Abstract
The most traditional method used to measure the lytic activity of cytotoxic T lymphocytes or natural killer (NK) cells is the chromium release assay (CRA). No study has been reported that systematically compares the traditional gamma counting method with various benchtop microplate scintillation formats to measure chromium release. Here we investigated the utilization of microplate beta counters in comparison with the traditional gamma counting method to quantitate antigen-specific cytolysis, lymphokine-activated killer (LAK) activity, and NK activity in the CRA. Supernatants from standard CRA (n = 7) were directly transferred to a 96-well microplate containing either a solid scintillant (Lumaplate) or a liquid scintillant (flexible beta plate). Samples were quantified by using two benchtop microplate beta counters, Wallac Microbeta Trilux (Lumalux and Trilux methods, respectively) and Packard TopCount instruments (TopCount method). These results were then compared with data from an identical assay run in parallel using the traditional gamma counting method (LKB). The lytic activity for influenza virus-stimulated effectors measured in the benchtop microplate beta counters using Lumalux and Trilux methods exhibited excellent correlations with the one measured in the traditional LKB (r = 0.967 and 0.968, respectively). The TopCount method demonstrated a similar correlation (r = 0.966). Similar findings were observed for LAK and NK activity. The 96-well microplate format, specifically the dry-scintillant Lumaplates, offers several advantages over the traditional gamma counting format. Most notable are the reductions in sample volume needed and in the total sample preparation and counting time. Furthermore, this system reduces the amount of dry and mixed radioactive waste generated while using the same instrument for gamma- and beta-emitting isotopes.
CD8 cytotoxic T lymphocytes (CTL) and natural killer (NK) cells play an important role in the control of microbial infections and tumors through cytotoxicity and cytokine production (12). Several methods for evaluating virus-specific CD8 T cells are available (11). CTL recognize and kill target cells expressing antigen-derived peptides presented by the class I major histocompatibility complex (12). The necessity of accurately evaluating vaccine strategies has promoted the development of new methods to detect and quantify virus-specific CD8 T-cell activity (6, 8, 11). The most traditional method used to measure the function of CTL or NK cells is the chromium release assay (CRA) (2, 13). This method has been performed with cells obtained from peripheral blood mononuclear cells (PBMC) isolated from freshly drawn blood, cryopreserved PBMC, and cell lines.
The CRA has proven extremely useful in determining functional CTL responses to cancer, viral infections, and vaccines (12, 13). Despite the disadvantages of having to use radioactive compounds, the requirement for high number of available cells, and time required in assay preparation, this assay still remains in widespread use.
Cytolysis of antigen-specific 51Cr-labeled targets in the standard CRA is determined by the quantification of 51Cr released into the supernatant collected from CTL cultures by using traditional gamma counters. Historically, 51Cr has been considered a gamma emitter, and until the advent of multidetector microplate scintillation counters, the use of gamma counters was favored as the counting method. Considering that 51Cr actually emits more beta particles than gamma particles, the utilization of the multidetector beta counter to quantitate 51Cr release presents an attractive alternative over the traditional gamma counter (4, 10). Simplified procedures utilizing benchtop microplate beta scintillation counters offer several advantages over the traditional method with regard to time and sample management (4, 10). In these procedures, culture supernatants are directly transferred to microplates retaining the original 96-well microplate format. Multiple wells are counted simultaneously, reducing the overall counting time (4, 10).
Several studies use one of these three systems, but there is no published literature that systematically compares the results obtained from each of the three instruments. This comparison is important for laboratories that may have access only to a benchtop microplate beta counter and want to initiate the use of CRA to measure CTL activity against cancer-specific or virus-specific antigens.
