood samples were collected and after lysis of red blood cells with buffered ammonium chloride, the resulting WBC pellet was washed with phosphate-buffered saline and stored at −80°C. For mRNA detection, RNA was isolated using a commercial kit (RNeasy; Qiagen, Basel, Switzerland), and reverse transcription was performed using random hexamers (A3500; Promega Corp., Madison, Wis.). Fluorogenic PCR of cDNA was performed using a set of Mx-specific primers (fw, 5′-AAC ACC TGA CCG CGT ACC A-3′; rev, 5′-GCA CGA AGA ACT GGA TGA TCA A-3′) and the internal probe (5′-[6-carboxy-fluorescein]-CAG GAA GTC AGC ACC CGC ATC TCC-[6-carboxy-tetramethyl-rhodamine]-3′) as described (38). Reactions were run on the ABI Prism 7700 sequence detector system (Applied Biosystems, Foster City, Calif.). To determine the actual cell number present in each individual sample, expression of the housekeeping 18S rRNA gene was measured. Primers and probe used to amplify the 18S rRNA were part of a predeveloped reagent kit (4310893E; Applied Biosystems). From the respective signals, the actual cell number present in each sample was calculated by using a previously established 18S rRNA standard curve (37, 38). This curve was used to normalize Mx signals measured by fluorogenic PCR to a fixed cell number. The normalized amounts of Mx mRNA expressed in the WBC samples were then compared with the day 0 value for the animal and finally used to calculate mean values and standard deviations for the six animals at each time point.
Immunoblot assays with WBC lysates were performed after discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of the proteins from 2 × 105 cells and using the anti-Mx MAb M143 (15, 32) in conjunction with chemiluminescence (38). Quantification of Mx signals was achieved by densitometry with the Chemilmager 5000 (Alpha Innotech Corp., San Leandro, Calif.) and setting the positive-control value at 100%. This control was obtained by culturing bovine WBC overnight in the presence of 1,000 IU of recombinant IFN-αB/D per ml (30, 38).
To determine the presence of Mx proteins in the skin of the calves, the MAb M143 was used in conjunction with the peroxidase-based EnVision system for primary antibody detection (K4001; DAKO Diagnostics AG, Zug, Switzerland). Skin biopsy specimens were fixed with 4% buffered formaldehyde and prepared for immunostaining by adopting a pronase digestion step before incubation with the primary antibody. Confirmatory immunostaining was achieved with a second MAb (2C12) to murine Mx1 protein, which recognizes a different epitope than does MAb M143 (15, 32). Immunoblots showed that this MAb recognizes bovine Mx protein, as does MAb M143 (data not shown).
RESULTS
The ncpBVDV strain 110/39 exhibits moderate pathogenicity for experimentally infected calves.
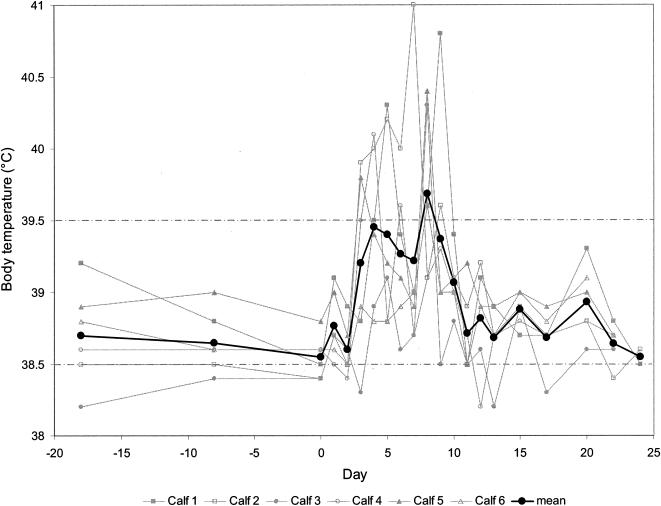
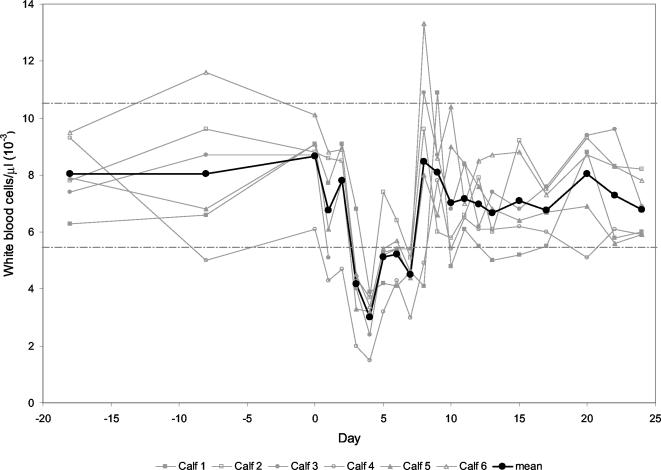
All six calves infected with ncpBVDV strain 110/93 showed moderate signs of a transient acute infection, namely, reduced general health condition and respiratory distress, as revealed by elevated body temperature, sporadic coughing, and nasal discharge. These events lasted from days 3 to 15, and full recovery was observed towards the end of the third week. The body temperature reached average values above 39°C between days 3 and 10, showing a biphasic trend with one peak at day 4 and a second peak at day 8 (Fig. 1). As expected for conventionally reared outbred calves, all parameters monitored showed considerable variability. The peripheral WBC counts declined from day 3 on, reached a nadir on day 4 with an average of 3 × 103 WBC/μl, and rebounded to preinfection levels by day 8 (Fig. 2). Three of the six animals subsequently displayed a short period of leukocytosis for 1 or 2 days before returning to physiological preinoculation values.
FIG. 1.
