Abstract
Purpose
To investigate associations of baseline CVD risk profile, dosing regimen and treatment duration with incident CVD events during androgen deprivation therapy with degarelix in patients with PCa.
Materials and Methods
Data from 1704 subjects who participated in 9 clinical trials was pooled for the analysis. Subjects received treatment with either monthly (20-240 mg) or 3-monthly doses (240-480 mg) of degarelix for an average of 22 months (up to 66 months). Endpoints were ischemic heart disease, cerebrovascular disorders, arterial thrombotic/embolic events, and claudication
Results
First-time CVD events were reported in 92 subjects in the year prior to study entry and 168 subjects after degarelix treatment. Event rates were similar before and after degarelix treatment (5.5 vs. 6.1 per 100 person-years; P=0.45) in the total population and in the subset of men without baseline CVD (5.6 vs. 4.3 per 100 person-years; p=0.11). In contrast, event rates were higher after degarelix treatment (5.3 to 10.5 events per 100 person-years; p=0.0013) in the subset of men with CVD at baseline. In multivariate analysis, CVD at baseline was the strongest independent predictor of events during treatment followed by older age, abstinence to alcohol and obesity (all p<0.05). Dose and schedule of degarelix treatment were not independently associated with CVD events.
Conclusions
In men with PCa, observed rates of CVD events were similar before and after degarelix treatment. Events during degarelix treatment were largely confined to those with pre-existing CVD and further modulated by age and modifiable risk factors.
Keywords: cardiovascular events, degarelix, long-term treatment, predictors, prostate cancer patients
Introduction
ADT with either a GnRH agonist or a GnRH blocker (antagonist) is the mainstay of systemic therapy for locally advanced and metastatic PCa [1]. ADT increases survival in some clinical settings [2], but has potential adverse effects [3]. The increasing use in locally advanced PCa draws attention to better understand the long-term benefits and harms of the treatment.
Most [4-10] but not all [11-13] population-based studies have reported greater CVD risk in those treated with GnRH agonists. Higher CVD incidence was observed with short-term (1-4 months) GnRH agonist exposure and the magnitude of this risk did not appear to increase with longer treatment duration [5]. On October 20, 2010, the U.S. FDA notified the manufacturers of GnRH agonists of the need to add new safety information to the Warnings and Precautions section of the drug labels. This new information warns about increased risk of diabetes and certain CVDs (heart attack, sudden cardiac death, and stroke) in men receiving GnRH agonists for PCa [14].
Several large populations based studies have evaluated the relationship between orchiectomy and CVD events in men with PCa. In contrast to the observations about GnRH agonists, most but not all studies reported no association between orchiectomy with increased risk of CVD events [4,5,10,11]. It is yet unknown whether the discordant observations are due to differences in mechanisms of action or dissimilarities between the patient populations [4]. Nonetheless, these observations raise the possibility that CVD risk may vary for different forms of ADT.
Similar to orchiectomy, GnRH blockers elicit prompt and sustained suppression of gonadal testosterone production [15]. This response is qualitatively different from that elicited by agonists that are frequently associated with an initial testosterone flare and microflares at repeated dosing [16]. In addition, recent studies revealing the presence of GnRH and functional GnRH receptors on lymphocytes [17,18], a dominant cellular type in atherosclerotic plaques [19-21], draws attention to other possible pathways by which agonists and blockers may elicit events with potential direct implications for CVD safety [22,23]. There is limited information about the cardiovascular safety of degarelix (or other GnRH blockers) in PCa patients.
We used data from prospective clinical trials to evaluate how baseline patient characteristics, the dose and duration of degarelix treatment influence the risk of CVD events during long-term exposure. The analyses were designed to address the following questions: 1) do rates of CVD events differ before and after degarelix treatment? 2) which baseline patient characteristics are associated with CVD events during degarelix treatment? 3) what is the relationship between degarelix dose and/or schedule with CVD events?
