Abstract
MicroRNAs are a primordial mechanism of gene expression control that appear to be crucial to cellular development and may play an important role in tumor development. Much is known about the genetics of medullary thyroid carcinomas, as approximately 25% are hereditary and harbor germ line activating mutations in the RET gene. Somatic RET mutations are also seen in roughly 50% of sporadic medullary thyroid carcinomas. Few studies, however, have evaluated the role of microRNA expression in these tumors. DNA and RNA were extracted from formalin-fixed paraffin-embedded tissue blocks of 15 medullary thyroid carcinomas [10 with RET mutations (3 hereditary) and 5 without RET mutations] and 5 non-tumor thyroid glands. miRNA expression of 754 targets was quantitated by real time PCR using the ABI OpenArray miRNA assay. Three miRNAs showed significant differential expression and were validated in a larger cohort of 59 cases by real-time PCR. Expression of potential downstream targets and upstream regulators were also investigated by real-time PCR. miR-375 and miR-10a were significantly overexpressed, while miR-455 was underexpressed in medullary thyroid carcinomas. Expression of all 3 miRNAs were validated in the larger cohort of cases (miR-375, p = 3.3×10−26; miR-10a, p = 5.6×10−14; miR-455, p = 2.4×10−4). No significant differences in miRNA expression were found between RET mutation positive and negative tumors nor between sporadic and hereditary tumors. Expression of the potential downstream targets of miR-375, YAP1 (a growth inhibitor) and SLC16a2 (a transporter of thyroid hormone), was downregulated in the tumors suggesting that miR-375 is a negative regulator of the expression of these genes. Thus, differential expression of miR-375, miR-10a and miR-455 may be important for tumor development and/or the reflect c-cell lineage of medullary thyroid carcinoma. Furthermore, the growth inhibitor YAP1 is identified as a potential important downstream target of miR-375.
Keywords: medullary thyroid carcinoma, microRNA, miR-375, miR-10a, miR-455, YAP1
Introduction
Medullary thyroid carcinoma is a neuroendocrine tumor with parafollicular (C-cell) differentiation that accounts for approximately 5% of all primary thyroid malignancies. It occurs in both hereditary (25%) and sporadic (75%) forms(Albores-Saavedra et al., 1985). Activating mutations in the RET proto-oncogene are responsible for hereditary medullary thyroid carcinoma (MEN2 syndrome and familial medullary thyroid carcinoma)(Carlson et al., 1994). Approximately 50% of sporadic cases also harbor activating RET mutations and, more recently, a subset of RET mutation negative cases have been shown to harbor RAS mutations(Boichard et al., 2012; Moura et al., 2011; Wells and Massimo Santoro, 2009). Although medullary thyroid carcinoma accounts for a small percentage of primary thyroid tumors, it is responsible for a disproportionately large number of thyroid cancer deaths due to its more aggressive behavior compared to well-differentiated papillary thyroid and follicular carcinoma. Overall, medullary thyroid carcinoma has a 10 year survival rate of 80% compared to 92% and 90% for papillary and follicular carcinomas, respectively(Gilliland et al., 1997). The prognosis of medullary thyroid carcinoma can be predicted by the particular RET mutation and also depends on stage at presentation(Gilliland et al., 1997; Modigliani et al., 1998). Locally advanced tumors and the presence of metastatic disease are associated with a 5-year survival of 40% or less (Modigliani et al., 1998).
MicroRNAs (miRNAs) are endogenous short single-stranded non-coding RNAs that are negative regulators of gene expression. They selectively bind to complementary 3’ UTR mRNAs and target them for either cleavage or translational repression (Zeng et al., 2003). To date, over 1000 human miRNAs have been identified. Although their functions have not been fully characterized, miRNAs are known to have important roles in regulating cell differentiation, proliferation and survival. It is estimated that 30% of human genes may be targeted by miRNAs (Lewis et al., 2005; N.-K. Liu and Xu, 2011; Xie et al., 2005). Furthermore, many studies have supported a role for miRNAs in human cancers (Bottoni et al., 2005; C. Croce, 2006; Garzon et al., 2006; Kumar et al., 2008; Lu et al., 2005; P. P. Medina et al., 2010; Takamizawa et al., 2004; Zhang et al., 2006). Through negative regulation of gene expression, miRNAs can function either as 'oncomiRs' to promote tumor growth and progression or as tumor suppressors (He et al., 2005). Altered miRNA expression has been detected in a variety of human cancers including esophageal, breast, gastric, colorectal, pancreatic and lung carcinoma (Lu et al., 2005; Nikitina et al., 2012).
Few studies have examined the role of miRNAs in medullary thyroid carcinoma (Abraham et al., 2011; Mian et al., 2012; Nikiforova et al., 2008). To date, only two miRNA array profiling studies of medullary thyroid carcinomas have been published (Abraham et al., 2011; Mian et al., 2012). Understanding miRNA expression patterns and their effects on gene expression may provide a better understanding of tumor development and progression and may also yield potential therapeutic targets. In this study, we evaluated the expression of over 700 miRNAs in a retrospective cohort of medullary thyroid carcinomas and found significant up-regulation of three key miRNAs compared to normal thyroid tissue. Several predicated targets of these miRNAs were evaluated and showed significant down-regulation, consistent with the effects of miRNA expression. These data suggest that expression of specific miRNAs is involved in medullary thyroid carcinoma, providing new insights to pathogenesis and possible treatment targets.