Here, we investigated the utilization of two different benchtop microplate beta counter instruments, the Microbeta Trilux microplate scintillation beta counter and the Packard TopCount counter, to quantitate 51Cr release from cytotoxicity assays run in parallel using influenza virus-infected Epstein-Barr virus (EBV)-transformed B-lymphoblastoid cell line (B-LCL) targets, the Daudi and K562 cell lines. 51Cr release was measured with the Microbeta Trilux scintillation counter by using a 96-well microplate format with either a liquid scintillant (flexible beta plates) or a solid scintillant (Lumaplate). Only the Lumaplate format was used to measure cytolysis with the TopCount instrument. These results were then correlated with data collected by traditional gamma counting from an assay run in parallel.
MATERIALS AND METHODS
Fresh PBMC were obtained from healthy normal volunteers by leukapheresis from the NIH Blood Transfusion Department under an National Institutes of Health Institutional Review Board-reviewed and -approved protocol. For our study, seven independent experiments were performed with cryopreserved PBMC from three different healthy anonymous volunteers. Cells were isolated by Ficoll-Hypaque (Amersham Biosciences, Piscataway, N.J.) gradient centrifugation. PBMC were washed three times in phosphate-buffered saline (Gibco BRL, Grand Island, N.Y.) and resuspended in culture medium (CM) containing RPMI 1640 medium (Gibco BRL) supplemented with penicillin-streptomycin (100 IU/ml, 100 μg/ml; Sigma-Aldrich, St. Louis, Mo.), l-glutamine (2 mM; Gibco BRL), and HEPES buffer (20 mM; Gibco BRL). PBMC were cryopreserved in CM containing 20% fetal bovine sera (FBS; HyClone, Logan, Utah) and 7.5% dimethyl sulfoxide (Sigma-Aldrich) by using a controlled-rate freezer. Cryopreserved PBMC were used to generate antigen-specific CTL effector cells and autologous EBV-transformed B-LCL from each donor to be used as target cells.
Generation of EBV target cells.
Cryopreserved PBMC were thawed, washed, and resuspended in CM plus 20% FBS (CM-20) at 6 × 106/ml for the generation of EBV-transformed B-LCL. To each well containing 3 × 106 cells, 0.5 ml of EBV supernatant obtained from B95.8 marmoset cell line (American Type Culture Collection, Manassas, Va.) and 2 μg of anti-CD3 monoclonal antibody (Pharmingen, San Diego, CA) per ml were added (9) EBV-transformed B-LCL were cryopreserved until needed. B-LCL were thawed and placed in CM plus 10% FBS (CM-10) for 3 days prior to CRA.
Preparation of targets for CRA.
Cultured EBV-transformed B-LCL were washed in CM and counted, and viability (>90%) was determined. B-LCL (2 × 106 cells) were incubated in a 200-μl volume with either CM (control target) alone or a 1:4 final dilution of influenza virus (American Type Culture Collection) at 37°C in 6% CO2 for 1 h, with gentle agitation every 20 min (1). B-LCL were washed and labeled with Na251CrO4 (100 μCi/106 cells; Perkin-Elmer, Boston, Mass.) for 2 h at 37°C in 6% CO2 with gentle agitation every 30 min. 51Cr-labeled B-LCL were washed three times in CM-10 and adjusted to 5 × 104 cells/ml. Cell viability was determined by trypan blue exclusion.
In some experiments (n = 4), K562 cells were used to determine NK activity and Daudi cells were used as targets for LAK activity (4, 7). PBMC cultured in the presence of interleukin-2 (IL-2; 5 IU/ml; Hoffman-LaRoche, Basel, Switzerland) were used as effectors for the NK cell and LAK assays. An aliquot of 2 × 106 cells was removed from each cell line, washed, and labeled with Na251CrO4 (100 μCi/106 cells; Perkin-Elmer) for 2 h at 37°C in 6% CO2 with gentle agitation every 30 min. 51Cr-labeled Daudi and K562 cells were then washed three times in CM-10 and adjusted to 5 × 104 cells/ml. Cell viability (>90%) was determined by trypan blue exclusion.
Generation and preparation of antigen-specific CTL effector cells.
