Abstract
Numerous studies provide detailed insight into the triggering and amplification mechanisms of the inflammatory response associated with prosthetic wear particles, promoting final dominance of bone resorption over bone formation in multiple bone multicellular units around an implant. In fact, inflammation is a highly regulated process tightly linked to simultaneous stimulation of tissue protective and regenerative mechanisms in order to prevent collateral damage of periprosthetic tissues. A variety of cytokines, chemokines, hormones and specific cell populations, including macrophages, dendritic and stem cells, attempt to balance tissue architecture and minimize inflammation. Based on this fact, we postulate that the local tissue homeostatic mechanisms more effectively regulate the pro-inflammatory/pro-osteolytic cells/pathways in patients with none/mild periprosthetic osteolysis (PPOL) than in patients with severe PPOL. In this line of thinking, ‘particle disease theory’ can be understood, at least partially, in terms of the failure of local tissue homeostatic mechanisms. As a result, we envision focusing current research on homeostatic mechanisms in addition to traditional efforts to elucidate details of pro-inflammatory/pro-osteolytic pathways. We believe this approach could open new avenues for research and potential therapeutic strategies.
Keywords: Homeostatic mechanisms, periprosthetic osteolysis, inflammatory response, tissue-appropriate response, osteoclasts, dendritic cells, macrophages, fibroblasts, lymphocytes, cytokines total hip arthroplasty, replacement
Introduction
Total hip arthroplasty (THA) is an effective and safe method for treating severe degenerative, post-traumatic and other end-stage diseases of the hip joint. However, with the extension of THAs to a younger and generally more active population, the expected time of service of THAs could be insufficient and the number of revision surgeries will therefore increase. The incidence of revision THAs in the USA increased from 9.5/100,000 to 15.2/100,000 between 1990 and 2002, and the projections for 2030 are even higher.1 The main reasons for failure of THA is aseptic loosening accompanied by osteolysis, followed by instability and infection; the latter two diagnoses occur during the early postoperative period, whereas aseptic loosening and osteolysis usually occur much later.2
Periprosthetic osteolysis refers to progressive insidious bone resorption associated at first with a well functioning THA. Importantly, periprosthetic osteolysis predates aseptic loosening in the majority of cases, which is, unfortunately, asymptomatic for a long time.3,4 As a result, severe bone defects can develop, at least in some patients, which eventually requires difficult revision surgery because the time for early revision was not apprehended.5 Such revision surgery often takes longer, is more expensive and is associated with an increased rate of complications compared with a less complicated early revision with minor bone defects. In addition, clinical outcome and survivorship in such difficult cases can be compromised.6 With this in mind, research on the pathophysiology of osteolysis is of paramount importance and well-justified. This review introduces new immunological findings into the pathophysiology of periprosthetic osteolysis around THA and suggests that failure of local protective/homeostatic mechanisms could contribute to periprosthetic osteolysis and aseptic loosening in addition to other biologic and non-biologic events.
Current concept of periprosthetic osteolysis
‘Ball in socket’ artificial hip joints were originally designed as low-friction arthroplasties, with low levels of removal of material from the softer bearing surfaces during each step. Historically, ultrahigh molecular weight polyethylene (UHMWPE) articulating against a metallic ball was the most commonly used bearing couple exhibiting wear rates from 0.01 to several millimeters per year.7 Recently, conventional UHMWPE was replaced by more wear-resistant highly cross-linked polyethylene and alternative bearing materials with only negligible wear rates in comparison with conventional UHMWPE.8 However, even when the loss of material is in the order of tenths of millimeters, the total number of polymer particles can achieve an order of hundreds of trillions.9 In addition, the hip and surrounding tissues must withstand permanent mechanical stresses and hydraulic pressures of joint fluid, which are both associated with use of the limb.10
Willert and Semlitsch were the first to introduce the concept of aseptic loosening and osteolysis as a result of periprosthetic tissue reaction to large amounts of prosthetic wear microparticles.11 In the following decades, researchers uncovered a number of basic pathogenetic reactions as components of a complex host response to chronic exposure of the hip to prosthetic debris and repetitive mechanical stresses.12–15 However, to date, a number of mechanisms/pathways still remain to be elucidated.
The term ‘particle disease’ was coined by Dr William Harris to stress the importance of particles generated by a prosthesis for induction of host response.16 The key concept in particle disease is that very small prosthetic particles (micrometers and less in size) stimulate periprosthetic cells to express pro-inflammatory/pro-osteoclastic cytokines and other substances that orchestrate increased accumulation/activity/survival of osteoclasts, and inhibit the osteogenic activity of osteoblasts.17,18 As a result, osteoresorption predominates over osteogenesis at the level of the bone multicellular units around the implant, leading, eventually, to macroscopic bone defects.19 The degree of bone loss according to this concept is at least in part a function of number, size and origin of prosthetic particles that influence the number and depth of deregulated resorption sites.
The expansion of particle disease across the prosthetic joint is facilitated by joint fluid that is abundantly synthesized by a synovial-like membrane composed of macrophages and fibroblasts. Accordingly, joint fluid washes the prosthetic microparticles from the articulating surfaces and transports signaling and inflammatory molecules, delivering them to adjacent bone sites.20 For this reason, particle disease can extend to new sites, contributing in this way to an overall expansion of osteolysis and weakening of the bone–implant interface (the effective joint space concept). Finally, compressed joint fluid can also induce direct bone resorption.10 In contrast, however, there are many patients that escape the above-mentioned fate despite having the same type of THA with comparable alignment and physical activity.21 In this line of thought, the key question is: How do these patients avoid developing periprosthetic osteolysis mechanically?