Materials and Methods
Case selection
The study was approved by the Human Research Protection Office of Washington University and the Institutional Review Board at the National Institute of Health. Archival, formalin-fixed, paraffin-embedded tissue blocks and slides from all available cases of medullary thyroid carcinoma from 1989 to 2009 were retrieved from the Department of Pathology and Immunology at the Washington University School of Medicine (St. Louis, MO). The hematoxylin and eosin (H&E)-stained sections were reviewed by the author (RDC) to confirm the diagnosis and determine suitability for inclusion in the study. Only cases with sufficient tumor tissue for RET mutation analysis and miRNA studies were included; tumor size was at least 5 × 5 mm and 2 mm in thickness. In total, 44 cases had sufficient material for testing. Ten RET mutation positive cases (including 5 with the most common mutation, M918T) and 5 RET mutation negative cases were selected at random for the initial miRNA array studies. Of note, none of these cases harbored RAS mutations, which have recently been described in a subset of RET mutation negative medullary thyroid carcinomas. Of the RET mutation positive cases, 3 were hereditary (MEN2A) and 7 were sporadic. Five non-tumor thyroid glands with the diagnosis of 'nodular hyperplasia' were selected at random for the miRNA array studies as controls. Tissue blocks corresponding to non-nodular areas of thyroid parenchyma were selected. For the validation studies, the remaining 29 medullary thyroid carcinomas (19 RET mutation positive, 8 of which were M918T) and 10 additional non-tumor thyroid glands were selected in a similar manner. Table 1 summarizes the clinical and molecular characteristics of the tumors evaluated on the miRNA array. Supplementary Table 1 summarizes the clinical and molecular characteristics of remaining cases used for the validation studies.
Table 1.
Clinical characteristics and RET mutation status of the 15 tumors selected for miRNA array profiling.
| Case | Age | Sex | RET mutation status | Hereditary? |
|---|---|---|---|---|
| 1 | 48 | F | ATG>ACG M918T | No |
| 2 | 66 | M | ATG>ACG M918T | No |
| 3 | 17 | M | ATG>ACG M918T | No |
| 4 | 32 | M | ATG>ACG M918T | No |
| 5 | 61 | M | ATG>ACG M918T | No |
| 6 | 44 | F | GTC>ATG V804M | No |
| 7 | 76 | M | TGC>CGC C634R | No |
| 8 | 26 | F | TGC>TAC C634Y | Yes (MEN2A) |
| 9 | 68 | M | TGC>TCC C618S | Yes (MEN2A) |
| 10 | 45 | M | TGC>GGC C609R | Yes (MEN2A) |
| 11 | 51 | F | No mutation | No |
| 12 | 59 | F | No mutation | No |
| 13 | 47 | M | No mutation | No |
| 14 | 58 | F | No mutation | No |
| 15 | 49 | M | No mutation | No |
M = Male; F = Female; MEN2A = Multiple endocrine neoplasia syndrome, type 2A
Nucleic Acid Extraction
Tissue blocks and slides were aligned and areas containing >70% tumor celluarity without significant necrosis or inflammation were selected and cored with 2 mm sterile punches. Total RNA was extracted from formalin fixed paraffin embedded tissue using the Ambion RecoverAll Kit (Ambion, Foster City, CA, USA) following the manufacturer’s instructions for RNA extraction. DNA and RNA quantity was determined by spectrophotometric methods using a Nanodrop 2000 (Thermoscientific, Wilmington, DE, USA).
RET and RAS mutation analysis
Targeted analysis of RET mutations in exons 10, 11, and 13–16 was performed by pyrosequencing as described previously (Tamburrino et al., 2012). RAS mutations were searched in genes H-, N-, and K-RAS by exome sequencing as previously described (Tamburrino et al., 2012).
miRNA array profiling
The expression levels of 754 miRNAs were profiled using the stem-loop RT-PCR-based TaqMan Openarray MicroRNA Panel (Life Technologies, Foster City, CA, USA) following manufacturer’s instructions. Briefly, 100ng total RNA was added to each well before the reverse transcription step using Megaplex RT primers (Life Technologies). Next, a preamplification step was performed using Megaplex PreAmp primers (Life Technologies). The diluted sample was then subjected to real-time PCR analysis using 754 unique miRNA primers on the TaqMan OpenArray platform. Ct (crossing threshold) values for each miRNA were calculated automatically using the supplied software.
qPCR of miRNA and mRNA
miRNAs with expression that was statistically significantly different between medullary thyroid carcinoma and non-tumor thyroid after correction for the number of tests performed (Bonferroni correction) were selected for validation in the larger cohort of cases. Validation of the these miRNAs in the larger cohort of cases (44 tumors and 15 non-tumor thyroid glands) was performed by real time PCR on a Life Technologies 7500 instrument using TaqMan miRNA assays with TaqMan® Universal Master Mix II according to standard protocol (Life Technologies, Foster City, CA, USA). To ensure sensitivity, 500ng of total RNA was added to each well before reverse transcription with a miRNA specific primer. One-fifteenth of the RT reaction was used in a qPCR reaction using the TaqMan 2× universal master mix and the following cycle conditions: 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds. All samples were run in duplicate and the resulting Ct values averaged.
