Abstract
Aim/Hypothesis. To examine whether children with DMT1 are less physically fit than healthy children and to assess whether an elevated level of HbA1c was associated with decreased physical fitness among children with diabetes. Methods. The study was conducted using case-control methodology. The cases were 100 children with T1DM, 7–17,9 years. Study subjects underwent a 6MWT, where distance measured, heart rate, and oxygen saturation was recorded. Results. Results of the 6MWT for children with T1DM and controls were 601.3 ± 86.1 meters versus 672.1 ± 60.6 meters, respectively (P < 0.001). The cases were divided into two subgroups, one with HbA1c levels >8% and one with HbA1c <8%. Results for both groups were inferior to the controls (P < 0.001). The posttest pulse rate in all subjects was higher than the pretest pulse rate (P < 0.001). Pulse oxygen levels were lower than controls at the pretest measurement (P < 0.001), and for both cases and controls, pulse oxygen levels decreased after test (P = 0.004). However, the change in oxygen saturation did not differ between the groups (P = 0.332). Conclusions. Children with T1D are less fit than matched controls. The level of HbA1c did not affect the physical fitness of children with T1D.
1. Introduction
Diabetes mellitus type 1 is a multifactorial autoimmune disease characterized by complete destruction of pancreatic beta cells and loss of insulin production, caused by multiple genetic and environmental influences [1].
Young people with type 1 diabetes (T1D) have been found to have decreased aerobic capacity and lower cardiorespiratory fitness levels compared to nondiabetic control subjects [2, 3].
Serum concentration of HbA1c is the “gold standard” for the assessment of both therapeutic efficacy and the risk of development of diabetic microvascular and macrovascular complications [4].
Various factors are associated with glycemic control including age, sex, diabetes duration, and management (frequency of blood glucose monitoring, insulin regimen, and dose adjustments), as well as those indirectly connected to diabetes care (family history, dietary, cultural habits, etc.) [5].
Physical activity also plays an important role of glycemic control. Nondiabetic individuals have a reduction in insulin secretion and an increase in glucose counterregulatory hormones that facilitate an increase in liver glucose production that matches skeletal muscle glucose uptake during exercise, and consequently glucose levels during physical activity remain stable [6, 7].
In patients with T1D the pancreas does not regulate insulin levels in response to exercise, and there may be impaired glucose counterregulation, making normal fuel regulation nearly impossible [8]. In T1D patients with poor glycaemic control, there is insufficient amount of insulin, and counterregulatory hormones induced by physical activity will cause a further increase in blood glucose levels. In contrast, increased amounts of insulin present in the circulation will reduce or even prevent the mobilization of glucose which can result with hypoglycemia [9]. Regular physical activity has a particularly positive effect by improving insulin sensitivity and allows for a better use of the synthesis of glycogen and fat as energy sources [7, 10].
The six-minute walk test (6MWT) is a quick, simple and inexpensive method of determining physical fitness. It is also an important clinical test used to determine the quality of life [11]. It measures the distance achieved during a smooth walk with a constant effort for six minutes on a flat, hard surface. This is a simple method for estimating the physical stamina during submaximal effort. It is routinely used to assess the condition of people with cardiovascular and pulmonary diseases [12]. Initially the test was twelve minutes long and was used to test the healthy population. Cooper used it in the study of physical fitness of American pilots [13]. The test has been used in evaluation of patients with chronic obstructive pulmonary disease and the American Thoracic Society has developed guidelines for its use [14, 15]. Additionally the 6MWT has been used for monitoring the course of disease and response to therapy in patients with rheumatism, pulmonary, cardiovascular, and endocrine diseases [14–25]. Factors that have been shown to affect the test results include age, gender, leg length, body weight, various diseases, and the motivation of the subjects undergoing testing [15]. In children the 6MWT is being increasingly used [26–28].
2. Objective
The objectives of this research are
investigate whether children with T1D are less physically fit than healthy children;
establishing the influence of HbA1c level on physical fitness.
3. Subjects and Methods
3.1. Patients
We examined all children (186 children) aged 7–18 from Split—Croatia and Mostar—Bosnia and Herzegovina who have type 1 diabetes.