Subjects and Methods
Safety database
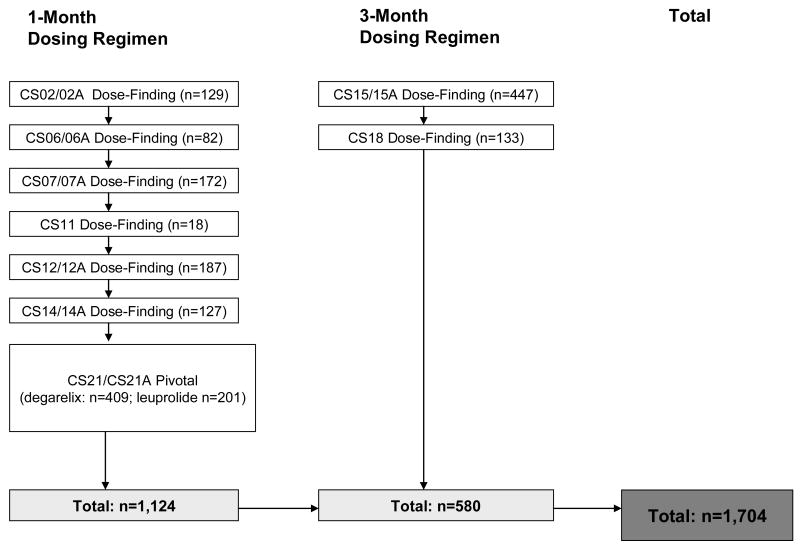
The present analysis is comprised of 6 completed Phase 2/3 studies (5 with safety extension) and one completed Phase 3 study with ongoing safety extension study on the 1-month depot (n=1124) and 2 completed Phase 2 studies (one with safety extension) on the 3-month depot formulation (n=580) (Fig.1). The Phase 3 pivotal trial was a randomized, active-controlled, parallel group trial (15). All other studies were uncontrolled open-label trials. The clinical trials were performed in accordance with the Declaration of Helsinki II and Good Clinical Practice guidelines. The respective study protocols were approved by independent Ethics Committees and Institutional Review Boards.
Figure 1.
Summary of degarelix studies in the safety database. “A” after the number of the trial denotes safety extension trial of the original trial.
Subjects
In each study, the primary inclusion criteria were male subjects ≥18 years of age with histologically confirmed adenocarcinoma of the prostate (33.2% localized, 28.6% locally advanced, 21.2% metastatic, and 17.0% not-classified), in whom ADT, except for neoadjuvant hormonal therapy, was indicated. None of the subjects had received long-term ADT before study entry. All subjects had baseline serum testosterone levels that were either within the age-specified normal range or above the lower limit of the normal range of the central laboratory. Inclusion and exclusion criteria [17] were similar across studies.
Baseline characteristics
Baseline evaluation included physical examination and medical history including assessment of demographic characteristics, lifestyle factors, and other major CVD risk factors. CVD history focused on the following three Standardized MedDRA Queries (ischemic heart diseases, cerebrovascular disorders, and other arterial embolic/thrombotic events), and an additional category created from selected MedDRA preferred terms (manifest macrovascular disease of the lower extremity, i.e. claudication intermittent). Based on the traditional risk factors and the presence or absence of CVD at baseline, each subject was assigned to one of the following three risk categories: 1) no major risk factors, 2) ≥1 risk factors but no CVD, and 3) CVD at baseline.
Testosterone measurements
Serum testosterone was typically measured at baseline and then monthly in all subjects. Serum testosterone was determined by a central laboratory (Esoterix Inc, Calabasas, CA, USA) using a validated liquid chromatography system with tandem mass spectrometry assay (lower limit of quantization: 3 ng/dl).
CVD end-points
Events prior to treatment were collected with dates as part of the medical history assessment. Events occurring after treatment initiation were collected typically at monthly visits with dates as adverse events or serious adverse events. CVD events were coded using MedDRA terms and grouped into one of the same categories used for the collection of medical history.
Statistical methods
CVD event rate before and after degarelix treatment was investigated by 1-Kaplan-Meier plots of time to events setting 1 year before treatment initiation as baseline. A change of hazards from the pre- to the post-treatment period was analyzed by the Likelihood Ratio test (χ2, df=1) associated with the two-parametric exponential model as described in Kim et al [24]. The corresponding hazard ratios (HR) and their 95% CI were inferred using the delta-method and standard maximum-likelihood theory. Distribution of patients between strata of baseline CVD risk was compared with Pearson chi-square test. We applied univariate and multivariate Cox proportional hazards models to identify predictors of incident events occurring during degarelix treatment. Serum testosterone and degarelix dose/schedule were both entered into the model as time-dependent covariates. All analyses have been performed at the 5% nominal significance level using the SAS version 9.2.
