Abstract
Acute hepatitis C virus (HCV) infection is underdiagnosed because most patients are asymptomatic. The majority of new infections occur among people who inject drugs (PWID), many of whom have a history of incarceration. In a previous pilot study, we identified symptomatic HCV cases, mainly among Caucasian inmates. We designed a cross-sectional study to evaluate whether risk factor-based screening of newly incarcerated inmates would enhance identification of asymptomatic acute HCV infection and elucidate any demographic shifts in HCV acquisition. From October 2006-March 2008, 6,342 inmates underwent health assessments and 3470 inmates (55%) were screened. The racial distribution was as follows: African-American 24.0%, Caucasian 49.5%, Hispanic 22.2%. One hundred seventy-one inmates (4.9%) were classified as high-risk. After further evaluation, 35 (20.5%) were diagnosed with acute HCV with a mean age of 29 years; 62.9% were female and 91% were Caucasian. No African-Americans were diagnosed with acute HCV. Our case-finding rate was 1.9 patients/month nearly a three-fold increase compared to our historical control period with a higher proportion of asymptomatic cases. We estimate a prevalence of ~1.0% [95% CI 0.7%–1.4%] of acute HCV infections among newly incarcerated inmates. Conclusions: Within the correctional system, systematic screening based on risk factors successfully identifies acute HCV infection among PWID, including asymptomatic patients. Our data also reflect changing nationwide patterns of injection drug use that vary by age, ethnicity, and race, leading to a marked reduction of acute HCV infections among African-Americans as compared to non-Hispanic whites. The nationwide implementation of this simple low-cost strategy in prison-based settings could identify more than 7,000 acute HCV infections among PWID and provide insight into changing epidemiologic trends and facilitate appropriate therapeutic and preventive interventions.
Keywords: Hepatitis C infection, HCV diagnosis, HCV screening, prisoners, inmates
Introduction
Most people who inject drugs (PWID) acquire hepatitis C virus (HCV) infection within the first years of unsafe injection practices (1, 2). National surveillance data demonstrate that PWIDs account for 46% of symptomatic acute HCV infections in the United States (3). However, recognition of newly acquired infection is uncommon since the majority of patients are asymptomatic. An additional barrier to HCV diagnosis among PWID is the sporadic and fragmented nature of their healthcare (4, 5).
From an epidemiologic and interventional viewpoint, the correctional system is an appropriate sentinel site to assess both chronic and acute HCV infections among PWID. The seroprevalence rates of chronic HCV infection among incarcerated populations range from 25 to 41 percent, approximately 20-fold higher than in the community (6, 7). Many inmates in state prisons are also at risk for acute infection; in one survey, 57 percent acknowledged using drugs in the month prior to their incarceration (6). Since the majority of inmates are released into the community within two years of sentencing, a meaningful impact on public health could be made through focused preventive and therapeutic measures within this hard-to-reach patient population (8). Yet, many correctional medical programs do not screen for HCV infection among persons at risk, despite surveillance recommendations by the Centers for Disease Control and Prevention (CDC) and the Institute of Medicine (9, 10).
In a prior pilot project, we identified 21 inmates with acute HCV infection over a 30–month period, the majority referred for symptomatic disease (11). Since most newly-infected persons have minimal symptoms, these cases likely represented the “tip of the iceberg” (12). Furthermore, most of these patients were Caucasian, although African-Americans made up approximately 25% of the prison population (13). We postulated that underdiagnosis of acute HCV infection in other racial/ethnic groups could be related to differences in injection drug use (IDU), lower rates of symptomatic disease, or poorer utilization of health care (11). Motivated by these pilot data, our objective was to determine whether active case finding, using a low-cost screening intervention for high-risk behaviors, would enhance identification of asymptomatic acute HCV infection among newly incarcerated PWID in a “real-life” setting, where health care resources are limited. Moreover, we aimed to elucidate the racial/ethnic profile of those at risk for acute HCV.
Methods
Study sites
This study was performed at two separate facilities: Massachusetts Correctional Institute (MCI)-Concord for male inmates and MCI-Framingham for female inmates. All admitted prisoners who underwent a medical evaluation were eligible for screening. Self-reported race/ethnicity data were collected upon incarceration.
Screening strategy
We designed a two-minute questionnaire focused on history of high-risk exposures and prior HCV serologic testing. From October 2006 to March 2008, healthcare providers from the University of Massachusetts correctional health program incorporated this screening tool as part of intake medical evaluations, after a brief educational seminar regarding the potential individual and public health benefits of identifying acute HCV.
