Abstract
An enzyme-linked immunosorbent assay (ELISA) and a rapid immunochromatographic test for detection of immunoglobulin G (IgG) antibodies in severe acute respiratory syndrome (SARS) patients were developed by utilizing the well-characterized recombinant proteins Gst-N and Gst-U274. The ELISA detected IgG antibodies to SARS-CoV in all 74 convalescent-phase samples from SARS patients while weakly cross-reacting to only 1 of the 210 control sera from healthy donors. This finding thus led to a kit sensitivity, specificity, and accuracy of 100, 99.5, and 99.6%, respectively. The test thus provided a positive predictive value (PPV) of 98.7% and a negative predictive value (NPV) of 100%. In addition, the ELISA gave a positive delta of 5.4 and a negative delta of 3.6, indicating an excellent differentiation between positives and negatives. The same recombinant proteins were also applied to a newly developed platform for the development of a 15-min rapid test. The resulting rapid test has an excellent agreement of 99.6%, with a kappa value of 1.00, with the ELISA. Again, this rapid test was able to detect 100% of the samples tested (n = 42) while maintaining a specificity of 99.0% (n = 210). The PPV and NPV for the rapid test thus reached 95.3 and 100%, respectively.
Severe acute respiratory syndrome (SARS) is a newly emerged disease of global significance because of its highly contagious nature in an increasingly globalized world. This was promptly recognized by the World Health Organization (WHO) when the SARS epidemic spread beyond its place of origin in Guangdong Province, China, and a global alert was declared for the first time in WHO history. The outbreaks affected over 8,098 people and spread to more than 29 countries and regions in a short period of 6 months, with a mortality rate of up to 15% (WHO summary report available at http://www.who.int/csr/sars/country/table2003_09_23/en). Obviously, this disease is not a limited problem but one with profound consequences affecting sectors beyond public health care.
Although it is now understood that a novel coronavirus, SARS-CoV, is the etiological agent for SARS (3, 5, 7, 10), more remains to be learned in terms of how to detect, diagnose, and treat the disease. Prompt identification and isolation of infected patients remain of paramount importance for disease control, since no drug or vaccine is available for this disease. With few options in the laboratory for detection, initial disease management relied on travel and contact history and presentation of symptoms. PCR tests developed with unprecedented speed are now available and can detect specific RNA of SARS-CoV, thus providing a laboratory method for diagnosis of SARS. WHO had, based on this method, revised its criteria for case definition. However, existing protocols for PCR were not without their limitations. The Singapore experiences showed that the positive predictive values (PPVs) provided by PCR varied from 56.7 to 83.8% when different clinical specimens were used (A. E. Ling, “SARS—the Singapore laboratory experience,” presented at the WHO Conference on SARS Research, Singapore, Republic of Singapore, 19 June 2003). In a very recent report (13), the reverse transcription-PCR (RT-PCR) protocols of two WHO SARS network laboratories were evaluated, and the findings confirmed similar shortcomings of RT-PCR. Therefore, the existing PCR cannot rule out the presence of the SARS virus when a negative result is obtained; neither can it exclude the possibility of false detection due to laboratory contamination (9). In the meantime, methods involving indirect immunofluorescence assay (IFA), classic tissue culture for viral isolation, and electronic microscopy of the cell culture for diagnosis are either time-consuming or technically very demanding. Alternative approaches are therefore critical for efficient management of the disease.
The enzyme-linked immunosorbent assay (ELISA) is one such alternative, because it can provide diagnostic information that is complementary to the data provided by PCR. In addition, a rapid point-of-care test such as an immunochromatographic test can also facilitate case diagnosis and disease management in remote areas where laboratory facilities are not readily available. In this study, we were able to demonstrate the potential of two serological tests developed by utilizing two recombinant proteins: Gst-N and Gst-U274. As reported elsewhere, Gst-N was derived from the nucleocapsid region, whereas Gst-U274 was derived from a unique protein of SARS-CoV (12a). Our study results suggested that the two serological tests (each with an excellent sensitivity of 100%) could provide confirmatory information for the diagnosis of SARS, especially for suspected cases initially screened by PCR as negative.