Rectal temperature of calves experimentally infected with ncpBVDV. The temperatures of the individual calves and the mean temperatures for the six calves are depicted from day 18 before infection (day −18) through day 0 (day of virus inoculation) and up to day 24 postinfection. The upper and lower physiological limits are indicated by the broken lines.
FIG. 2.
WBC counts of calves experimentally infected with ncpBVDV. The WBC counts (103) of individual calves and the mean values for the six calves are depicted from day 18 before infection (day −18) through day 0 (day of virus inoculation) and up to day 24 postinfection. The upper and lower physiological limits are indicated by the broken lines.
Inoculation of calves with ncpBVDV is followed by transient viremia, virus shedding, and seroconversion.
A WBC-associated viremia was observed in four of six inoculated animals with one to four successful virus isolations in cell culture between days 4 and 9. Infectious ncpBVDV could be isolated from nasal swabs in five of six animals with one to four positive cultures between days 5 and 10. Anti-NS3 antibodies became apparent in five animals by day 21, while the remaining calf was found positive at the end of the observation period (data not shown). All animals, irrespective of virus shedding, showed a comparable rise of neutralizing serum antibodies by day 17 (data not shown). Together, these data clearly indicate that oral inoculation with ncpBVDV strain 110/39 led to a transient systemic infection in each of the animals. This was reflected by moderate clinical signs lasting for 2 to 3 weeks, transient viremia and virus shedding, and the appearance of antiviral serum antibodies towards the end of the observation period (P. Schaller, A. Arquint, and E. Peterhaus, unpublished data).
Transient infection with ncpBVDV is paralleled by a temporary rise of the Hp and SAA APPs.
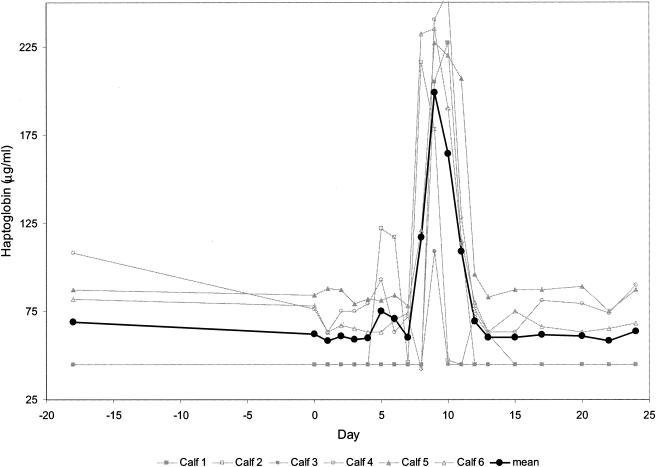
The two proteins Hp and SAA, considered the major bovine APPs, were upregulated during the course of the infection, both showing a biphasic response. Hp preinoculation values showed a certain variability, namely, levels at or below the detection limit of the assay (45 μg/ml) in three animals and comparatively high values, approximating 75 μg/ml, in the remaining three animals. Mean serum Hp levels were elevated from days 8 to 11 (Fig. 3). An approximately fourfold rise of this APP occurred in five of six animals. In calf 3, Hp levels above 100 μg/ml were observed only once (day nine). Identical results were obtained with the MAb-based capture ELISA (data not shown).
FIG. 3.
Levels of Hp in the sera of calves experimentally infected with ncpBVDV detected by using a commercial test kit. This enzymatic test is based on the peroxidase activity of hemoglobin which is preserved at low pH after binding of Hp. The detection limit of the test was determined to be 45 μg/ml. The Hp levels for the individual calves and the mean values for the six calves are depicted from day 18 before infection (day −18) through day 0 (day of virus inoculation) and up to day 24 postinfection.
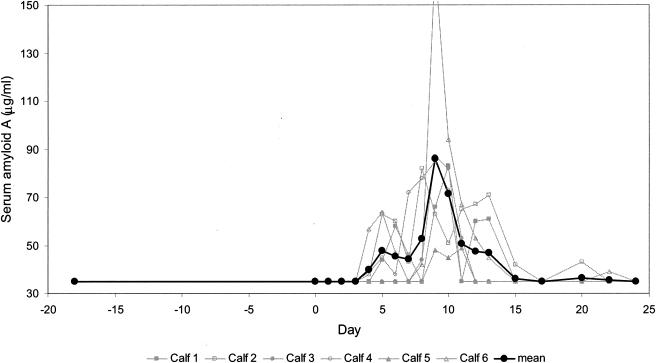
Mean SAA levels peaked between days 4 and 13 (Fig. 4). Calf 5 displayed SAA levels that remained close to the preinfection baseline level, i.e., below the detection limit of the test (38 μg/ml). Calf 6 showed the greatest SAA response, with a threefold increase reached by day 9. The remaining three animals displayed maximal SAA concentrations approximating 80 μg/ml. To conclude, it was observed that the levels of the SAA and Hp APPs increase during the acute stage of the infection, with a diagnostic window lasting from days 8 to 11 for Hp and from days 4 to 13 for SAA.
FIG. 4.
Levels of SAA in the sera of calves experimentally infected with ncpBVDV detected by using a commercial test kit. This test works on the basis of a solid-phase sandwich ELISA. The detection limit of the test was determined to be 35 μg/ml. The SAA levels for the individual calves and the mean values for the six calves are depicted from day 18 before infection (day −18) through day 0 (day of virus inoculation) and up to day 24 postinfection.
Expression of the Mx gene in WBC is upregulated during transient infection with ncpBVDV.
In vitro, the Mx gene is rapidly upregulated in WBC upon stimulation with IFN-α/β or infection with certain viruses, as shown previously (38). It was therefore of interest whether an infection with ncpBVDV in vivo is accompanied by upregulation of the Mx gene, which would be a useful indicator for the ongoing infection.