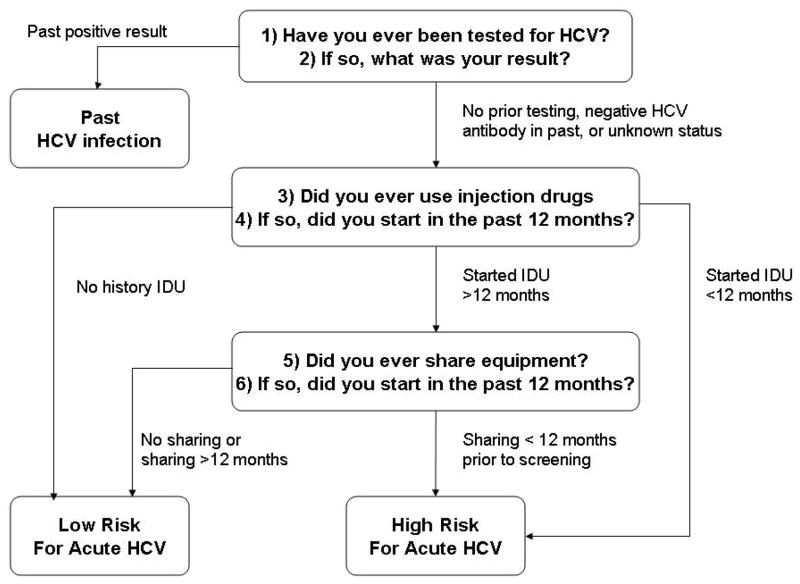
To limit the burden on healthcare providers, the screening questionnaire was comprised of only six questions (Figure 1). The first two addressed whether the inmate had prior HCV serologic testing. If the subject self-reported a history of a positive HCV test, the individual was considered likely to have past infection and was referred to the medical service. If the subject reported no prior testing, unknown status, or prior HCV seronegativity, additional questions were posed regarding new behavioral risk factors within 12 months prior to incarceration (initiation of IDU, sharing of needles/paraphernalia (14). If the patient denied risk factors for HCV acquisition, he/she was classified as low-risk. If he/she reported recent initiation of IDU and/or sharing of needles or paraphernalia, the patient was classified as “high-risk.” In addition, inmates who were initially diagnosed with HCV during the current incarceration, or who had new seroconversion, were considered high-risk for acute infection.
Figure 1. Schema for determining risk for acute HCV infection.
Inmates who self-reported a history of HCV seropositivity were considered to have past infection
HCV: hepatitis C virus
IDU: injection drug use
In-depth evaluation of high-risk individuals
Inmates identified as “high-risk” underwent in-depth interviews with the study personnel, either a registered nurse or an infectious disease specialist. Historical data were collected in the following domains: (1) symptoms consistent with hepatitis (right-sided quadrant pain, nausea, vomiting, fatigue, jaundice, dark urine, and loss of appetite), (2) specific risk behaviors, and (3) temporal changes in behaviors. If the inmate reported recent HCV testing, medical records were requested after permission was granted. In order to evaluate elevations in aminotransferases, the inmate was also asked about alcohol intake prior to incarceration.
In addition, we performed laboratory testing including alanine aminotransferase (ALT), HCV antibody (EIA 2.0, Abbott Laboratories), HCV RNA levels (bDNA, Chiron), HIV antibody (Genetic Systems HIV-1 Western Blot, BioRad or OraQuick ADVANCE rapid antibody test, OraSure Technologies), and hepatitis A virus (HAV) and hepatitis B virus (HBV) serologies (ie, HAV total antibody, HBV core antibody, HBV surface antigen, HBV surface antibody). High-risk inmates were immunized for hepatitis A and B, as needed.
Definitions of HCV infection
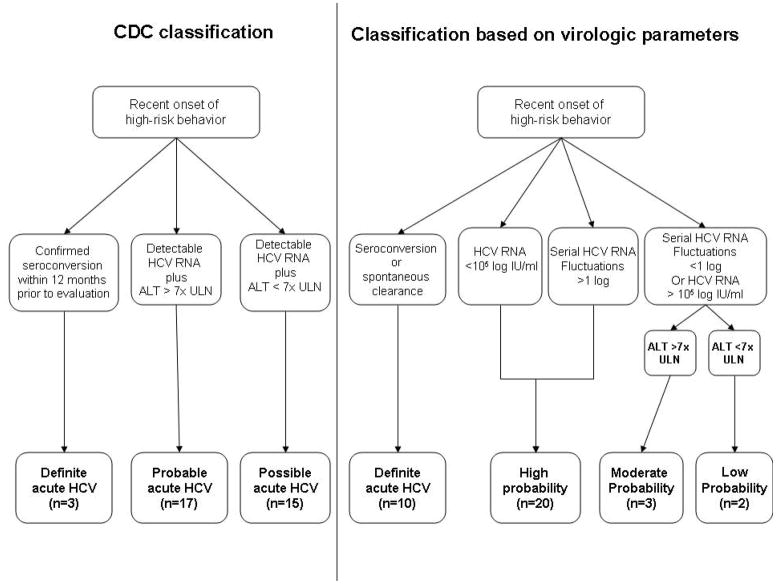
Patients were categorized according to their probability of having acute viral hepatitis using two parallel approaches as previously reported (Figure 2) (15). We utilized an ALT level >7x upper limit of normal (ULN) as our diagnostic threshold, as defined by the CDC (16). Patients with recent onset of high risk-taking behaviors were categorized in terms of probability of acute HCV infection as follows: a) HCV seronegative patients with viremia at baseline and subsequent seroconversion had “definite” acute HCV; b) patients who were HCV-seropositive with a documented seronegative status within six months and an ALT>7x ULN also had “definite” acute HCV; c) those without documentation of prior testing within 6 months who were HCV-seropositive with detectable HCV-RNA and ALT>7x ULN had “probable” HCV infection; d) those without documentation of prior testing within 6 months who were HCV-seropositive with detectable HCV-RNA and ALT<7x ULN had “possible” HCV infection.
Figure 2. Two parallel approaches for diagnosis of acute HCV infection.