MATERIALS AND METHODS
Recombinant proteins.
The materials and methods used for obtaining the recombinant proteins are described in detail elsewhere (12a). Briefly, both Gst-N and Gst-U274 used in the study were expressed as Gst fusion proteins in Escherichia coli and purified with glutathione-Sepharose beads (Amersham Pharmacia). Protein Gst-N was derived from nucleocapsid of the SARS-CoV with a highly conserved motif (amino acids 111 to 118) found in all coronaviruses (12) deleted, whereas Gst-U274 was from a unique SARS-CoV protein. For this study, the Gst-U274 protein was further purified with a Superdex S-200 HR10/30 column on an AKTA fast protein liquid chromatography (FPLC) system (Amersham). The buffer used contained 20 mM Tris-HCl (pH 7.5), 100 mM NaCl, 6 M urea, and 1 mM β-mercaptoethanol; the flow rate was 0.5 ml/min; and fractions of 1 ml were collected. Fractions 12 and 13 were combined and dialyzed against phosphate-buffered saline (PBS) overnight with at least three changes of buffer.
Serum specimens.
Seventy-four convalescent-phase serum samples were collected from SARS patients admitted to the Tan Tock Seng Hospital or the Singapore General Hospital. Ninety-one control serum samples were obtained from healthly local donors who worked at the Institute of Molecular and Cell Biology, Singapore, Republic of Singapore. All specimens were collected with consent, and patient samples were collected at 16 to 65 days from the onset of symptoms. In addition, 119 sera from healthy donors purchased from BioClinical Partners, Inc. (Franklin, Mass.), were included in the study as additional healthy controls.
ELISA.
The Gst-N and GstT-U274 proteins were prediluted in carbonate buffer (pH 9.6) at final concentrations of 0.1 and 0.15 μg/ml, respectively, prior to plate coating. The plates were prepared as described in reference 4. Briefly, the 96-well polystyrene microtiter plates (Immuno 1B; Themo Labsystem, Franklin, Mass.) were coated with the protein mixtures at a volume of 100 μl per well by incubation overnight (16 to 18 h) at room temperature. The plates were washed five times with PBS-Tween 80 (PBST), and nonspecific binding sites were blocked with 200 μl (per well) of a Tris-based diluent for 1 h at room temperature. The plates were further washed another five times before 10 μl of serum in 200 μl of Tris-based diluent (containing 1% each bovine serum albumin [BSA] and skim milk powder) was added. Subsequently, the plates were incubated for 30 min at 37°C, followed by six washes with PBST. Horseradish peroxidase-conjugated goat anti-human immunoglobulin G (IgG; 1:500 dilution) was added at 100 μl per well, and this mixture was incubated for 30 min at 37°C. The plates were then washed six times in PBST and color development proceeded with the addition of the enzyme substrate tetramethylbenzidine (TMB) at 100 μl per well. After a 15-min incubation in the dark at 37°C, the reaction was stopped by adding 100 μl of 1 N HCl per well. The optical densities (OD) were measured at 450 nm with a 620-nm reference filter.
Rapid immunochromatographic test.
The membrane-based immunochromatographic test device consisted of a chromatography strip, a separator, and an absorbent pad, all housed in a cassette as described previously (6).
The chromatography strip was prepared separately according to the procedure detailed in reference 6, with slight modification before assembly into the device. Briefly, a nitrocellulose membrane with an average pore size of 8 μm (Whatman, Ann Arbor, Mich.) was sprayed with the Gst-N and the Gst-U274 recombinant antigens in two separate lines, at concentrations of 0.1 and 0.15 mg/ml, respectively, with a BioDot (Irvine, Calif.) spraying machine. The membrane was dried for 10 min before being immersed for 1 min in a blocking buffer consisting of Milli-Q purified water with 6.7% StabilCoat (SurModics, Inc.), 0.05% Triton X-100, and 0.5% casein. The blocked membrane was then dried at 37°C for 60 min before being affixed to a membrane backing.
The reagent-bearing pad was prepared using a porous matrix. The porous matrix was sprayed with goat anti-human IgG antibodies that were labeled with colloidal gold particles 25 to 30 nm in diameter. This reagent-bearing pad was then dried at 37°C for 2 h prior to incorporation into the device.