Engh et al.22 estimated that both wear and patient propensity to osteolysis may together account for 53% of the variance in the total area of osteolysis. Recently, the concept of individual susceptibility to periprosthetic osteolysis has been introduced, but the key factors that are involved are still poorly understood. Single nucleotide polymorphism (SNP) is a common form of variation in the human genome indicating that a single base change in the DNA sequence (genotype) could influence the amount/functionality of secreted proteins and, in this way, could influence the presentation of osteolysis (phenotype). Wilkinson et al.23 were the first to publish a study on the association between polymorphisms in the gene encoding for TNF-α and the risk of periprosthetic osteolysis in THA. After this introductory work several articles were published nominating other molecules as candidates involved in the processes of aseptic loosening/osteolysis.24–26 Structurally and functionally these include receptors, intracellular mediators, enzymes, cytokines and other proteins. A recently published systematic review on genetically-determined susceptibility to aseptic loosening of THA revealed several areas of potential agreement (SNPs of TNF-238A allele, IL1RA +2018C allele, polymorphisms in genes for IL-6, MMP-1, etc.), but also several sources of heterogeneity between studies, showing the need for large, multi-centre prospective studies that should provide stronger evidence for genetic predisposition to osteolysis and aseptic loosening.27
The problem is also that we know only a little on the good long-term adaptation on the implant. Although autopsy retrieval studies of well-fixed and loose components have described the bone–implant interface at the histopathological level,28,29 the pathophysiological and immunological parameters around well-functioning THA in the long term have not been elucidated. We can only translate data from in vivo or in vitro models of THA to the human situation30–32 and compare these with data from analyses of tissues from failed THAs. Unfortunately, tissues retrieved during revision surgery reflect late stages of a process that, in the majority of cases, reflects a long lag period from the time at which the local homeostasis was first disrupted.33
Early events after implantation of THA
Immediately after the joint implant is placed inside the body, a series of events is initiated. Surgical trauma could be considered the first insult to the periprosthetic tissues inducing localized necrosis and ischemia followed by an inflammatory response (Figure 1). The second insult might be associated with the physical and chemical properties of an implant. Unfortunately, limited information is known about such interactions between the implant and the surrounding host tissues, both local and remote. However, it is believed that by-products from these host–implant interactions directly influence the behavior of periprosthetic cells and remote tissues even though current biomaterials per se are biologically inert.34Third, early synovitis induced by prosthetic wear particles may further alter the periprosthetic environment after several months of ambulation.35 When the above steps occur, periprosthetic osteolysis might develop, subsequently leading eventually to aseptic loosening and vice versa if the inflammatory response is averted, the corresponding tissue architecture and metabolism can be restored. With regards to the long-term resistance of the bone–implant interface to osteolysis, it is also important that the residual ‘postsurgical’ empty spaces around an implant are filled by bone instead of fibrous tissue.22 Recently, it has been proposed that part of the inter-individual variance in the risk of early prosthetic migration and development of late aseptic loosening might be explained by patient-specific differences in regenerative processes around the implant early postoperatively.36
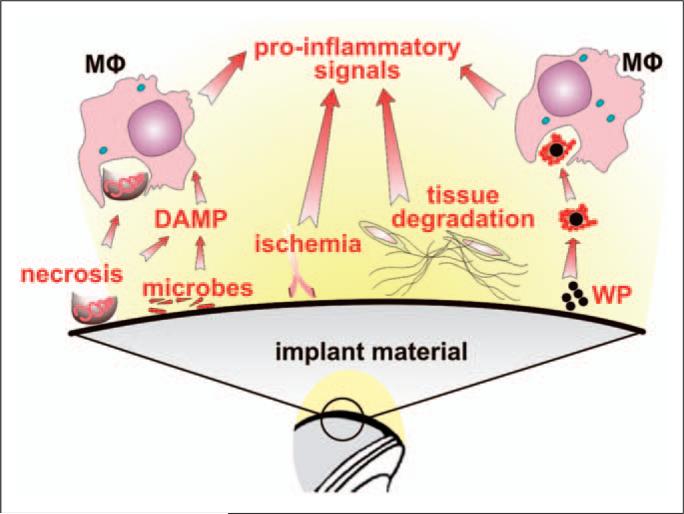
Figure 1.
Initial triggers of inflammatory response to total hip arthroplasty (THA). Immediately after implantation of THA into the bone bed several factors can trigger an inflammatory response which can contribute to tissue damage, poor initial fixation and periprosthetic osteolysis. The main factors are: (i) tissue necrosis, ischemia and degradation of tissues sensed mainly by macrophages (MΦ), other cells and the complement system; (ii) microbial remnants (PAMPS/MAMPS) from direct contamination or from the blood; (iii) micromotion at the implant-host interface and formation of a synovial interface-like membrane around the implant leading to activation of fibroblasts and other mesenchymal cells, and to release of osteoclast-activating pro-inflammatory factors; (iv) prosthetic wear particles (WP), liberated from the articulating and/or non-articulating surfaces, together with adsorbed host proteins (shown as a red string around the wear particles) are sensed by MΦ, triggering inflammation.
Concept of local tissue homeostasis
The term ‘homeostasis’ was coined by Claude Bernard in 1865 to describe the constancy of the internal environment in healthy individuals. Metchnikoff introduced the concept into immunology, together with the term ‘physiological inflammation’ to stress active maintenance of tissue harmony. Current theory predicts the existence of at least four basic components of an inflammatory response (Figure 2): inducers of inflammation; their sensors; inflammatory mediators secreted by sensors after stimulation by inducers; and effectors influencing the tissues affected by inflammation.37 The type of inflammatory response depends on the characteristics of inflammatory inducers. Briefly, in the case of sepsis, bacteria are phagocytosed for killing but also detected by pattern-recognition receptors (PRRs) located on the cell surface (e.g. TLRs 1, 2, 5, 6, 11) or the endosomal component (e.g. TLRs 3, 7, 8, 9) of leukocytes, and of tissue cells that, after stimulation, secrete inflammatory chemokines and cytokines to orchestrate innate and adaptive immunity responses to eradicate bacterial invasion. In the case of sterile inflammation, the danger signals (cellular by-products, protein covered particles, etc.),38 are detected by tissue-resident monocyte/macrophage lineage cells and other cells participating in innate host defense that initiate accordingly the resorption of necrotic tissues, encapsulation of large foreign bodies, phagocytosis of sterile particles and also the triggering of adaptive immune responses. Taken together, pure activation of inflammatory sensors should lead to the release of a number of inflammatory mediators and enzymes that should lead, in turn, to the damage of tissues affected by inflammation. In order to prevent this, protective and regenerative mechanisms are activated simultaneously with activation of sensors of inflammation, ensuring the restoration of original tissue architecture and normal metabolism once inducers are excluded.37 Unfortunately, little is known about these homeostatic mechanisms with respect to both the adaptation and mal-adaptation on THA resulting eventually in periprosthetic osteolysis and aseptic loosening.
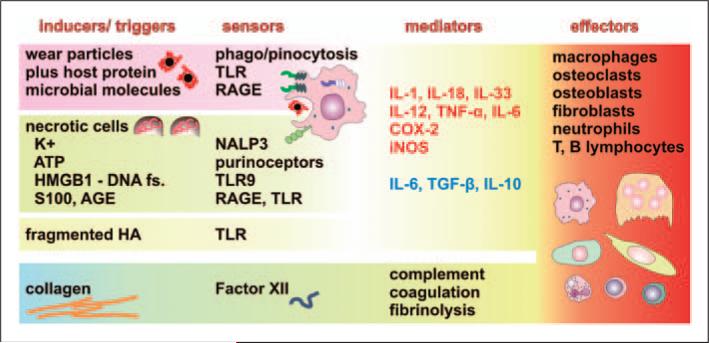
Figure 2.
Components of inflammatory response ‘inducers—sensors—mediators—effectors’ potentially associated with periprosthetic osteolysis and aseptic loosening. The host response is initiated by triggers (inducers), which are of both exogenous (wear particles, bacteria or microbial remnants) and endogenous (cells, extracellular matrix and bodily fluids) origin. Wear particles with adsorbed host proteins are phago-/pinocytosed (leading to a foreign body reaction) and sensed by pattern recognizing receptors, such as TLRs and receptor for advanced glycation end products (RAGE). Trauma-associated necrosis leads to the release of cellular components, such as ATP, K+ ions, fragmented DNA, members of the S100 calcium-binding protein family, advanced glycation end products (AGE) and others, effectively triggering inflammation after binding to, and activating, respective sensors, such as purinoreceptors, P2X7, NACHT, Leucine-Rich Repeat- and PYD-domains Containing Protein 3 (NALP3 or cryopyrin), high mobility group box 1 protein (HMGB1; the complex with DNA that can stimulate TLR9), RAGE and others, expressed mostly by macrophages. Trauma also leads to the breakdown of extracellular matrix components, such as hyaluronan (HA), fragments of which are sensed by TLRs. Receptors, or sensors, activate macrophages to release the pro-inflammatory factors IL-1, IL-12, IL-18, IL-33, TNF-α, cyclooxygenase 2 (COX-2) and inducible nitric oxide synthetase (iNOS), but also the anti-inflammatory factors IL-10 and TGF-β. IL-6 has multiple activities depending on differentiation and activation status, and receptor expression on target cells, such as osteoclasts; therefore, it could both suppress osteolysis but facilitate osteoclast formation. In addition to the above-mentioned cellular receptors, soluble factors, such as Factor XII, sense extracellular matrix components presented by collagen leading to activation of coagulation, fibrinolysis and complement, which could substantially contribute to the recruitment of inflammatory cells. The above-mentioned inflammatory mediators contribute to the activation or differentiation of several cell lines participating in periprosthetic osteolysis and aseptic loosening of THA. Anti-inflammatory factors associated with bone homeostasis are in blue, pro-inflammatory factors or those associated with bone resorption are in red.