Potential downstream mRNA targets of miRNA inhibition and one upstream mRNA regulator of miRNA expression were selected based on a combination of miRNA target prediction (using the TargetScan database, targetscan.org) and literature review. The selected miRNA targets included: NCOR2, MDM4, RASD1, YAP1 and SLC16a2. One upstream miRNA regulator was also included: ASH1. Validation of potential downstream mRNA targets of the differentially expressed miRNAs was performed using realtime PCR. To ensure adequate mRNA abundance, preamplification of cDNA was performed using the Taqman PreAmp Master Mix Kit (Life Technologies) using the recommended protocol. Briefly, 500ng of total RNA was reverse transcribed using random hexamer primers. Preamplification was performed by 14 cycles of 95°C denaturation for 15 seconds followed by 4 minutes of annealing and extension at 60°C using a dilute mixture of the supplied TaqMan miRNA expression primers. The resulting products were then diluted 15 fold and 1uL added to individual 20uL miRNA expression reactions using specific TaqMan primers/probes. Amplification was performed using 40 cycles of 95°C extension followed by 1 minute of 60°C annealing and extension.
Statistical analysis
Unpaired two-tailed Student t-tests were used to determine significant differential expression of miRNAs between medullary thyroid carcinoma and non-tumor thyroid tissue, RET mutation positive and negative tumors, RET M918T mutation and all other RET mutation harboring tumors, and hereditary and sporadic tumors. Multiple comparison corrections were estimated by multiplying the number of tests performed by the p-value (Bonferroni correction, p-value multiplier of 754). Unpaired two-tailed Student t-tests were used to determine mRNA expression differences between medullary thyroid carcinoma and non-tumor thyroid tissue. Statistical analysis was performed using Excel (Microsoft Corporation, Seattle, WA, USA).
Results
Seventy-one human miRNAs were differently expressed in medullary thyroid carcinoma compared to non-tumor thyroid tissue on the ABI Openarray miRNA based on a p-value cut off of 0.05. The top 10 differentially expressed genes are shown in Table 2. However, after multiple comparison correction, only three microRNAs retained statistical significance between medullary thyroid carcinoma and non-tumor thyroid tissue, including miR-375 (485 fold increase in expression, p=0.0003), miR-10a (23 fold increase in expression, p=0.024), and miR-455 (4 fold decrease in expression, p=0.036). There were no significant differences in miRNA expression between RET mutation positive and negative medullary thyroid carcinoma or between M918T and all other RET mutation harboring tumors nor were there significant differences in miRNA expression between hereditary and sporadic medullary thyroid carcinoma after multiple comparison correction (data not shown).
Table 2.
Top 10 most differentially expressed miRNAs between medullary thyroid carcinoma and non-tumor thyroid tissue.
| miRNA | p-value (uncorrected) | Direction change in tumor |
|---|---|---|
| hsa-miR-375 | 5.07 ×10−7* | upregulated |
| hsa-miR-10a | 3.23 ×10−5* | upregulated |
| hsa-miR-455 | 4.80 ×10−5* | downregulated |
| hsa-miR-409-3p | 9.32 ×10−5 | upregulated |
| hsa-miR-190b | 0.000103 | upregulated |
| hsa-miR-642 | 0.000382 | upregulated |