After examining medical history from the study children with cardiorespiratory disease and anemia were excluded. Also, patients with blood glucose levels were excluded (below 4, 0 and above 14 mmol/L), just prior to exercise testing or who tested positive for ketone.
The study group consisted of 100 children with T1D aged 7–18 years from the Mostar—Bosnia and Hercegovina and Split—Croatia, without clinical cardiopulmonary disease or anemia.
The control group consisted of the same number of healthy individuals from primary and secondary school, of equal age and sex, with a similar height (not greater than 2 cm), weight (not greater than 2 kg), length of the lower extremities, BMI, and who were not acutely ill a month before the test. Table 1 presents the demographic and anthropometric measures for the cases and controls.
Table 1.
Presentation of the groups studied in relation to body height, weight, length of the lower extremities, and BMI.
| Variables | Mean ± standard deviation | P* | |
|---|---|---|---|
| Test group | Control group | ||
| Number of participants | 100 | 100 | |
| Height (cm) | 159.9 ± 14.3 | 160.1 ± 13.9 | 0.942 |
| Weight (kg) | 51.1 ± 15.0 | 50.98 ± 14.7 | 0.964 |
| Lower extremities length (cm) |
78.9 ± 8.1 | 78.8 ± 7.5 | 0.964 |
| BMI (kg/m2) | 19.4 ± 3.5 | 19.5 ± 3.3 | 0.955 |
*Student t-test.
At a predetermined arrangement with the principal and class, children were provided with informational flyers that were submitted to parents for review and eventual approval. Signed approval was given by parents and the participant. The study was approved by the Ethics Committees of the University Hospital Mostar and the University Hospital Split. The diagnosis of T1D was made based on the criteria of the American Diabetes Association [29].
3.2. Anthropometric and Other Measurements
The participants weight (kg) and height (cm), body mass index (BMI—weight in kg divided by height in meters squared), heart rate (beats per minute), blood pressure (mm Hg), the lower segment of the body from the upper edge of the symphysis to the floor in upright position (cm), and blood oxygen saturation SpO2 (%) (CMS-50E fingertip Pulse Oximeter OLED) were determined in order to match participants and compare their performance.
In a group of type 1 diabetic patients HbA1c was measured within ±10 days of 6MWT by spectrophotometery (DCA 2000+, Siemens, Germany). Capillary blood glucose (CBG) level measured by a glucose meter was performed before and after the 6MWT. All patients had breakfast and an insulin injection administered 2 h to 2,5 h earlier.
3.3. 6MWT
The test consisted of the six-minute walking on a flat, hard surface 20 meters long (20 meters forward and 20 meters back). Participants used the measuring wheel (Nedo GmbH & Co. KG, Dornstetten, Germany) which was held by hand. The measuring wheel has different handle lengths (240, 370, and 560 mm) that were changed depending on the height of participant. Each participant chose the handle of appropriate length. Participant had not engaged in serious physical activity for at least two hours before the test.
The test was performed in a separate room, and a dial on the wheel was covered during the performance test to rule out the possibility of competition between participants.
On the floor a distance of 20 meters was measured and marked by cones. Each participant was given the opportunity to experience the wheel and select the optimal handle size. Then he was given instructions for the test which read: “The purpose of this test is to walk as fast as you can in six minutes and cross as many meters you can. You will walk around the set cones. You are allowed to slow down and stop, if necessary, and lean on the wall and when you can, continue. It is forbidden to run. Again, the purpose is to cross as many meters as you can. If you're ready, go!” Time was measured by a stopwatch, and every minute the respondents were informed by standard phrases: after the first minute, “Well done, you have five minutes to the end,” after another minute, “Keep it up, you got four more minutes,” after three minutes, “Well done, you're half-way,” after four minutes, “Keep it up, you got two more minutes,” and after five minutes, “Just keep going, you have another minute.” No other words of encouragement as well as body language were used to avoid the possibility of encouraging the participant to speed up or slow down. The examiner at all times stood in the middle of the walking track to control the accuracy of the test. After six minutes the participant was asked to stop. Immediately after that participant was placed in a sitting position where blood oxygen saturation and heart rate were measured. Then we noted how many meters the participant had crossed in six minutes.
