Abstract
Dexamethasone (DEX) is often given for the treatment of rheumatoid arthritis and clinical dosing regimens of DEX have often been based empirically. This study tests whether the inflammation processes in a rat model of rheumatoid arthritis alters the clearance and volume of distribution of DEX when compared with healthy controls. Groups of healthy and arthritic male Lewis rats received either a low (0.225 mg/kg) or high (2.25 mg/kg) intramuscular dose of DEX. Arthritis was induced by intradermal injection of type II porcine collagen in incomplete Freund's adjuvant emulsion at the base of the tail. DEX was dosed in the arthritic animals 22 days post arthritis induction. Plasma DEX concentrations were determined by HPLC. Plasma concentration versus time data were analysed by non-compartmental analysis and pharmacokinetic model fitting using the population pharmacokinetic software NONMEM V. A linear bi-exponential pharmacokinetic model with extravascular input described the data for both healthy and arthritic animals. Clearance was the only parameter determined statistically different between both groups (healthy=1.05 l/h/kg, arthritic=1.19 l/h/kg). The steady-state volume of distribution for both groups was 4.85 l/kg. The slight difference in clearance was visibly undetectable and unlikely to produce meaningful changes in DEX disposition in arthritic rats.
Keywords: dexamethasone, pharmacokinetics, arthritis, collagen
Introduction
Glucocorticoids were discovered for the treatment of rheumatoid arthritis (RA) in 1949 and today their synthetic analogs, corticosteroids, remain a mainstay in treatment of the disease [1–3]. Dosing of these compounds has often been based empirically. Dexamethasone (DEX) is very potent in this class of drugs having a fluorinated structure and increased affinity for the glucocorticoid receptor [2–5]. While the kinetics of DEX in healthy individuals and patients with arthritis are well characterized, the pharmacokinetics (PK) of this drug in various animal models of rheumatoid arthritis has not been examined.
Animal models of RA are valuable as a means to quantify disease progression and drug effect as measured by specific molecular biomarkers and disease endpoints. Although not a complete model of the genetically complex disease in humans, these models offer insight into a specific pathology of RA and chronic inflammation [6]. Collagen-induced arthritis in the Lewis rat is a recent and clinically relevant model of RA [6–8]. Rats can be utilized for destructive tissue sampling and a rich set of time dependent drug-effect data may be generated. Before DEX concentrations can be related to the effect in these arthritis models, its PK need to be appreciated. Additionally, such PK data may lend insight into the mechanisms of drug distribution and elimination potentially relevant to the clinical scenario.
Inflammation affects many organs throughout the body. Chronic inflammation can possibly alter the clearance, distribution, and unbound and total plasma concentrations of DEX. Garg et al. showed a 2.4-fold decrease in clearance and a reduction of 10–30% in the plasma protein binding of prednisolone elevating both total and free concentrations of the steroid in animals with carrageen-induced chronic inflammation. Acute and chronic inflammatory conditions in rats have caused changes in liver expression of enzymes such as cytochrome P450 isozymes and p-glycoprotein relevant to the in vivo metabolism and distribution of DEX [3,9–12]. Piquette-Miller showed that hepatic PGP expression was reduced 50–70% following administration of LPS in the rat. Assenat et al. and Pascussi et al. showed that TNF-α, IL-1β and IL-6 inhibit constitutive androstane receptor (CAR) and pregnane x-receptor expression (PXR), reducing CYP450 expression in primary human hepatocytes and that NF-κB interferes with the glucocorticoid response element located in the promoter regions of CAR and PXR genes. Vuppugalla noted an acute inhibitory effect of nitric oxide on cytochrome P450 activity. Furthermore, Ling et al. demonstrated reduced clearance of verapamil by cytochrome P450 isozymes in early phase adjuvant induced arthritis in the rat. Wang et al. showed a redistribution of macromolecules from blood to inflamed tissue during adjuvant arthritis in the rat, potentially explaining the decrease in plasma protein binding observed by Garg et al. for prednisolone. Thus inflammation in an animal model of arthritis could reduce the clearance, alter the distribution and decrease the plasma protein binding of DEX [13–19].
