Abstract
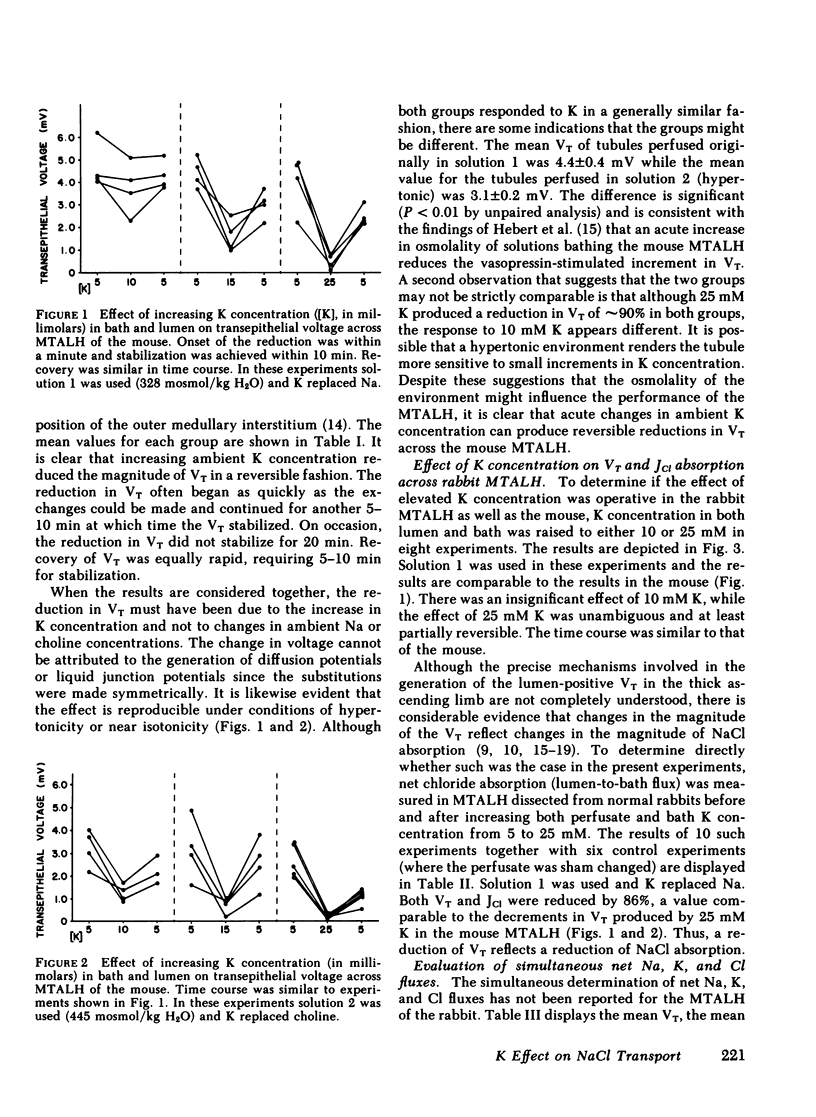
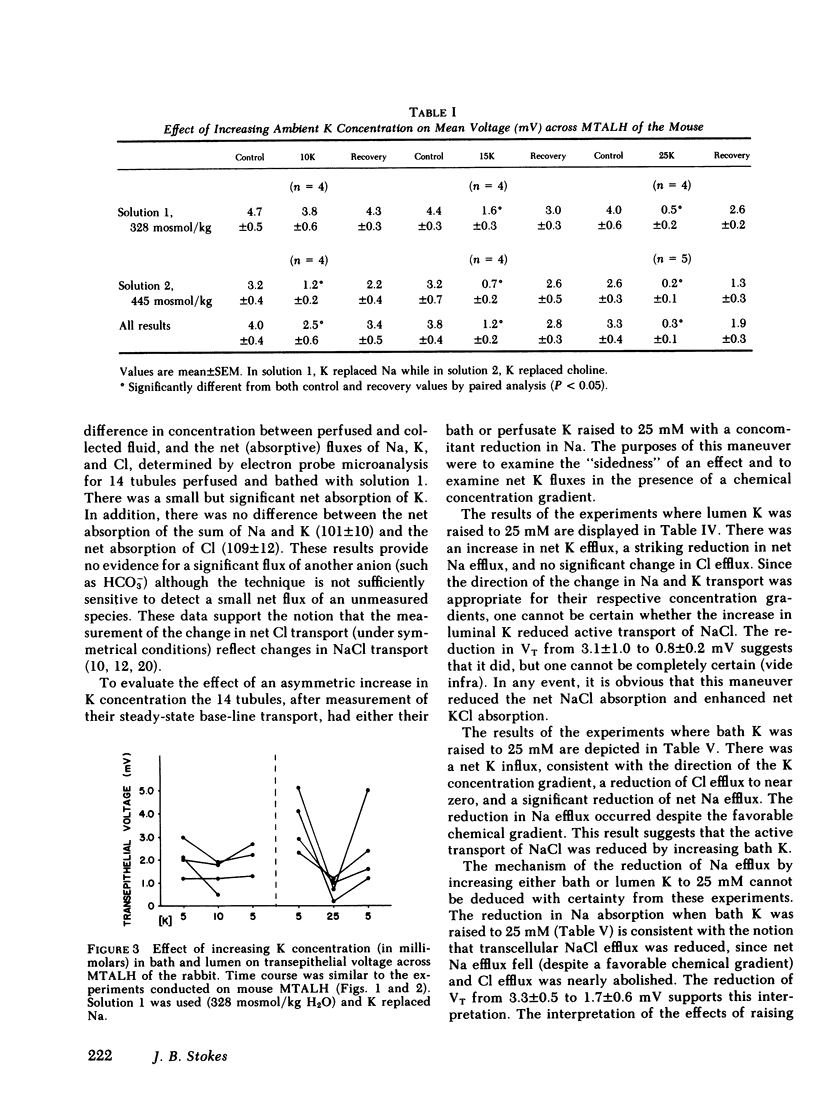
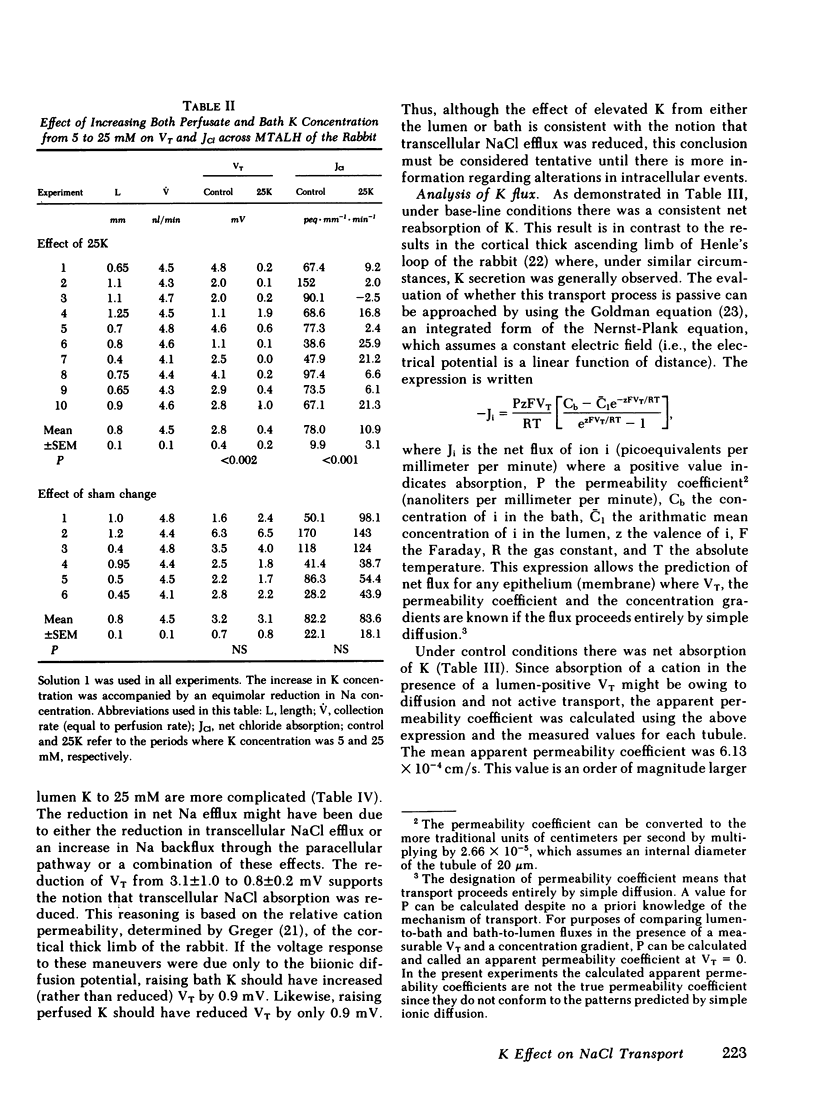
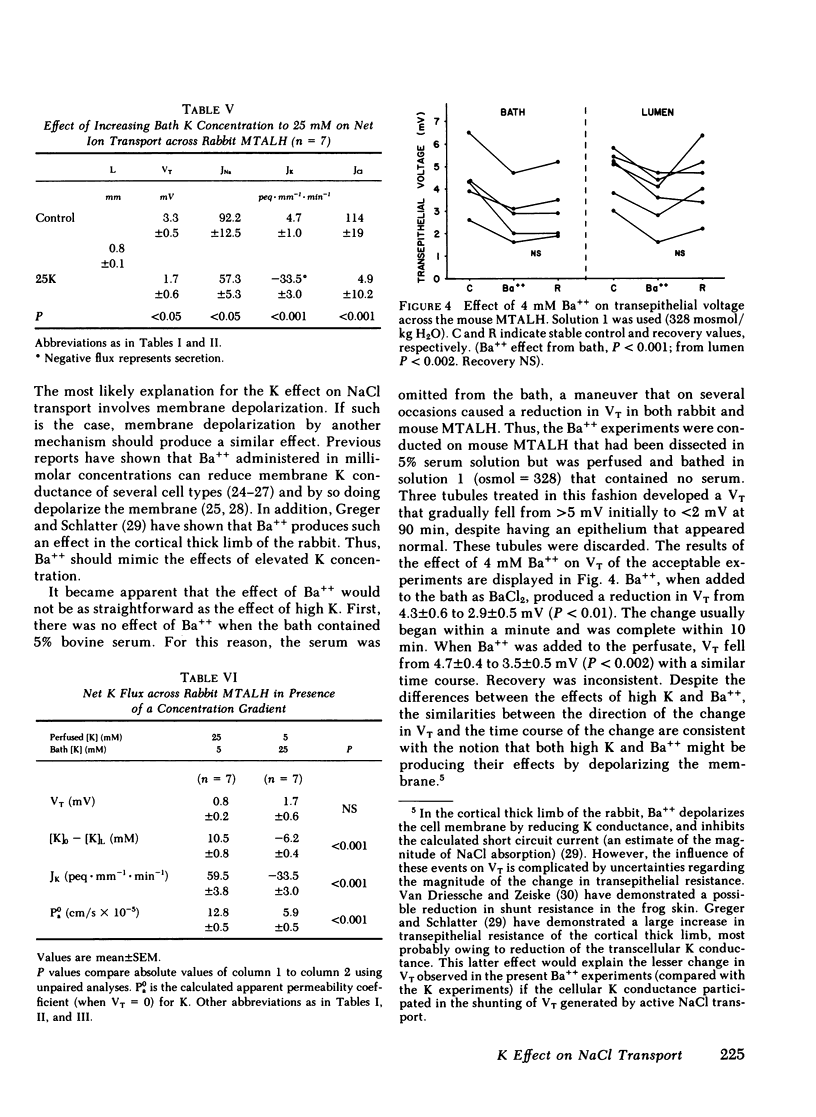
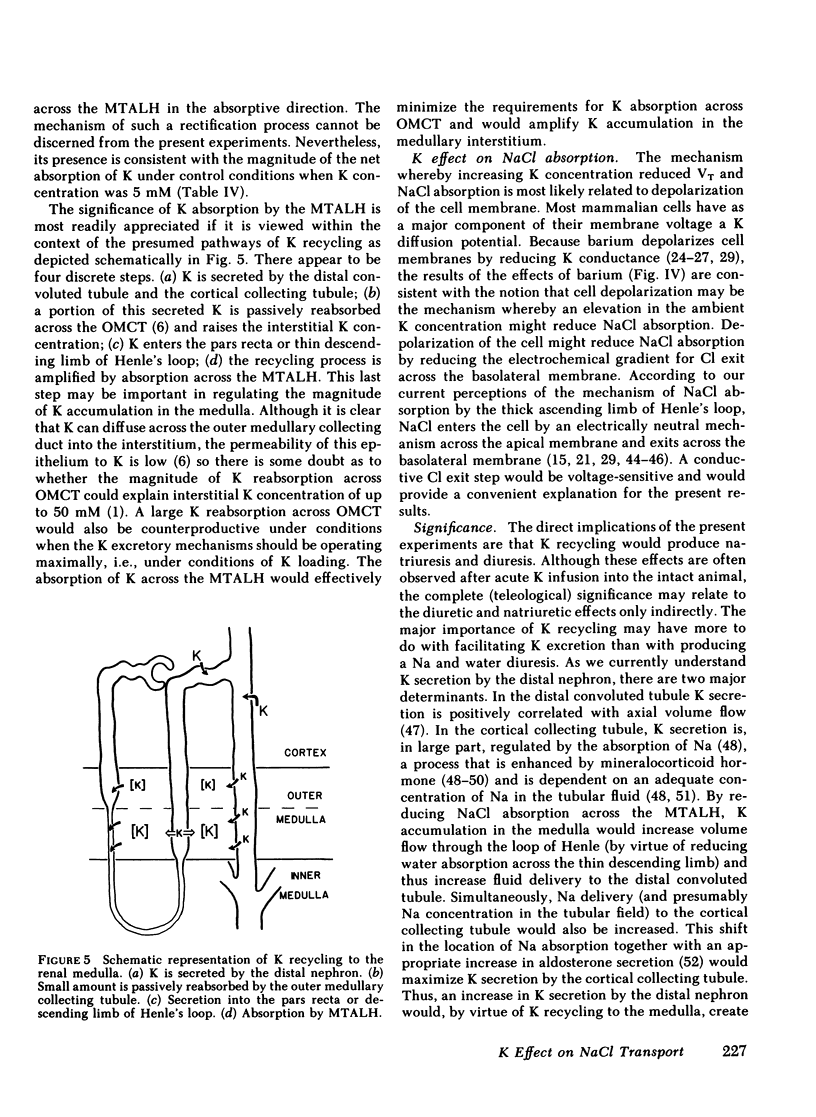
The consequences of K recycling and accumulation in the renal medulla were examined by measuring the effect of elevated K concentration on ion transport by the medullary thick ascending limb of Henle's loop. Perfused and bathed in vitro, thick limbs from both mouse and rabbit displayed a graded, reversible reduction of transepithelial voltage after increasing K concentration from 5 to 10, 15, or 25 mM. The effect was reproducible whether osmolality was 328 or 445 mosmol/kg H2O, and whether K replaced Na or choline. Net chloride absorption and transepithelial voltage were reduced by almost 90% when ambient K concentration was 25 mM. When either lumen or bath K was increased to 25 mM, net Na absorption was reduced. There was spontaneous net K absorption when perfusate and bath K concentration was 5 mM. Analysis of transepithelial K transfer after imposition of chemical gradients demonstrated rectification in the absorptive direction. Absorption of K by this segment provides a means to maintain high medullary interstitial concentration. Accumulation of K in the outer medulla, by reducing NaCl absorption, would increase volume flow through the loop of Henle and increase Na and water delivery to the distal nephron. K recycling thus might provide optimum conditions for K secretion by the distal nephron.