| hsa-miR-20b | 0.000719 | downregulated |
| hsa-miR-99b* | 0.000748 | upregulated |
| hsa-miR-30a-3p | 0.000834 | downregulated |
| hsa-miR-889 | 0.00103 | upregulated |
Retained significance after correction for the number of tests performed
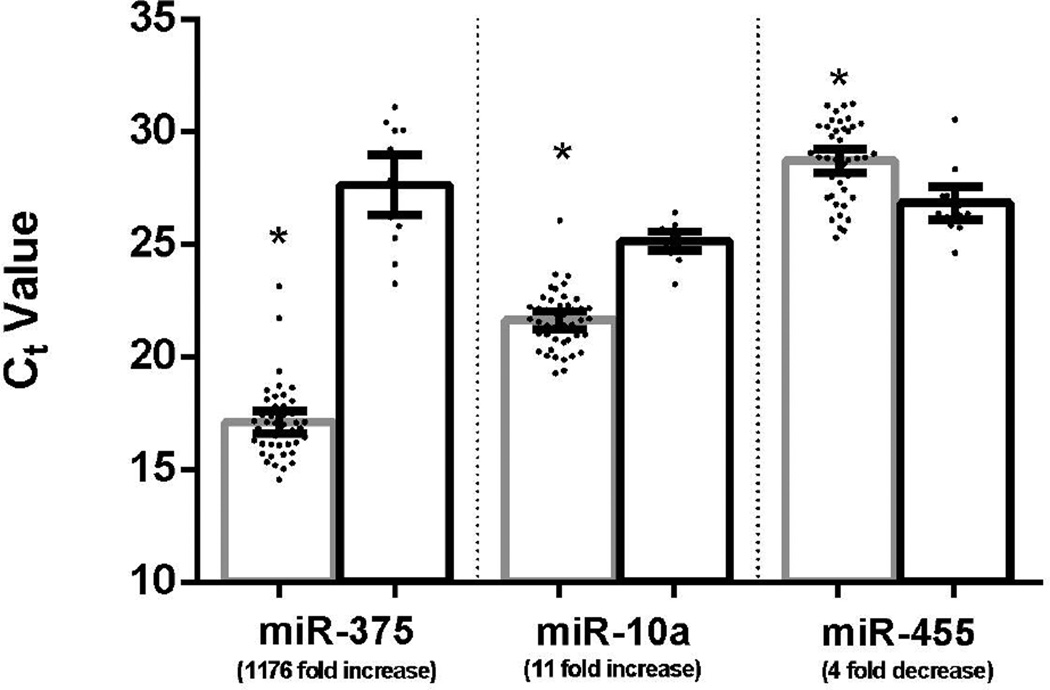
The altered expression of miR-375, miR-10a, and miR-455 in medullary thyroid carcinoma was validated in the larger cohort of cases (44 tumors and 15 non-tumor thyroid glands) by qPCR. The original 15 tumors and 5 non-tumor thyroid glands run on the initial miRNA array studies were included in this larger group. The expression patterns identified on the miRNA array were confirmed in the larger set of cases (Figure 1). By qPCR, miR-375 showed an 1176 fold higher level in medullary thyroid carcinoma compared to non-tumor thyroid glands (Ct value of 17.4 in tumor versus 27.6 in non-tumor, p<3.3×10−26). For miR-10a, there was approximately an 11-fold higher expression level in medullary thyroid carcinoma compared to non-tumor thyroid glands (Ct value of 21.6 for tumor versus 25.1 for non-tumor, p<5.6×10−14). miR-455 showed 4 fold lower expression in medullary thyroid carcinoma (Ct value of 28.7 for tumor versus 26.8 for non-tumor, p=2.4×10−4).
Figure 1. Validation of miR-375, miR-10a and miR-455 expression by realtime PCR.
Differential expression of miR-375, miR-10a and miR-455 between medullary thyroid carcinoma and non-tumor thyroid tissue was confirmed in the larger cohort of 59 cases by realtime PCR (* indicates p-value < 0.05). Ct value comparison between tumor (gray) and non-tumor (black) are shown. Error bars demonstrate 95% confidence intervals.
Next, mRNA expression of potential downstream targets and upstream regulators of miR-375 and miR-10a was investigated. miR-375 and miR-10a were selected for further investigation because these were the two most upregulated miRNAs in medullary thyroid carcinoma and review of the literature also supported their role in human malignancy (De Souza Rocha Simonini et al., 2010; Nishikawa et al., 2011; Poy et al., 2009). For miR-375, potential downstream targets (YAP1, SLC16a2 and RASD1) and an upstream regulator (ASH1) were selected for evaluation. YAP1 was selected because it is a growth inhibitor that has previously been shown to be negatively regulated by miR-375 in small cell carcinoma of the lung, another type of neuroendocrine carcinoma (Nishikawa et al., 2011). Furthermore, miR-375 inhibition of YAP1 was shown to be regulated by ASH1 (Nishikawa et al., 2011). SLC16a2 was chosen because it was the top predicted target of miR-375 (targetscan.org) with a known role in the transport of thyroid hormone (Kinne et al., 2011; Poy et al., 2009).Thus, one would anticipate lower expression of SLC16a2 in medullary thyroid carcinoma compared to non-tumor thyroid tissue, since only the latter is composed predominately of follicular cells, which have a role in thyroid hormone transport. RASD1 is another top predicted target with a known role in human malignancy(De Souza Rocha Simonini et al., 2010). For miR-10a, the potential downstream targets MDM4 (murine double minute 4) and NCOR2 (nuclear receptor co-repressor 2) were chosen because of their known miR-10a-mediated down regulation in acute myeloid leukemia and neuroblastoma (Foley et al., 2011; Ovcharenko et al., 2011).