Theoretically, several cell populations could play a role in the regulation of the inflammatory response to prosthetic particles (Figure 3). The most important include monocyte/macrophage lineage cells (homeostatic monocytes, regulatory macrophages), regulatory dendritic cells, tissue-resident fibroblasts and lymphocytes (lymphoid cells, Treg lymphocytes, etc.) that continuously monitor tissue homeostasis. Regarding bone, the key role is played by osteocytes, osteoblasts and their precursors.39 Additionally, neuronal cells, including their non-adrenergic, non-cholinergic (NANC) actions, should play a role in sensing and effector functions related to tissue homeostasis.40
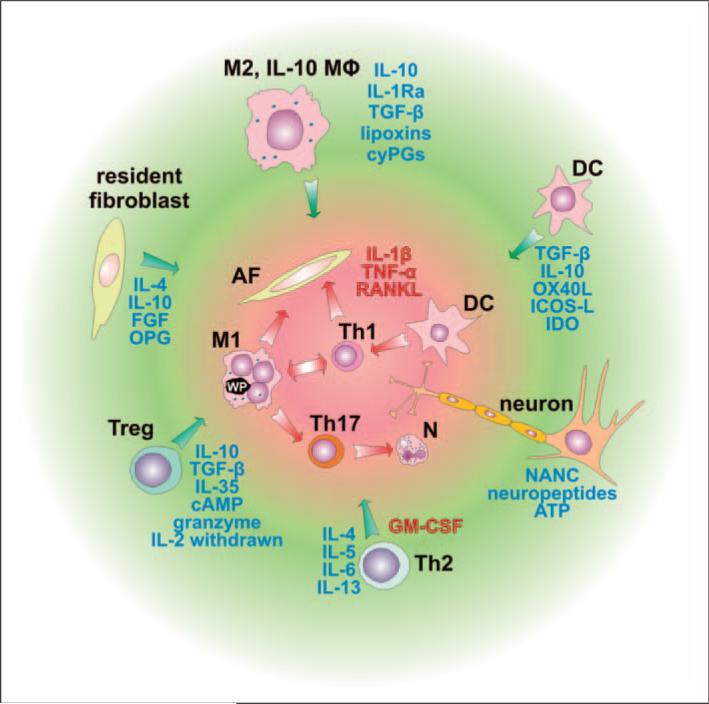
Figure 3.
Cell populations involved in the suppression of the inflammatory response. Cells involved in the inflammatory reaction, such as classically-activated macrophages (M1), Th1 cells, Th17 cells, activated fibroblasts, dendritic cells (DC) and neutrophils, contribute to bone resorption mostly through IL-1β, TNF-α and receptor activator of NF-κB ligand (RANKL) signaling. Activity of the above-mentioned cells is controlled and suppressed by several factors secreted predominantly from immune cells represented by macrophages stimulated through the IL-4, IL-13, α-tocopherol, IgG-containing immune complexes (IC), apoptotic cells or prostaglandins, leading to the ‘alternatively activated’ healing phenotype (M2), from regulatory IL-10-secreting macrophages (IL-10 MΦ), DCs, regulatory T cells (Treg) and Th2 cells. Depending on the modulating properties of signals they receive, DCs could play a pro-inflammatory role by activating pro-inflammatory Th1 or Th17 cells, or an anti-inflammatory role by activating regulatory T cells (Treg) that suppress immune reactions. Furthermore, inflammatory cells could be suppressed by resident fibroblasts and neurons. AF, activated fibroblast; cyPGs, cyclopentenone prostaglandins; GM-CSF, granulocyte-macrophage colony stimulating factor; OPG, soluble receptor for RANKL – osteoprotegerin; FGF, fibroblast growth factor; OX40L, tumor necrosis factor ligand superfamily member 4; ICOS-L, inducible T-cell co-stimulator ligand, IDO, indolamine 2,3 dioxygenase; NANC, non-adrenergic non-cholinergic neurotransmitters. Anti-inflammatory factors leading to bone remodeling are in blue, pro-inflammatory factors or those associated with bone resorption are in red.
When sensing tissue damage cells express many genes to regulate/terminate inflammation and induce/orchestrate tissue repair and remodeling. Recent studies focus on the potential role of hematopoietic stem cells in the balance of the immune response and maintenance of the tissue homeostasis.41 An important question is whether distinct, phenotypically-stable cell subpopulations exist for all types of tissue responses. Currently, it seems more probable that macrophages, as well as other cells (e.g. fibroblasts), can adopt different context-dependent phenotypes that either promote or inhibit inflammation/tissue damage based on the type of trigger and prevailing signaling network.42,43 Accordingly, steady state macrophages exhibit a predominantly anti-inflammatory phenotype. After a particular stimulus, they switch to key organizers of the inflammatory response but they simultaneously co-induce anti-inflammatory protective feedback mechanisms, which are essential for preventing redundant tissue damage. The type, magnitude and subsequent effect of the protective and homeostatic responses depend on the type and extent of tissue injury, and individually-determined characteristics of the homeostatic mechanism.37,44 Under current surgical experience, the extension of intraoperative injury is comparable among cases after THA, therefore implicating the general ability to achieve tissue homeostasis in a majority of patients early after surgery. Therefore, dysregulation is a problem.
Local tissue homeostasis is regulated by complex cross-talk between stem cells, already differentiated cells, the local microenvironment and the whole organism.45 The repair and remodeling of inflamed and otherwise damaged tissue is a complex process that involves highly-arranged actions of many different cell types, and appropriate chemokines, cytokines, growth factors and extracellular matrix proteins (Figure 4). However, the cellular and molecular regulators of tissue repair and remodeling are still not well understood.37 A key role is played by resident tissue cells that are involved in both sensing the damage and restoring homeostasis. Homeostatic chemokines and their receptors play a role in the recruitment of stem cells and creation of the niche for regenerative cells.46 Similarly to the process of inflammation, the protective and reparative processes are also tightly controlled at the genetic and epigenetic level.47,48
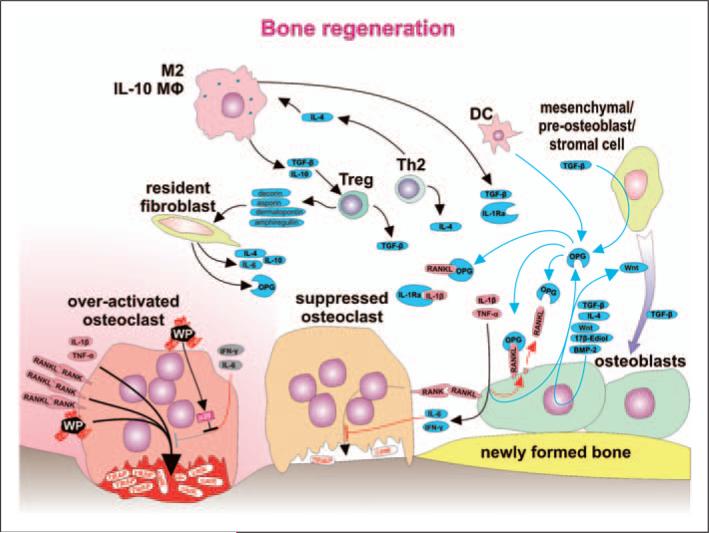
Figure 4.