This study compares the PK of DEX in a rat model of rheumatoid arthritis with the kinetics of DEX in healthy rats. The time course of plasma concentrations is presented for both healthy and arthritic rats receiving 2.25 and 0.225 mg/kg intramuscular (IM) DEX. A PK model was developed to test for differences in parameters between treatment groups. Lewis rats were examined at the occurrence of peak peripheral joint edema.
Methods
Materials
Dexamethasone sodium phosphate was obtained from Henry Schein, Inc. (Melville, NY). Type II porcine collagen dissolved to 2 mg/ml in 0.5 m acetic acid was purchased from Chondrex, Inc. (Redmond, WA). Incomplete Freund's adjuvant was purchased from Sigma Aldrich (St Louis, MO).
Animals
Forty-eight male Lewis rats, age 6–9 weeks, were purchased from Harlan Sprague Dawley, Inc. weight matched to approximately 150 g. Animals were housed individually in the University Laboratory Animal Facility and acclimatized for 1 week under constant temperature (22°C), humidity (72%) and circadian light cycle (12 h light/12 h dark). Rats had free access to rat chow and water. All protocols followed Principles of Laboratory Animal Care (National Institute of Health publication 85-23, revised 1985) and were approved by the University at Buffalo Institutional Animal Care and Use Committee.
Induction of collagen induced arthritis
The induction of collagen induced arthritis (CIA) in Dark Agouti and Lewis rats followed protocols supplied by Chondrex, Inc. Porcine collagen type II (2 mg/ml) in 0.05 m acetic acid was emulsified with incomplete Freund's adjuvant using an electric homogenizer (Virtis) equipped with a blade of 10 mm diameter. Equal amounts of collagen (2 mg/ml) and Incomplete Freund's Adjuvant (IFA) were mixed in an ice water bath, adding the collagen drop-wise to the IFA at low speed. The Virtis speed was increased to 30 000 rpm for 2.5 min, then 0 rpm for 2.5 min, and a final mix at 30 000 rpm for 2.5 min. The emulsion was ready when it appeared to be a stiff white substance that congealed instead of dissipating when dropped in water. Thirty-two rats were anesthetized with ketamine/xylazine (75/10 mg/kg) and received 0.2 ml of collagen emulsion by intra-dermal injection at the base of the tail. Booster injections were administered on day 7 of the study with 0.1 ml emulsion at the same injection site. Disease status was measured by monitoring hind paw swelling and body weight on study days 9, 12, 15, 17, 19, 20, 21, 22 and 23.
Dexamethasone pharmacokinetic study
The PK of DEX was compared in arthritic rats and healthy controls. Healthy and arthritic male Lewis rats were dosed intramuscularly with either 0.27 or 2.7 mg/kg dexamethasone phosphate in saline (equivalent to 0.225 and 2.25 mg/kg dexamethasone). Animals with CIA were dosed 22 days post disease induction immediately after the peak edema in the hind paw was observed. On study day 21 arthritic rats underwent surgery to externally cannulate the jugular vein. Healthy animals underwent cannulation 1 week after arrival and 1 day prior to dosing. During surgery the rats were anesthetized with ketamine/xylazine (75/10 mg/kg). External cannulas were kept clear and functional with EDTA in saline (1.5 mg/ml).
To supply the desired number of sample times without taking too much blood from each rat, two sets of sampling schedules were used (A and B). Thirty-two rats were used in the study. Each disease group (CIA, healthy) had two dose levels (0.27, 2.7 mg/kg). Each dose level had two sampling schedules (A, B) with four rats in each group. Collagen was administered to 32 rats to ensure CIA developed in 16 animals, expecting an incidence of about 60%. Four rats that had developed arthritis by study day 17 were assigned to each dosing schedule for each dose level for a total of eight rats to be serial sampled for each dose level. Animals were considered to have developed arthritis if hind paw edema was clearly visible and swollen more than 150%, as measured by digital calipers, in both paws. Animals in dosing schedule A were sampled at 10 and 30 min, 1, 2, 4 and 8 h post dose with an additional 24 h time point in the high dose group. Animals in dosing schedule B were sampled at 20 and 45 min, 1.5, 3, 6 and 12 h post dose with an additional 24 h time point in the high dose group. Rats were killed at their last sample time by aortal exsanguinations under ketamine/xylazine anesthesia. Blood was collected in syringes containing sufficient EDTA to yield 1.5 mg/ml (4 mm) final concentration [20]. Samples were centrifuged at 1800 × g for 10 min at 4°C. Plasma was collected and stored at –20°C.