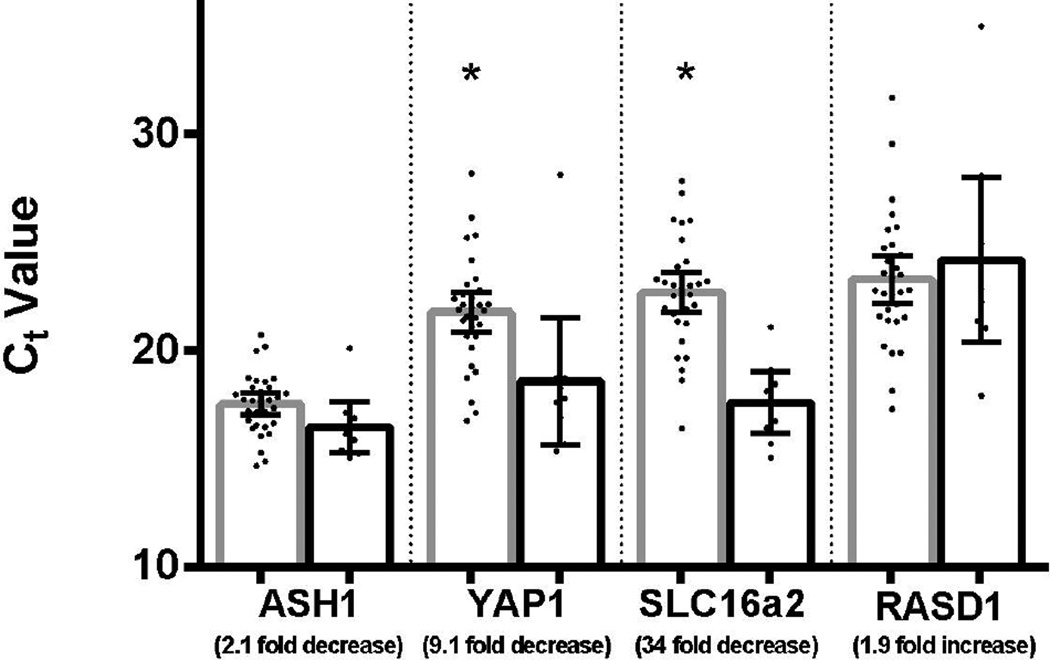
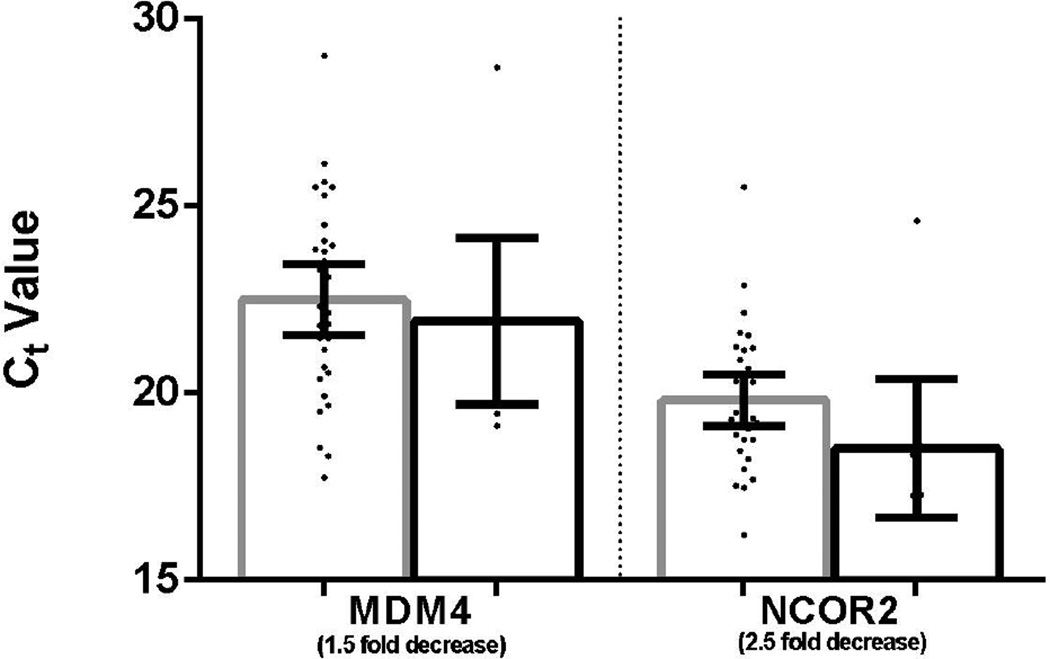
Of these mRNAs, the miR-375 predicted targets YAP1 and SLC16a2 showed decreased expression in medullary thyroid carcinoma relative to non-tumor thyroid tissue suggesting that they may be down-regulated by miR-375 (p=0.005and p=1.9×106, respectively; Figure 2). The potential upstream regulator of miR-375, ASH1, showed no significant difference in expression. Expression of the predicted miR-375 target, RASD1, also was not different between the two groups. Nor were there significant differences in expression of the potential downstream targets of miR-10a (MDM4 and NCOR2) in a subset of the cases evaluated (31 tumors and 9 non-tumor thyroid glands) showing the most differential expression of miR10a (Figure 3). The expression levels of the predicted miRNA targets and regulator in medullary thyroid carcinoma and non-tumor thyroid tissue are summarized in Table 3.
Figure 2. Expression of predicted mRNA targets and regulator of miR-375 by realtime PCR.
The predicted targets of miR-375, YAP1 and SCL6a2, show decreased expression in medullary thyroid carcinoma compared to non-tumor thyroid tissue, as expected (* indicates p-value < 0.05). However, there is no difference in expression of ASH1 or RASD1. Ct value comparison between tumor (gray) and non-tumor (black) are shown. Error bars demonstrate 95% confidence intervals.
Figure 3. Expression of predicted mRNA targets of miR-10a by realtime PCR.
There is no difference in expression of the miR-10a predicted targets MDM4 and NCOR2 between medullary thyroid carcinoma and non-tumor thyroid tissue. Ct value comparison between tumor (gray) and non-tumor (black) are shown. Error bars demonstrate 95% confidence intervals.
Table 3.