Bone regeneration is orchestrated with a substantial contribution from immune cells. Within the bone multicellular unit (BMU), suppression of the inflammatory response is associated with a change from net bone resorption toward bone remodeling. Osteoclasts stimulated by RANKL, IL-1β, TNF-α and wear particles pump protons (H+), move toward the bone surface and secrete bone destructing cathepsin K (catK) and tartarate-resistant acid phosphatase (TRAP). A decrease in the level of the stimulating factors is a consequence of anti-inflammatory activity of several immune cell types (M2, IL-10-secreting macrophages, DC, Treg cells, Th2 cells), as well as non-immune cells, such as resident-tissue fibroblasts and mesenchymal/pre-osteoblast/stromal cells. Anti-inflammatory activity is mediated by soluble factors IL-4, IL-10, TGF-β, soluble receptor for IL-1 (IL-1Ra), and OPG secreted and acting within the area of BMU. Strong immunosuppressive Treg cells differentiate under the influence of TGF-β and IL-10 secreted from M2 and IL-10-secreting macrophages and specific populations of DC. Further, Treg cells secrete decorin, asporin, dermatopontin and amphiregullin contributing to a reparative bone remodeling. Bone remodeling is further supported by stimulation of osteoblasts by 17β-estradiol, differentiation factor Wnt and bone morphogenetic protein 2 (BMP-2)—some of them acting as autocrine factors. IFN-γ secreted by Th1 and NK cells or IL-6 secreted by T cells and macrophages contribute to bone remodeling by activation of osteoblasts to produce RANKL inhibitor OPG. The number of osteoblasts increases as osteoblast precursors differentiate under the influence of sphingosine-1-phosphate produced by mature osteoclasts. The mechanisms above and TGF-β, together with other factors, contribute to the recruitment and differentiation of pre-osteoblasts toward mature bone remodeling osteoblasts. Furthermore, IFN-γ and IL-6 suppress bone resorption by acting directly upon osteoclasts. Nevertheless, activated osteoclasts are not responsive to IFN-γ and IL-6 because intracellular response pathways are blocked by over-expressed p38.
In this context of THA, the inability to resolve chronic high-grade inflammation can lead to irreversible tissue damage with formation of suboptimal tissues at the bone implant interface (e.g. fibrous, fibro-granulomatous tissue instead of bone). Based on this, chronic unresolved inflammation could be a major driver of aseptic loosening via particle disease.44 Because the vast majority of patients avoid this outcome, a balance between the inflammatory response and regulatory mechanisms must exist as a basic prerequisite for maintaining local tissue homeostasis around a THA. For this reason, processes leading to osteolysis and aseptic loosening can also be considered to be problems of mal-adaptation to prosthetic degradation products and/or to the implant in general.
Inducers of ‘particle disease’
Regardless of the type of inducer, the goal of the inflammatory response is to eliminate the inducers from the affected tissues and restore the pre-insult homeostatic state. In the case of THA, the tissue-specific monocyte subpopulations (particularly monocytes, macrophages and dendritic cells) ingest wear/corrosive particles, apoptotic/necrotic cells and orchestrate recruitment of new monocytes from the circulation.49 When larger foreign bodies are present, the tissue-resident monocytes and fibroblasts create a granulomatous limiting membrane around the foreign bodies to separate them from the rest of the host tissues.50 However, the inducers of periprosthetic inflammation cannot be completely eliminated in the case of THA; thus, the tissues are under continuous activation, not only by newly generated particles, but also by tissue alarmins, chemokines/cytokines, hypoxia and necrosis. Despite this, the majority of patients with THA exhibit something akin to ‘tolerance’ that could be defined as a lack of or low inflammatory/ adverse responsiveness to the prosthesis-related stimuli.
Regarding the control of prosthesis-related inducers, the most important factor appears to be reduction of the wear particle load, facilitated by the use of newer articulations or modern technologies of prosthetic surface treatment. This issue is described in detail elsewhere.51,52 Biological inducers of periprosthetic inflammation are less well described and some of the most important ones include by-products of cell necrosis, extracellular matrix damage, hypoxia and reactive oxygen species, and by-products of microbial remnants (all liberating alarmins).
Cell necrosis can affect both macrophages and other cells. Necrosis results from hypoxia and unfavorable mechanical conditions, but the most important cause seems to be the influence of large foreign particle load. Inorganic particles per se are undigestable; they can destabilize the phagolysosomal membrane leading to leakage of their content. Metallic particles (corrosion products) are cytotoxic and, in some cases, genotoxic.53 Taken together, these stimuli can lead, separately or combined, to irreparable DNA damage and to cell death eventually. Molecules released from dying cells together with breakdown products of the extracellular matrix are considered important inducers of inflammation. In contrast, ingestion of apoptotic cells is associated with the release of inflammation-resolving cytokines, such as IL-10 and TGF-β.44 As a result, the ratio of necrotic cells to apoptotic cells around the implant could play a significant role in relation to the maintenance of periprosthetic tissue homeostasis.54
Hypoxia appears to be an important factor in aseptic loosening. Interface membranes can suffer from hypoxia because the tissue is hypovascular and caused by the chronic local hypoxia–reperfusion injury, which is at least partially caused by implant loading. There is also increased oxygen consumption by local inflammatory cells.55 A hypoxic environment induces the expression of hypoxia-inducible factors (HIF-1α, HIF-2α) and some heat shock proteins that adapt gene expression and cell metabolism to lower oxygen availability within the hypoxic tissue. For this reason, decreased proliferation is a fundamental physiological response to hypoxia in many cell types.56 Osteoclasts, tissue macrophages and fibroblasts are relatively well adapted to hypoxic conditions. In contrast, osteoblastic cell lines display decreased growth, differentiation and mineralization capacity under hypoxic conditions contributing to the overall functional predominance of osteoclasts over osteoblasts. Hypoxic osteoblasts and other cells induce, in turn, angiogenesis via increased secretion of vascular endothelial growth factor (VEGF).57 Recently, it was shown that there was increased expression of VEGF in fibroblasts from failed THA compared with controls with osteoarthritis.58 An important role in the transduction of hypoxic signal to bone–implant interface may be attributed to osteocytes via increased expression of HIF and osteopontin, leading eventually to osteocyte-induced osteoclastic-mediated bone resorption.59