3.4. Statistical Analysis
Statistical analysis included the Kolmogorov-Smirnov test for symmetry of distribution of continuous variables. For a description of their assembly and disintegration the arithmetic mean and standard deviation was used. Comparison of two normally distributed independent variables was performed using Student's t-test. Comparison of continuous variables measured at multiple time points was made by the test of repeated measurements. Unlike the distribution of nominal variables an ordinal χ 2 test was used. The possibility of a type I error was set at α < 0.05 and differences between groups were accepted as statistically significant at P < 0.05.
For statistical analysis we used SPSS for Windows (version 13.0, SPSS Inc. Chicago, IL, USA) and Microsoft Excel (version 11, Microsoft Corporation, Redmond, WA, USA).
4. Results
The study and control groups included 49 girls (49.0%) and 51 boys (51.0%), and there were no significant differences in sex ratio (χ 2 test = 0.040, df = 1, P = 0.841). The average age was 13.0 ± 2.9 years (mean ± SD), range 7.0 to 17.9 years.
Test and control groups did not differ significantly in average height (Student t-test = 0.073, P = 0.942), in average body weight (Student t-test = 0.046, P = 0.964), in the average length of the lower extremities (Student t-test = 0.045, P = 0.964), and in mean body mass index (Student's t-test = 0.043, P = 0.955) (Table 1).
Combining both cases and controls, the average value of the 6MWT was 636.7 ± 82.3 meters, range (360.3–808.0) meters.
The cases had significantly shorter distance measured in meters compared to the control group (Student t-test = 6.718, P < 0.001) (Table 2).
Table 2.
Presentation of the groups studied in relation to the mileage meter.
| Variables | Mean ± standard deviation | P* | |
|---|---|---|---|
| Test group | Control group | ||
| Number of participants | 100 | 100 | |
| Crossed distance | 601.3 ± 86.1 m | 672.1 ± 60.6 m | <0.001 |
*Student t-test = 6.718; P < 0.001.
According to the values of HbA1c test group was divided into two subgroups. The first group consisted of children with the values of HbA1c <7.9% (n = 40). In the second group children with the values of HbA1c ≥8.0% (n = 60) were included. Both subgroups were compared with their pairs in the control group.
Children with lower levels of HbA1c of 8.0% exceeded by significantly shorter distance the corresponding control group (Student's t-test = 4.109, P < 0.001) (Table 3).
Table 3.
Presentation of the groups studied with HbA1c levels lower than 8.0% and their controls in relation to the mileage meter.
| Variables | Mean ± standard deviation | P* | |
|---|---|---|---|
| Test group | Control group | ||
| Number of participants | 40 | 40 | |
| Crossed distance | 599.8 ± 86.7 m | 669.2 ± 62.3 m | <0.001 |
*Student t-test = 4.109; P < 0.001.
Children with levels of HbA1c ≥8.0% walked a significantly shorter distance than the corresponding control group (Student t-test = 5.279, P < 0.001) (Table 4).
Table 4.
Presentation of the groups studied with the level of HbA1c ≥8.0% and their controls in relation to the mileage meter.
| Variables | Mean ± standard deviation | P* | |
|---|---|---|---|
| Test group | Control group | ||
| Number of participants | 60 | 60 | |
| Crossed distance | 602.4 ± 86.4 m | 674.1 ± 59.9 m | <0.001 |
*Student t-test = 5.279; P < 0.001.
Observing the entire sample, cases, and control groups pretest heart rate was significantly slower (88.3 ± 14.7), compared to the heart rate after the test (131.2 ± 20.7) (F(1.198) = 931.573; P < 0.001).
There were no significant differences in heart rate before and after the test between the two groups (F(1.198) = 1.733, P = 0.190) (Figure 1).
Figure 1.