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Taylor S. R. Interaction of barium ions with potassium channels in squid giant axons. Biophys J. 1980 Jun;30(3):473–488. doi: 10.1016/S0006-3495(80)85108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrascue J. F., Dobyan D. C., Jamison R. L. Potassium recycling in the renal medulla: effects of acute potassium chloride administration to rats fed a potassium-free diet. Kidney Int. 1981 Sep;20(3):348–352. doi: 10.1038/ki.1981.145. [DOI] [PubMed] [Google Scholar]
- Battilana C. A., Dobyan D. C., Lacy F. B., Bhattacharya J., Johnston P. A., Jamison R. L. Effect of chronic potassium loading on potassium secretion by the pars recta or descending limb of the juxtamedullary nephron in the rat. J Clin Invest. 1978 Nov;62(5):1093–1103. doi: 10.1172/JCI109215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandis M., Keyes J., Windhager E. E. Potassium-induced inhibition of proximal tubular fluid reabsorption in rats. Am J Physiol. 1972 Feb;222(2):421–427. doi: 10.1152/ajplegacy.1972.222.2.421. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Cannon P. J., Ames R. P., Laragh J. H. Relation between potassium balance and aldosterone secretion in normal subjects and in patients with hypertensive or renal tubular disease. J Clin Invest. 1966 Jun;45(6):865–879. doi: 10.1172/JCI105402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal J., Duchesneau D. Effect of potassium on proximal tubular function. Am J Physiol. 1978 May;234(5):F381–F385. doi: 10.1152/ajprenal.1978.234.5.F381. [DOI] [PubMed] [Google Scholar]
- Dobyan D. C., Lacy F. B., Jamison R. L. Suppression of potassium-recycling in the renal medulla by short-term potassium deprivation. Kidney Int. 1979 Dec;16(6):704–709. doi: 10.1038/ki.1979.186. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Field M., Schultz S. G. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol. 1979 Jan;236(1):F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good D. W., Wright F. S. Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am J Physiol. 1979 Feb;236(2):F192–F205. doi: 10.1152/ajprenal.1979.236.2.F192. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Kurg M. B., Obloff J. The nature of transtubular Na and K transport in isolated rabbit renal collecting tubules. J Clin Invest. 1970 Oct;49(10):1815–1826. doi: 10.1172/JCI106399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger R. Cation selectivity of the isolated perfused cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1981 Apr;390(1):30–37. doi: 10.1007/BF00582707. [DOI] [PubMed] [Google Scholar]
- Greger R. Chloride reabsorption in the rabbit cortical thick ascending limb of the loop of Henle. A sodium dependent process. Pflugers Arch. 1981 Apr;390(1):38–43. doi: 10.1007/BF00582708. [DOI] [PubMed] [Google Scholar]
- Greger R. Coupled transport of Na+ and Cl- in the thick ascending limb of Henle's loop of rabbit nephron. Scand Audiol Suppl. 1981;14 (Suppl):1–15. [PubMed] [Google Scholar]
- Greger R., Schlatter E. Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1981 Nov;392(1):92–94. doi: 10.1007/BF00584588. [DOI] [PubMed] [Google Scholar]
- Hall D. A., Varney D. M. Effect of vasopressin on electrical potential difference and chloride transport in mouse medullary thick ascending limb of Henle's loop. J Clin Invest. 1980 Oct;66(4):792–802. doi: 10.1172/JCI109917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert S. C., Culpepper R. M., Andreoli T. E. NaCl transport in mouse medullary thick ascending limbs. I. Functional nephron heterogeneity and ADH-stimulated NaCl cotransport. Am J Physiol. 1981 Oct;241(4):F412–F431. doi: 10.1152/ajprenal.1981.241.4.F412. [DOI] [PubMed] [Google Scholar]