Activation of PRRs by danger-associated molecular patterns (DAMPS),60 either pathogen-associated molecular patterns (PAMPs)61 or microbial-associated molecular patterns (MAMPs), and/or endogenous alarmins,62 in periprosthetic tissues can alter local tissue homeostasis at any time postoperatively.63–65 It was also suggested that hydrophobic molecules are likely to be strong stimulators of innate host response through PRRs.60 Bearing this in mind, it is interesting that polyethylene is rather a hydrophobic material. In addition, it has been shown that polymeric alkane structures released during UHMWPE breakdown can directly activate PRRs (TLR1 and TLR2 signaling pathways), while UHMWPE particles phagocytosed by periprosthetic cells could induce endosomal destabilization and inflammasome activation.66 Taken together, PRRs have a strong potential to trigger inflammation via several pathways, including differentiation of macrophages toward M1 populations, multinuclear foreign body giant cells and, eventually, osteoclasts that resorb bone.50 On the other hand, stimulation of TLRs under specific conditions can induce tissue renewal and repair.67
Regulation of particle disease effectors
The effectors of an inflammatory response are periprosthetic cells, the functional states of which are specifically affected by the inflammatory mediators.68 Hundreds of genes are activated by inducers of particle disease in macrophages, fibroblasts, osteoblasts and probably also in other bone-marrow and endothelial cells.32,69,70 In turn, expression of these genes determines the cellular responses to particular stimuli.68
Importantly, together with a set of effector inflammatory molecules, a set of regulators is co-activated to control the intensity and extension of the local inflammatory response (negative regulators of inflammation). These can be distinguished as signal- and gene-specific regulators. The first category consists of regulators that inhibit signal transduction by PRRs and other inflammatory pathways [e.g. IL-10; IL-1R-associated kinase M (IRAK-M); suppressor of cytokine signaling (SOCS) proteins]. For example, SOCS3 interacts with JAK/STAT signaling pathways decreasing the responsiveness of M0 macrophages to IFN-γ.71 Recently, a potential role of IRAK-M was demonstrated to be involved in the regulation of inflammation induced by prosthetic particles.72 The second category comprises transcriptional repressors (basal repressors and inducible repressors) or other negative regulators that modulate gene expression (e.g. microRNAs, long-non coding RNAs).47
Regarding the termination of inflammation and/or adaptation of sensors on chronic input of inducers, an important role could be played by the anti-inflammatory cytokines (IL-10, as well as Th2 cell cytokines, such as IL-4, IL-5 and IL-13) together with very effective resolution-inducing lipoxins (Figure 4). These bind to the receptors expressed, for instance, on macrophages and their precursors, diminishing the TNF-α and IL-1β activation of NF-κβ complex, simultaneously stimulating anti-inflammatory pathways.44,73
Tissue-preserving response to THA
Until now, we have assumed that the immune–inflammatory system alone dictates the direction and fate of periprosthetic tissue reactions after stimulation by prosthetic by-products. However, the concept of tissue-appropriate immunity stresses the role of local tissues in the control of the effector mechanisms that prevent self-destruction.74 On this basis, tissue-resident cells might influence the response to a stimulus in order to maintain the health of the affected tissues by secreting homeostatic chemokines and soluble anti-inflammatory factors, modifying local and systemic cell activities (Figure 5).
Figure 5.
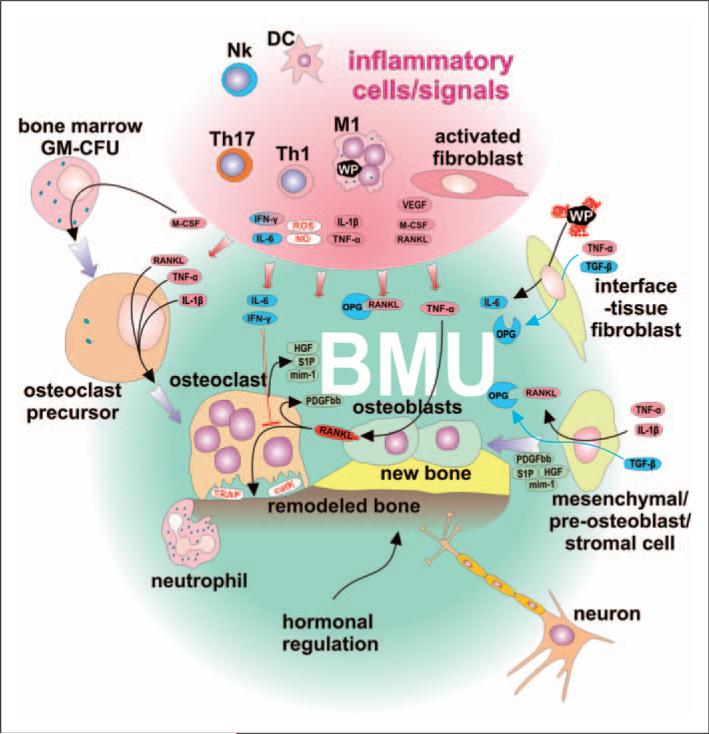
The BMU is self regulatory and not affected by pro-inflammatory signals produced by activated immune cells and fibroblasts. Classically-activated M1 macrophages and subpopulations of DCs secrete Th1- and Th17-stimulating cytokines, as well as toxic (reactive) nitrogen and oxygen species, and COX-2. Stimulated Th1 lymphocytes secrete RANKL, one of the most important osteoclast-activating cytokines. M1 further secrete cytokines IL-1β and TNF-α, thus inducing pre-osteoclast differentiation and activation leading to increased bone resorption. IL-1β and TNF-α stimulate osteoblasts to secrete RANKL, thus creating positive feedback for bone resorption. Furthermore, inflammatory-activated fibroblasts support bone resorption by secretion of RANKL and stimulation of pre-osteoclast differentiation by M-CSF. Although the BMU is exposed to the above factors, it has its own regulatory mechanisms to help ensure homeostasis: the BMU is covered by a canopy of cells so that BMUs can undergo activation–resorption-formation cycles in bone remodeling compartments. Osteoclasts exposed to IL-6 and IFN-γ do not respond to RANKL-mediated activation. Furthermore, IL-6 is secreted by fibroblasts after exposure to wear particles. Osteoclasts, upon stimulation by RANKL, secrete platelet-derived growth factor bb (PDGFbb), which induces the proliferation of pre-osteoblasts leading to an increase in the number of osteoblast precursors. Furthermore, osteoclasts produce sphingosine 1-phosphate (S1P), myb-induced myeloid protein-1 (mim-1) and hepatocyte growth factor (HGF), collectively contributing to pre-osteoblasts migration and osteoblast survival. Attenuation of osteoclast activity leads to a decrease in the production of PDGFbb and S1P-induced differentiation of pre-osteoblasts toward active osteoblasts and bone remodeling. Physiologically-activated interface tissue fibroblasts/tissue-resident fibroblasts respond to TNF-α together with TGF-β by secretion of OPG. In addition, BMU neurons could sense local inflammation and modulate activity of both immune and BMU cells through ATP and neuropeptide mediators, and further activate the neuroendocrine system. All the above factors are most prominent early after total hip arthroplasty surgery or when the inflammatory response is more quiescent.