Results
Baseline characteristics
Table 1 summarizes the baseline characteristics for the total population and subcategories classified by CVD and/or major risk factors.
Exposure
Mean duration of degarelix treatment was 22 months. Distribution of subjects according to treatment duration or average monthly degarelix across the 3 groups of baseline CVD risk is summarized in Table 2.
Long-term testosterone suppression
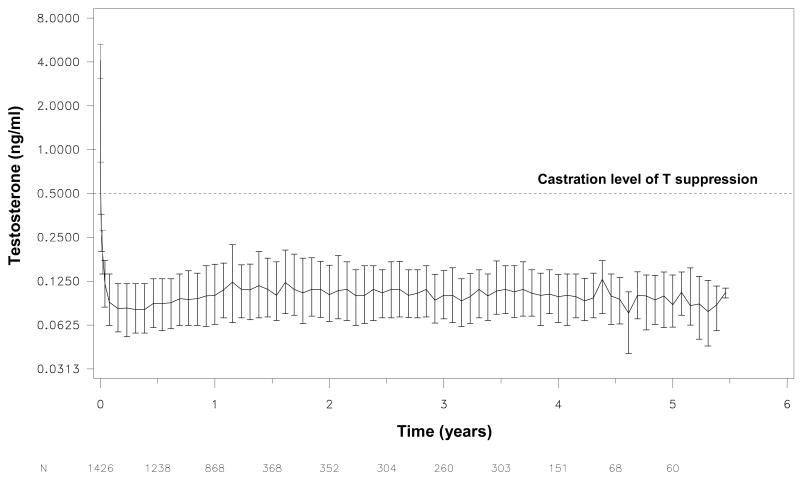
Figure 2 summarizes the pattern of testosterone suppression over time achieved by regularly dosed degarelix (n=1426). As highlighted by the figure, degarelix maintained testosterone suppression below 0.5 ng/ml throughout the observation period.
Figure 2.
Long-term testosterone suppression in studies with regular degarelix dosing as per protocol (n=1462). Results shown are medians ± inter-quartile range.
CVD events before and after initiation of degarelix treatment
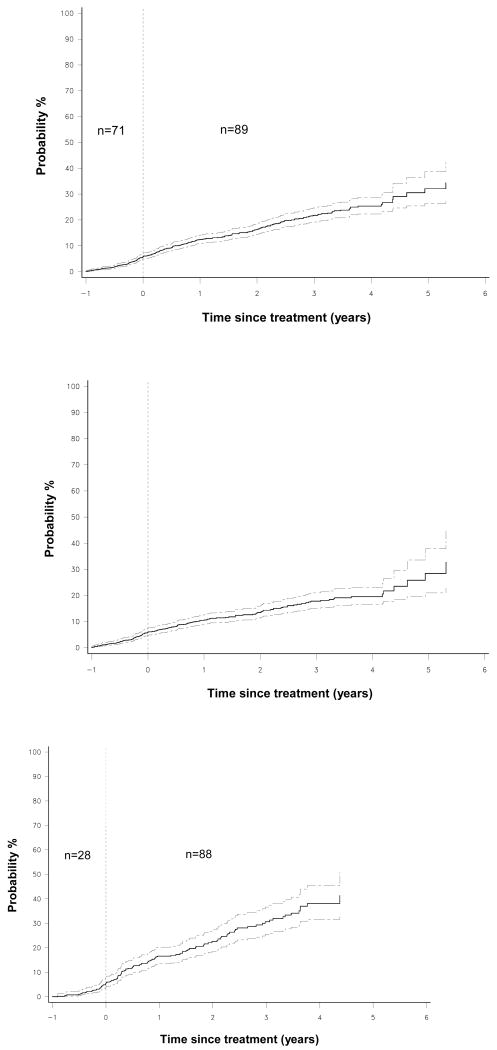
Figure 3 shows 1-Kaplan-Meier plot of time to first new CVD event in the total population starting 1 year prior to degarelix treatment. Event rates before and during treatment were virtually identical (5.5 versus 6.1 events per 100 person-years; HR 1.10, 95% CI 0.85-1.42, p=0.45). In subjects without established CVD, event rates were numerically lower after initiation of degarelix treatment (5.6 vs. 4.3 per 100 person-years; HR 0.77, 95% CI 0.56-1.06; p=0.11) (Fig. 3), whereas in the 498 subjects with established CVD event rates seemed to increase after treatment initiation (5.3 to 10.5 events per 100 person-years; HR 1.98, 95% CI 1.28-3.08; p=0.0013) (Fig. 3). Importantly, 23 of the 26 CVD events occurred in the second half of the pre-treatment year also illustrated by a marked increase in the slope of the curve starting from Month -6 (Fig. 3). When taking event rates during the 6 months prior treatment as the reference period no significant further increase was seen after treatment initiation (8.9 vs. 10.7 events per 100 person-years, HR 1.20, 95% CI 0.76-1.90, p=0.42) despite the relatively high number of events (n=91) that have emerged during this period.