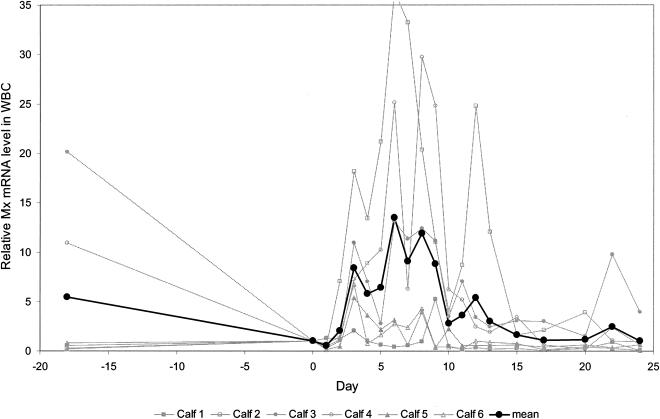
Using fluorogenic PCR with a newly designed set of bovine Mx-specific primers and probe, a baseline level of expression of the Mx gene in WBC was seen even before infection. During infection with ncpBVDV, gene expression was upregulated from day 2 onward (Fig. 5). Between days 3 and 9, the amount of mRNA was on average >10-fold upregulated relative to the amount on day 0, with a maximum on day 6 (average increase, 13.5-fold). One animal even showed a >35-fold upregulation on day 6. After day 10, mRNA levels were still upregulated up to fivefold, with a second minor peak on day 12 (average increase, 5.41-fold) before declining to preinoculation values towards the end of the observation period. This experiment was repeated with WBC samples stored at −80°C for more than 1 year, and the results obtained in the two trials were essentially identical.
FIG. 5.
Relative expression level of Mx mRNA in the WBC of calves experimentally infected with ncpBVDV as determined by fluorogenic PCR. The values for the individual calves and the mean values for the six calves are depicted from day 18 before infection (day −18) through day 0 (day of virus inoculation) and up to day 24 postinfection. Threshold cycle values obtained were normalized with respect to the expression of the housekeeping 18S rRNA determined in parallel. This allowed to delineate the result according to a specified number of cells. Relative values were calculated by the method of Livak and Schmittgen (37) and compared with the individual animal's value obtained at day 0 (day of virus inoculation).
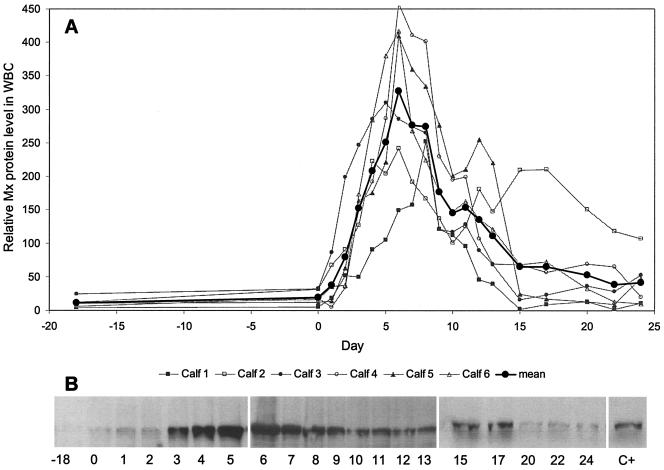
Mx protein expression in WBC was analyzed using an immunoblot assay with a MAb and densitometric evaluation of immunoreactive bands. A baseline level of expression was seen even before infection (Fig. 6). From day 1 onward, Mx protein expression increased dramatically, and the highest levels coincided with corresponding high levels of Mx mRNA expression on day 6. Compared to the positive control, which was defined as 100%, average Mx protein levels on day 0 were 20% and increased to about 325% on day 6. By day 20, Mx protein expression in WBC reached preinoculation levels, as seen with the corresponding mRNA.
FIG. 6.
Relative Mx protein levels in the WBC of calves experimentally infected with ncpBVDV. Samples of lysed WBC (2 × 105 cell equivalents) were evaluated by immunoblot assay after SDS-PAGE and using a Mx-specific MAb (M143). Serologically reactive Mx bands were recorded by chemiluminescence followed by densitometry. The values for the individual calves and the mean values for the six calves are depicted from day 18 before infection (day −18) through day 0 (day of virus inoculation) and up to day 24 postinfection. (A) Relative Mx protein levels in the WBC of individual calves and mean Mx protein levels for the six calves. (B) Original Mx SDS-PAGE profile of calf 6. A positive-control (C+) sample was prepared by culturing WBC from a healthy cow overnight in the presence of 1,000 IU of recombinant IFN (rIFN-αB/D) per ml and using 2 × 105 cell equivalents for the immunoblot assay (30, 38). The densitometric result obtained with the positive control was arbitrarily defined as 100%. The individual densitometric recordings of the calves are depicted in proportion to this positive-control value.
It is possible that skin is suited for sensitive detection of Mx protein.
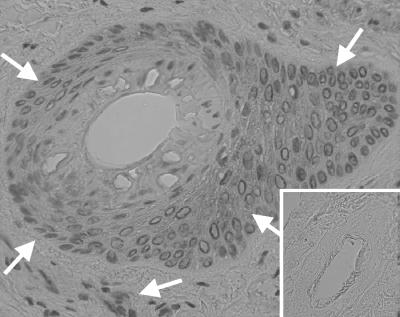
The presence of Mx protein in the skin of the ncpBVDV-infected animals was analyzed by immunohistochemistry on formaldehyde-fixed skin biopsy specimens. From day 1 onward, positive signals were obtained, and the strongest signals were seen from days 4 to 9 (Table 1). The staining pattern (Fig. 7) consisted of intracytoplasmic granular to homogenous red staining in basal cells of the epidermis and epithelial cells of hair follicle sheaths, in some endothelial and muscle cells of the vessel walls, and in solitary dermal fibroblasts.