Expression patterns of predicted mRNA targets and regulator of miRNA in medullary thyroid carcinoma.
| Change relative to non-tumor thyroid |
p-value | Ct Tumor | Ct Non-Tumor | |
|---|---|---|---|---|
| miR-375 targets | ||||
| YAP1 | 9 fold decrease | 0.005 | 21.8 | 18.6 |
| SLC16a2 | 34 fold decrease | 1.9×10−6 | 22.7 | 17.6 |
| RASD1 | Not significant | 0.50 | 23.3 | 24.2 |
| miR-375 regulator | ||||
| ASH1 | Not significant | 0.056 | 17.5 | 16.5 |
| miR-10a targets | ||||
| MDM4 | Not si gnificant | 0.58 | 22.5 | 21.9 |
| NCOR2 | Not significant | 0.09 | 19.8 | 18.5 |
Ct = Crossing time
Discussion
The role of miRNAs in the development and progression of medullary thyroid carcinomas has not been well examined. To our knowledge, only two other array-based studies have evaluated miRNA expression in medullary thyroid carcinoma (Lu et al., 2005; Xie et al., 2005). Nikiforova et al (2008) were the first to investigate miRNA expression in 2 cases of medullary thyroid carcinoma as part of a larger study of all types of thyroid tumors (Nikiforova et al., 2008). Although only 2 cases were evaluated, one of the top 10 most upregulated miRNAs in medullary thyroid carcinoma compared to non-tumor thyroid was miR-10a (Nikiforova et al., 2008), which we have also found to be upregulated. miR-375, the most significantly upregulated miRNA in our study, was not included in their smaller panel of 158 miRNAs. A subsequent study of miRNA expression in medullary thyroid carcinoma that utilized a larger array of over 1000 miRNAs and evaluated 45 tumors did find miR-375 to be among a panel of miRNAs (miR-375, miR-183 and miR-9*) significantly over expressed in sporadic compared to hereditary carcinomas (Abraham et al., 2011). Furthermore, miR-375 expression was associated with more aggressive behavior including residual disease, lymph node metastases, distant metastases, and mortality (Abraham et al., 2011). Mian et al (2012) confirmed overexpression of miR-375 in both medullary thyroid carcinoma and c-cell hyperplasia but was not able to document a difference in miR-375 expression between hereditary or sporadic tumors or any correlation with worse patient outcome (Mian et al., 2012). Similarly, we found no significant difference in miR-375 expression between hereditary and sporadic tumors, although our number of hereditary tumors was small (3 hereditary tumors used in the array subset and 11 hereditary tumors in the validation set). We also did not find differential miRNA expression by RET mutation status after stringent correction for the number of tests performed. The only miRNA found to be significantly underexpressed by medullary thyroid carcinoma in our study was miR-455. Alteration in miR-455 expression in medullary thyroid carcinoma was not previously reported in any of the other studies (Abraham et al., 2011; Mian et al., 2012; Nikiforova et al., 2008).
The effect of altered miRNA expression on target mRNA expression has not been previously investigated in medullary thyroid carcinoma. Here, we investigated several potential downstream targets and one potential upstream regulator of miR-375 and miR-10a, the two overexpressed miRNAs in our study of medullary thyroid carcinoma. Of these, we found the potential targets of miR-375, YAP1 and SLC16a2, to have lower expression levels in medullary thyroid carcinoma compared to non-tumor thyroid glands. This is compatible with miR-375 mediated inhibition of gene expression.
It is not surprising that SLC16a2 is down regulated in medullary thyroid carcinomas relative to non-tumor thyroid tissue. SLC16a2 is known to encode the thyroid hormone transporter MCT8. This transporter is critical for thyroid hormone transport and inactivating mutations in SLC16a2 result in Allan-Herndon-Dudley syndrome (AHDS), an X-linked syndrome that results in severe mental retardation (Kinne et al., 2011). Higher expression of SLC16a2 in non-tumor thyroid tissue likely simply reflects the overwhelming predominance of follicular cells in this tissue, which are involved in thyroid hormone synthesis and transport. In contrast, medullary thyroid carcinoma is derived from C-cells which do not play a role in thyroid hormone synthesis or transport. Thus, differential expression likely simply represents different cell lineage. Unfortunately, although normal C-cells would be the ideal control to compare medullary thyroid carcinoma miRNA expression to, they are simply not available for this due to their scarcity in normal thyroid tissue and the inability to isolate them from tissue specimens.
On the other hand, expression of YAP1 (Yes-associated protein 1) has a greater likelihood of being related to tumor development or progression in medullary thyroid carcinoma. YAP1 is a transcriptional coactivator that can exert both oncogenic and tumor suppressive activities and is a known target of miR-375 (Nishikawa et al., 2011). Recently, it was shown that ASH1 mediated activation of miR-375 was important for neuroendocrine differentiation in small cell carcinoma of the lung (Nishikawa et al., 2011). ASH1 knockouts led to apoptosis of neuroendocrine cells, and ASH1 introduction into adenocarcinoma cell lines caused neuroendocrine differentiation (Nishikawa et al., 2011). Furthermore, the miR-375 target YAP1 caused lineage specific cell inhibition of proliferation when virally transfected into small cell carcinoma cell lines but not in adenocarcinoma cell lines (Nishikawa et al., 2011). Thus, it appears that ASH1 induces miR-375 expression, which in turn inhibits YAP1 and leads to tumor cell proliferation in a pathway specific to lung tumors with neuroendocrine differentiation (Nishikawa et al., 2011). It is possible that miR-375 and YAP1 have similar roles in medullary thyroid carcinoma, which, like small cell carcinoma, is also of neuroendocrine lineage, even though we did not find ASH1 to be over expressed in medullary thyroid carcinoma. Further investigation of the role of YAP1 in medullary thyroid carcinoma is warranted.