Cryopreserved PBMC were thawed, washed, resuspended in CM-20 at 3 × 106 cells/ml, and incubated with influenza virus at a final concentration of 1:100, as previously described (1). PBMC incubated in CM alone were used as control effectors. Cultures were incubated at 37°C in 6% CO2 for 7 days. On day 3, IL-2 was added to each well at 5 IU/ml. Cultured PBMC were harvested on day 7 and resuspended in CM-10 at 5 × 106 cells/ml. Viability was ascertained as described above.
CRA.
Autologous EBV-transformed B-LCL were used as target cells for the influenza virus-specific CTL assays. Equal volumes of target (5 × 104 cells/ml) and effector cells were added to triplicate wells of 96-well tissue culture plates, and 1:2 serial dilutions of effectors were made, producing effector-to-target (E:T) ratios of 100:1, 50:1, 25:1, and 12.5:1 (9). Spontaneous release of 51Cr was assessed by the incubation of target cells in medium alone, and maximum release of 51Cr was determined by the incubation of target cells in 0.1% Triton X-100 (Sigma-Aldrich). CRA plates were set up in duplicate to run traditional gamma counting in parallel with the other counting platforms. After a 4-h incubation of the effector cells with the target cells at 37°C in 6% CO2, supernatants were collected following brief centrifugation.
For the traditional measurement of 51Cr release, the supernatant was collected from one of the duplicate CRA culture plates into filters of supernatant collection system harvesting frames (Molecular Devices Skatron, Sunnyvale, Calif.) and transferred to polystyrene tubes to be counted with the LKB 1272 Clinigamma counter (Wallac). This method (LKB) was run in parallel with other methods adapted from manufacturers' recommendations.
The supernatant from the second duplicate CRA culture plate was collected and transferred to a new 96-well microplate (supernatant plate), and aliquots were taken from this plate for each of the following procedures. For the methods using the Lumaplates (Perkin-Elmer), an aliquot of 50 μl was directly transferred from the supernatant plate to the Lumaplate and allowed to air dry in a hood overnight. Lumaplates are opaque-walled 96-well microplates containing a thin layer of solid scintillant on the bottom of the wells. Plates were sealed and counted in the Packard TopCount counter (Perkin-Elmer) for 1 min per well with dual detectors using a 0-to-256 channel window with the instrument protocol set for solid scintillant conditions (TopCount method). The isothermal counting chamber of the instrument was set at 19°C. The same Lumaplates were then counted on the Microbeta Trilux instrument (Perkin-Elmer) (Lumalux method) for 1 min per well with dual detectors using a 5-to-170 channel window. All detectors were normalized according to manufacturers' suggestions.
The Lumaplate and tube transfer methods were then run in parallel with the method using the 96-well flexible beta plates (Perkin-Elmer) by using a nonvolatile liquid scintillant, Optiphase SuperMix (Perkin-Elmer). Flexible beta plates are constructed of polyethylene terephthalate and contain a printed gridline pattern on the surface to separate each well. An aliquot of 25 μl of CRA supernatant was transferred from the supernatant plate to the flexible beta plate containing 150 μl of Optiphase SuperMix. The plate was sealed, shaken vigorously for 15 min, and then counted on the Microbeta Trilux instrument as described above (Trilux method).
Optical cross talk was minimized by the use of the opaque-walled Lumaplates and the printed gridlines on the flexible beta plates. Isotopic cross talk was corrected for by the software protocol for each instrument. However, for chromium, the beta events are concentrated in one part of the spectrum and the high-energy gamma events that give rise to the cross talk problem are located at the higher end of the spectrum. The channel window setting of 5 to 170 minimizes the effect of cross talk. The cross talk of 1 μCi of 51Cr in flexible beta plates run in the Microbeta Trilux instrument was 0.06%.
Percent specific lysis for 51Cr release was determined with the following equation: [(experimental 51Cr release − spontaneous 51Cr release)/(maximum 51Cr release − spontaneous 51Cr release)] × 100.