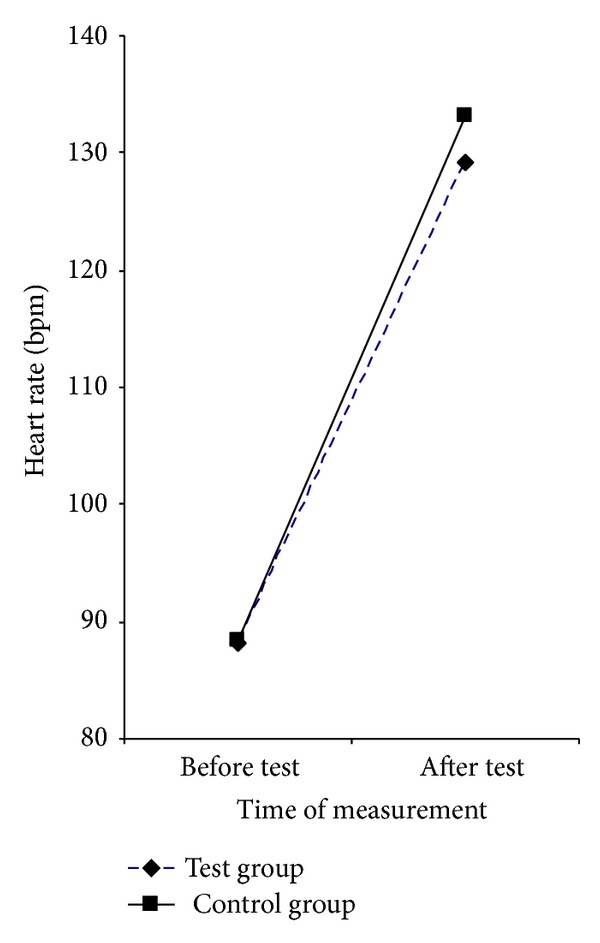
Presentation of the groups studied in relation to the value of the heart rate before and after the test.
Observing the entire sample, cases, and control group, oxygen saturation (%) before the test was significantly higher (98.8 ± 1.0) compared to the oxygen saturation (%) after the test (98.6 ± 1.0) (F(1.198) = 8.529, P = 0.004).
A downward trend in the oxygen saturation between the two groups, before and after the test, showed no statistically significant difference (F(1.198) = 0.948, P = 0.332).
Taking into consideration the overall value of oxygen saturation before and after the test, the test group had significantly lower values of saturation (98.3 ± 1.1) compared to the control group (99.1 ± 1.1) (F(1.198) = 51.238, P < 0.001) (Figure 2).
Figure 2.
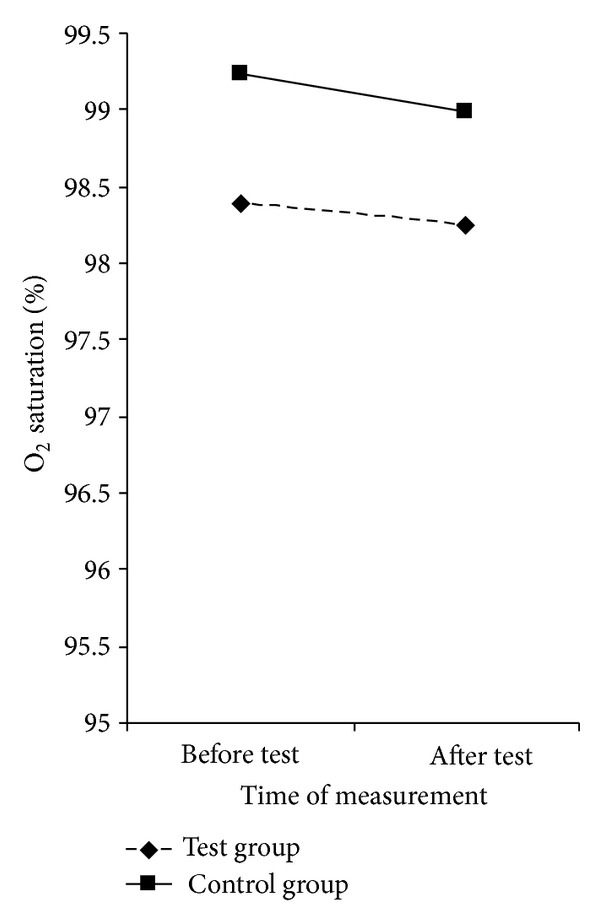
Presentation of the groups studied compared to the value of oxygen saturation before and after the test.