Figure 3.
1-Kaplan-Meier plots of time to first new CVD event in the total population (upper panel), in subjects with no CVD (middle panel) and in subjects with established CVD (lower panel) starting 1 year prior degarelix treatment. Dotted lines indicate 95% confidence intervals. Event rates were comparable before and after degarelix treatment in the total population, decreased in subjects without CVD, but virtually increased in patients with established CVD. However, the acceleration in event rate started 6 month prior treatment initiation with no significant further increase captured after treatment initiation. Numbers indicate new CVD events in the respective time-intervals.
CVD risk per baseline CVD profile at treatment initiation
During the treatment period 193 subjects reported a total of 262 CVD events. In 177 subjects these were all primary event(s), whereas in 16 subjects these were secondary events. Of the 262 reported events, the most common ones were ischemic cardiac events (n=117) followed by cerebrovascular events (n=63), other arterial thrombotic/embolic events (n=54) and claudication intermittent (n=28).
Of the 193 subjects, 128 (66%) discontinued medication within a median period of 102 days (IQR 18.5-287.5 days), whereas 65 subjects continued receiving medication and completed the study following a median time of 658 days (IQR 250-1012 days) after the event. Of those who discontinued, 49 subjects died within a median period of 23 days (IQR 1-119 days) following the CVD event.
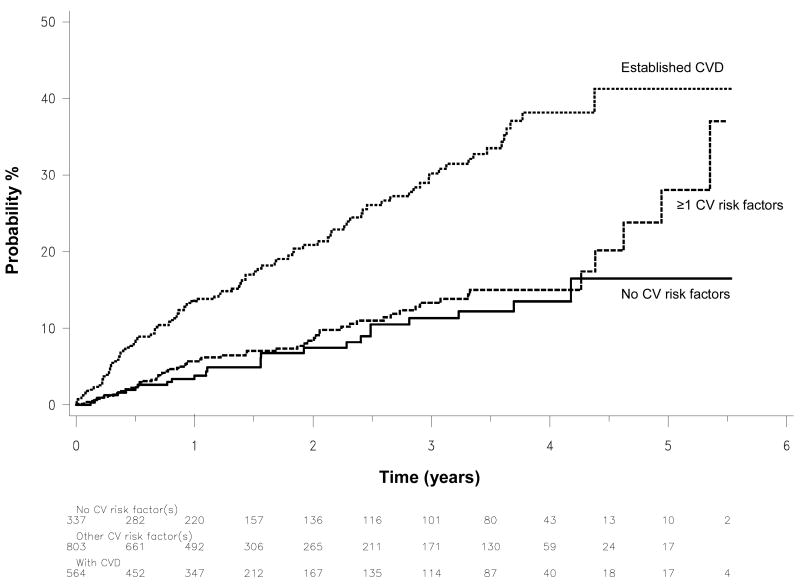
The rate of events in subjects with CVD at baseline (HR 3.06, 95% CI 1.98-4.73, p<0.0001), but not in those with ≥1 CVD risk factors (HR 1.25, 95% CI 0.79-1.99, p=0.28) was significantly increased compared with controls (Fig. 4). Adjusting for the differences in age between the risk groups did not change these findings with corresponding HRs of 2.83 (95% CI 1.83-4.39, p<0.0001) and 1.20 (95% CI 0.78-1.95, p=0.38).