All experiments were performed in triplicate wells. Results are expressed as mean percent specific lysis ± standard deviation or mean counts per minute of triplicate wells. Percent spontaneous release in all of the assays was ≤12.4% for any target. Background levels (mean counts per minute ± standard deviation) for empty wells and tubes (n = 22) were 62.8 ± 16.2 for the LKB method, 3.7 ± 4.0 for Trilux, 2.0 ± 1.2 for TopCount, and 2.2 ± 9.1 for Lumalux.
Statistical analysis.
Data obtained from other counting platforms were correlated with data collected by the traditional gamma counting procedure using the Pearson correlation coefficient. Spearman correlation coefficients yielded nearly identical findings and are therefore not reported. The nonparametric Mann-Whitney test was used to determine statistical differences between counting methods. A P value of <0.05 was considered significant.
RESULTS
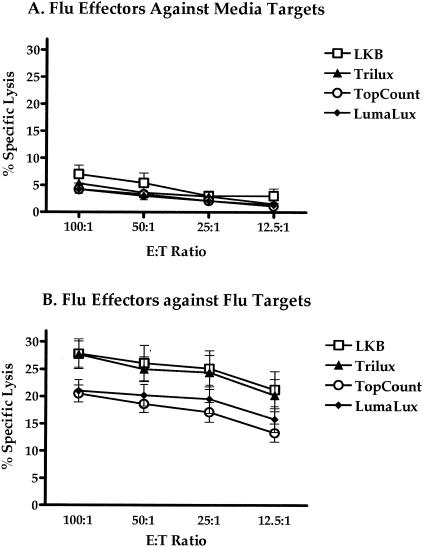
Influenza virus-specific CD8 T-cell effector activity was evaluated with autologous influenza virus-infected B-LCL or uninfected (medium control) targets (Fig. 1). Antigen-specific cytolytic responses (n = 7) were measured at E:T ratios of 100:1, 50:1, 25:1, and 12.5:1 against uninfected (Fig. 1A) or influenza virus-infected (Fig. 1B) targets. The percent specific lysis was measured from the 96-well Lumaplates using the Wallac Microbeta Trilux and the Packard TopCount, from the 96-well flexible beta plates using the Microbeta Trilux instrument, or by the traditional CRA tube transfer method using the traditional gamma counter. The mean ranges for spontaneous and maximum release of each type of target (medium, influenza virus, Daudi, and K562) are expressed for each of the counting methods (Table 1). The highest range was observed with the Lumaplates read on the Microbeta Trilux (Lumalux) for both the maximum and spontaneous release, followed by the Lumaplates read with the Packard TopCount instrument, traditional gamma counting (LKB), and finally the flexible beta plates with liquid scintillation read on the Microbeta Trilux instrument.
FIG. 1.
Comparison of CTL responses of influenza virus-stimulated effectors to uninfected (A) or influenza-infected EBV transformed B-LCL (B) targets evaluated by the quantification of chromium-51 release using the benchtop microplate beta counters Microbeta Trilux and TopCount against traditional gamma counting. Microbeta Trilux readings were taken from both solid (Lumalux) and liquid (Trilux) scintillation formats. Data are CTL responses from seven individual experiments, as specified in Materials and Methods.
TABLE 1.
Spontaneous and maximum release ranges for uninfected and influenza virus-infected targets as well as NK and LAK targets for each counting method
| Target (no. of specimens) | Avg cpm range obtained witha:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Trilux
|
TopCount
|
Lumalux
|
LKB
|
|||||
| Spontaneous | Maximum | Spontaneous | Maximum | Spontaneous | Maximum | Spontaneous | Maximum | |
| Medium (7) | 205-343 | 2,141-3,802 | 646-1,042 | 9,957-16,101 | 956-1,818 | 14,276-25,818 | 537-743 | 4,684-7,077 |
| Influenza virus (7) | 158-406 | 1,992-3,783 | 520-1,393 | 8,617-18,618 | 833-1,988 | 13,115-26,205 | 386-917 | 4,250-8,809 |
| Daudi (4) | 137-641 | 2,695-6,580 | 422-1,929 | 11,438-28,738 | 534-3,265 | 18,234-42,160 | 344-1,672 | 6,153-15,437 |
| K562 (4) | 240-285 | 5,811-6,625 | 704-876 | 25,903-30,773 | 1,103-1,507 | 38,428-45,982 | 550-697 | 13,059-15,832 |
Determined as described in Materials and Methods.