5. Discussion
In our study, we compared the physical fitness of children with T1D with controls. The results of our study indicate that children with T1D are less physically fit than a matched set of healthy control children (Table 1).
Physical activity has a positive effect on health [30]. The ability of trained muscle to take and oxidize free fatty acid reduces blood lipid levels. The positive effect on blood pressure significantly reduces the overall risk of cardiovascular disease [31]. Maintaining fitness and ideal body weight promotes self-esteem and self-satisfaction [32]. All these effects are particularly important in patients with diabetes who have chronic hyperglycemia as they are exposed to additional risks. Regular physical activity improves insulin sensitivity and reduces the daily need for exogenous insulin. In children with T1D the frequency of regular physical activity was associated with lower HbA1c without increasing the risk of severe hypoglycemia [33].
Despite the positive effect that physical activity has on blood glucose, controlled studies have not confirmed a long-term improvement in metabolic control in patients with T1D. Physical activity may be just one element in the complex therapy of diseases. Another possible explanation is that patients avert the risk of hypoglycemia before physical activity by underdosing insulin or by injecting carbohydrate, and this offsets the benefits of exercise [34].
The 6MWT is a reliable test for the assessment of physical fitness [11]. In many countries all over the world the standards of normal values for different age groups have been established [26–28, 35]. The test can be used to monitor the progress of disease and response to therapy in patients with various diseases. Dos Santos Alves et al. used the 6MWT for monitoring lung capacity in patients with idiopathic scoliosis [16]. Otto Lelieveld used the 6MWT in children with juvenile idiopathic arthritis [17]. However, the most common use of 6MWT is in pulmonary and cardiovascular diseases [18–23]. Novak et al. applied 6MWT in diabetics who have neuropathy as a complication of their primary disease and showed that patients with severe leg pain have more difficulty in walking than patients with mild pain or no pain which significantly affects their quality of life [24] 6MWT represents a practical and reliable assessment tool for exercise performance in overweight and obese children and adolescents [36, 37].
To the best of our knowledge in the literature, we have not found studies that used the 6MWT in children with T1D. We found results documented from one pilot study. Physical condition of seven type 1 diabetic girls aged 8–10 years was given exercise scheme activities and examined with a 6MWT before and three months after [25].
Thanks to the precise regulation in nondiabetic individuals glucose levels during physical activity remain stable [6, 7]. In T1D the pancreas does not regulate insulin levels in response to exercise, and there may be impaired glucose contrarregulation. In poor controlled diabetic patients, there is an insufficient amount of insulin, and contraregulatory hormones in physical activity cause a further rise in blood glucose levels. In contrast, the increased amount of insulin present in the circulation will reduce or even prevent the mobilization of glucose which can result in hypoglycemia; it may explain the weaker results of physical fitness of the group with T1D [38]. Although we hypothesized that that subjects with well-controlled diabetes would perform better on the 6MWT than those with poorly controlled diabetes [39], our results did not confirm this (Tables 2 and 3). The study group was divided into two groups: well and poorly controlled. A HbA1c of 8.0% was taken as the dividing boundary. Then these groups were compared with their controls. It is known that HbA1c reflects levels of glycemia over the preceding 4–12 weeks [7]. The reason why is this value taken as the limit comes in the following facts. From the clinical side of view, we can say that the value of HbA1c of 7.9% and less is acceptable especially if it is known that the majority of respondents were in puberty, when many adolescents experience a deterioration in metabolic control. One of the reasons for this is the greater insulin resistance at puberty [40].