ALT: alanine aminotransferase test
CDC: Centers for Disease Control and Prevention
HCV: hepatitis C virus
ULN: upper limit of normal
In our second approach, we performed serial monitoring of HCV RNA to assess for virologic fluctuations (>1 log) and low-level viremia (<100,000 copies/mL) and/or clearance, which are highly suggestive of acute infection. In this “dynamic” model, patients with recent onset of high risk-taking behaviors were categorized in terms of probability of acute HCV infection as follows: a) patients who had spontaneous clearance were classified as having “definite” acute HCV infection b) patients with HCV-RNA fluctuations >1 log were defined as “high probability”; c) patients with HCV-RNA fluctuations <1 log were classified as “moderate probability” or “low probability” based on whether their peak ALT was greater or less than 7x ULN; d) patients with any single HCV-RNA level <105 IU/ml were defined as having “high probability” of acute infection. All patients diagnosed with acute HCV did not have any evidence of recent hepatitis A or hepatitis B infections. All those diagnosed with acute HCV infection became candidates for antiviral therapy, as previously reported (17).
A diagnosis of past infection was based on patient self-report, a high-risk period that exceeded 12 months prior to screening, or a confirmed history of HCV (through medical records or past laboratory testing). Spontaneous clearance was defined as a non-detectable HCV RNA level, as determined by a molecular assay (Versant HCV RNA v3.0 assay bDNA; Bayer Diagnostics, lower limit of detection <615 IU/ml) on two occasions at least 4 weeks apart, or on a single occasion after a prior positive HCV RNA level, without any treatment intervention.
Historical control period
From November 2001 to May 2004, we provided educational seminars on acute HCV infection and requested that all medical providers, within the eighteen sites of the Massachusetts Department of Corrections (DOC) refer any patient with symptoms of hepatitis or significant aminotransferase elevations. During this historical control period, 21 inmates were diagnosed with acute HCV infection, the majority had symptomatic disease (67 percent) (11). Risk factor-based screening was not performed.
Data analysis
During the risk factor-based screening period, we measured the rates of identification of past versus acute HCV infection by dividing the number of cases by the number of months. We subsequently compared demographic and clinical features of individuals with acute HCV infection during this timeframe to those identified during the historical control period (11). Fisher’s exact test was used to generate relative risks or odds ratios for categorical variables; student’s t test was used when necessary to compare continuous variables. The prevalence of acute HCV was calculated by dividing the number of cases by the number of individuals screened; a modified Wald methodology was used for calculation of the confidence interval of a proportion. GraphPad Prism-4 (GraphPad Software, San Diego, CA) was utilized for analysis.
Ethical issues
The protocol was approved a priori by the Human Research Review Committee of Lemuel Shattuck Hospital, which includes a prisoner advocate. University of Massachusetts Correctional Health approved the use of the screening form during the inmate intake examination as part of standard medical care. Those with suspected acute HCV infection gave written informed consent for an ongoing parallel immunology/virology study (18).
Role of the Funding Source
Funding for this study was provided by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (Hepatitis C Cooperative Center U19 AI066345 to GML and AYK, K23 AI054379 to AYK, Harvard University Center for AIDS Research P30 AI060354). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Results
Classification of screening forms
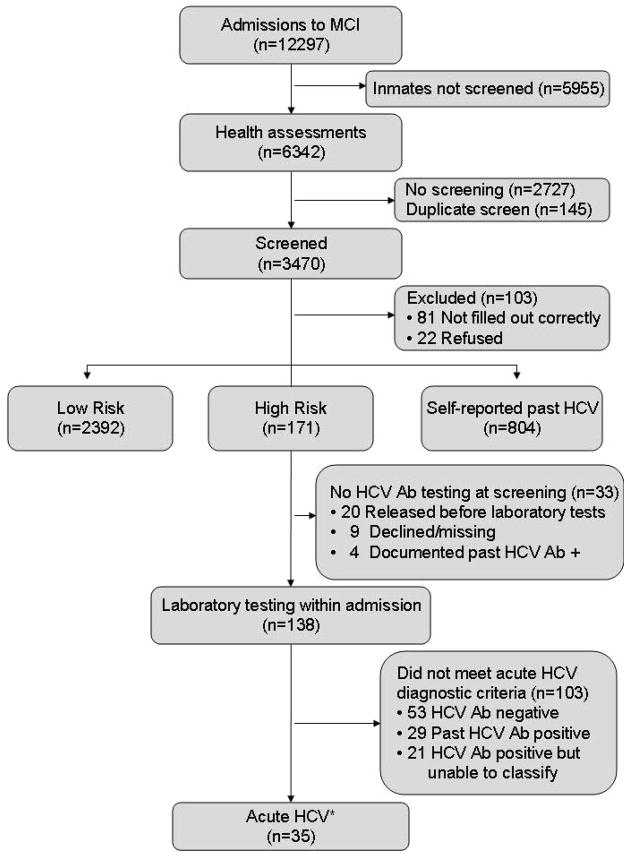
During an 18-month period, 6,034 men and 6,263 women were admitted to MCI-Concord and MCI-Framingham, respectively. Of these 12,297 inmates, 6,342 (52%) underwent health assessments within seven days of admission and 3470 inmates (55%) were screened (Figure 3). Primary reasons for lack of screening were understaffing, provider turnover, and unavailable forms during the medical intake; 22 male inmates (0.6%) refused screening while none of the women refused. Overall, 4.9% were classified as high risk, 68.9% were low risk, and 23.2% self-reported past HCV infection (Table 1). Women were more likely than men to self-report a past positive HCV infection (OR 4.1, 95% CI 3.4–4.8) and more women than men were classified in the high-risk category for acute infection (OR 3.6, 95% CI 2.6–5.0).