- Hebert S. C., Culpepper R. M., Andreoli T. E. NaCl transport in mouse medullary thick ascending limbs. II. ADH enhancement of transcellular NaCl cotransport; origin of transepithelial voltage. Am J Physiol. 1981 Oct;241(4):F432–F442. doi: 10.1152/ajprenal.1981.241.4.F432. [DOI] [PubMed] [Google Scholar]
- Hebert S. C., Culpepper R. M., Andreoli T. E. NaCl transport in mouse medullary thick ascending limbs. III. Modulation of the ADH effect by peritubular osmolality. Am J Physiol. 1981 Oct;241(4):F443–F451. doi: 10.1152/ajprenal.1981.241.4.F443. [DOI] [PubMed] [Google Scholar]
- Hermsmeyer K. Ba2+ and K+ alteration of K+ conductance in spontaneously active vascular muscle. Am J Physiol. 1976 Apr;230(4):1031–1036. doi: 10.1152/ajplegacy.1976.230.4.1031. [DOI] [PubMed] [Google Scholar]
- Jamison R. L., Bennett C. M., Berliner R. W. Countercurrent multiplication by the thin loops of Henle. Am J Physiol. 1967 Feb;212(2):357–366. doi: 10.1152/ajplegacy.1967.212.2.357. [DOI] [PubMed] [Google Scholar]
- Jamison R. L., Lacy F. B., Pennell J. P., Sanjana V. M. Potassium secretion by the decending limb or pars recta of the juxtamedullary nephron in vivo. Kidney Int. 1976 Apr;9(4):323–332. doi: 10.1038/ki.1976.38. [DOI] [PubMed] [Google Scholar]
- Johnston P. A., Battilana C. A., Lacy F. B., Jamison R. L. Evidence for a concentration gradient favoring outward movement of sodium from the thin loop of Henle. J Clin Invest. 1977 Feb;59(2):234–240. doi: 10.1172/JCI108633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M., Bohrer N. K. Effect of potassium-induced diuresis on renal concentration and dilution. Am J Physiol. 1967 Jun;212(6):1365–1375. doi: 10.1152/ajplegacy.1967.212.6.1365. [DOI] [PubMed] [Google Scholar]