Corticosteroid extraction and HPLC analysis
Rat plasma was thawed and 0.1–1.0 ml aliquots were extracted with methylene chloride in 7 ml glass Pyrex tubes (Corning Glass Works, Corning, NY). Tubes were shaken for 45 min before washing the methylene chloride phase with 0.5 ml of 0.1n sodium hydroxide. The NaOH phase was removed following centrifugation and the methylene chloride phase was washed twice with 0.5 ml water, discarding the water after each wash. Methylene chloride was evaporated off with purified air, leaving a residue that was reconstituted in 110 μl of mobile phase. Chromatography conditions involved a mobile phase of 600 ml methylene chloride, 350 ml heptane, 10 ml glacial acetic acid and 54 ml ethanol, a Zorbax normal phase column, Waters model 1515 isocratic pump and Waters model 2487 dual λ absorbance detector [21]. The lower limit of quantification was 5 ng/ml with an intra-day coefficient of variation of less than 10%.
Pharmacokinetic data analysis
Plasma concentrations of DEX were modeled as a function of time with a linear two-compartment mammillary PK model with first-order absorption input. Initial estimates were obtained by non-compartmental analysis using WinNonlin 5.0 (Pharsight Corp., Mountain View, CA). The Bailer–Satterthwaite method was used for determination of area under the curve (AUC0–24) [22,23]. Both serial and sparse samples were available for analysis and this method permits a T-test comparison for sparse data using observed sample times versus nominal sample times, potentially reducing random error depending on the difference of the observed and nominal sampling times. The method employs a two-sided t-test to compare values of AUC0–24 between treatment groups for the same dose (Eq. (1))
| (1) |
where AUCi is for healthy animals, AUCj is for arthritic animals, and s2(AUC) is the variance of AUC0–24 for the respective groups. Intramuscular bioavailability (F) was determined previously and a value of 0.86 was used in the final model [24].
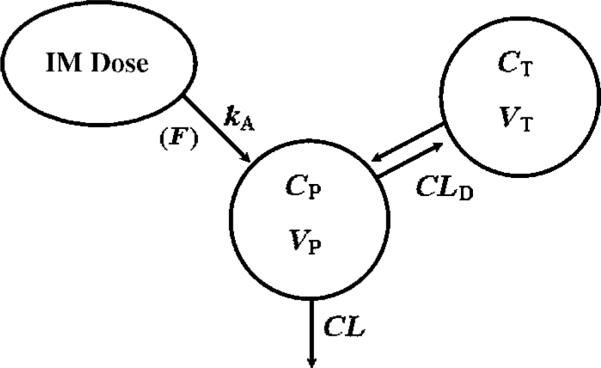
Model selection was made based on visual inspection of the data, goodness of fit plots and the Akaike Information Criterion (AIC). Figure 1 is a schematic of the final PK model used to fit the concentration—time data. Equations and initial conditions describing the amounts of DEX in each compartment are
| (2) |
| (3) |
| (4) |
where Aabs indicates the amount at the absorption site, Aplasma is the amount of DEX in plasma, AT is the amount in peripheral tissues, ka is the first-order absorption rate constant for i.m. administration, CL is the clearance from the plasma compartment, CLD is the inter-compartmental clearance, VP is the volume of distribution in plasma, VT is the peripheral volume of distribution, and F is the i.m. bioavailability (0.86).
Figure 1.