Figure 3. Flow of patients, classified by screening status.
Ab: antibody
HCV: hepatitis C virus
MCI: Massachusetts Correctional Institute
Table 1.
Characteristics of the population screened for high-risk behaviors
| Total (n=3470) | Low-risk for acute HCV (n=2392) | Self-reported past HCV (n=804) | High-risk for acute HCV (n=171) | Acute HCV Cases (n=35) | ||
|---|---|---|---|---|---|---|
| No IVDU (n= 2096) | Past IVDU >12 months (n=277) | |||||
| Male Sex, No. (%) | 2241(64.6) | 1584 (75.6) | 169 (61.0) | 313 (38.9) | 60 (35.1) | 13 (37.1) |
| Age, Mean (SD) | 34 (9.7) | 34 (9.8) | 34 (9.4) | 36 (9.7) | 30 (7.0) | 29 (5.5) |
| Race/Ethnicity | ||||||
| Black, NH, No (%) | 834 (24.0) | 679 (32.4) | 28 (10.1) | 85 (10.6) | 5 (2.9) | 0 (0.0) |
| White, NH, No (%) | 1719 (49.5) | 793 (37.8) | 198 (71.5) | 534 (66.4) | 150 (87.7) | 32 (91.4) |
| Hispanic, No (%) | 771 (22.2) | 526 (25.1) | 44 (15.9) | 156 (19.4) | 12 (7.0) | 2 (5.7) |
| American Indian, No (%) | 8 (0.23) | 6 (0.29) | 0 (0.0) | 0 (0.0) | 2 (1.2) | 0 (0.0) |
| Asian, No (%) | 22 (0.63) | 22 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0.0) |
| Other, No (%) | 116 (3.3) | 70 (3.3) | 7 (2.5) | 29 (3.6) | 2 (1.2) | 1 (2.9) |
The 35 cases of acute HCV were identified from within the high risk group. 22 inmates refused the screening questionnaire, 81 questionnaires were incomplete. These inmates were not classified for probability of HCV infection.
Investigation of high-risk individuals
Our systematic screening efforts identified 171 high-risk men and women (9.5 persons/month). Further evaluation of these individuals led to a diagnosis of acute HCV infection in 35 patients (Figure 3) (15). Using the total number of inmates who were classified as high-risk as the denominator (n=171), the minimum prevalence of acute HCV was 20.5% [95% CI 0.14 to 0.26] (Figure 3). Among high-risk individuals, rates of acute HCV infection were similar between males (21.7%) and females (19.8%), suggesting that high-risk classification had a similar positive predictive value, regardless of gender. Using the total screened as the denominator (n=3470), the prevalence of acute HCV among newly incarcerated inmates was 1.0% [95% CI 0.7%–1.4%].
Thirty-three high-risk individuals were released prior to testing. Of the 138 high-risk inmates who did undergo laboratory testing, 50 were HCV seropositive, but could not be classified as having acute infection for the following reasons: a) the history of risk behavior exceeded 12 months, prior HCV seropositivity was documented, or HCV RNA was undetectable (n=29) or b) the inmate was released prior to an in-depth interview (n=21), including one inmate with an ALT>7xULN (Figure 3). These incomplete cases were not included in our estimates for acute HCV seroprevalence.
Demographics and risk factors for HCV acquisition
The high-risk classification was significantly more prevalent among Caucasians than their African-American counterparts (OR=16, 95% CI: 6.5–39.1). To determine if the racial distribution of cases was due to differential application of the screening strategy, we ascertained race and ethnicity of 3395 of 3470 (97.8%) screened individuals (Table 1). African-Americans constituted 32.0% of all male inmates screened, but only 0.6% of those classified as “high risk.” African-American females represented 9.6% of those screened, but only 2.3% of those classified as “high risk” (Table 1).
Thirty of 35 patients with acute HCV infection stated that their high-risk behavior included sharing needles for the first time or sharing with a new partner, while 5 of 35 patients disclosed that they shared other drug paraphernalia (i.e. cookers, cotton), a known risk for HCV transmission (19).
Comparison of screening and historical control periods
Prior to implementation of the screening questionnaire, we identified 21 individuals with acute HCV infection over a 30-month period from multiple healthcare sites within the Massachusetts DOC; the average rate of identification of HCV infection by referral alone was 0.7 cases/month (11). In contrast, the current screening effort yielded 35 cases over an 18-month period from only two sites within this same institutional system; this average rate of 1.94 cases/month represented nearly a three-fold increase in our case-finding rate. Most importantly, acute cases identified through screening were twice as likely to be asymptomatic (48.6%) compared to those identified during the historical control period (33.3%; relative risk 2.0, p=0.09) (12).
Age and demographic distribution of self-reported HCV among recently incarcerated inmates
Inmates who self-reported a positive test for HCV were classified as having past infection, per the algorithm described in the methods. We observed that similar racial/ethnicity trends noted for acute HCV infection also applied to a past history of HCV. While African-Americans made up 23.0% of all inmates screened under 30 years of age, they only comprised 4.2% of those self-reporting past HCV infection; by contrast non-Hispanic Caucasians made up 48.2% of inmates screened under 30 years of age, but comprised 78.2% of those with past infection.