TABLE 1.
Mx protein expression in the skin of calves transiently infected with ncpBVDV
| Calf | Mx protein expression on daya:
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −18 | −8 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 15 | 17 | 20 | 22 | 24 | |
| 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| 3 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 |
| 4 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 3 | 2 | 3 | 2 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 |
| 5 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 2 | 1 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| 6 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 1 | 3 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
Mx protein expression as revealed by indirect immunostaining on formaldehyde-fixed tissue sections. Immunostaining was scored from 0 (no staining) to 3 (strong intracytoplasmic staining) and scores of 2 and 3 are shown in boldface type. The skin of calves was tested from day 18 before infection (day −18) through day 0 (day of virus inoculation) and up to day 24 postinfection.
FIG. 7.
Expression of Mx protein in the skin of a calf (calf 4) after infection with ncpBVDV. Sections from a formaldehyde-fixed skin biopsy specimen, taken at day 7 postinfection, were subjected to immunostaining by using the primary anti-Mx MAb M143 with the EnVision system (DAKO) for primary antibody detection in the presence of AEC (3-amino-9-ethylcarbazole) chromogen. Note the intracytoplasmic staining, which is preferentially associated with epithelial cells from hair follicles, sometimes with the basal cell layer of the epidermis, and occasionally with endothelial cells. Arrows demarcate regions of intense immunostaining. The insert depicts the lack of specific staining in a section from a skin biopsy specimen taken 8 days before viral infection. Magnification of the micrograph, ×40.
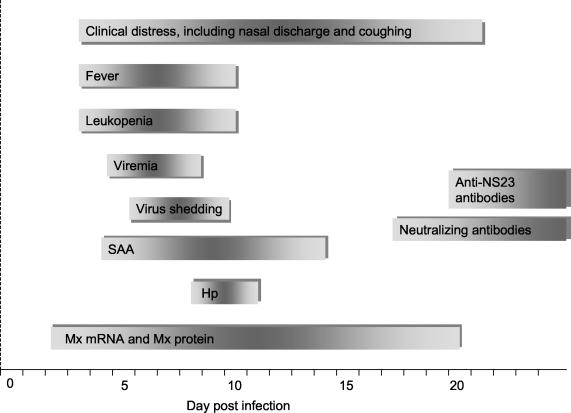
Figure 8 depicts a schematic summary of the time-ordered manifestation of the clinical, virological, and immunological parameters addressed in this study.
FIG. 8.
Schematic summary of clinical, virological, and immunological events in six calves during the course of transient infection with ncpBVDV. The length of the bar represents the duration of the event and depicts the time-ordered manifestation of the various parameters studied.
DISCUSSION
The present study characterized the temporal appearance of effector proteins of the innate immune response during a transient infection of calves with ncpBVDV and evaluated the use of these parameters as early indicators of viral infection. This was achieved by determining the products of the IFN-α/β-regulated Mx gene in WBC (38) and in skin and the levels of the two major bovine APPs Hp and SAA in serum (12). These parameters were compared with clinical and hematological data as well as virological findings (viremia and viral excretion) and the adaptive humoral immune response (Fig. 8).
By using previously established methods to detect bovine Mx mRNA and protein (38), it was observed that transient infection of calves with a ncpBVDV strain resulted in a significant increase of Mx mRNA and Mx protein in WBC. The upregulation of WBC-associated Mx gene products started as early as 24 h and not later than 48 h postinfection, and peak levels were reached at day 6 postinfection. The signals for Mx mRNA and Mx protein remained elevated for a 3-week period, reflecting a long-lasting action of endogenously produced IFN-α/β. A baseline level of Mx expression in WBC was present in all six calves, suggesting that the Mx gene is constitutively expressed at low levels in the WBC of clinically healthy calves.
Upregulation of Mx gene products was the first parameter to respond to virus inoculation. It preceded all other observed parameters of infection, i.e., virus shedding, hematological changes, the onset of the adaptive immune response, and even the onset of clinical signs. In the current trial, it was possible to compare preinoculation levels of Mx mRNA with the transcript levels during the acute phase of the infection for each individual animal. This allowed us to detect increased expression in the individual, despite significant differences in animals in the baseline expression of Mx. So far it is not known whether the observed differences in baseline Mx expression were due to the manifestation of other, subclinical infections or to the fact that the calves were outbred and of different breeds.
The differences in the animals' individual expression levels of the Mx gene products, in particular of Mx mRNA, could be explained in several ways. As stated above, the outbred genetic background, age differences, and the conventional rearing conditions may at least in part account for the variations between individual calves. In contrast to findings in humans (3, 45), it is not sufficiently known yet how the different bovine WBC subsets express the Mx gene upon activation of the IFN-α/β system. In humans, mainly monocytes and lymphocytes have been shown to express the Mx gene, whereas Mx expression in granulocytes was much lower (45). Assuming that this expression pattern is also valid for ruminants and considering the fact that the observed leukopenia was mainly lymphopenia (data not shown), the relative number of Mx-expressing cells would then be even lower during the acute phase of the infection, making the observed Mx upregulation even more impressive. This could be monitored further by establishing a flow cytometric protocol for Mx expression in bovine blood leukocytes.
Under our experimental conditions, we compared mRNA levels for each animal to its day 0 value. Because this approach is not feasible under field conditions, we need to better characterize the nature of a baseline Mx expression. Some of this information can be obtained by repeatedly testing a larger number of healthy cattle and analyzing Mx expression levels for confounding parameters, such as age, sex, and breed. In this process, Mx protein levels may turn out to provide a more robust parameter than mRNA levels. This information will be essential to develop Mx expression protocols as a routine assay that can be applied under field conditions.