A chromatographic card was prepared by affixing a 0.1% Triton X-100-treated porous matrix to one end of the nitrocellulose strip and the reagent-bearing pad to the other end on the same membrane backing. This assembly was then cut into a strip approximately 4 by 56 mm2 in size.
An assay device was assembled by placing an absorbent pad at the bottom half of the cassette and then a separator, followed by one unit of the chromatographic strip before closing the top half of the cassette. In addition, a reagent-releasing wash buffer was also prepared with Milli-Q purified water with 50 mM NaH2PO4, 300 mM NaCl, and 0.1% sodium dodecyl sulfate (pH 8.0).
When testing, 25 μl of undiluted serum sample was added to the specimen window of the assay device. The sample was allowed to migrate laterally and cover part of the membrane. When the sample reached the indicator in the viewing window in approximately 30 s, three drops of reagent-releasing washing buffer were added to the buffer window to release the colloidal gold-labeled goat anti-human IgG antibodies. The separator was then removed by pulling the protruding end to allow the chromatographic element and the absorbent pad to come into contact. The colloidal gold-labeled goat anti-human IgG antibodies were then allowed to migrate across the chromatographic strip completely.
The result can be read in typically 2 to 15 min through the viewing window. A negative result will be indicated by the appearance of a control line only, whereas with a positive result, the control line and either or both of the test lines appear in the viewing window (Fig. 1).
FIG. 1.
Examples of the assembled rapid immunochromatographic test devices with their separators (transparent tabs) at the “removed” (after assay) position. To the left is a device after an assay with a sample from a patient, and to the right is another after an assay with a sample from a healthy control. The three lines in the viewing window for the positive sample represent (from top to bottom) the control, Gst-N, and Gst-U274, respectively. The negative sample generated the control line only.
Statistical analysis.
The kappa statistic was used to measure the strength of agreement between the results by the new rapid test and the new ELISA. Kappa statistic values of >0.75, 0.40 to 0.75, and <0.40 represent excellent agreement, good to fair agreement, and poor agreement, respectively (11). For the data analysis for ELISA, an arbitrary cutoff value of 0.45 was selected but confirmed with the delta values (2), which could provide an indication of an optimal differentiation between the positive and negative populations.
RESULTS
ELISA.
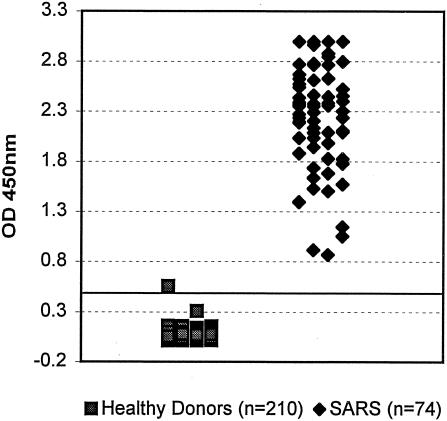
When tested at a sample dilution of 1:20, the ELISA detected IgG antibodies to SARS-CoV in 100% (n = 74) of the convalescent-phase samples from the SARS patients, with a mean ± standard deviation OD value of 2.32 ± 0.54 (Table 1 and Fig. 2). In contrast, only 1 initial-positive sample was found by the test out of the 210 control sera from the healthy donors with a mean ± standard deviation OD value of 0.05 ± 0.05 (P < 0.001) (Fig. 2). The test thus provided a PPV of 98.7% and a negative predictive value (NPV) of 100% (Table 1). In addition, the ELISA gave a positive delta of 5.4 and a negative delta of 3.6, indicating excellent differentiation between positives and negatives. Furthermore, and as shown in Fig. 3, when titration curves were obtained with seven samples from the SARS patients by using this new ELISA, the reactivity end point was found at dilutions ranging from >1:40 to 1:640 with different samples.
TABLE 1.