- Kokko J. P. Membrane characteristics governing salt and water transport in the loop of Henle. Fed Proc. 1974 Jan;33(1):25–30. [PubMed] [Google Scholar]
- Nagel W. Inhibition of potassium conductance by barium in frog skin epithelium. Biochim Biophys Acta. 1979 Apr 4;552(2):346–357. doi: 10.1016/0005-2736(79)90289-x. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G., Helman S. I. Transport characteristics of renal collecting tubules: influences of DOCA and diet. Am J Physiol. 1977 Dec;233(6):F544–F558. doi: 10.1152/ajprenal.1977.233.6.F544. [DOI] [PubMed] [Google Scholar]
- Ramsay A. G., Gallagher D. L., Shoemaker R. L., Sachs G. Barium inhibition of sodium ion transport in toad bladder. Biochim Biophys Acta. 1976 Jul 1;436(3):617–627. doi: 10.1016/0005-2736(76)90445-4. [DOI] [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Membrane characteristics regulating potassium transport out of the isolated perfused descending limb of Henle. Kidney Int. 1973 Nov;4(5):326–330. doi: 10.1038/ki.1973.124. [DOI] [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest. 1973 Mar;52(3):612–623. doi: 10.1172/JCI107223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S., Imai M. Effects of vasopressin on water and NaCl transport across the in vitro perfused medullary thick ascending limb of Henle's loop of mouse, rat, and rabbit kidneys. Pflugers Arch. 1980 Feb;383(3):215–221. doi: 10.1007/BF00587521. [DOI] [PubMed] [Google Scholar]
- Schafer J. A., Troutman S. L., Andreoli T. E. Volume reabsorption, transepithelial potential differences, and ionic permeability properties in mammalian superficial proximal straight tubules. J Gen Physiol. 1974 Nov;64(5):582–607. doi: 10.1085/jgp.64.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. J., Burg M. B. Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am J Physiol. 1978 Dec;235(6):F576–F585. doi: 10.1152/ajprenal.1978.235.6.F576. [DOI] [PubMed] [Google Scholar]