Finally, it should be noted that the fluorogenic PCR experiments performed were based on the assumption that the housekeeping 18S rRNA transcription rate and integrity were not influenced by the ongoing viral infection (56). Ronni et al. (44), working with influenza virus and human lung epithelial cells, showed that expression of glyceraldehyde-3-phosphate dehydrogenase and β-actin mRNA sharply decreased during ongoing viral infection. These aspects will have to be considered in future experiments.
The results obtained clearly show that measuring the expression of Mx gene products in WBC is a suitable tool to monitor an ongoing infection with ncpBVDV even during the incubation period. Since expression of the Mx gene is believed to be under the strict control of IFN-α/β (50), the present Mx findings imply that infection with the virus strain under study was paralleled by endogenous synthesis and signaling of IFN-α/β. This observation is in agreement with a recent report showing that transient infection of young calves with ncpBVDV is paralleled by a IFN-α/β response (8). However, we cannot rule out the possibility that ncpBVDV could activate the Mx gene directly, as seen with some RNA viruses (21, 31, 44, 46). This issue has to be further addressed by determining the expression of IFN-α/β rather than Mx gene products during infection with ncpBVDV.
From several in vitro studies, it was concluded that ncpBVDV does not induce IFN-α/β (1, 11, 39, 48) and is therefore able to establish persistence in the bovine fetus when infection takes place between days 30 and 120 of gestation. Further support for this theory comes from a recent in vivo study where it was shown that fetuses infected with ncpBVDV in this early phase of gestation were not able to produce IFN-α/β (9). On the other hand, an in vivo study showed that ncpBVDV did induce IFN-α/β in immunocompetent calves, leading to the idea that the developmental stage of the host's innate immune system may play an important role in the establishment of persistence (8). Our findings that infection with a ncpBVDV strain leads to an upregulation of the Mx gene are therefore consistent with recent publications (8, 18) showing an IFN-α/β response in calves upon infection with ncpBVDV.
The bovine trophoblast has been found to resist infection with BVDV for up to 30 days of gestation (57). This phenomenon is possibly linked to the reported antiviral effect of IFN-τ, a constitutively expressed cytokine of the ruminant trophoblast (10). Although IFN-τ resembles IFN-α/β in structure as well as in many of its biological properties, it is not induced by virus and is instead produced constitutively by the embryonic trophectoderm during the period immediately prior to implantation (10). In conclusion, it is intriguing to learn that infection of the immature bovine fetus with ncpBVDV, specifically between days 30 and 120 of gestation, may ultimately lead to viral persistence because the virus evades both the host's innate and adaptive immune responses.
From day 1 postinfection with ncpBVDV, distinct levels of Mx protein were expressed in the skin of the individual calves. As all animals remained negative for BVDV antigen in skin at all times (4), increased Mx expression in skin was likely due to the systemic action of endogenously produced IFN-α/β rather than Mx expression due to local BVDV replication and concomitant IFN-α/β generation. This would be in line with the findings of al-Masri and coworkers (3) who reported a strong expression of Mx protein in the skin of humans during parenteral treatment with IFN-α2b.
Further studies are necessary to evaluate the sensitivity of skin compared to WBC to monitor an upregulation of Mx gene products as an indicator for the systemic action of endogenously produced IFN-α/β.
APPs have been proposed as valuable indicators of the manifestation and severity of pathological conditions in humans and animals. Levels of APPs are preferentially elevated during acute bacterial infections, notably during bovine mastitis (13) and less pronounced or even missing during viral infections (6, 23, 24, 33). Godson et al. (20) reported on the sequential infection of cattle with bovine herpesvirus 1 and Pasteurella multocida and the concomitant rise of Hp, which was prompt and distinct after the secondary bacterial infection. At the same time, it was recognized that the maximum level of Hp in serum was a valuable prognostic factor and a mirror of the disease severity. In the present study, the levels of Hp and SAA in serum remained relatively low and increased later than Mx gene products did. In addition, peak levels of APPs recorded were of shorter duration than those observed with the Mx gene products. In a recent study, Hp and SAA levels also peaked between days 8 and 9 during experimental infection with BVDV, but the levels reached were generally higher than in our study (18). This difference can be explained by the different viral strain used and/or the different genetic background of the animals. After infection of 1- to 2-week-old calves with another viral pathogen (bovine respiratory syncytial virus), Heegaard and coworkers (27) recorded maximal SAA and Hp levels between days 6 and 8. The maximal SAA and Hp levels were between days 4 to 13 for SAA and days 8 to 11 for Hp in the present study. It was further noted that the levels of SAA recorded during this study compared to infection with bovine respiratory syncytial virus (27) were of similar magnitude, whereas Hp levels observed in the present study remained generally low. These differences could be explained either by the different virus-induced pathologies, by the ever possible comanifestation of secondary pathogens, or by the age of the animals. By considering the assumption that Hp levels were a reflection of the severity of clinical distress (12, 20, 27), the symptoms recorded during the present study would be consistent with a minor and transient health impairment.
This study finally indicated that Mx mRNA and Mx protein were more widely applicable indicators to monitor the ongoing infection with ncpBVDV than were Hp and SAA. In view of a possible use of Mx gene products as early and sensitive indicators for the manifestation of viral infections, three basic questions need to be addressed, namely (i) the likely contribution of IFN-τ to the systemic upregulation of the Mx gene in pregnant ruminants (61), (ii) the possible manifestation of IFN-κ in the skin of cows as observed in humans (34, 40), and (iii) the ever possible virus-specific interruption of the cascade of IFN-α/β induction, signaling, and downstream regulation of dependent effector genes (19, 35). In any case, the upregulation of Mx gene products as an indicator for endogenously produced IFN-α/β and of APPs as an indicator for an inflammatory process can be valuable tools to monitor the health status of cattle.