Performance of individual markers and the new serological tests with sera from SARS patients and healthy donors
| Test format | SARS patients
|
Healthy controls
|
PPV (%) | NPV (%) | ||
|---|---|---|---|---|---|---|
| No. positive/ total | % Sensi- tivity | No. positive/ total | % Speci- ficity | |||
| ELISA (combined)a | 74/74 | 100 | 1/210 | 99.5 | 98.7 | 100 |
| Rapid test | ||||||
| Gst-N | 42/42 | 100 | 2/210 | 99.0 | 95.3 | 100 |
| Gst-U274 | 36/42 | 85.7 | 0/210 | 100 | 100 | 97.2 |
| Combined | 42/42 | 100 | 2/210 | 99.0 | 95.3 | 100 |
Gst-N plus Gst-U274.
FIG. 2.
Scatter chart of OD values obtained with sera from both SARS patients and healthy controls tested for IgG antibody to SARS-CoV by using the newly developed ELISA.
FIG. 3.
Titration curves of seven SARS patient samples obtained with the ELISA.
Rapid immunochromatographic test.
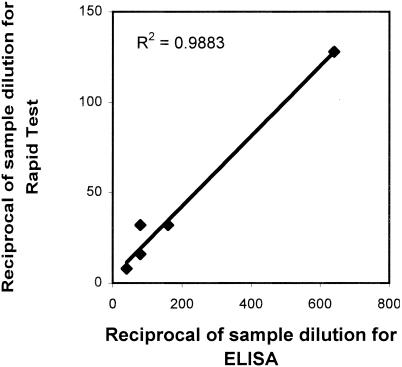
When tested with undiluted samples, the Gst-N and the Gst-U274 proteins used in the rapid test reacted to IgG antibodies in 100% (42 of 42) and 85.7% (36 of 42), respectively, of the sera from SARS patients who met the WHO criteria for SARS (Table 1). Thus, the overall detection rate for the new test was 100% (42 of 42) (Table 1). Only the Gst-N protein in the rapid test was found to cross-react with 2 of the 210 sera from the healthy controls. The test was therefore shown to have specificity, PPV, and NPV of 99.0, 95.3, and 100%, respectively, with the tested populations (Table 1). When the results were compared, the rapid test and the ELISA gave an excellent agreement of 99.6%, with a kappa statistic of 1.00 (Table 2). In addition, an excellent correlation (R2 = 0.988) was found when the reactivity end points of the rapid test (at dilutions ranging from 1:8 to 1:128) and the ELISA were compared by using the same seven patient samples (Fig. 4).
TABLE 2.
Agreement between the new rapid test and the new ELISA with sera from SARS patients and healthy controls
| Rapid test | No. of ELISA results
|
% Agreement | Kappa statistica | |
|---|---|---|---|---|
| Positive | Negative | |||
| Positive | 43 | 1 | 99.6 | 1.00 |
| Negative | 0 | 208 | 100 | |
A kappa statistic of ≥0.75 represents excellent agreement, 0.40 to 0.75 represents good-to-fair agreement, and <0.40 represents poor agreement (11).
FIG. 4.
Correlation between the ELISA and the rapid test when comparing the dilutions at which reactivity end points were obtained with the seven SARS patient samples.
DISCUSSION
Recent studies have suggested the potential of a SARS-CoV nucleocapsid protein as a diagnostic reagent for the detection of SARS (8, 12a). However, the application of this protein in developing a viable diagnostic test remained to be fully demonstrated. The follow-up study presented here provides results to fill in the gap. The newly developed ELISA in this study utilizing the two Gst-N and Gst-U274 recombinant proteins was able to detect all 74 samples tested. This result thus has demonstrated the capability of the new test for the detection of IgG antibodies to SARS-CoV using the convalescent-phase samples ≥16 days from the onset of the disease. In addition, the new test was found to be highly specific, with only 1 initial false positive out of the 210 control subject samples tested. Furthermore, the reactivity end points obtained with the ELISA suggested satisfactory sensitivity by a different parameter. Although the results were not obtained with identical samples from a previous preliminary study (Y. C. Wang, SARS Diagnostics: Scientific and Regulatory Challenges Workshop, 2003; U.S. Food and Drug Administration, http://www.fda.gov/ohrms/dockets/dockets/03n0281/03n0281.htm), the range of dilutions from >1:40 to 1:640 for the reactivity end points is nevertheless a good indication of sensitivity comparable to if not better than those previously studied. All of these findings suggested that the ELISA be ready for application at least for the exclusion of SARS in testing patients as advocated by the Centers for Disease Control and Prevention (CDC). The CDC recommended that the case definition for exclusion of SARS include the absence of antibody to the virus in convalescent-phase serum obtained >21 days after onset of illness (1).