As shown in Fig. 1B, the lytic activity (specific lysis) against influenza virus-infected targets measured by the LKB method had a mean range of 27.8% lysis at an E:T ratio of 100:1 to 21.2% lysis at 12.5:1. The Trilux method showed a similar mean range of 27.7 to 20.2%. The TopCount method produced a mean range of 20.5 to 13.2%, while the Lumalux results ranged from 21.0 to 15.8%. The highest mean range of lytic activity compared to the LKB method was observed with Trilux, followed by Lumalux and finally TopCount. Although Trilux and LKB methods demonstrated similar ranges of lytic activity against influenza virus-infected targets for all E:T ratios, the differences seen in comparison with the Lumalux and TopCount methods were not statistically significant except when LKB was compared to TopCount at the 12.5:1 E:T ratio (P = 0.03, Mann-Whitney). LKB also showed the highest mean percentage of specific lysis against uninfected targets when compared to the alternative methods (Fig. 1A). Of the three alternative methods compared with the traditional LKB method, Trilux not only produced the highest mean range of lytic activity but also produced the lowest range of counts per minute, as represented in Table 1.
The net percent specific lysis of influenza virus-specific responses relative to the medium control ranged from 22.3% at an E:T ratio of 100:1 to 18.7% at an E:T ratio of 12.5:1 with Trilux, 16.3 to 12.2% with TopCount, and 18.1 to 14.5% with Lumalux, compared to 20.9 to 18.0% with LKB.
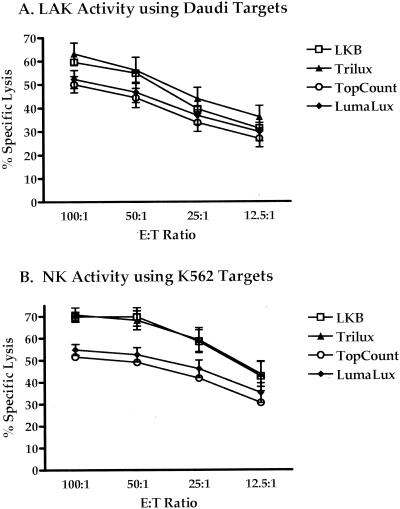
LAK and NK activity (n = 4) was measured against Daudi (Fig. 2A) and K562 (Fig. 2B) cell lines by the four methods. LAK activity against Daudi targets measured with the LKB method had a mean range of 59.5% lysis at an E:T ratio of 100:1 to 31.2% lysis at an E:T ratio of 12.5:1 and 69.9 to 42.5% for NK activity against K562 targets. The TopCount method showed mean lytic ranges of 50.0 to 26.8% and 51.6 to 30.5% for LAK and NK activity, respectively. Trilux produced ranges of 63.1 to 36.0% for Daudi and 70.7 to 43.6% for K562 cell lysis. Mean ranges of 52.3 to 29.7% lysis for Daudi and 54.9 to 35.0% lysis with K562 targets were observed with the Lumalux method. Both NK and LAK activity corresponded well with the trend of lytic activity observed with the influenza virus-infected targets. Results demonstrated higher percentages with the LKB and Trilux methods than with the TopCount and Lumalux methods, but all followed similar patterns of titration. However, statistically significant differences were observed only between LKB and TopCount methods against K562 targets at E:T ratios of 100:1 to 25:1 (P = 0.03) and between LKB and Lumalux at ratios of 100:1 and 50:1 (P = 0.03). No significant differences were seen with the Daudi targets.