In conclusion, the results of this study provided several lessons to be learned (Fig. 8), namely, that infection of calves with ncpBVDV is transient and paralleled by a prompt innate immune response. This is reflected by a short elevation of the levels of the Hp and SAA APPs in serum and an early and longer lasting activation of the Mx gene as observed with WBC and skin samples. The innate immune response is followed by a sustained adaptive immune response, as reflected by the appearance of antiviral serum antibodies. Together, it seems likely that both the innate and adaptive immune responses contributed to the successful control of this viral infection. The Mx findings indirectly prove that ncpBVDV induces an IFN-α/β response, even though the possibility of a direct Mx induction by the virus has to be ruled out by further experiments. APP levels remained relatively low and were possibly a reflection of the mild clinical distress induced in the experimentally infected calves, and finally, products of the Mx gene are of prospective value to monitor the endogenous action of IFN-α/β in cattle and other animals as well. This possibility is currently being addressed in a field study.
Acknowledgments
We thank Jovan Pavlovic for supplying anti-Mx MAbs; Justin D. Radolf, Uwe Müller-Doblies, and Hans-Peter Hefti for valuable discussions and critical reading of the manuscript; Rita Castro for excellent technical support; and Adrian Hehl for help in using a densitometer.
This study was financially supported by the University of Zurich.
REFERENCES
- 1.Adler, B., H. Adler, H. Pfister, T. W. Jungi, and E. Peterhans. 1997. Macrophages infected with cytopathic bovine viral diarrhea virus release a factor(s) capable of priming uninfected macrophages for activation-induced apoptosis. J. Virol. 71:3255-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi, M., J. Fah, N. Hurt, C. E. Samuel, D. Thomis, L. Bazzigher, J. Pavlovic, O. Haller, and P. Staeheli. 1989. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol. Cell. Biol. 9:5062-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.al-Masri, A. N., T. Werfel, D. Jakschies, and P. von Wussow. 1997. Intracellular staining of Mx proteins in cells from peripheral blood, bone marrow and skin. Mol. Pathol. 50:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arquint, A. 2003. Immunhistologische Untersuchungen an Hautbiopsien bei akuter BVD (Bovine Virus Diarrhoe). Thesis, University of Zurich, Zurich, Switzerland.
- 5.Baigent, S. J., G. Zhang, M. D. Fray, H. Flick-Smith, S. Goodbourn, and J. W. McCauley. 2002. Inhibition of beta interferon transcription by noncytopathogenic bovine viral diarrhea virus is through an interferon regulatory factor 3-dependent mechanism. J. Virol. 76:8979-8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann, H., and J. Gauldie. 1994. The acute phase response. Immunol. Today 15:74-80. [DOI] [PubMed] [Google Scholar]
- 7.Canal, C. W., M. Strasser, C. Hertig, A. Masuda, and E. Peterhans. 1998. Detection of antibodies to bovine viral diarrhoea virus (BVDV) and characterization of genomes of BVDV from Brazil. Vet. Microbiol. 63:85-97. [DOI] [PubMed] [Google Scholar]
- 8.Charleston, B., L. S. Brackenbury, B. V. Carr, M. D. Fray, J. C. Hope, C. J. Howard, and W. I. Morrison. 2002. Alpha/beta and gamma interferons are induced by infection with noncytopathic bovine viral diarrhea virus in vivo. J. Virol. 76:923-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charleston, B., M. D. Fray, S. Baigent, B. V. Carr, and W. I. Morrison. 2001. Establishment of persistent infection with noncytopathic bovine viral diarrhoea virus in cattle is associated with a failure to induce type I interferon. J. Gen. Virol. 82:1893-1897. [DOI] [PubMed] [Google Scholar]
- 10.Demmers, K. J., K. Derecka, and A. Flint. 2001. Trophoblast interferon and pregnancy. Reproduction 121:41-49. [DOI] [PubMed] [Google Scholar]
- 11.Diderholm, H., and Z. Dinter. 1966. Interference between strains of bovine virus diarrhea virus and their capacity to suppress interferon of a heterologous virus. Proc. Soc. Exp. Biol. Med. 121:976-980. [DOI] [PubMed] [Google Scholar]
- 12.Eckersall, P. D. 2000. Recent advances and future prospects for the use of acute phase proteins as markers of disease in animals. Rev. Méd. Vét. 151:577-584. [Google Scholar]
- 13.Eckersall, P. D., F. J. Young, C. McComb, C. J. Hogarth, S. Safi, A. Weber, T. McDonald, A. M. Nolan, and J. L. Fitzpatrick. 2001. Acute phase proteins in serum and milk from dairy cows with clinical mastitis. Vet. Rec. 148:35-41. [DOI] [PubMed] [Google Scholar]
- 14.Flint, S. J., L. W. Enquist, R. M. Krug, V. R. Racaniello, and A. M. Skalka. 2000. Principles of virology. Molecular biology, pathogenesis and control, 1st ed. ASM Press, Washington, D.C.