The same recombinant proteins of Gst-N and Gst-U274 used in ELISA were also applied to the rapid immunochromatographic test but were applied separately as two different testing markers. Intrinsically, the newly developed rapid immunochromatographic test could provide not only indications for case detection but also details of maybe clinical significance, should a correlation be established later. Although the biological function of the unique protein is yet to be unraveled, the Gst-U274 recombinant was found to be serologically reactive and unique to SARS-CoV (12a). In addition, the protein was found in some cases to give a higher reaction signal when tested for the IgM antibodies to SARS-CoV (12a). Because of this kind of finding, the usefulness of the protein should not be ruled out prematurely, even though the result obtained here with the rapid test indicated its limitation for case detection with the 42 convalescent-phase samples included in this study. Nevertheless, Gst-U274 alone detected a fair proportion (85%) of the patient samples tested, and further studies of the rapid test using acute-phase specimens might prove rewarding.
Both the recombinant-based ELISA and rapid immunochromatographic test developed in this study were found to be highly sensitive and specific with the samples tested. When the results were compared, the two tests gave an excellent agreement of 99.6%, with a kappa statistic of 1.00 (Table 2), and an excellent correlation, with an R2 of 0.988 in relation to their reactivity end points with the seven samples tested (Fig. 4). This suggested that although the immunochromatographic test was a simple and rapid test that needs no special training to use, the performance of the test was fully compatible with that of ELISA. This should be a valuable addition to current options for combating SARS, especially in areas where laboratory facilities are not available.
In the recent outbreak in Singapore, the total number of probable cases of SARS was initially reported as 206. However, the number was finally adjusted to 238 after reclassification with a serological test, the IFA. The confirmation of an additional 32 cases suggested the importance of serological tests and the necessity for retesting, especially for samples with results initially negative by PCR. However, although antibody to SARS-CoV can be detected in some cases during the acute phase of the disease (1), IgG antibody is believed to reach its peak value around 60 days after onset of obvious symptoms (9). Therefore, even though they were found to be sensitive with the convalescent-phase sera tested, the utility of the serological tests developed here for earlier detection has yet to be established and may very well be limited. In this aspect, further development of the tests to include the capability to detect IgM antibody is warranted, because IgM antibody is believed to appear much earlier and reach its peak value around 14 days after the onset of the disease (9).
Acknowledgments
We thank Hoe Nam Leong, Yee Sin Leo, and Ai Ee Ling for providing the SARS patient sera.
This project was partially supported by an EDB (Economic Development Board of Singapore) grant under its Innovation Development Scheme.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2003. Updated interim surveillance case definition for severe acute respiratory syndrome (SARS)—United States, April 29, 2003. Morb. Mortal. Wkly. Rep. 52:1-3. [PubMed] [Google Scholar]
- 2.Crofts, N., W. Maskill, and D. Gust. 1988. Evaluation of enzyme-linked immunosorbent assays: a method of data analysis. J. Virol. Methods 22:51-59. [DOI] [PubMed] [Google Scholar]
- 3.Drosten, C., S. Gunther, W. Preiser, S. van der Werf, H. R. Brot, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova, R. A. Fouchier, A. Berger, A. M. Burguiere, J. Cinatl, M. Eickmann, N. Escriou, K Grywna, S. Kramme, J. C. Manuguerra, S. Muller, V. Rickerts, M. Sturmer, S. Vieth, H. D. Klenk, A. D. Osterhaus, H. Schmitz, and H. W. Doerr. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 384:1967-1976. [DOI] [PubMed] [Google Scholar]
- 4.Feng, P., S. H. Chan, M. Y. R. Soo, D. X. Liu, M. Guan, E. R. Ren, and H. Z. Hu. 2001. Antibody response to Epstein-Barr virus Rta protein in patients with nasopharyngeal carcinoma. Cancer 92:1872-1880. [DOI] [PubMed] [Google Scholar]
- 5.Fouchier, R. A. M., T. Kuiken, M. Schutten, G. van Amerongen, G. J. J. van Doornum, B. G. van den Hoogen, M. Peiris, W. Lim, K. Stohr, and A. D. M. E. Osterhaus. 2003. Aetiology: Koch's postulates fulfilled for SARS virus. Nature 423:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan, M., H. Y. Chen, T. P. Chow, A. R. Pereira, and P. K. Mun. November 2001. Assay devices and methods of analyte detection. U.S. patent 6,316,205.