There are several possible explanations for why we did not observe down regulation of any of the other potential miRNA targets examined by real-time PCR analysis. One explanation is that the miRNAs are inducing translational repression in these targets rather than degradation so that the mRNA is still measurable by PCR, although it is not functional. Translational repression is a well-known and frequent consequence of small RNA interference (J. Liu et al., 2005). Alternatively, the role of miRNA may be to 'fine-tune' mRNA expression leading to only subtle differences in mRNA expression levels (Sevignani et al., 2006). Lastly, it is possible that lower quality of mRNA obtained from formalin-fixed paraffin embedded tissue had an effect on the measurement of mRNA levels by PCR. Formalin-fixed paraffin embedded tissue appears to be an excellent substrate to analyze miRNA expression, which is in short ~20bp segments, but since mRNA is longer than miRNA, it may be more affected by the cross-linking effects of formalin.
In summary, utilizing a large miRNA array, we found that miR-375 and miR-10a are overexpressed, while miR-455 is underexpressed in medullary thyroid carcinoma. miRNA expression did not appear to vary by RET mutation status. Furthermore, we found that YAP1, a growth inhibitor that is known to be downregulated in small cell lung carcinoma by miR-375, is also downregulated in medullary thyroid carcinoma. Thus, miR-375 mediated downregulation of YAP1 may be important for tumor development or progression in medullary thyroid carcinomas as well. Further investigation of this pathway is warranted.
Supplementary Material
Highlights.
We examined the expression of over 700 miRNAs in medullary thyroid carcinoma (MTC)
miR-375 and miR-10a were over expressed and miR-455 was under expressed in MTC
A known target of miR-375 and a growth inhibitor, YAP1, was down regulated in MTC
Inhibition of YAP1 by miR-375 may be important for MTC development or progression
Acknowledgements
We thank Xiaopei Zhu M.D. for technical assistance with the PCR experiments and James S Lewis Jr. for critical review of the manuscript. We also thank Chris Sawyer and the Washington University Genome Technology Access Core (GTAC) for their assistance running PCR arrays. The GTAC is partially supported by NCI Cancer Center Support Grant #P30 CA91842 to the Siteman Cancer Center and by ICTS/CTSA Grant# UL1RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest: The authors have no conflict of interest to declare.
References
- Abraham D, Jackson N, Gundara JS, Zhao J, Gill AJ, Delbridge L, Robinson BG, Sidhu SB. MicroRNA profiling of sporadic and hereditary medullary thyroid cancer identifies predictors of nodal metastasis, prognosis, and potential therapeutic targets. Clinical Cancer Research. 2011;17:4772–4781. doi: 10.1158/1078-0432.CCR-11-0242. [DOI] [PubMed] [Google Scholar]
- Albores-Saavedra J, LiVolsi VA, Williams ED. Medullary carcinoma. Seminars in diagnostic pathology. 1985;2:137–146. [PubMed] [Google Scholar]
- Boichard A, Croux L, Al Ghuzlan A, Broutin S, Dupuy C, Leboulleux S, Schlumberger M, Bidart JM, Lacroix L. Somatic RAS mutations occur in a large proportion of sporadic RET-negative medullary thyroid carcinomas and extend to a previously unidentified exon. The Journal of clinical endocrinology and metabolism. 2012;97:E2031–E2035. doi: 10.1210/jc.2012-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottoni A, Piccin D, Tagliati F, Luchin A, Zatelli MC, degli Uberti EC. miR-15a and miR-16-1 down-regulation in pituitary adenomas. Journal of Cellular Physiology. 2005;204:280–285. doi: 10.1002/jcp.20282. [DOI] [PubMed] [Google Scholar]
- Carlson KM, Dou S, Chi D, Scavarda N, Toshima K, Jackson CE, Wells SA, Jr, Goodfellow PJ, Donis-Keller H. Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:1579–1583. doi: 10.1073/pnas.91.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. MicroRNAs in leukemia. Clinical Advances in Hematology & Oncology. 2006;4:577–578. [PubMed] [Google Scholar]
- De Souza Rocha Simonini P, Breiling A, Gupta N, Malekpour M, Youns M, Omranipour R, Malekpour F, Volinia S, Croce CM, Najmabadi H, Diederichs S, Sahin O, Mayer D, Lyko F, Hoheisel JD, Riazalhosseini Y. Epigenetically deregulated microRNA-375 is involved in a positive feedback loop with estrogen receptor alpha in breast cancer cells. Cancer Research. 2010;70:9175–9184. doi: 10.1158/0008-5472.CAN-10-1318. [DOI] [PubMed] [Google Scholar]