Whether activated macrophages can be deactivated and the mechanisms responsible for deactivation are topics discussed elsewhere.73,75 Theoretically, there are at least two possibilities leading to the deactivation of macrophages. The first is associated with a decrease in stimulation at the signaling level (e.g. IFN-γ and other cytokines, LPS and other bacterial stimuli). The second could be associated with the activity of tissue-resident or migrating cells inducing negative modulation of macrophage pro-inflammatory activities and macrophage apoptosis.76 In addition, another important group of resident-tissue cells (NK cells) could play an important role in attenuation of local inflammatory status. Recently, it was demonstrated that NK cells retrieved from periprosthetic tissues lose their ability to express IFN-γ in response to IL-12/IL-18 in comparison with blood NK cells.77
Activated fibroblasts perpetuate inflammation via inappropriate expression of survival molecules leading to the retention of activated cells in affected tissues or via ectopic secretion of chemokines supporting recruitment of new cells as a fuel for continuation of inflammation.43 They also express several tissue degrading and ‘osteoclastogenic’ cytokines in periprosthetic membranes, including M-CSF, VEGF or RANKL, contributing together to the suppression of osteoblast function and to predominance of bone resorption over osteogenesis.78 Based on these observations, it seems inevitable that deactivated and quiescent fibroblasts could, like macrophages, significantly contribute to the resolution of particle-induced inflammation. Recent studies have revealed that inflammation is not generic but contextual.73 Therefore, tissue-resident fibroblasts may be able to switch ongoing inflammation to a stage of resolution. In this connection, it is important to know which mechanisms induce fibroblast anti-inflammatory and regenerative activities. First, fibroblasts could act as a source of anti-inflammatory and regenerative cytokines, such as IL-4, IL-10 and fibroblast growth factors (FGFs). Second, fibroblasts can provide an anti-inflammatory stromal microenvironment in the periprosthetic interface membrane involving a plethora of cell-to-cell and, perhaps. intra-, juxta-, auto- and paracrine interactions.43
On one hand, lymphocytes, together with other cells, enhance osteoclast differentiation, stimulate the formation of foreign body giant cells and exhibit many other activities contributing to tissue damage. On the other hand, Th0 T cells can be driven (polarized) in favor of Th2, Th3 and Treg responses instead of Th1 and Th17 responses79 or a delayed type of hypersensitivity.80 The homeostatic cells, including lymphocytes, express many genes encoding molecules associated with tissue repair/remodeling, such as extracellular matrix proteins decorin, asporin or dermatopontin, and strong regulators of tissue repair and remodeling, such as amphiregullin.81 Unfortunately, clinical strategies are not yet available to modify the above processes and induce/maintain localized tissue homeostasis.
Directions for future research
Efforts need to be channeled toward determining the key tissue protective mechanisms that go beyond long-term host tolerance to prosthetic particles and biomechanical stresses signaling via osteocytes and integrins. Potentially, the most strategically important responses are those associated with activation of tissue-protective ligands and receptors that control the induction and amplification of inflammatory responses in periprosthetic tissues. It is also important to determine the type and extent of cross-talk between pro-inflammatory and tissue-resident homeostatic cells that control the damaging processes associated with particle disease. Similarly, a degree of cross-talk between damaged tissue and cells initiating the repair process, with restoration of tissue homeostasis, should be a target for future research. Knowledge on the context-dependent regulation of monocytes and fibroblasts in the periprosthetic tissues may offer new therapeutic potential in the clinical management of particle-induced periprosthetic osteolysis. If this avenue of research is successful, new strategies could be developed combining biomimetic material engineering with hematopoietic stem cell self-renewal and homeostatic chemokines for restoration and maintenance of functional tissues around total joint arthroplasties.
Conclusion
Periprosthetic osteolysis is currently considered a multifactorial complication of total joint arthroplasty in which prosthesis-related factors act in concert with genetically- and environmentally-determined host responses. Although there is little evidence that currently supports the role of protective and homeostatic mechanisms in non-destructive local host tissue response to prosthetic stimuli in successful THAs, it is clear that such a role should exist. Assuming their existence, it may be postulated that, in the absence of a key part of the negative feedback loop, the pro-inflammatory chemokines/cytokines develop a severe inflammatory microenvironment associated with the predominance of activated macrophages, fibroblasts, synoviocytes, lymphocytes and osteoclasts over osteoblasts, resident fibroblasts and other homeostatic repair cells. In this line, periprosthetic osteolysis can be understood as a result of failure of local tissue homeostatic mechanisms. This disturbance can be localized at the level of sensors, regulators and also effectors of ‘particle disease’. We believe that precise identification of the deregulated signaling pathways, mediators and cellular differentiation programs that contribute to periprosthetic osteolysis will facilitate the development of selective, targeted therapeutic strategies, including a new generation of mimetic, self-diagnosing and multifunctional prosthetic surfaces.
Acknowledgements
We apologize to many authors whose important works could not be cited owing to space limitations.
Funding
This study was supported by Internal Grant Agency Ministry of Health Czech Republic NT/11049-5, Sigrid Jusélius Foundation, ORTON Orthopaedic Hospital, Finska Läkaresällskapet and the Danish Council for Strategic Research, the Ellenburg Chair in Surgery, Stanford University, and research project MSM6198959223 of Ministry of Education, Youth and Sports Czech Republic.
References
- 1.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007. 89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 2.Ulrich SD, Seyler TM, Bennett D, Delanois RE, Saleh KJ, Thongtrangan I, et al. Total hip arthroplasties: What are the reasons for revision? Int Orthop. 2008;32:597–604. doi: 10.1007/s00264-007-0364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallo J, Kaminek P, Zapletalova J, Cechova I, Spicka J, Ditmar R. Is osteolysis associated with a stable total hip replacement asymptomatic? Acta Chir Orthop Traumatol Cech. 2004;71:20–25. [PubMed] [Google Scholar]
- 4.Maloney W, Rosenberg A. What is the outcome of treatment for osteolysis? J Am Acad Orthop Surg. 2008;16(Suppl. 1):S26–S32. doi: 10.5435/00124635-200800001-00007. [DOI] [PubMed] [Google Scholar]
- 5.Deirmengian GK, Zmistowski B, O'Neil JT, Hozack WJ. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am. 2011;93:1842–1852. doi: 10.2106/JBJS.J.01197. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Cimbrelo E, Garcia-Rey E, Cruz-Pardos A. The extent of the bone defect affects the outcome of femoral reconstruction in revision surgery with impacted bone grafting: a five-to 17-year follow-up study. J Bone Joint Surg Br. 2011;93:1457–1464. doi: 10.1302/0301-620X.93B11.27321. [DOI] [PubMed] [Google Scholar]