- 7.Ksiazek T. G., D. Erdman, C. S. Goldsmith, S. R. Zaki, T. Peret, S. Emery, S. Tong, C. Urbani, J. A. Comer, W. Lim, P. E. Rollin, S. F. Dowell, A. E. Ling, C. D. Humphrey, W. J. Shieh, J. Guarner, C. D. Paddock, P. Rota, B. Fields, J. DeRisi, J. Y. Yang, N. Cox, J. M. Hughes, J. W. LeDuc, W. J. Bellini, L. J. Anderson, and the SARS Working Group. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966. [DOI] [PubMed] [Google Scholar]
- 8.Lin, Y., X. Shen, R. F. Yang, Y. X. Li, Y. Y. Ji, Y. Y. He, M. D. Shi, W. Lu, T. L. Shi, J. Wang, H. X. Wang, H. L. Jiang, J. H. Shen, Y. H. Xie, Y. Wang, G. Pei, B. F. Shen, J. R. Wu, and B. Sun. 2003. Identification of an epitope of SARS-coronavirus nucleocapsid protein. Cell Res. 13:141-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nie, Q. H., X. D. Luo, and W. L. Hui. 2003. Advances in clinical diagnosis and treatment of severe acute respiratory syndrome. World J. Gastroenterol. 9:1139-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peiris, J. S., S. T. Lai, L. L. Poon, Y. Guan, L. Y. Yam, W. Lim, J. Nicholls, W. K. Yee, W. W. Yan, M. T. Cheung, V. C. Cheng, K. H. Chan, D. N. Tsang, R. W. Yung, T. K. Ng, K. Y. Yuen, and SARS Study Group. 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pottumarthy, S., A. J. Morris, A. C. Harrison, and V. C. Wells. 1999. Evaluation of the tuberculin gamma interferon assay: potential to replace the Mantoux skin test. J. Clin. Microbiol. 37:3229-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rota, P. A., M. S. Oberste, S. S. Monroe, W. A. Nix, R. Campagnoli, J. P. Icenogle, S. Penaranda, B. Bankamp, K. Maher, M. H. Chen, S. Tong, A. Tamin, L. Lowe, M. Frace, J. L. DeRisi, Q. Chen, D. Wang, D. D. Erdman, T. C. Peret, C. Burns, T. G. Ksiazek, P. E. Rollin, A. Sanchez, S. Liffick, B. Holloway, J. Limor, K. McCaustland, M. Olsen-Rasmussen, R. Fouchier, S. Gunther, A. D. Osterhaus, C. Drosten, M. A. Pallansch, L. J. Anderson, and W. J. Bellini. 2003. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300:1394-1399. [DOI] [PubMed] [Google Scholar]
- 12a.Tan, Y. J., P. Y. Goh, B. C. Fielding, S. Shen, C. F. Chou, J. L. Fu, H. N. Leong, Y. S. Leo, E. E. Ooi, A. E. Ling, S. G. Lim, and W. J. Hong. 2004. Profile of antibody responses against severe acute respiratory syndrome coronavirus recombinant proteins and their potential use as diagnostic markers. Clin. Diagn. Lab. Immunol. 11:362-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yam, W. C., K. H. Chan, L. L. M. Poon, Y. Guan, K. Y. Yuen, W. H. Seto, and J. S. M. Peiris. 2003. Evaluation of reverse transcription-PCR assays for rapid diagnosis of severe acute respiratory syndrome associated with a novel coronavirus. J. Clin. Microbiol. 41:4521-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]