FIG. 2.
Lytic activity of cultured PBMC in the presence of IL-2 assayed for LAK activity against Daudi targets (A) and for NK activity against K562 targets (B) measured from cytotoxicity assays. Lytic activity from 51Cr released into cell culture supernatants was measured with the microplate readers Microbeta Trilux and TopCount and by traditional gamma counting (LKB). Microbeta Trilux readings were done in the presence of solid (Lumalux) and liquid (Trilux) scintillation cocktails. CTL responses are means from seven experiments.
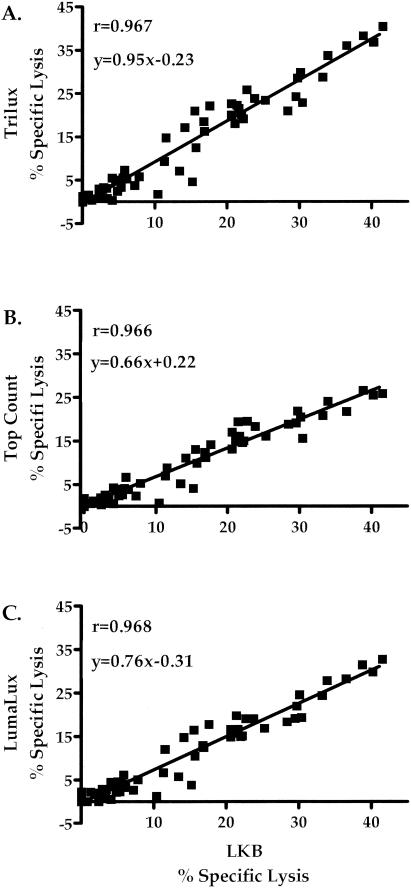
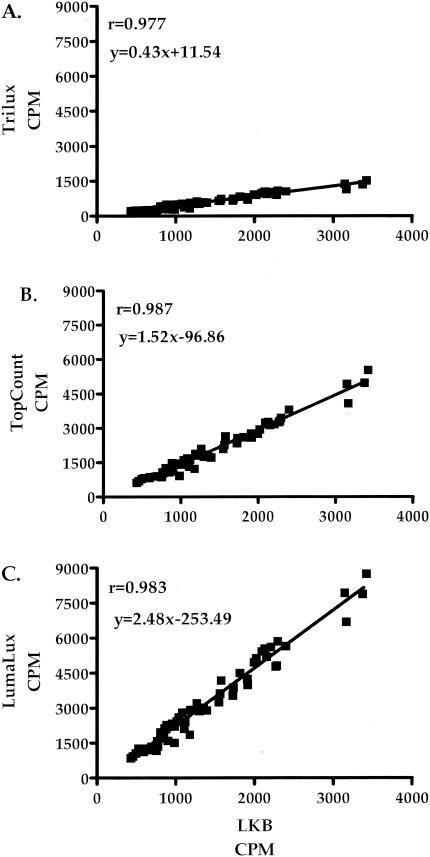
The correlation of percent specific lysis and counts per minute for influenza virus-stimulated effector cells against medium control and influenza virus-infected targets for each of the methods is shown in Fig. 3 and 4. There was a strong correlation between percentage specific lysis (Fig. 3) and counts per minute (Fig. 4) for each of the methods evaluated compared with the traditional LKB method. The Trilux method (Fig. 3A and 4A) demonstrated correlation values of 0.967 for specific lysis and 0.977 for counts per minute. The TopCount method (Fig. 3B and 4B) showed correlation values of 0.966 for specific lysis and 0.987 for counts per minute. The Lumalux method (Fig. 3C and 4C) also demonstrated a strong correlation with the LKB method, with values of 0.968 for specific lysis and 0.983 for counts per minute. Similarly strong correlations were found when analysis was restricted for influenza virus-specific responses against influenza virus-infected targets (r = 0.922, 0.927, and 0.930 for Trilux, TopCount, and Lumalux, respectively). Comparison of percentage specific lysis within the separate E:T ratios tested for each method also exhibited a strong correlation with the LKB method, with r values of ≥0.957 at 100:1, ≥0.950 at 50:1, ≥0.984 at 25:1, and ≥0.956 at 12.5:1.