Pharmacokinetic model for dexamethasone. Symbols are defined in the text and Table 2
Concentrations of DEX in plasma were generated from
| (5) |
The model was implemented in NONMEM VI using the ADVAN4 subroutine and TRANS3 parameterization. The model was fitted to data from each group independently and then simultaneously using the FOCE with interaction module in NONMEM VI. For the simultaneous fitting conditional coefficients (FDi) were added that corrected CL, CLD, V, VSS and ka to the disease state values for animals with arthritis. Equation (6) describes the value that NONMEM implemented for pharmacokinetic parameters (CL, CLD, V, VSS, ka) specified by Pi, in terms of the healthy population expected values (θi), correction coefficient for disease state (FDi), and inter-animal variation (ηi) on plasma clearance (CL).
| (6) |
A χ2 model discrimination test was done using differences in the minimum value of the objective function before and after removal of a model parameter to determine whether each disease coefficient had a significant impact on the model fitting. Significant differences between healthy and diseased animals were tested in terms of clearance and volume of distribution. If model parameters did not yield a difference in MVOF of greater than 3.86, they were removed and the disease was determined not to have a significant effect on that parameter [25].
Results and Discussion
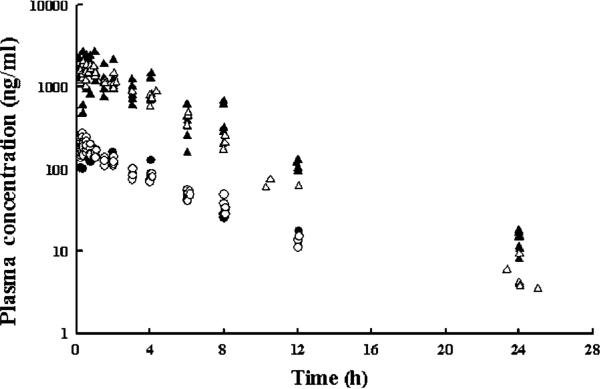
Plasma PK of total DEX after i.m. administration was compared in normal and healthy arthritic rats. The concentration—time profiles for both healthy and arthritic rats receiving either 0.225 or 2.25 mg/kg i.m. DEX are shown in Figure 2. Dexamethasone absorption from the intramuscular site was rapid as the time of maximum concentration (tmax) was 0.5 h for all doses. Following peak concentrations, concentration data appeared to decline mono-exponentially in parallel between the dose groups. There appears to be little, if any, difference between the plasma concentrations of DEX in the healthy and the arthritic animals. The 12 and 24 h high dose concentrations from arthritic rats appear lower than the data from the healthy rats. This is in agreement with the results of the non-compartmental analysis for the high dose groups in Table 1 where the apparent clearance is larger for the arthritic animals with high dose. However, the apparent clearance appeared to be the same for the lower dose levels.
Figure 2.
Plasma concentration-time profiles of high (triangles) and low-dose (circles) dexamethasone in healthy (solid symbols) and arthritic (open symbols) rats
Table 1.
Pharmacokinetic noncompartmental measures for i.m. dexamethasone
| Parameter | Definition | Healthy |
Arthritic |
||
|---|---|---|---|---|---|
| Dose, mg/kg: 2.25 | 0.225 | 2.25 | 0.225 | ||
| λZ (h–1) | Terminal slope | 0.157 | 0.164 | 0.165 | 0.187 |
| Cmax (μg/l) | Maximum concentration | 1540 | 133 | 1460 | 168 |
| AUC0–Tlast (μg h/l) | Area under curve | 7700a (752)b | 668a (21.8)b | 6030a (112)b | 683a (16.7)b |
| AUC0–∞ (μg h/l) | Area under curve | 7760 | 733 | 6050 | 734 |
| AUC0–∞/Dose | Dose-normalized AUC | 3450 | 3260 | 2690 | 3040 |
| CL/F (l/h/kg) | Apparent clearance | 1.09 | 1.16 | 1.41 | 1.16 |
| VP/F (l/kg) | Apparent central volume | 5.82 | 5.91 | 7.12 | 5.18 |
Bailer–Satterthwaite area under curve.
Standard error.
Non-compartmental analysis did not indicate any differences in the AUC0–24 between treatment groups for the same dose level. The Bailer–Satterthwaite AUC0–24 values and standard deviations are presented in Table 1. The tobs value from Equation (1) was 0.518 for the low dose and 0.220 for the high dose values. The critical t-values were determined based on the Satterthwaite degrees of freedom for each dosing group to be 2.57 and 2.78 for the low and high doses [23,26]. In either dose group the tobs was less than the critical t-value. Therefore, no statistical differences were concluded in AUC0–24 values between treatments for both doses (α=0.05). Dose normalization of the AUC0–∞ yielded similar values.