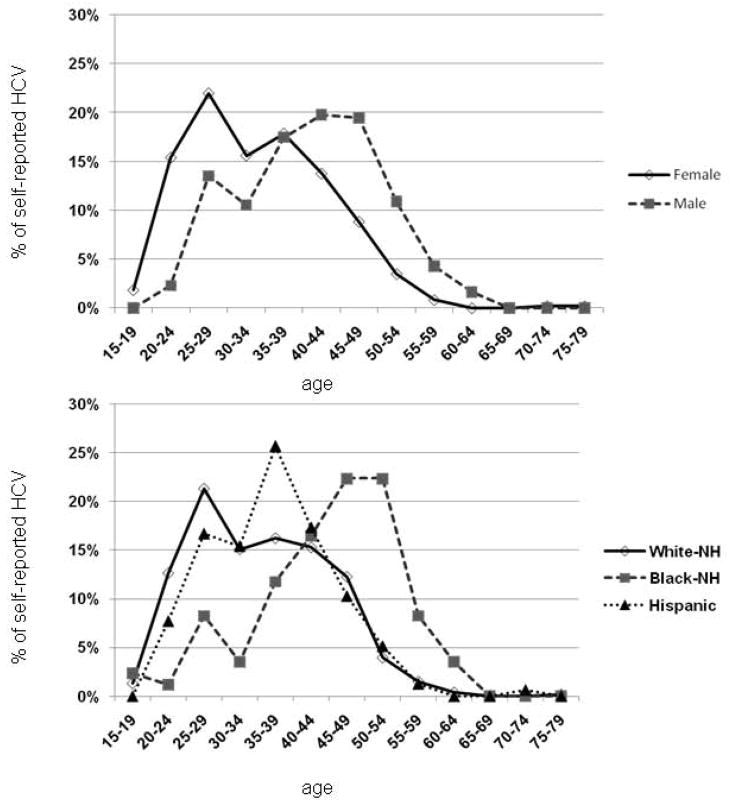
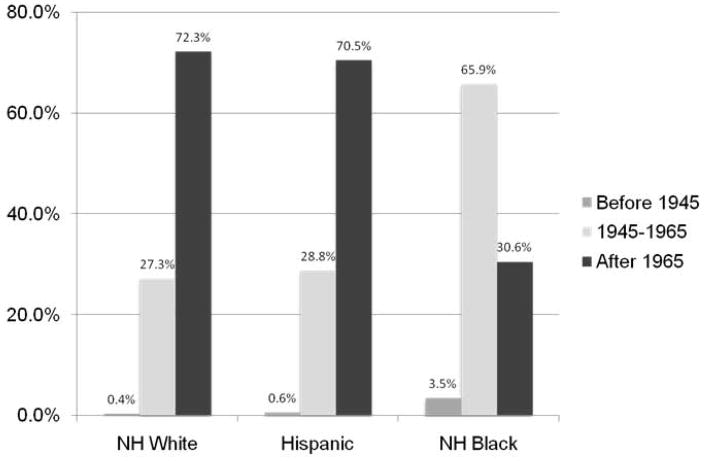
In Figure 4, we report the distribution of self-reported HCV by age, stratified by gender (A) and race (B). Only the age distribution of the African-Americans inmates resembled the expected shape from National Health and Nutritional Examination Survey (NHANES) data (20); in contrast, the age distribution of the other groups reflected the 2011 Massachusetts Department of Public Health (DPH) report showing an increase in confirmed HCV cases among Caucasians (21). The CDC estimates that approximately three-fourths of community patients living with chronic HCV in the US were born within the birth cohort ranging from 1945 to 1965 (20). However, within the prison population, only 31.9% of the inmates who reported past HCV infection were born within this birth cohort while 67.3% percent were born after 1965 and 0.8% were born before 1945. The age distribution of self-reported HCV would suggest that baby-boomer targeted screening in this incarcerated setting would only capture 27.3% and 28.8% of infections among non-Hispanic Caucasians and Hispanics, respectively, while finding 66% of infections among African-Americans (Figure 5).
Figure 4. Distribution of self-reported HCV cases among Massachusetts inmates by age group, stratified by gender (A) and race/ethnicity (B).
HCV: hepatitis C virus
NH: non-Hispanic
Figure 5. Percentage of inmates reporting past HCV infection, by age cohort, stratified by race.
HCV: Hepatitis C virus
NH: non-Hispanic
DISCUSSION
The correctional system provides a window into the enormity of the HCV epidemic with a unique opportunity to target PWID, who comprise a significant proportion of inmates (22) Using simple historical questions applied systematically, we identified approximately 1 case of acute HCV infection for every 100 persons screened, including asymptomatic patients. Previously a large CDC-sponsored surveillance study of symptomatic acute HCV infection demonstrated that 72% of patients had a history of incarceration (3). Given that 739,132 individuals were newly incarcerated in prisons nationwide in 2008 (23), 7,000 new diagnoses of acute HCV infection could potentially be identified if screening strategies were systematically adopted.