- 7.Dumbleton JH, Manley MT, Edidin AA. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty. 2002;17:649–661. doi: 10.1054/arth.2002.33664. [DOI] [PubMed] [Google Scholar]
- 8.Kurtz SM, Gawel HA, Patel JD. History and systematic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene. Clin Orthop Relat Res. 2011;469:2262–2277. doi: 10.1007/s11999-011-1872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallo J, Slouf M, Goodman SB. The relationship of polyethylene wear to particle size, distribution, and number: A possible factor explaining the risk of osteolysis after hip arthroplasty. J Biomed Mater Res B Appl Biomater. 2010;94:171–177. doi: 10.1002/jbm.b.31638. [DOI] [PubMed] [Google Scholar]
- 10.Aspenberg P, van der Vis H. Fluid pressure may cause periprosthetic osteolysis. Particles are not the only thing. Acta Orthop Scand. 1998;69:1–4. doi: 10.3109/17453679809002344. [DOI] [PubMed] [Google Scholar]
- 11.Willert HG, Semlitsch M. Reactions of the articular capsule to wear products of artificial joint prostheses. J Biomed Mater Res. 1977;11:157–164. doi: 10.1002/jbm.820110202. [DOI] [PubMed] [Google Scholar]
- 12.Maloney WJ, Jasty M, Harris WH, Galante JO, Callaghan JJ. Endosteal erosion in association with stable uncemented femoral components. J Bone Joint Surg Am. 1990;72:1025–1034. [PubMed] [Google Scholar]
- 13.Goodman SB, Chin RC, Chiou SS, Schurman DJ, Woolson ST, Masada MP. A clinical-pathologic-biochemical study of the membrane surrounding loosened and nonloosened total hip arthroplasties. Clin Orthop Relat Res. 1989 Jul;:182–187. [PubMed] [Google Scholar]
- 14.Tallroth K, Eskola A, Santavirta S, Konttinen YT, Lindholm TS. Aggressive granulomatous lesions after hip arthroplasty. J Bone Joint Surg Br. 1989;71:571–575. doi: 10.1302/0301-620X.71B4.2768299. [DOI] [PubMed] [Google Scholar]
- 15.Santavirta S, Konttinen YT, Bergroth V, Eskola A, Tallroth K, Lindholm TS. Aggressive granulomatous lesions associated with hip arthroplasty. Immunopathological studies. J Bone Joint Surg Am. 1990;72:252–258. [PubMed] [Google Scholar]
- 16.Harris WH. Osteolysis and particle disease in hip replacement. A review. Acta Orthop Scand. 1994;65:113–123. doi: 10.3109/17453679408993734. [DOI] [PubMed] [Google Scholar]
- 17.Konttinen YT, Zhao D, Beklen A, Ma G, Takagi M, Kivela-Rajamaki M, et al. The microenvironment around total hip replacement prostheses. Clin Orthop Relat Res. 2005 Jan;:28–38. doi: 10.1097/01.blo.0000150451.50452.da. [DOI] [PubMed] [Google Scholar]
- 18.Ren PG, Irani A, Huang Z, Ma T, Biswal S, Goodman SB. Continuous infusion of UHMWPE particles induces increased bone macrophages and osteolysis. Clin Orthop Relat Res. 2011;469:113–122. doi: 10.1007/s11999-010-1645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallo J, Raska M, Mrazek F, Petrek M. Bone remodeling, particle disease and individual susceptibility to periprosthetic osteolysis. Physiol Res. 2008;57:339–349. doi: 10.33549/physiolres.931140. [DOI] [PubMed] [Google Scholar]
- 20.Schmalzried TP, Jasty M, Harris WH. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am. 1992;74:849–863. [PubMed] [Google Scholar]
- 21.Gallo J, Havranek V, Cechova I, Zapletalova J. Influence of demographic, surgical and implant variables on wear rate and osteolysis in ABG I hip arthroplasty. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:135–141. doi: 10.5507/bp.2006.021. [DOI] [PubMed] [Google Scholar]
- 22.Engh CA, Hooten JP, Jr, Zettl-Schaffer KF, Ghaffarpour M, McGovern TF, Bobyn JD. Evaluation of bone ingrowth in proximally and extensively porous-coated anatomic medullary locking prostheses retrieved at autopsy. J Bone Joint Surg Am. 1995;77:903–910. doi: 10.2106/00004623-199506000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson JM, Wilson AG, Stockley I, Scott IR, Macdonald DA, Hamer AJ, et al. Variation in the TNF gene promoter and risk of osteolysis after total hip arthroplasty. J Bone Miner Res. 2003;18:1995–2001. doi: 10.1359/jbmr.2003.18.11.1995. [DOI] [PubMed] [Google Scholar]
- 24.Gallo J, Mrazek F, Petrek M. Variation in cytokine genes can contribute to severity of acetabular osteolysis and risk for revision in patients with ABG 1 total hip arthroplasty: a genetic association study. BMC Med Genet. 2009;10:109. doi: 10.1186/1471-2350-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon A, Kiss-Toth E, Stockley I, Eastell R, Wilkinson JM. Polymorphisms in the interleukin-1 receptor antagonist and interleukin-6 genes affect risk of osteolysis in patients with total hip arthroplasty. Arthritis Rheum. 2008;58:3157–3165. doi: 10.1002/art.23863. [DOI] [PubMed] [Google Scholar]
- 26.Mrazek F, Gallo J, Stahelova A, Petrek M. Functional variants of the P2RX7 gene, aseptic osteolysis, and revision of the total hip arthroplasty: a preliminary study. Hum Immunol. 2010;71:201–205. doi: 10.1016/j.humimm.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Del Buono A, Denaro V, Maffulli N. Genetic susceptibility to aseptic loosening following total hip arthroplasty: a systematic review. Br Med Bull. 2012;101:39–55. doi: 10.1093/bmb/ldr011. [DOI] [PubMed] [Google Scholar]
- 28.Fornasier V, Wright J, Seligman J. The histomorphologic and morphometric study of asymptomatic hip arthroplasty. A postmortem study. Clin Orthop Relat Res. 1991;271:272–282. [PubMed] [Google Scholar]
- 29.Kwong LM, Jasty M, Mulroy RD, Maloney WJ, Bragdon C, Harris WH. The histology of the radiolucent line. J Bone Joint Surg Br. 1992;74:67–73. doi: 10.1302/0301-620X.74B1.1732269. [DOI] [PubMed] [Google Scholar]
- 30.Skurla CP, James SP. Assessing the dog as a model for human total hip replacement: analysis of 38 postmortem-retrieved canine cemented acetabular components. J Biomed Mater Res B Appl Biomater. 2005;73:260–270. doi: 10.1002/jbm.b.30204. [DOI] [PubMed] [Google Scholar]
- 31.Ma T, Ortiz SG, Huang Z, Ren P, Smith RL, Goodman SB. In vivo murine model of continuous intramedullary infusion of particles–a preliminary study. J Biomed Mater Res B Appl Biomater. 2009;88:250–253. doi: 10.1002/jbm.b.31175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Warrak AO, Olmstead M, Schneider R, Meinel L, Bettschart-Wolfisberger R, Akens MK, et al. An experimental animal model of aseptic loosening of hip prostheses in sheep to study early biochemical changes at the interface membrane. BMC Musculoskelet Disord. 2004;5:7. doi: 10.1186/1471-2474-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shanbhag AS, Kaufman AM, Hayata K, Rubash HE. Assessing osteolysis with use of high-throughput protein chips. J Bone Joint Surg Am. 2007;89:1081–1089. doi: 10.2106/JBJS.F.00330. [DOI] [PubMed] [Google Scholar]
- 34.Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 35.Cooper HJ, Ranawat AS, Potter HG, Foo LF, Koob TW, Ranawat CS. Early reactive synovitis and osteolysis after total hip arthroplasty. Clin Orthop Relat Res. 2010;468:3278–3285. doi: 10.1007/s11999-010-1361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aspenberg P, Wagner P, Nilsson KG, Ranstam J. Fixed or loose? Dichotomy in RSA data for cemented cups. Acta Orthop. 2008;79:467–473. doi: 10.1080/17453670710015445. [DOI] [PubMed] [Google Scholar]
- 37.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 39.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 40.Konttinen Y, Imai S, Suda A. Neuropeptides and the puzzle of bone remodeling. State of the art. Acta Orthop Scand. 1996;67:632–639. doi: 10.3109/17453679608997772. [DOI] [PubMed] [Google Scholar]