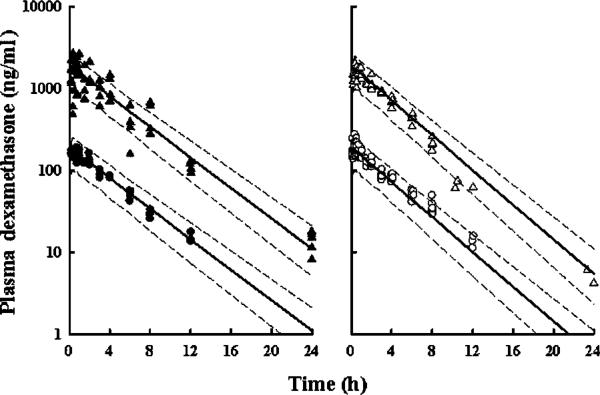
Initial inspection of tmax for each dose, linearity of the AUC values between doses, and the mono-exponential decline of the data suggested that the one-compartment mammillary model with first-order absorption was appropriate. However, a two-compartment linear model fitting yielded a lower value of AIC. The two-compartment model also better fitted the initial short distribution phase that is apparent in the arthritic low dose data (Figure 3, Panel B). Unlike corticosterone and prednisolone, DEX does not bind nonlinearly to transcortin [3,27]. Rather DEX is primarily bound linearly to albumin at about 70% in rats. This observation supports linear DEX kinetics. On the other hand, in humans plasma DEX concentrations exhibit a bi-exponential decline owing to a slower conversion of the phosphate ester prodrug to DEX [24]. In rats this process occurs so rapidly that it is not readily observed in the concentration—time profiles given the slightly delayed i.m. absorption.
Figure 3.
Model fitting of low and high-dose plasma dexamethasone in healthy (Panel A) and arthritic rats (Panel B). Solid lines indicate the median population prediction of 1000 simulated individuals with the final population mean parameters for each dosing and treatment group. Dashed lines represent the 5 (lower) and 95 (upper) percentiles for each predicted plasma concentration time course
Figure 3 shows the final two-compartment model fitting in both healthy (Panel A) and arthritic (Panel B) rats. Final PK parameter estimates are presented in Table 2. Clearance was the only model parameter that tested different between arthritic and healthy groups using the χ2 model discrimination test by change in the MVOF with and without conditional disease factors present in the fitted model. The change in MVOF for clearance was 35.1 lower with the presence of a disease correction factor (FDCL=1.13). Despite a significant difference noted by the NONMEM model fittings, the actual change in value of clearance is minor (1.13-fold increase).
Table 2.
Model pharmacokinetic parameter estimates for i.m. dexamethasone
| Parameter | Definition | Estimate | SE % |
|---|---|---|---|
| CLHealthy (l/h/kg) | Clearance in healthy Lewis rats | 1.05 | 4.08 |
| CLArthritic (l/h/kg) | Clearance in arthritic Lewis rats | 1.19 | 3.00 |
| CLD (l/h/kg) | Distributional clearance | 7.20 | 42.5 |
| VP (l/kg) | Central volume of distribution | 3.41 | 2.83 |
| VSS (l/kg) | Steady-state volume of distribution | 4.85 | 3.17 |
| ka (1/h) | Intramuscular absorption rate constant | 5.78 | 10.0 |
| η CL | Interanimal variation of clearance | 0.0565 | 45.0 |
| CCV | Slope of random error | 0.215 | 18.3 |
These results were unexpected as earlier studies suggested a potential decrease in clearance and an increase in the plasma protein binding of DEX. A decrease in clearance would suggest that concentrations of DEX would remain elevated at later times. Instead it was observed that concentrations were lower at the later times and the clearance of DEX was in fact slightly higher than the healthy animals. If acute phase response proteins such as α1-acid glycoprotein, α2-macroglobulin, C-reactive protein, serum amyloid-A and others were elevated, it is possible that plasma DEX binding may no longer appear linear. In this case, one might expect a distinct early distribution phase to be noted for the lower doses. This may explain why, for the arthritic animals, a slight early distribution phase was noted. Additional doses would be required to confirm this and resolve parameters in a model sufficient to characterize this behavior. Due to only having data from two doses, variation in the data and the slightness of this distribution phase in the low arthritic dose, the two-compartment linear model exhibited the lowest AIC of all tested models. The availability of plasma in these animals was too limited to yield a thorough evaluation of DEX protein binding in plasma.