Correctional systems can serve as sentinel sites for monitoring epidemiologic trends among PWID as they pass between prison walls and the communities where they live. As in our pilot study (11), we continued to find high rates of acute HCV infection primarily among young Caucasian men and women. These trends were not explained by inadequate sampling of other racial/ethnic groups. Our results are also consistent with several lines of national and local epidemiologic data that demonstrate changing racial trends in HCV acquisition. Recently published data by the CDC indicate that acute hepatitis C infection occurs more often in non-Hispanic whites than blacks (0.27 per 100,000 versus 0.11 per 100,000 population, respectively) (24). The Massachusetts DPH also reported a significant increase in HCV cases among white adolescents and young adults who reported IDU (21). These data reflect changing nationwide patterns of IDU that vary by age, ethnicity, and race, including a marked reduction of acute HCV infections among African-Americans as compared to non-Hispanic whites (25–29). The underpinning of these racial/ethnic trends should be explored to inform future prevention efforts (30).
In addition, we noted higher rates of discovery of acute and self-reported HCV among females compared to males, consistent with epidemiologic surveys by Massachusetts DPH from community-based surveillance; a 2011 report on newly-acquired HCV demonstrated that 58% of the cases <25 years of age occurred among women. Furthermore, a blinded Massachusetts DPH serosurvey conducted in 2000 within the same institutions also noted higher HCV seroprevalence rates among women compared to men (44% vs. 27%, respectively) (31), as observed in other prison cohorts (32, 33). Explanations for gender differences were not elucidated by our study, but are likely influenced by the dominant reasons for incarceration (e.g., drug-related offenses and prostitution among women vs. more violent offenses among men) (34, 35).
In 2006, the CDC discontinued its 25-year long surveillance system for acute HCV owing to low numbers of new symptomatic cases and a lack of resources to expand community-based testing sites (3). However, we have demonstrated that a real-life intervention targeted within correctional settings is feasible and has great potential for case identification among PWID, including asymptomatic individuals(36, 37). This streamlined questionnaire meets the mandate to seek and find HCV within difficult-to-reach populations, voiced by the CDC and the Institute of Medicine (6, 10). Furthermore, although some suggest that the incidence of cases had declined through 2006 (36), it has been difficult to fully capture trends among PWID due to their fragmented care. Moreover, new epidemics of HCV reported in young Caucasian drug-initiates (21, 29) likely render the CDC’s estimate of acute infections as conservative. A jail or prison-based surveillance system may help to elucidate the true burden of new infections among PWID (3, 22).
Our questionnaire enhanced the case-finding rate compared to a historical control period (11) including the identification of asymptomatic patients, who are less likely to spontaneously clear viremia (38). Identification of such individuals is particularly important since early treatment leads to high rates of sustained virologic clearance (39) and may decrease the risk of transmission to others upon release to the community. We, and others, have previously demonstrated that antiviral treatment for acute HCV infection is feasible and as successful in the correctional setting as it is in the community (17, 40). Although treatment efficacy rates for chronic HCV genotype-1 infection are now improved with the addition of specifically-targeted antiviral agents (41), these are at increased cost and toxicity compared to therapeutic interventions for acute infection (39). In addition to therapy, the structured environment of the prison system offers numerous opportunities for mental health assessments, HIV testing and counseling regarding prevention, hepatitis A and B immunizations and harm reduction programs to decrease risk of re-infection (6, 30, 42). These interventions were well-received with over 90 percent acceptance (data not shown).
The age distribution of patients with self-reported HCV infection in our prison population is distinct from that seen in the 1998–2008 NHANES survey (20). Persons born from 1945 to 1965 accounted for over three-fourths of all HCV-infected patients living in the United States; males were twice as likely to be infected as females and African-Americans exhibited the highest seroprevalence rates (20). In stark contrast, 68 percent of inmates with self-reported HCV infection were born outside this time period. Although we did not have laboratory confirmation of these past HCV infections, one study found that the predictive value of self-reported anti-HCV seropositivity among PWID was 94%, lending credence to our findings (5). Thus, our observations suggest that risk-factor based screening of incarcerated adults, in addition to community screening of those born in the birth cohort, would be effective complementary strategies for national HCV testing and treatment initiatives.
Our findings may be influenced by several limitations. Firstly, initial screening was not performed by trained research staff, which enhances the probability of errors. Secondly, only ~28% of all newly incarcerated inmates were screened; however, the racial/ethnic distribution of those screened was similar to the overall population. Importantly, we have likely underestimated the true prevalence of acute HCV for several reasons: (1) high-risk inmates who did not undergo complete evaluation were not included as potential cases, even if they had abnormal aminotransferases. (2) inmates may have underreported IDU due to stigma, fears of recrimination or loss of confidentiality (43), (3) inmates may have incorrectly reported their HCV serostatus, and (4) inmates already found to be seropositive would have been classified as having past infection, but may, in fact, have been recently infected or reinfected (44). We also could not determine the prevalence of acute HCV among low-risk individuals due to limitations in resources.
Our real-life screening approach should be validated in additional healthcare settings, such as emergency rooms, opiate substitution clinics, detoxification clinics, needle exchange programs, and other correctional facilities. As one modeling study suggests, this risk factor-based screening approach might also be cost-effective in finding new diagnoses (45). Implementation of screening protocols for acute HCV in high-risk populations represents a promising component of a comprehensive nationwide strategy for HCV prevention and surveillance (10). Furthermore, identification of those with chronic HCV infection in the prison setting would provide a golden opportunity for evaluating liver disease and providing therapeutic interventions (22).