FIG. 3.
Correlation of specific lysis of influenza virus-stimulated effectors against influenza virus-infected and control uninfected targets obtained by traditional gamma counting with values using flexible plates read with the Microbeta Trilux counter (A) and Lumaplates read with the TopCount (B) or Microbeta Trilux (C) counter.
FIG. 4.
Correlation of mean counts per minute of influenza virus-stimulated effectors against influenza virus-infected and control uninfected targets obtained by traditional gamma counting with those obtained from the Microbeta Trilux instrument using a solid (Lumalux) and a liquid (Trilux) scintillant and from the TopCount instrument using solid-scintillant Lumaplates.
Measurements of NK activity against K562 targets also showed excellent correlation (specific lysis, r ≥ 0.975; counts per minute, r ≥ 0.962) between instruments for the methods used, as did LAK activity against Daudi targets (specific lysis, r ≥ 0.979; counts per minute, r ≥ 0.990).
DISCUSSION
Several studies suggest that CTL and NK cells play a central role in the eradication of infectious diseases and cancer by the immune system (3, 5). Chromium-51 is a good radioactive label for standard cytotoxicity assays and is generally nontoxic as Na2CrO4 (2, 13). Other advantages of chromium use include easy uptake by cells, relatively low spontaneous release, simple and sensitive detection, and stable target cell morphology or characteristics (2). The major disadvantages of chromium are associated with the use, waste generation, and disposal of radioactivity, which can be significantly reduced with the methods evaluated using the benchtop microplate beta counters (TopCount and MicroBeta Trilux).
Here, we compared influenza virus-specific CTL, LAK, and NK cell responses assessed by chromium-51 release measured with three different instruments using either transfer tubes or 96-well microplates containing either a solid or liquid scintillant. Cytotoxicity results obtained with the different benchtop microplate beta counters correlated very well between instruments and with the traditional gamma counting system. However, the traditional LKB method produced the highest percent specific lysis with the uninfected and influenza virus-infected EBV targets as well as the K562 targets. The Trilux method showed results very similar to those of the LKB method and higher lytic activity with the Daudi targets. With the LKB method, the CRA traditionally yields a 200-μl volume of supernatant that is collected into the harvesting filters. This volume is 4 to 8 times higher than the amount required for each of the alternative methods which demonstrated similar patterns of lytic activity. This advantage over the LKB method allows a reduction in sampling size requirements. In addition, the use of the microplate format to count chromium release in cytotoxicity assays eliminates potential errors associated with tube manipulation. Samples prepared in the 96-well plate format can be counted directly in that format, ensuring correct positioning of samples, and each plate replaces the need for 96 tubes. Maintaining the microplate format facilitates the possibility of automated liquid sample handling in the cases when liquid scintillation cocktail is used. The benchtop microplate beta counters also allow the utilization of the same equipment for detection of gamma- and beta-emitting isotopes. Some of these counters also have the ability to increase the number of detectors to allow up to 12 wells to be read simultaneously, greatly reducing the overall counting time and optimizing data output.
With increasing concerns over the safety and disposal of liquid scintillation cocktails, solid scintillation counting using Lumaplates represents a further attractive alternative to liquid counting in a microplate format by reducing the amount of mixed radioactive and chemical liquid waste generated. The lower volume size required with benchtop microplate beta counter formats also allows assay cultures to be scaled down, reducing total cell numbers and sampling sizes and minimizing the amount of radioactive waste generated. These features make the TopCount and Lumalux methods suitable for high-throughput settings.
However, the applicability of the microplate methods to the analysis of cytolytic responses to weak inducers of CTL activity or from patients with compromised immune systems needs to be further examined.