Earlier studies indicated that clearance may be decreased and the protein binding of DEX may be altered [14,15]. Results of these studies, however, were presented for no longer than the first 7 days of disease induction. Our animals were dosed on day 22 post induction immediately after the peak paw swelling. Since the mature state of collagen-induced model of arthritis is viewed as localized to the joints [6], it is possible that any acute phase response relevant to DEX PK occurred much earlier in the time course of progression and had diminished by 22 days post induction. Only a single point in the disease time course was evaluated. The kinetics of DEX at other time points of the disease progression should be established prior to evaluation of pharmacodynamic effects of the drug at those times.
Inter-animal variation was tested on clearance and volume parameters. In the final model, variation in the clearance of DEX best explained the inter-animal differences. A simple constant coefficient of variation model accounted for the random effects observed in the data.
Acknowledgements
Supported by NIH Grant GM24211 and by a Fellowship from Amgen Inc. for J. C. Earp.
References
- 1.Neeck G. Fifty years of experience with cortisone therapy in the study and treatment of rheumatoid arthritis. Ann N Y Acad Sci. 2002;966:28–38. doi: 10.1111/j.1749-6632.2002.tb04199.x. [DOI] [PubMed] [Google Scholar]
- 2.Czock D, Keller F, Rasche FM, Haussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2005;44:61–98. doi: 10.2165/00003088-200544010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Evans WE, Schentag JJ, Jusko WJ. Applied pharmacokinetics: principles of therapeutic drug monitoring. 1992. p. 1.p. v. (various pagings)
- 4.Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol. 2003;43:1216–1227. doi: 10.1177/0091270003258651. [DOI] [PubMed] [Google Scholar]
- 5.Mager DE, Pyszczynski NA, Jusko WJ. Integrated QSPR—pharmacodynamic model of genomic effects of several corticosteroids. J Pharm Sci. 2003;92:881–889. doi: 10.1002/jps.10343. [DOI] [PubMed] [Google Scholar]
- 6.Holmdahl R, Lorentzen JC, Lu S, et al. Arthritis induced in rats with nonimmunogenic adjuvants as models for rheumatoid arthritis. Immunol Rev. 2001;184:184–202. doi: 10.1034/j.1600-065x.2001.1840117.x. [DOI] [PubMed] [Google Scholar]
- 7.Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- 8.Stuart JM, Cremer MA, Townes AS, Kang AH. Type II collagen-induced arthritis in rats. Passive transfer with serum and evidence that IgG anticollagen antibodies can cause arthritis. J Exp Med. 1982;155:1–16. doi: 10.1084/jem.155.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meijer OC, Karssen AM, de Kloet ER. Cell- and tissue-specific effects of corticosteroids in relation to glucocorticoid resistance: examples from the brain. J Endocrinol. 2003;178:13–18. doi: 10.1677/joe.0.1780013. [DOI] [PubMed] [Google Scholar]
- 10.Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. Absence of the mdr1a P-glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest. 1995;96:1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danielson PB. The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans. Curr Drug Metab. 2002;3:561–597. doi: 10.2174/1389200023337054. [DOI] [PubMed] [Google Scholar]
- 12.Waxman DJ, Attisano C, Guengerich FP, Lapenson DP. Human liver microsomal steroid metabolism: identification of the major microsomal steroid hormone 6 beta-hydroxylase cytochrome P-450 enzyme. Arch Biochem Biophys. 1988;263:424–436. doi: 10.1016/0003-9861(88)90655-8. [DOI] [PubMed] [Google Scholar]