Figure 4.
1-Kaplan-Meier plots of time to first new CVD event during degarelix treatment in subjects stratified according to baseline CVD risk profile.
Individual predictors of CVD events during degarelix treatment
In age-adjusted univariate analyses, obesity (HR 1.53, 95% CI 1.07-2.17, p=0.02), treated type 2 diabetes (HR 1.57, 95% CI 1.02-2.45, p=0.04), treated hyperlipidemia (HR 1.49, 95% CI 1.12-2.28, p=0.03) were all associated with increased, whereas regular alcohol consumption with decreased risk (HR 0.67, 95% CI 0.50-0.89, p=0.005) of CVD events. The association with current smoking was only borderline significant (HR 1.34, 95% CI 0.93-1.94, p=0.13).
In multivariate analyses (Table 3), age, obesity and CVD at baseline were all independently associated with increased, whereas regular alcohol consumption with decreased hazards of CVD events (all p<0.05). Current smoking (p=0.08) and treated type 2 diabetes (p=0.13) were of borderline statistical significance only as many of the subjects having these risk factors had already CVD as well.
Discussion
We used prospective data to evaluate the potential relationship between degarelix and CVD events. The main findings were as follows: 1) rates of events were similar before and after initiation of degarelix treatment in the total population, 2) events during degarelix treatment were more common in men who had established CVD already at baseline, 3) other traditional CVD risk factors including older age, obesity, smoking, and abstinence from alcohol were also associated with greater risk during the treatment period, 4) neither degarelix dosing regimen nor treatment duration was independently associated with CVD risk.
In contrast to GnRH agonists, most studies have found no association between orchiectomy and risk of CVD events [4,5,10,11]. In the largest (n=73,196) reported observational study [5], for example, GnRH agonists were consistently associated greater risks for coronary heart disease, cerebrovascular events and sudden cardiac death (HRs 1.16, 1.11, and 1.16, respectively, all p<0.001), whereas no increased risk for any of these events was associated with orchiectomy (HRs 0.99, 0.94, 1.01, respectively, all p>0.4). Several observations suggest that degarelix, like orchiectomy, is not associated with CVD. First, degarelix treatment was not associated with increases in the rate of first new events in the total population. Second, degarelix treatment was associated with an apparent decrease rather than increase in the risk of primary CVD events in subjects without CVD. Third, when adjusted for effects on serum testosterone (i.e. direct drug effect), the relatively large variation in mean monthly doses of degarelix was not independently associated with relative increases in the risk of CVD events.
In men with established CVD before study entry, the annual rate of events was lower before than after degarelix treatment. Notably, the event rate increased 6 month prior the initiation of degarelix treatment (Fig. 3) with no apparent increases in response to treatment initiation. The rate of CVD events tended to decline one year after treatment initiation and hence did not suggest either sustained or cumulative increase in CVD risk. Finally, the observation that the risk increased in subjects with, but appeared to decrease in those without CVD argues against a consistent treatment-related adverse effect. Taken together, these observations suggest that degarelix may not be causally related to CVD events.
Recent report from the pivotal Phase 3 trial [25] indicated that changes of the QT interval and incidence of cardiac arrhythmias (ADT-related complications) were comparable between degarelix and leuprolide-treated patients. When regarding CVD events discussed herein the low incidence and the relatively small number of patients (∼200) per arm posed obvious limitations to make statistically robust comparisons. Nevertheless, it is still noteworthy pointing out that the incidence of the most common event (ischemic heart disease) was lower in patients receiving the approved monthly dose of degarelix (n=8, 4%) compared with those receiving leuprolide (n=21, 10%). Clearly, a larger dataset from prospective randomized clinical trials is needed to further explore this apparent signal favouring the GnRH antagonist.