- 15.Flohr, F., S. Schneider-Schaulies, O. Haller, and G. Kochs. 1999. The central interactive region of human MxA GTPase is involved in GTPase activation and interaction with viral target structures. FEBS Lett. 463:24-28. [DOI] [PubMed] [Google Scholar]
- 16.Fray, M. D., G. E. Mann, and B. Charleston. 2001. Validation of an Mx/CAT reporter gene assay for the quantification of bovine type-I interferon. J. Immunol. Methods 249:235-244. [DOI] [PubMed] [Google Scholar]
- 17.Gabay, C., and I. Kushner. 1999. Acute phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340:448-454. [DOI] [PubMed] [Google Scholar]
- 18.Ganheim, C., C. Hulten, U. Carlsson, H. Kindahl, R. Niskanen, and K. P. Waller. 2003. The acute phase response in calves experimentally infected with bovine viral diarrhoea virus and/or Mannheimia haemolytica. J. Vet. Med. Ser. B 50:183-190. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Sastre, A. 2002. Mechanisms of inhibition of the host interferon alpha/beta-mediated antiviral responses by viruses. Microbes Infect. 4:647-655. [DOI] [PubMed] [Google Scholar]
- 20.Godson, D. L., M. Campos, S. K. Attah-Poku, M. J. Redmond, D. M. Cordeiro, M. S. Sethi, R. J. Harland, and L. A. Babiuk. 1996. Serum haptoglobin as an indicator of the acute phase response in bovine respiratory disease. Vet. Immunol. Immunopathol. 51:277-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goetschy, J. F., H. Zeller, J. Content, and M. A. Horisberger. 1989. Regulation of the interferon-inducible IFI-78K gene, the human equivalent of the murine Mx gene, by interferons, double-stranded RNA, certain cytokines, and viruses. J. Virol. 63:2616-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 23.Gruys, E., M. J. Obwolo, and M. J. M. Toussaint. 1994. Diagnostic significance of the major acute phase proteins in veterinary clinical chemistry: a review. Vet. Bull. 64:1009-1018. [Google Scholar]
- 24.Gruys, E., A. M. van Ederen, S. P. M. Alsemgeest, H. C. Kalsbeek, and T. Wensing. 1993. Acute-phase protein values in blood of cattle as indicator of animals with pathological processes. Arch. Lebensmittelhyg. 44:107-111. [Google Scholar]
- 25.Haller, O., M. Frese, and G. Kochs. 1998. Mx proteins: mediators of innate resistance to RNA viruses. Rev. Sci. Tech. 17:220-230. [DOI] [PubMed] [Google Scholar]
- 26.Hamers, C., P. Dehan, B. Couvreur, C. Letellier, P. Kerkhofs, and P. P. Pastoret. 2001. Diversity among bovine pestiviruses. Vet. J. 161:112-122. [DOI] [PubMed] [Google Scholar]
- 27.Heegaard, P. M., D. L. Godson, M. J. Toussaint, K. Tjornehoj, L. E. Larsen, B. Viuff, and L. Ronsholt. 2000. The acute phase response of haptoglobin and serum amyloid A (SAA) in cattle undergoing experimental infection with bovine respiratory syncytial virus. Vet. Immunol. Immunopathol. 77:151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hefti, H. P., M. Frese, H. Landis, C. Di Paolo, A. Aguzzi, O. Haller, and J. Pavlovic. 1999. Human MxA protein protects mice lacking a functional alpha/beta interferon system against La Crosse virus and other lethal viral infections. J. Virol. 73:6984-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horadagoda, N. U., K. M. Knox, H. A. Gibbs, S. W. Reid, A. Horadagoda, S. E. Edwards, and P. D. Eckersall. 1999. Acute phase proteins in cattle: discrimination between acute and chronic inflammation. Vet. Rec. 144:437-441. [DOI] [PubMed] [Google Scholar]
- 30.Horisberger, M. A., and K. de Staritzky. 1987. A recombinant human interferon-alpha B/D hybrid with a broad host-range. J. Gen. Virol. 68:945-948. [DOI] [PubMed] [Google Scholar]
- 31.Hug, H., M. Costas, P. Staeheli, M. Aebi, and C. Weissmann. 1988. Organization of the murine Mx gene and characterization of its interferon- and virus-inducible promoter. Mol. Cell. Biol. 8:3065-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kochs, G., C. Janzen, H. Hohenberg, and O. Haller. 2002. Antivirally active MxA protein sequesters La Crosse virus nucleocapsid protein into perinuclear complexes. Proc. Natl. Acad. Sci. USA 99:3153-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kushner, I., and D. L. Rzewnicki. 1994. The acute phase response: general aspects. Bailliere's Clin. Rheumatol. 8:513-530. [DOI] [PubMed] [Google Scholar]
- 34.LaFleur, D. W., B. Nardelli, T. Tsareva, D. Mather, P. Feng, M. Semenuk, K. Taylor, M. Buergin, D. Chinchilla, V. Roshke, G. Chen, S. M. Ruben, P. M. Pitha, T. A. Coleman, and P. A. Moore. 2001. Interferon-kappa, a novel type I interferon expressed in human keratinocytes. J. Biol. Chem. 276:39765-39771. [DOI] [PubMed] [Google Scholar]
- 35.Levy, D. E., and A. Garcia-Sastre. 2001. The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev. 12:143-156. [DOI] [PubMed] [Google Scholar]
- 36.Levy, D. E., I. Marie, and A. Prakash. 2003. Ringing the interferon alarm: differential regulation of gene expression at the interface between innate and adaptive immunity. Curr. Opin. Immunol. 15:52-58. [DOI] [PubMed] [Google Scholar]