In summary, our results indicate that supernatants from CRA can be counted in solid or liquid scintillant with Microbeta Trilux or TopCount Instruments and that each of these methods correlates significantly with the traditional gamma counting method.
Acknowledgments
This project was funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400.
We thank Mohammad Ishaq for providing access to the Packard TopCount instrument and Alan Doty and Richard Jones for their technical support and recommendations for the methods outlined here.
REFERENCES
- 1.Blazevic, V., L. A. Pinto, C. M. Trubey, and G. M. Shearer. 2000. Alloantigenic stimulation bypasses CD28-B7 costimulatory blockade by an interleukin-2-dependent mechanism. J. Leukoc. Biol. 67:817-824. [DOI] [PubMed] [Google Scholar]
- 2.Brunner, K. T., J. Mauel, J. C. Cerottini, and B. Chapuis. 1968. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology 14:181-196. [PMC free article] [PubMed] [Google Scholar]
- 3.Herberman, R. B. 2002. Cancer immunotherapy with natural killer cells. Semin. Oncol. 29:27-30. [DOI] [PubMed] [Google Scholar]
- 4.Hillman, G. G., N. Roessler, R. S. Fulbright, J. E. Pontes, and G. P. Haas. 1993. 51Cr-release assay adapted to a 96-well format sample reading. BioTechniques 15:744-749. [PubMed] [Google Scholar]
- 5.Kagi, D., and H. Hengartner. 1996. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr. Opin. Immunol. 8:472-477. [DOI] [PubMed] [Google Scholar]
- 6.Lee-MacAry, A. E., E. L. Ross, D. Davies, R. Laylor, J. Honeychurch, M. J. Glennie, D. Snary, and R. W. Wilkinson. 2001. Development of a novel flow cytometric cell-mediated cytotoxicity assay using the fluorophores PKH-26 and TO-PRO-3 iodide. J. Immunol. Methods 252:83-92. [DOI] [PubMed] [Google Scholar]
- 7.Lucey, D. R., L. A. Pinto, F. R. Bethke, J. Rusnak, G. P. Melcher, F. N. Hashemi, A. L. Landay, H. A. Kessler, R. J. Paxton, K. Grabstein, and G. M. Shearer. 1997. In vitro immunologic and virologic effects of interleukin 15 on peripheral blood mononuclear cells from normal donors and human immunodeficiency virus type 1-infected patients. Clin. Diagn. Lab. Immunol. 4:43-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michel, N., P. Ohlschlager, W. Osen, E. J. Freyschmidt, H. Guthohrlein, A. M. Kaufmann, M. Muller, and L. Gissmann. 2002. T cell response to human papillomavirus 16 E7 in mice: comparison of Cr release assay, intracellular IFN-gamma production, ELISPOT and tetramer staining. Intervirology 45:290-299. [DOI] [PubMed] [Google Scholar]
- 9.Pinto, L. A., J. Sullivan, J. A. Berzofsky, M. Clerici, H. A. Kessler, A. L. Landay, and G. M. Shearer. 1995. ENV-specific cytotoxic T lymphocyte responses in HIV seronegative health care workers occupationally exposed to HIV-contaminated body fluids. J. Clin. Investig. 96:867-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potter, C. G., F. Gotch, G. T. Warner, and J. Oestrup. 1987. Lymphocyte proliferation and cytotoxic assays using flat-bed scintillation counting. J. Immunol. Methods 105:171-177. [DOI] [PubMed] [Google Scholar]
- 11.Shacklett, B. L. 2002. Beyond 51Cr release: new methods for assessing HIV-1-specific CD8+ T cell responses in peripheral blood and mucosal tissues. Clin. Exp. Immunol. 130:172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zinkernagel, R. M. 1996. Immunology taught by viruses. Science 271:173-178. [DOI] [PubMed] [Google Scholar]
- 13.Zinkernagel, R. M., and P. C. Doherty. 1974. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature 248:701-702. [DOI] [PubMed] [Google Scholar]