Acknowledgments
Dr. Kim had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We especially thank the individuals who consented to take part in this study. We acknowledge Arthur Brewer, Thomas Groblewski, and Warren Ferguson of University of Massachusetts Medical School Correctional Health and the providers at MCI-Framingham and MCI-Concord for their support, especially Patricia Casella, Jessica Laprel, Jennifer O’Keefe, Laura Smith at MCI-Framingham and Rosalie Berry, Amie Dunbar, Jessica Fabry, Deirdre Kells, Khalid Mohammed, Joanne Pomerancz, and Edith Quintinella at MCI-Concord. We thank Daniel Church, Kimberly Page and Rochelle Walensky for careful review of the manuscript. This study was presented in part at the 45th Annual Meeting of the Infectious Disease Society of America, San Diego, CA, October 4–7, 2007 and at the 60th Annual Meeting of the American Association for the Study of Liver Diseases, Boston, MA, October 30-November 3, 2009.
References
- 1.Hahn JA, Page-Shafer K, Lum PJ, Ochoa K, Moss AR. Hepatitis C virus infection and needle exchange use among young injection drug users in San Francisco. Hepatology. 2001;34:180–187. doi: 10.1053/jhep.2001.25759. [DOI] [PubMed] [Google Scholar]
- 2.Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol. 2008;168:1099–1109. doi: 10.1093/aje/kwn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams IT, Bell BP, Kuhnert W, Alter MJ. Incidence and transmission patterns of acute hepatitis C in the United States, 1982–2006. Arch Intern Med. 2011;171:242–248. doi: 10.1001/archinternmed.2010.511. [DOI] [PubMed] [Google Scholar]
- 4.Edlin BR, Kresina TF, Raymond DB, Carden MR, Gourevitch MN, Rich JD, Cheever LW, et al. Overcoming barriers to prevention, care, and treatment of hepatitis C in illicit drug users. Clin Infect Dis. 2005;40 (Suppl 5):S276–285. doi: 10.1086/427441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagan H, Campbell J, Thiede H, Strathdee S, Ouellet L, Kapadia F, Hudson S, et al. Self-reported hepatitis C virus antibody status and risk behavior in young injectors. Public Health Rep. 2006;121:710–719. doi: 10.1177/003335490612100611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinbaum C, Lyerla R, Margolis HS. Prevention and control of infections with hepatitis viruses in correctional settings. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2003;52:1–36. quiz CE31-34. [PubMed] [Google Scholar]
- 7.Fox RK, Currie SL, Evans J, Wright TL, Tobler L, Phelps B, Busch MP, et al. Hepatitis C virus infection among prisoners in the California state correctional system. Clin Infect Dis. 2005;41:177–186. doi: 10.1086/430913. [DOI] [PubMed] [Google Scholar]
- 8.Bureau of Justice. [Accessed May 30, 2012];2011 http://bjs.ojp.usdoj.gov/index.cfm?ty=pbdetail&iid=2056.
- 9.Spaulding A, Greene C, Davidson K, Schneidermann M, Rich J. Hepatitis C in state correctional facilities. Prev Med. 1999;28:92–100. doi: 10.1006/pmed.1998.0418. [DOI] [PubMed] [Google Scholar]
- 10.Institute of Medicine. Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 11.McGovern BH, Wurcel A, Kim AY, Schulze zur Wiesch J, Bica I, Zaman MT, Timm J, et al. Acute hepatitis C virus infection in incarcerated injection drug users. Clin Infect Dis. 2006;42:1663–1670. doi: 10.1086/504327. [DOI] [PubMed] [Google Scholar]
- 12.Thomas DL. Acute hepatitis C: a window of opportunity. Clin Infect Dis. 2006;42:1671–1673. doi: 10.1086/504333. [DOI] [PubMed] [Google Scholar]
- 13.Massachusetts Department of Correction Research and Planning Division. 2007 Court Commitments to the Massachusetts Department of Correction, Publication No 08-133-03. 2008. [Google Scholar]
- 14.Hagan H, Pouget ER, Williams IT, Garfein RL, Strathdee SA, Hudson SM, Latka MH, et al. Attribution of hepatitis C virus seroconversion risk in young injection drug users in 5 US cities. J Infect Dis. 2010;201:378–385. doi: 10.1086/649783. [DOI] [PubMed] [Google Scholar]
- 15.McGovern BH, Birch CE, Bowen MJ, Reyor LL, Nagami EH, Chung RT, Kim AY. Improving the diagnosis of acute hepatitis C virus infection with expanded viral load criteria. Clin Infect Dis. 2009;49:1051–1060. doi: 10.1086/605561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wasley A, Miller JT, Finelli L. Surveillance for Acute Viral Hepatitis --- United States, 2005. MMWR. 2007;56:1–24. [PubMed] [Google Scholar]
- 17.McGovern BH, Nagami EH, Birch CE, Bowen MJ, Reyor LL, Chung RT, Kim AY. Rate of sustained virologic response in relation to baseline hepatitis C virus (HCV) RNA level and rapid virologic clearance in persons with acute HCV infection. J Infect Dis. 2009;200:877–881. doi: 10.1086/605444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox AL, Page K, Bruneau J, Shoukry NH, Lauer GM, Kim AY, Rosen HR, et al. Rare birds in North America: acute hepatitis C cohorts. Gastroenterology. 2009;136:26–31. doi: 10.1053/j.gastro.2008.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagan H, Thiede H, Weiss NS, Hopkins SG, Duchin JS, Alexander ER. Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public Health. 2001;91:42–46. doi: 10.2105/ajph.91.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 21.CDC. Hepatitis C virus infection among adolescents and young adults ---Massachusetts, 2002–2009. MMWR Morb Mortal Wkly Rep. 2011;60:537–541. [PubMed] [Google Scholar]