- Foley NH, Bray I, Watters KM, Das S, Bryan K, Bernas T, Prehn JH, Stallings RL. MicroRNAs 10a and 10b are potent inducers of neuroblastoma cell differentiation through targeting of nuclear receptor corepressor 2. Cell Death and Differentiation. 2011;18:1089–1098. doi: 10.1038/cdd.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon R, Fabbri M, Cimmino A, Calin GA, Croce CM. MicroRNA expression and function in cancer. Trends in Molecular Medicine. 2006;12:580–587. doi: 10.1016/j.molmed.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer. 1997;79:564–573. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinne A, Schulein R, Krause G. Primary and secondary thyroid hormone transporters. Thyroid Res. 2011;4(Suppl 1):S7. doi: 10.1186/1756-6614-4-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA, Jacks T. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3903–3908. doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nature Cell Biology. 2005;7:719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N-K, Xu X-M. MicroRNA in central nervous system trauma and degenerative disorders. Physiological genomics. 2011;43:571–580. doi: 10.1152/physiolgenomics.00168.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- Mian C, Pennelli G, Fassan M, Balistreri M, Barollo S, Cavedon E, Galuppini F, Pizzi M, Vianello F, Pelizzo MR, Girelli ME, Rugge M, Opocher G. MicroRNA profiles in familial and sporadic medullary thyroid carcinoma: preliminary relationships with RET status and outcome. Thyroid. 2012;22:890–896. doi: 10.1089/thy.2012.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modigliani E, Cohen R, Campos JM, Conte-Devolx B, Maes B, Boneu A, Schlumberger M, Bigorgne JC, Dumontier P, Leclerc L, Corcuff B, Guilhem I. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. The GETC Study Group. Groupe d’étude des tumeurs à calcitonine. Clinical endocrinology. 1998;48:265–273. doi: 10.1046/j.1365-2265.1998.00392.x. [DOI] [PubMed] [Google Scholar]
- Moura MM, Cavaco BM, Pinto AE, Leite V. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. The Journal of clinical endocrinology and metabolism. 2011;96:E863–E868. doi: 10.1210/jc.2010-1921. [DOI] [PubMed] [Google Scholar]
- Nikiforova MN, Tseng GC, Steward D, Diorio D, Nikiforov YE. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. Journal of Clinical Endocrinology and Metabolism. 2008;93:1600–1608. doi: 10.1210/jc.2007-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitina EG, Urazova LN, Stegny VN. MicroRNAs and human cancer. Experimental Oncology. 2012;34:2–8. [PubMed] [Google Scholar]
- Nishikawa E, Osada H, Okazaki Y, Arima C, Tomida S, Tatematsu Y, Taguchi A, Shimada Y, Yanagisawa K, Yatabe Y, Toyokuni S, Sekido Y, Takahashi T. miR-375 is activated by ASH1 and inhibits YAP1 in a lineage-dependent manner in lung cancer. Cancer Research. 2011;71:6165–6173. doi: 10.1158/0008-5472.CAN-11-1020. [DOI] [PubMed] [Google Scholar]
- Ovcharenko D, Stolzel F, Poitz D, Fierro F, Schaich M, Neubauer A, Kelnar K, Davison T, Muller-Tidow C, Thiede C, Bornhauser M, Ehninger G, Brown D, Illmer T. miR-10a overexpression is associated with NPM1 mutations and MDM4 downregulation in intermediate-risk acute myeloid leukemia. Experimental Hematology. 2011;39:1030 e7–1042 e7. doi: 10.1016/j.exphem.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P, Zavolan M, Stoffel M. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5813–5818. doi: 10.1073/pnas.0810550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevignani C, Calin GA, Siracusa LD, Croce CM. Mammalian microRNAs: a small world for fine-tuning gene expression. Mammalian Genome. 2006;17:189–202. doi: 10.1007/s00335-005-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Research. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- Tamburrino A, Molinolo AA, Salerno P, Chernock RD, Raffeld M, Xi L, Gutkind JS, Moley JF, Wells SA, Jr, Santoro M. Activation of the mTOR pathway in primary medullary thyroid carcinoma and lymph node metastases. Clinical Cancer Research. 2012;18:3532–3540. doi: 10.1158/1078-0432.CCR-11-2700. [DOI] [PubMed] [Google Scholar]
- Wells SA, Santoro Massimo. Targeting the RET pathway in thyroid cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:7119–7123. doi: 10.1158/1078-0432.CCR-08-2742. [DOI] [PubMed] [Google Scholar]
- Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha V, Lindblad-Toh K, Lander ES, Kellis M. Systematic discovery of regulatory motifs in human promoters and 3’ UTRs by comparison of several mammals. Nature. 2005;434:338–345. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Yi R, Cullen BR. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9779–9784. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Huang J, Yang N, Greshock J, Megraw MS, Giannakakis A, Liang S, Naylor TL, Barchetti A, Ward MR, Yao G, Medina A, O’Brien-Jenkins A, Katsaros D, Hatzigeorgiou A, Gimotty PA, Weber BL, Coukos G. microRNAs exhibit high frequency genomic alterations in human cancer. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9136–9141. doi: 10.1073/pnas.0508889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.