- 13.Assenat E, Gerbal-Chaloin S, Larrey D, et al. Interleukin 1beta inhibits CAR-induced expression of hepatic genes involved in drug and bilirubin clearance. Hepatology. 2004;40:951–960. doi: 10.1002/hep.20387. [DOI] [PubMed] [Google Scholar]
- 14.Garg V, Hon YY, Jusko WJ. Effects of acute and chronic inflammation on the pharmacokinetics of prednisolone in rats. Pharm Res. 1994;11:541–544. doi: 10.1023/a:1018966516195. [DOI] [PubMed] [Google Scholar]
- 15.Ling S, Jamali F. Effect of early phase adjuvant arthritis on hepatic P450 enzymes and pharmacokinetics of verapamil: an alternative approach to the use of an animal model of inflammation for pharmacokinetic studies. Drug Metab Dispos. 2005;33:579–586. doi: 10.1124/dmd.104.002360. [DOI] [PubMed] [Google Scholar]
- 16.Pascussi JM, Gerbal-Chaloin S, Pichard-Garcia L, et al. Interleukin-6 negatively regulates the expression of pregnane X receptor and constitutively activated receptor in primary human hepatocytes. Biochem Biophys Res Commun. 2000;274:707–713. doi: 10.1006/bbrc.2000.3219. [DOI] [PubMed] [Google Scholar]
- 17.Piquette-Miller M, Pak A, Kim H, Anari R, Shahzamani A. Decreased expression and activity of P-glycoprotein in rat liver during acute inflammation. Pharm Res. 1998;15:706–711. doi: 10.1023/a:1011962818051. [DOI] [PubMed] [Google Scholar]
- 18.Vuppugalla R, Mehvar R. Short-term inhibitory effects of nitric oxide on cytochrome P450-mediated drug metabolism: time dependency and reversibility profiles in isolated perfused rat livers. Drug Metab Dispos. 2004;32:1446–1454. doi: 10.1124/dmd.104.001487. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Miller SC, Sima M, et al. The arthrotropism of macromolecules in adjuvant-induced arthritis rat model: a preliminary study. Pharm Res. 2004;21:1741–1749. doi: 10.1023/b:pham.0000045232.18134.e9. [DOI] [PubMed] [Google Scholar]
- 20.Samtani MN, Jusko WJ. Stability of dexamethasone sodium phosphate in rat plasma. Int J Pharm. 2005;301:262–266. doi: 10.1016/j.ijpharm.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haughey DB, Jusko WJ. Analysis of methylprednisolone, methylprednisone and corticosterone for assessment of methylprednisolone disposition in the rat. J Chromatogr. 1988;430:241–248. doi: 10.1016/s0378-4347(00)83159-x. [DOI] [PubMed] [Google Scholar]
- 22.Bailer AJ. Testing for the equality of area under the curves when using destructive measurement techniques. J Pharmacokinet Biopharm. 1988;16:303–309. doi: 10.1007/BF01062139. [DOI] [PubMed] [Google Scholar]
- 23.Nedelman JR, Gibiansky E, Lau DT. Applying Bailer's method for AUC confidence intervals to sparse sampling. Pharm Res. 1995;12:124–128. doi: 10.1023/a:1016255124336. [DOI] [PubMed] [Google Scholar]
- 24.Samtani MN, Jusko WJ. Comparison of dexamethasone pharmacokinetics in female rats after intravenous and intramuscular administration. Biopharm Drug Dispos. 2005;26:85–91. doi: 10.1002/bdd.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahlby U, Jonsson EN, Karlsson MO. Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn. 2001;28:231–252. doi: 10.1023/a:1011527125570. [DOI] [PubMed] [Google Scholar]
- 26.Satterthwaite FE. An approximate distribution of estimates of variance components. Biometrics Bull. 1946;2:110–114. [PubMed] [Google Scholar]
- 27.Samtani MN, Pyszczynski NA, Dubois DC, Almon RR, Jusko WJ. Modeling glucocorticoid-mediated fetal lung maturation: I. Temporal patterns of corticosteroids in rat pregnancy. J Pharmacol Exp Ther. 2006;317:117–126. doi: 10.1124/jpet.105.095851. [DOI] [PubMed] [Google Scholar]