In vitro experiments by Tanriverdi et al [18] on lymphocytes from men showed that the native GnRH-I and GnRH agonist increased, whereas a GnRH blocker (cetrorelix) blocked the activation and proliferative response of these immune cells. T lymphocytes are the predominant inflammatory cells infiltrating atherosclerotic plaques [19-21] and their activation may have direct implications for the rupture of the plaque and related thromboembolic complications [22,23]. Accordingly, the opposing effect of GnRH agonists and blockers on T lymphocyte activation and proliferation in plaques seem to offer a plausible mechanism that could differentiate different forms of ADT in terms of associated CVD risk and hence is worthy of further exploration.
In the general population, older age, hypertension, smoking, diabetes, and elevated cholesterol are associated with greater risk for CVD events [26]. In the current analyses, older age, obesity, type 2 diabetes, treated hyperlipidemia, and abstinence from alcohol were all associated with CVD events during degarelix treatment. When analyzing them in a multivariate model, inclusion of CVD at baseline in the model changed the direct association of these risk factors with CVD events, yet older age, obesity and drinking habits remained independent modulators of the risk. The similarities between risk factors identified from studies of the general population and men receiving degarelix supports a strategy to reduce CVD risk in PCa survivors using guidelines established for the general population [27].
The large number of informative events is a major strength of the current analyses compared with previous reports on considerably fewer events [4]. An additional strength is that the study reports on a broad range of effective degarelix doses, which together with the close monitoring of serum testosterone allowed addressing the direct CVD impact of degarelix. As potential limitation, the before/after analysis compared medical history-based retrospective time-to-event analyses with adverse event-based prospective time-to-event analyses. However, the nature of the clinical events and the reasonable short retrospective timeframes (1 year) to look back for events minimizes recall bias and makes the analysis reasonably precise, while also controlling for the confounding effect of aging on atherosclerotic events.
In summary, CVD event rates were similar before and after degarelix treatment in the general population of PCa patients. Established CVD, age, and modifiable risk factors were the main drivers of CVD events during treatment. Additional preclinical and clinical research (randomized comparative trials) is warranted to further explore and confirm the herein hypothesized role of extrapituitary GnRH receptors in the apparent different CVD risk profile associated with different forms of medical castration.
Acknowledgments
The authors gratefully acknowledge Jens-Kristian Jensen for his dedicated support with the statistical programming. Dr. Smith is supported by an NIH Midcareer Investigator Award (5K24CA121990) and competitive research awards from the Prostate Cancer Foundation.
References
- 1.Van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71:1001–6. doi: 10.1016/j.urology.2007.12.070. [DOI] [PubMed] [Google Scholar]
- 2.Cookson MS, Sarosdy MF. Hormonal therapy for metastatic prostate cancer: issues of timing and total androgen ablation. South Med J. 1994;87:1–6. doi: 10.1097/00007611-199401000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Isbarn H, Boccon-Gibod L, Carroll PR, et al. Androgen deprivation therapy for the treatment of prostate cancer: consider both benefits and risks. Eur Urol. 2009;55:62–75. doi: 10.1016/j.eururo.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine GN, D'Amico AV, Berger P, et al. American Heart Association Council on Clinical Cardiology and Council on Epidemiology and Prevention, the American Cancer Society, and the American Urological Association Androgen-Deprivation Therapy in Prostate Cancer and Cardiovascular Risk. Circulation. 2010;121:833–840. doi: 10.1161/CIRCULATIONAHA.109.192695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 6.Saigal CS, Gore JL, Krupski TL, et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 7.D'Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–2425. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 8.Tsai HK, D'Amico AV, Sadetsky N, et al. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 9.D'Amico AV, Chen MH, Renshaw AA, et al. Causes of death in men undergoing androgen suppression therapy for newly diagnosed localized or recurrent prostate cancer. Cancer. 2008;113:3290–3297. doi: 10.1002/cncr.23970. [DOI] [PubMed] [Google Scholar]
- 10.Keating NL, O'Malley AJ, Freedland SJ, et al. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alibhai SM, Duong-Hua M, Sutradhar R, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;278:3452. 2009. doi: 10.1200/JCO.2008.20.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roach M, III, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008;26:585–591. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 13.Studer UE, Whelan P, Albrecht W, et al. Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891. J Clin Oncol. 2006;24:1868–1876. doi: 10.1200/JCO.2005.04.7423. [DOI] [PubMed] [Google Scholar]
- 14.FDA Drug Safety Communication: Update to Ongoing Safety Review of GnRH Agonists and Notification to Manufacturers of GnRH Agonists to Add New Safety Information to Labeling Regarding Increased Risk of Diabetes and Certain Cardiovascular Diseases. http://www.fda.gov/Drugs/DrugSafety/ucm229986.htm.