The difference in heart rate frequency was statistically significant when comparing values before and after the test, which means that all respondents gave their maximum during the performance test (Figure 1). It is also very important to note that there was no difference in heart rate frequency between the groups suggesting that both groups were equally motivated and goal-directed in the test. The better result of physical fitness in the control group is not a result of greater effort than the test group. Given that the cases were free of vascular complications of diabetes, the difference in oxygen saturation between the groups was surprising (Figure 2). This could be explained by possible functional and structural abnormalities in the peripheral blood vessels caused by diabetes and poor cardiorespiratory fitness of these patients, although the trend of oxygen saturation in both groups remained the same. The question remains whether the reduced performance of children with T1D is associated with lower levels of physical activity or is it a result of their illness [3, 41]. Children with T1D have been found to have decreased aerobic capacity measured by VO2 max and also to have lower heart rate at exercise exhaustion compared to nondiabetic control subjects. The authors postulated that individuals with T1D had decreased lung ventilation associated with decreased maximal O2 consumption and exercising capacity [2]. Children with T1D aged 5–14 years had reduced cardiorespiratory fitness levels compared to nondiabetic control children [3]. Recent research in patients with type 2 diabetes reports less blood flow to the periphery in physical activity [42]. As the oxygen saturation is measured at the fingertip, this may explain our result.
6. Conclusions
We found that children with T1D are less physically fit than matched healthy controls as measured by the 6MWT.
We also found that the level of HbA1c did not affect the physical fitness of children with T1D.
Future research is needed to confirm these results and should investigate whether the reduced physical fitness in children with T1D is attributable to physiological changes resulting from the diabetes pathology itself.
Conflict of Interests
All previously mentioned authors disclose that there was not any financial or personal conflict of interests that might influence their work. All previously listed authors state that the submitted paper has not been previously published or is not under review in any other journal.
Ackowledgments
The authors thank the colleagues I. Unić and Z. Bilinovac, nurses N. Cvjetkovic, L. Božić, and B. Karačić from the Department of Pediatrics, Clinical Hospital Mostar and Split. Also they want to thank B. Petrov for his contribution in statistical analysis.
References
- 1.Atkinson MA, Maclaren NK. Mechanisms of disease: the pathogenesis of insulin-dependent diabetes mellitus. The New England Journal of Medicine. 1994;331(21):1428–1436. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 2.Komatsu WR, Lima Gabbay MA, Castro ML, et al. Aerobic exercise capacity in normal adolescents and those with type 1 diabetes mellitus. Pediatric Diabetes. 2005;6(3):145–149. doi: 10.1111/j.1399-543X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- 3.Williams BK, Guelfi KJ, Jones TW, Davis EA. Lower cardiorespiratory fitness in children with type 1 diabetes. Diabetic Medicine. 2011;28(8):1005–1007. doi: 10.1111/j.1464-5491.2011.03271.x. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial-Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. Journal of Pediatrics. 1994;125(2):177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 5.Haugstvedt A, Wentzel-Larsen T, Rokne B, Graue M. Psychosocial family factors and glycemic control among children aged 1-15 years with type 1 diabetes: a population-based survey. BMC Pediatrics. 2011;11, article 118 doi: 10.1186/1471-2431-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riddell MC, Perkins BA. Type 1 diabetes and vigorous exercise: applications of exercise physiology to patient management. Canadian Journal of Diabetes. 2006;30(1):63–71. [Google Scholar]