- 41.King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11:685–692. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckley CD. Why does chronic inflammation persist: An unexpected role for fibroblasts. Immunol Lett. 2011;138:12–14. doi: 10.1016/j.imlet.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 45.Perez-Losada J, Balmain A. Stem-cell hierarchy in skin cancer. Nat Rev Cancer. 2003;3:434–443. doi: 10.1038/nrc1095. [DOI] [PubMed] [Google Scholar]
- 46.Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. 2011;11:597–606. doi: 10.1038/nri3049. [DOI] [PubMed] [Google Scholar]
- 47.O'Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol. 2012;30:295–312. doi: 10.1146/annurev-immunol-020711-075013. [DOI] [PubMed] [Google Scholar]
- 48.Karouzakis E, Gay RE, Gay S, Neidhart M. Epigenetic control in rheumatoid arthritis synovial fibroblasts. Nat Rev Rheumatol. 2009;5:266–272. doi: 10.1038/nrrheum.2009.55. [DOI] [PubMed] [Google Scholar]
- 49.Goodman SB, Ma T. Cellular chemotaxis induced by wear particles from joint replacements. Biomaterials. 2010;31:5045–5050. doi: 10.1016/j.biomaterials.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anttila A, Lappalainen R, Heinonen H, Santavirta S, Konttinen YT. Superiority of diamondlike carbon coating on articulating surfaces of artificial hip joints. New Diamond Frontier Carbon Technol. 1999;9:283–288. [Google Scholar]
- 52.Callaghan JJ, Cuckler JM, Huddleston JI, Galante JO. How have alternative bearings (such as metal-on-metal, highly cross-linked polyethylene, and ceramic-on-ceramic) affected the prevention and treatment of osteolysis? J Am Acad Orthop Surg. 2008;16(Suppl. 1):S33–S38. doi: 10.5435/00124635-200800001-00008. [DOI] [PubMed] [Google Scholar]
- 53.Catelas I, Jacobs JJ. Biologic activity of wear particles. Instr Course Lect. 2010;59:3–16. [PubMed] [Google Scholar]
- 54.Landgraeber S, Jaeckel S, Loer F, Wedemeyer C, Hilken G, Canbay A, et al. Pan-caspase inhibition suppresses polyethylene particle-induced osteolysis. Apoptosis. 2009;14:173–181. doi: 10.1007/s10495-008-0297-3. [DOI] [PubMed] [Google Scholar]
- 55.Santavirta S, Takagi M, Gomez-Barrena E, Nevalainen J, Lassus J, Salo J, et al. Studies of host response to orthopedic implants and biomaterials. J Long Term Eff Med Implants. 1999;9:67–76. [PubMed] [Google Scholar]
- 56.Hubbi ME, Luo W, Baek JH, Semenza GL. MCM proteins are negative regulators of hypoxia-inducible factor 1. Mol Cell. 2011;42:700–712. doi: 10.1016/j.molcel.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knowles HJ, Athanasou NA. Acute hypoxia and osteoclast activity: a balance between enhanced resorption and increased apoptosis. J Pathol. 2009;218:256–264. doi: 10.1002/path.2534. [DOI] [PubMed] [Google Scholar]
- 58.Waris V, Sillat T, Waris E, Virkki L, Mandelin J, Takagi M, et al. Role and regulation of VEGF and its receptors 1 and 2 in the aseptic loosening of total hip implants. J Orthop Res. doi: 10.1002/jor.22138. Epub ahead of print 23 April 2012. DOI: 10.1002/jor.22138. [DOI] [PubMed] [Google Scholar]
- 59.Gross TS, King KA, Rabaia NA, Pathare P, Srinivasan S. Upregulation of osteopontin by osteocytes deprived of mechanical loading or oxygen. J Bone Miner Res. 2005;20:250–256. doi: 10.1359/JBMR.041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 61.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 63.Greenfield EM, Beidelschies MA, Tatro JM, Goldberg VM, Hise AG. Bacterial pathogen-associated molecular patterns stimulate biological activity of orthopaedic wear particles by activating cognate Toll-like receptors. J Biol Chem. 2010;285:32378–32384. doi: 10.1074/jbc.M110.136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearl JI, Ma T, Irani AR, Huang Z, Robinson WH, Smith RL, et al. Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials. 2011;32:5535–5542. doi: 10.1016/j.biomaterials.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takagi M, Tamaki Y, Hasegawa H, Takakubo Y, Konttinen L, Tiainen VM, et al. Toll-like receptors in the interface membrane around loosening total hip replacement implants. J Biomed Mater Res A. 2007;81:1017–1026. doi: 10.1002/jbm.a.31235. [DOI] [PubMed] [Google Scholar]
- 66.Maitra R, Clement CC, Scharf B, Crisi GM, Chitta S, Paget D, et al. Endosomal damage and TLR2 mediated inflammasome activation by alkane particles in the generation of aseptic osteolysis. Mol Immunol. 2009;47:175–184. doi: 10.1016/j.molimm.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Medzhitov R, Shevach EM, Trinchieri G, Mellor AL, Munn DH, Gordon S, et al. Highlights of 10 years of immunology in Nature Reviews Immunology. Nat Rev Immunol. 2011;11:693–702. doi: 10.1038/nri3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 69.Fujii J, Yasunaga Y, Yamasaki A, Ochi M. Wear debris stimulates bone-resorbing factor expression in the fibroblasts and osteoblasts. Hip Int. doi: 10.5301/hip.2011.7977. Epub ahead of print 11 April 2011. DOI: 511AE461-986F-4E4E-91C3-C511BBA83406. [DOI] [PubMed] [Google Scholar]
- 70.Garrigues GE, Cho DR, Rubash HE, Goldring SR, Herndon JH, Shanbhag AS. Gene expression clustering using self-organizing maps: analysis of the macrophage response to particulate biomaterials. Biomaterials. 2005;26:2933–2945. doi: 10.1016/j.biomaterials.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 71.Grom AA, Mellins ED. Macrophage activation syndrome: advances towards understanding pathogenesis. Curr Opin Rheumatol. 2010;22:561–566. doi: 10.1097/01.bor.0000381996.69261.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Hou C, Yu S, Xiao J, Zhang Z, Zhai Q, et al. IRAK-M in macrophages around septically and aseptically loosened hip implants. J Biomed Mater Res A. 2012;100:261–268. doi: 10.1002/jbm.a.33258. [DOI] [PubMed] [Google Scholar]
- 73.Liddiard K, Rosas M, Davies LC, Jones SA, Taylor PR. Macrophage heterogeneity and acute inflammation. Eur J Immunol. 2011;41:2503–2508. doi: 10.1002/eji.201141743. [DOI] [PubMed] [Google Scholar]
- 74.Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011;11:221–230. doi: 10.1038/nri2940. [DOI] [PubMed] [Google Scholar]
- 75.Ma J, Chen T, Mandelin J, Ceponis A, Miller NE, Hukkanen M, et al. Regulation of macrophage activation. Cell Mol Life Sci. 2003;60:2334–2346. doi: 10.1007/s00018-003-3020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Valledor AF, Comalada M, Santamaria-Babi LF, Lloberas J, Celada A. Macrophage proinflammatory activation and deactivation: a question of balance. Adv Immunol. 2010;108:1–20. doi: 10.1016/B978-0-12-380995-7.00001-X. [DOI] [PubMed] [Google Scholar]
- 77.Huss RS, Huddleston JI, Goodman SB, Butcher EC, Zabel BA. Synovial tissue-infiltrating natural killer cells in osteoarthritis and periprosthetic inflammation. Arthritis Rheum. 2010;62:3799–3805. doi: 10.1002/art.27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koreny T, Tunyogi-Csapo M, Gal I, Vermes C, Jacobs JJ, Glant TT. The role of fibroblasts and fibroblast-derived factors in periprosthetic osteolysis. Arthritis Rheum. 2006;54:3221–3232. doi: 10.1002/art.22134. [DOI] [PubMed] [Google Scholar]
- 79.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thyssen JP, Jakobsen SS, Engkilde K, Johansen JD, Soballe K, Menne T. The association between metal allergy, total hip arthroplasty, and revision. Acta Orthop. 2009;80:646–652. doi: 10.3109/17453670903487008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]