- 22.Spaulding AC, Thomas DL. Screening for HCV infection in jails. JAMA. 2012;307:1259–1260. doi: 10.1001/jama.2012.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabol WJ. Bureau of Justice Statistics. 2009. Prisoners in 2008; p. 8. [Google Scholar]
- 24.CDC. Summary of notifiable diseases--United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;59:1–111. [PubMed] [Google Scholar]
- 25.Des Jarlais DC, Perlis T, Arasteh K, Torian LV, Hagan H, Beatrice S, Smith L, et al. Reductions in hepatitis C virus and HIV infections among injecting drug users in New York City, 1990–2001. AIDS. 2005;19 (Suppl 3):S20–25. doi: 10.1097/01.aids.0000192066.86410.8c. [DOI] [PubMed] [Google Scholar]
- 26.Broz D, Ouellet LJ. Racial and ethnic changes in heroin injection in the United States: implications for the HIV/AIDS epidemic. Drug Alcohol Depend. 2008;94:221–233. doi: 10.1016/j.drugalcdep.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lelutiu-Weinberger C, Pouget ER, Des Jarlais DD, Cooper HL, Scheinmann R, Stern R, Strauss SM, et al. A meta-analysis of the hepatitis C virus distribution in diverse racial/ethnic drug injector groups. Soc Sci Med. 2009;68:579–590. doi: 10.1016/j.socscimed.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Substance Abuse and Mental Health Services Administration. NSDUH Series H-38A, HHS Publication No SMA 10-4586 Findings. 2010. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings Office of Applied Studies. [Google Scholar]
- 29.Notes from the field: hepatitis C virus infections among young adults - rural Wisconsin, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:358. [PubMed] [Google Scholar]
- 30.Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J Infect Dis. 2011;204:74–83. doi: 10.1093/infdis/jir196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eastman E, Rappaport E, DeMaria A, Werner B. Validation of self-reported risk as a method for identifying anti-hepatitis C positive individuals at time of intake physical examination at Massachusetts Department of Correction facilities. 129th Annual Meeting of the American Public Health Association; 2001; Atlanta, GA. 2001. [Google Scholar]
- 32.Calzavara L, Ramuscak N, Burchell AN, Swantee C, Myers T, Ford P, Fearon M, et al. Prevalence of HIV and hepatitis C virus infections among inmates of Ontario remand facilities. CMAJ. 2007;177:257–261. doi: 10.1503/cmaj.060416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poulin C, Alary M, Lambert G, Godin G, Landry S, Gagnon H, Demers E, et al. Prevalence of HIV and hepatitis C virus infections among inmates of Quebec provincial prisons. CMAJ. 2007;177:252–256. doi: 10.1503/cmaj.060760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doherty MC, Garfein RS, Monterroso E, Latkin C, Vlahov D. Gender differences in the initiation of injection drug use among young adults. J Urban Health. 2000;77:396–414. doi: 10.1007/BF02386749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boutwell AE, Allen SA, Rich JD. Opportunities to address the hepatitis C epidemic in the correctional setting. Clin Infect Dis. 2005;40 (Suppl 5):S367–372. doi: 10.1086/427455. [DOI] [PubMed] [Google Scholar]
- 36.CDC. [Accessed July 7, 2011]; http://www.cdc.gov/hepatitis/PDFs/disease_burden.pdf.
- 37.Cox AL, Netski DM, Mosbruger T, Sherman SG, Strathdee S, Ompad D, Vlahov D, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis. 2005;40:951–958. doi: 10.1086/428578. [DOI] [PubMed] [Google Scholar]
- 38.Maheshwari A, Ray S, Thuluvath PJ. Acute hepatitis C. Lancet. 2008;372:321–332. doi: 10.1016/S0140-6736(08)61116-2. [DOI] [PubMed] [Google Scholar]
- 39.Corey KE, Mendez-Navarro J, Gorospe EC, Zheng H, Chung RT. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat. 2010;17:201–207. doi: 10.1111/j.1365-2893.2009.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clin Infect Dis. 2009;49:561–573. doi: 10.1086/600304. [DOI] [PubMed] [Google Scholar]
- 41.Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429–2438. doi: 10.1056/NEJMcp1006613. [DOI] [PubMed] [Google Scholar]
- 42.Grebely J, Thomas DL, Dore GJ. HCV reinfection studies and the door to vaccine development. J Hepatol. 2009;51:628–631. doi: 10.1016/j.jhep.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Macalino GE, Dhawan D, Rich JD. A missed opportunity: hepatitis C screening of prisoners. Am J Public Health. 2005;95:1739–1740. doi: 10.2105/AJPH.2004.056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, Tobler L, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis. 2009;200:1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutton AJ, Edmunds WJ, Gill ON. Estimating the cost-effectiveness of detecting cases of chronic hepatitis C infection on reception into prison. BMC Public Health. 2006;6:170. doi: 10.1186/1471-2458-6-170. [DOI] [PMC free article] [PubMed] [Google Scholar]