- Seldin D. W., Rosin J. M., rector F. C., Jr Evidence against bicarbonate reabsorption in the ascending limb, particularly as disclosed by free-water clearance studies. Yale J Biol Med. 1975 Sep;48(4):337–347. [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N., Schneider M. F., Harris E. J. Decreased K+ conductance produced by Ba++ in frog sartorius fibers. J Gen Physiol. 1967 Jul;50(6):1565–1583. doi: 10.1085/jgp.50.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes J. B. Effect of prostaglandin E2 on chloride transport across the rabbit thick ascending limb of Henle. Selective inhibitions of the medullary portion. J Clin Invest. 1979 Aug;64(2):495–502. doi: 10.1172/JCI109487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes J. B., Ingram M. J., Williams A. D., Ingram D. Heterogeneity of the rabbit collecting tubule: localization of mineralocorticoid hormone action to the cortical portion. Kidney Int. 1981 Sep;20(3):340–347. doi: 10.1038/ki.1981.144. [DOI] [PubMed] [Google Scholar]
- Stokes J. B., Kokko J. P. Inhibition of sodium transport by prostaglandin E2 across the isolated, perfused rabbit collecting tubule. J Clin Invest. 1977 Jun;59(6):1099–1104. doi: 10.1172/JCI108733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes J. B. Na and K transport across the cortical and outer medullary collecting tubule of the rabbit: evidence for diffusion across the outer medullary portion. Am J Physiol. 1982 May;242(5):F514–F520. doi: 10.1152/ajprenal.1982.242.5.F514. [DOI] [PubMed] [Google Scholar]