- 15.Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–8. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- 16.Zinner NR, Bidair M, Centeno A, et al. Similar frequency of testosterone surge after repeat injections of goserelin (Zoladex) 3.6 mg and 10.8 mg: results of a randomized open-label trial. Urology. 2004;64:1177–81. doi: 10.1016/j.urology.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 17.Chen HF, Jeung EB, Stephenson M, et al. Human peripheral blood mononuclear cells express gonadotropin-releasing hormone (GnRH), GnRH receptor, and interleukin-2 receptor gamma-chain messenger ribonucleic acids that are regulated by GnRH in vitro. J Clin Endocrinol Metab. 1999;84:743–50. doi: 10.1210/jcem.84.2.5440. [DOI] [PubMed] [Google Scholar]
- 18.Tanriverdi F, Gonzalez-Martinez D, Hu Y, et al. GnRH-I and GnRH-II have differential modulatory effects on human peripheral blood mononuclear cell proliferation and interleukin-2 receptor gamma-chain mRNA expression in healthy males. Clin Exp Immunol. 2005;142:103–10. doi: 10.1111/j.1365-2249.2005.02904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Businaro R, Digregorio M, Riganò R, et al. Morphological analysis of cell subpopulations within carotid atherosclerotic plaques. Ital J Anat Embryol. 2005;110(2 Suppl 1):109–15. [PubMed] [Google Scholar]
- 20.Lee WH, Ko YH, Kim DI, Lee BB, et al. Prevalence of foam cells and helper-T cells in atherosclerotic plaques of Korean patients with carotid atheroma. Korean J Intern Med. 2000;15:117–21. doi: 10.3904/kjim.2000.15.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbate A, Bussani R, Liuzzo G, et al. Sudden coronary death, fatal acute myocardial infarction and widespread coronary and myocardial inflammation. Heart. 2008;94:737–42. doi: 10.1136/hrt.2007.115329. [DOI] [PubMed] [Google Scholar]
- 22.de Boer OJ, van der Wal AC, Verhagen CE, et al. Cytokine secretion profiles of cloned T cells from human aortic atherosclerotic plaques. J Pathol. 1999;188:174–9. doi: 10.1002/(SICI)1096-9896(199906)188:2<174::AID-PATH333>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Halvorsen B, Otterdal K, Dahl TB, et al. Atherosclerotic plaque stability--what determines the fate of a plaque? Prog Cardiovasc Dis. 2008;51:183–94. doi: 10.1016/j.pcad.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Kim DY, Woodroofe M, Wu Y. Testing for a change in the Hazard Rate with Staggered Entry. Communications in Statistics - Theory and Methods. 2004;33:2041–2058. [Google Scholar]
- 25.Smith MR, Klotz L, Persson BE, et al. Cardiovascular Safety of Degarelix: Results From a 12-Month, Comparative, Randomized, Open Label, Parallel Group Phase III Trial in Patients With Prostate Cancer. J Urol. 2010;184:2313–9. doi: 10.1016/j.juro.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson S, Johnston A, Robson J, et al. Predicting coronary risk in the general population--is it necessary to measure high-density lipoprotein cholesterol? J Cardiovasc Risk. 2003;10:137–41. doi: 10.1097/01.hjr.0000060844.48106.ff. [DOI] [PubMed] [Google Scholar]
- 27.Saylor PJ, Keating NL, Smith MR. Prostate cancer survivorship: prevention and treatment of the adverse effects of androgen deprivation therapy. J Gen Intern Med. 2009;24(2):S389–94. doi: 10.1007/s11606-009-0968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]