- 7.Thorell A, Hirshman MF, Nygren J, et al. Exercise and insulin cause GLUT-4 translocation in human skeletal muscle. American Journal of Physiology. 1999;277(4):E733–E741. doi: 10.1152/ajpendo.1999.277.4.E733. [DOI] [PubMed] [Google Scholar]
- 8.Robertson K, Adolfsson P, Scheiner G, Hanas R, Riddell MC. Exercise in children and adolescents with diabetes. Pediatric Diabetes. 2009;10(12):154–168. doi: 10.1111/j.1399-5448.2009.00567.x. [DOI] [PubMed] [Google Scholar]
- 9.Jensen J, Rustad PI, Kolnes AJ, Lai YC. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Frontiers in Physiology. 2011;2:p. 112. doi: 10.3389/fphys.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Graaf M, de Haan JH, Smits P, Mulder AH, Heerschap A, Tack CJ. The effect of acute exercise on glycogen synthesis rate in obese subjects studied by 13C MRS. European Journal of Applied Physiology. 2011;111(2):275–283. doi: 10.1007/s00421-010-1650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119(1):256–270. doi: 10.1378/chest.119.1.256. [DOI] [PubMed] [Google Scholar]
- 12.Balke B. A simple field test for the assessing of physical fitnes. Report. Civil Aeromedical Research Institute (U.S.) 1963;53:1–8. [PubMed] [Google Scholar]
- 13.Cooper KH. A means of assessing maximal oxygen intake. Correlation between field and treadmill testing. Journal of the American Medical Association. 1968;203(3):201–204. [PubMed] [Google Scholar]
- 14.McGavin CR, Gupta SP, McHardy GJR. Twelve minute walking test for assessing disability in chronic bronchitis. British Medical Journal. 1976;1(6013):822–823. doi: 10.1136/bmj.1.6013.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. American Journal of Respiratory and Critical Care Medicine. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 16.dos Santos Alves VL, Stirbulov R, Avanzi O. Impact of a physical rehabilitation program on the respiratory function of adolescents with idiopathic scoliosis. Chest. 2006;130(2):500–505. doi: 10.1378/chest.130.2.500. [DOI] [PubMed] [Google Scholar]
- 17.Lelieveld OTHM, Takken T, van der Net J, Van Weert E. Validity of the 6-minute walking test in juvenile idiopathic arthritis. Arthritis Care and Research. 2005;53(2):304–307. doi: 10.1002/art.21086. [DOI] [PubMed] [Google Scholar]
- 18.Lama VN, Flaherty KR, Toews GB, et al. Prognostic value of desaturation during a 6 minute walk test in idiopathic interstitial pneumonia. American Journal of Respiratory and Critical Care Medicine. 2003;168(9):1084–1090. doi: 10.1164/rccm.200302-219OC. [DOI] [PubMed] [Google Scholar]
- 19.Sivaranjini S, Vanamail P, Eason J. Six minute walk test in people with tuberculosis sequelae. Cardiopulmonary Physical Therapy Journal. 2010;21:5–10. [PMC free article] [PubMed] [Google Scholar]
- 20.Basaran S, Guler-Uysal F, Ergen N, Seydaoglu G, Bingol-Karakoç G, Ufuk Altintas D. Effects of physical exercise on quality of life, exercise capacity and pulmonary function in children with asthma. Journal of Rehabilitation Medicine. 2006;38(2):130–135. doi: 10.1080/16501970500476142. [DOI] [PubMed] [Google Scholar]
- 21.Mattiello R, Sarria EE, Stein R, et al. Functional capacity assessment during exercise in children and adolescents with post-infectious bronchiolitis obliterans. Jornal de Pediatria. 2008;84(4):337–343. doi: 10.2223/JPED.1807. [DOI] [PubMed] [Google Scholar]
- 22.Provencher S, Chemla D, Hervé P, Sitbon O, Humbert M, Simonneau G. Heart rate responses during the 6-minute walk test in pulmonary arterial hypertension. European Respiratory Journal. 2006;27(1):114–120. doi: 10.1183/09031936.06.00042705. [DOI] [PubMed] [Google Scholar]
- 23.Olsson LG, Swedberg K, Clark AL, Witte KK, Cleland JGF. Six minute corridor walk test as an outcome measure for the assessment of treatment in randomized, blinded intervention trials of chronic heart failure: a systematic review. European Heart Journal. 2005;26(8):778–793. doi: 10.1093/eurheartj/ehi162. [DOI] [PubMed] [Google Scholar]
- 24.Novak P, Burger H, Marincek C, Meh D. Influence of foot pain on walking ability of diabetic patients. Journal of Rehabilitation Medicine. 2004;36(6):249–252. doi: 10.1080/16501970410029816. [DOI] [PubMed] [Google Scholar]