- Stokes J. B. Potassium secretion by cortical collecting tubule: relation to sodium absorption, luminal sodium concentration, and transepithelial voltage. Am J Physiol. 1981 Oct;241(4):F395–F402. doi: 10.1152/ajprenal.1981.241.4.F395. [DOI] [PubMed] [Google Scholar]
- ULLRICH K. J., KRAMER K., BOYLAN J. W. Present knowledge of the counter-current system in the mammalian kidney. Prog Cardiovasc Dis. 1961 Mar;3:395–431. doi: 10.1016/s0033-0620(61)80001-7. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Zeiske W. Ba2+-induced conductance fluctuations of spontaneously fluctuating K+ channels in the apical membrane of frog skin (Rana temporaria). J Membr Biol. 1980 Aug 21;56(1):31–42. doi: 10.1007/BF01869349. [DOI] [PubMed] [Google Scholar]
- Vander A. J. Direct effects of potassium on renin secretion and renal function. Am J Physiol. 1970 Aug;219(2):455–459. doi: 10.1152/ajplegacy.1970.219.2.455. [DOI] [PubMed] [Google Scholar]
- Work J., Troutman S. L., Schafer J. A. Transport of potassium in the rabbit pars recta. Am J Physiol. 1982 Mar;242(3):F226–F237. doi: 10.1152/ajprenal.1982.242.3.F226. [DOI] [PubMed] [Google Scholar]
- Wright F. S. Sites and mechanisms of potassium transport along the renal tubule. Kidney Int. 1977 Jun;11(6):415–432. doi: 10.1038/ki.1977.60. [DOI] [PubMed] [Google Scholar]
- Wright F. S., Strieder N., Fowler N. B., Giebisch G. Potassium secretion by distal tubule after potassium adaptation. Am J Physiol. 1971 Aug;221(2):437–448. doi: 10.1152/ajplegacy.1971.221.2.437. [DOI] [PubMed] [Google Scholar]
- de Rouffignac C., Morel F. Micropuncture study of water, electrolytes, and urea movements along the loops of henle in psammomys. J Clin Invest. 1969 Mar;48(3):474–486. doi: 10.1172/JCI106005. [DOI] [PMC free article] [PubMed] [Google Scholar]