- 37.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 38.Müller-Doblies, D., M. Ackermann, and A. Metzler. 2002. In vitro and in vivo detection of Mx gene products in bovine cells following stimulation with alpha/beta interferon and viruses. Clin. Diagn. Lab. Immunol. 9:1192-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura, S., T. Shimazaki, K. Sakamoto, A. Fukusho, Y. Inoue, and N. Ogawa. 1995. Enhanced replication of orbiviruses in bovine testicle cells infected with bovine viral diarrhoea virus. J. Vet. Med. Sci. 57:677-681. [DOI] [PubMed] [Google Scholar]
- 40.Nardelli, B., L. Zaritskaya, M. Semenuk, Y. H. Cho, D. W. LaFleur, D. Shah, S. Ullrich, G. Girolomoni, C. Albanesi, and P. A. Moore. 2002. Regulatory effect of IFN-kappa, a novel type I IFN, on cytokine production by cells of the innate immune system. J. Immunol. 169:4822-4830. [DOI] [PubMed] [Google Scholar]
- 41.Pavlovic, J., O. Haller, and P. Staeheli. 1992. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. J. Virol. 66:2564-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavlovic, J., and P. Staeheli. 1991. The antiviral potentials of Mx proteins. J. Interferon Res. 11:215-219. [DOI] [PubMed] [Google Scholar]
- 43.Rinaldo, C. R., Jr., D. W. Isackson, J. C. Overall, Jr., L. A. Glasgow, T. T. Brown, S. I. Bistner, J. H. Gillespie, and F. W. Scott. 1976. Fetal and adult bovine interferon production during bovine viral diarrhea virus infection. Infect. Immun. 14:660-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ronni, T., S. Matikainen, T. Sareneva, K. Melen, J. Pirhonen, P. Keskinen, and I. Julkunen. 1997. Regulation of IFN-alpha/beta, MxA, 2′,5′-oligoadenylate synthetase, and HLA gene expression in influenza A-infected human lung epithelial cells. J. Immunol. 158:2363-2374. [PubMed] [Google Scholar]
- 45.Ronni, T., K. Melen, A. Malygin, and I. Julkunen. 1993. Control of IFN-inducible MxA gene expression in human cells. J. Immunol. 150:1715-1726. [PubMed] [Google Scholar]
- 46.Ronni, T., T. Sareneva, J. Pirhonen, and I. Julkunen. 1995. Activation of IFN-alpha, IFN-gamma, MxA, and IFN regulatory factor 1 genes in influenza A virus-infected human peripheral blood mononuclear cells. J. Immunol. 154:2764-2774. [PubMed] [Google Scholar]
- 47.Rossi, C. R., and G. K. Kiesel. 1983. Characteristics of the polyriboinosinic acid:polyribocytidylic acid assay for noncytopathogenic bovine viral diarrhea virus. Am. J. Vet. Res. 44:1916-1919. [PubMed] [Google Scholar]
- 48.Schweizer, M., and E. Peterhans. 2001. Noncytopathic bovine viral diarrhea virus inhibits double-stranded RNA-induced apoptosis and interferon synthesis. J. Virol. 75:4692-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-281. [DOI] [PubMed] [Google Scholar]
- 50.Simon, A., J. Fah, O. Haller, and P. Staeheli. 1991. Interferon-regulated Mx genes are not responsive to interleukin-1, tumor necrosis factor, and other cytokines. J. Virol. 65:968-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steel, D. M., and A. S. Whitehead. 1994. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol. Today 15:81-88. [DOI] [PubMed] [Google Scholar]
- 52.Swasdipan, S., H. Bielefeldt-Ohmann, N. Phillips, P. D. Kirkland, and M. R. McGowan. 2001. Rapid transplacental infection with bovine pestivirus following intranasal inoculation of ewes in early pregnancy. Vet. Pathol. 38:275-280. [DOI] [PubMed] [Google Scholar]
- 53.Swasdipan, S., M. McGowan, N. Phillips, and H. Bielefeldt-Ohmann. 2002. Pathogenesis of transplacental virus infection: pestivirus replication in the placenta and fetus following respiratory infection. Microb. Pathog. 32:49-60. [DOI] [PubMed] [Google Scholar]
- 54.Taniguchi, T., and A. Takaoka. 2002. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr. Opin. Immunol. 14:111-116. [DOI] [PubMed] [Google Scholar]
- 55.Tautz, N., G. Meyers, and H. J. Thiel. 1998. Pathogenesis of mucosal disease, a deadly disease of cattle caused by a pestivirus. Clin. Diagn. Virol. 10:121-127. [DOI] [PubMed] [Google Scholar]
- 56.Thellin, O., W. Zorzi, B. Lakaye, B. De Borman, B. Coumans, G. Hennen, T. Grisar, A. Igout, and E. Heinen. 1999. Housekeeping genes as internal standards: use and limits. J. Biotechnol. 75:291-295. [DOI] [PubMed] [Google Scholar]
- 57.Thiel, H. J., P. G. W. Plagemann, and V. Moennig. 1996. Pestiviruses, p. 1059-1073. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 58.Thur, B., M. Hilbe, M. Strasser, and F. Ehrensperger. 1997. Immunohistochemical diagnosis of pestivirus infection associated with bovine and ovine abortion and perinatal death. Am. J. Vet. Res. 58:1371-1375. [PubMed] [Google Scholar]
- 59.Thur, B., K. Zlinszky, and F. Ehrensperger. 1996. Immunohistochemical detection of bovine viral diarrhea virus in skin biopsies: a reliable and fast diagnostic tool. Zentralbl. Vetmed. Reihe B 43:163-166. [DOI] [PubMed] [Google Scholar]
- 60.von Wussow, P., D. Jakschies, H. K. Hochkeppel, C. Fibich, L. Penner, and H. Deicher. 1990. The human intracellular Mx-homologous protein is specifically induced by type I interferons. Eur. J. Immunol. 20:2015-2019. [DOI] [PubMed] [Google Scholar]
- 61.Yankey, S. J., B. A. Hicks, K. G. Carnahan, A. M. Assiri, S. J. Sinor, K. Kodali, J. N. Stellflug, and T. L. Ott. 2001. Expression of the antiviral protein Mx in peripheral blood mononuclear cells of pregnant and bred, nonpregnant ewes. J. Endocrinol. 170:R7-R11. [DOI] [PubMed] [Google Scholar]