- 25.Niewiadomska M, Radziejewska M, Horodnicka-Józwa A, Petriczko E. Agility in treatment of children with type 1 diabetes: pilot study. Pediatric Endocrinology, Diabetes, and Metabolism. 2010;16(2):89–93. [PubMed] [Google Scholar]
- 26.Lammers AE, A.A. Hislop AAH, Flynn Y, Haworth SG. The 6-minute walk test: normal values for children of 4–11 years of age. Archives of Disease in Childhood. 2008;93(6):464–468. doi: 10.1136/adc.2007.123653. [DOI] [PubMed] [Google Scholar]
- 27.Li AM, Yin J, Au JT, et al. Standard reference for the six-minute-walk test in healthy children aged 7 to 16 years. American Journal of Respiratory and Critical Care Medicine. 2007;176(2):174–180. doi: 10.1164/rccm.200607-883OC. [DOI] [PubMed] [Google Scholar]
- 28.Geiger R, Strasak A, Treml B, et al. Six-minute walk test in children and adolescents. Journal of Pediatrics. 2007;150(4):395–399. doi: 10.1016/j.jpeds.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 29.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association. Physical activity/exercise and diabetes. Diabetes Care. 2004;27:58–62. [Google Scholar]
- 31.Giannini C, Mohn A, Chiarelli F. Physical exercise and diabetes during childhood. Acta Biomedica. 2006;77(1):18–25. [PubMed] [Google Scholar]
- 32.He XZ, Baker DW. Body mass index, physical activity, and the risk of decline in overall health and physical functioning in late middle age. American Journal of Public Health. 2004;94(9):1567–1573. doi: 10.2105/ajph.94.9.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen KF, Price TB, Bergeron R. Regulation of net hepatic glycogenolysis and gluconeogenesis during exercise: impact of type 1 diabetes. Journal of Clinical Endocrinology and Metabolism. 2004;89(9):4656–4664. doi: 10.1210/jc.2004-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herbst A, Bachran R, Kapellen T, Holl RW. Effects of regular physical activity on control of glycemia in pediatric patients with type 1 diabetes mellitus. Archives of Pediatrics and Adolescent Medicine. 2006;160(6):573–577. doi: 10.1001/archpedi.160.6.573. [DOI] [PubMed] [Google Scholar]
- 35.Soares MR, Pereira CADC. Six-minute walk test: reference values for healthy adults in Brazil. Jornal Brasileiro de Pneumologia. 2011;37(5):576–583. doi: 10.1590/s1806-37132011000500003. [DOI] [PubMed] [Google Scholar]
- 36.Tuttle LJ, Sinacore DR, Cade WT, Mueller MJ. Lower physical activity is associated with higher intermuscular adipose tissue in people with type 2 diabetes and peripheral neuropathy. Physical Therapy. 2011;91(6):923–930. doi: 10.2522/ptj.20100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geiger R, Willeit J, Rummel M, et al. Six-minute walk distance in overweight children and adolescents: effects of a weight-reducing program. Journal of Pediatrics. 2011;158(3):447–451. doi: 10.1016/j.jpeds.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Elloumi M, Makni E, Ounis OB, et al. Six-minute walking test and the assessment of cardiorespiratory responses during weight-loss programmes in obese children. Physiotherapy Research International. 2011;16(1):32–42. doi: 10.1002/pri.470. [DOI] [PubMed] [Google Scholar]
- 39.McCrimmon RJ, Sherwin RS. Hypoglycemia in type 1 diabetes. Diabetes. 2010;59(10):2333–2339. doi: 10.2337/db10-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Souto DL, Paes de Miranda M. Physical excercises on glycemic control in type 1 diabetes mellitus. Nutricion Hospitalaria. 2011;26(3):425–429. doi: 10.1590/S0212-16112011000300001. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, Kim DJ, Jang HC, Choi SH. Epidemiology of micro and macrovascular complications of type 2 diabetes in Korea. Diabetes & Metabolism Journal. 2011;35:571–577. doi: 10.4093/dmj.2011.35.6.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joshi D, Shiwalkar A, Cross MR, Sharma SK, Vachhani A, Dutt C. Continuous, non-invasive measurement of the haemodynamic response to submaximal exercise in patients with diabetes mellitus: evidence of impaired cardiac reserve and peripheral vascular response. Heart. 2010;96(1):36–41. doi: 10.1136/hrt.2009.177113. [DOI] [PMC free article] [PubMed] [Google Scholar]


