Summary
Background
Chemokines ligands of CCR3 including eotaxin/CC chemokine ligand 11 (CCL11) may contribute to the pathogenesis of asthma. These chemokines and a growth factor (TGF-β) may be involved in the process of airway remodelling.
Objective
We analysed the effects of TGF-β on the expression of CCR3 ligands in human airway smooth muscle (HASM) cells and investigated the mechanisms.
Methods
HASM cells were cultured and treated with TGF-β and Th2 cytokines IL-4 or IL-13. Expression of mRNA was analysed by real-time PCR. Secretion of CCL11 into the culture medium was analysed by ELISA. Transcriptional regulation of CCL11 was analysed by luciferase assay using CCL11 promoter-luciferase reporter plasmids.
Results
IL-4 or IL-13 significantly up-regulated the expression of mRNAs for CCL11 and CCL26. TGF-β alone did not increase the expression of chemokine mRNAs, but enhanced the induction of only CCL11 by IL-4 or IL-13 among CCR3 ligands. Activity of the CCL11 promoter was stimulated by IL-4, and this activity was enhanced by TGF-β. Activation by IL-4 or IL-4 plus TGF-β was lost by mutation of the binding site for signal transducers and activators of transcription-6 (STAT6) in the promoter. Cooperative activation by IL-4 and TGF-β was inhibited by mutation of the binding site for nuclear factor-κB (NF-κB) in the promoter. Pretreatment with an inhibitor of NF-κB and glucocorticoid fluticasone propionate significantly inhibited the expression of CCL11 mRNA induced by IL-4 plus TGF-β, indicating the importance of NF-κB in the cooperative activation of CCL11 transcription by TGF-β and IL-4.
Conclusion
These results indicate that Th2 cytokines and TGF-β may contribute to the pathogenesis of asthma by stimulating expression of CCL11. The transcription factors STAT6 and NF-κB may play pivotal roles in this process.
Keywords: airway smooth muscle cells, asthma, CCL11, eotaxin, NF-κB, TGF-β
Introduction
Asthma is a chronic disease of the airway characterized by the infiltration of inflammatory cells, desquamation of the epithelium, hypersecretion of mucus, and thickening of the airway wall. Hyperplasia and hypertrophy of airway smooth muscle (ASM) cells are important aspects of airway remodelling, and these pathological features may be involved in airway narrowing, airway hyperresponsiveness, and disease severity [1–4].
ASM cells are believed to be involved in constriction of the airway in patients with asthma, but recent data have also implicated production of cytokines and chemokines, including CC chemokine ligand (CCL) 11/eotaxin. Expression of CCL11 in the smooth muscle of patients with asthma has been reported [5]. CCL11 is a C-C chemokine first identified during the purification of an eosinophil chemoattractant from bronchoalveolar lavage fluid of ovalbumin-sensitized and -challenged guinea-pigs [6]. CCL11 binds to its receptor, C-C chemokine receptor 3 (CCR3). Other CCR3 ligands include CCL24/eotaxin-2/MPIF-2/CKβ-6, CCL26/eotaxin-3, and CCL13/monocyte chemoattractant protein (MCP)-4. CCR3 is expressed by cells involved in allergic disease, such as eosinophils, basophils, a subset of Th2 cells, and mast cells. CCL11, CCL13, CCL24, and CCL26 are potent chemoattractants for these cells and are highly expressed in the airway of patients with asthma [7, 8].
The roles of chemokine ligands of CCR3 in ASM cells in vivo have not been well characterized. However, one role may be in the chemoattraction of mast cells to the smooth muscle layer. Brightling et al. [9] reported that mast cells infiltrate the smooth muscle layer in patients with asthma. Ochi et al. [10] showed that CCL11 induces the migration of mast cells. Mast cells are known to secrete chemical mediators and cause constriction of smooth muscle. The proliferation of ASM cells is thought to be mediated by mast cell-derived tryptase, chemical mediators, such as histamine and leukotrienes, and cytokines, such as TGF-β and TNF-α. Interaction of ASM cells and mast cells may be involved in the pathophysiology of asthma.
We have recently confirmed that TNF-α and IL-4 cooperatively stimulate the expression of CCL11, CCL13, and CCL26 in ASM cells [11], In this study, we investigated the regulation of expression of CCR3 ligands by the growth factor TGF-β and Th2 cytokines, such as IL-4 and LL-13, in ASM cells. We next investigated the mechanism of regulation of CCL11 by these cytokines.
Materials and methods
Reagents
Recombinant cytokines IL-4, IL-13, TNF-α, and TGF-β1 were purchased from R&D Systems (Tokyo, Japan). BAY 11-7085, an inhibitor of nuclear factor-kappaB (NF-κB) that inhibits IκBα kinase, was from Calbiochem (San Diego, CA USA). The glucocorticoid fluticasone propionate and the long acting β2-agonist salmeterol were provided by GlaxoSmithKline.
Cell culture
Human ASM (HASM) cells were purchased from Cambrex (Baltimore, MD, USA) and cultured in SmBM medium with SmGM-2 SingleQuots (Cambrex) containing insulin, fibroblast growth factor, gentamicin, 5% fetal bovine serum, and epidermal growth factor at 37 °C with 5% CO2 in humidified air. Confluent cells at passages 2–5 were stimulated with cytokines as described below.
Purification of RNA and real-time polymerase chain reaction
Total RNA was extracted from cells with reagent Isogen (Nippon-Gene, Tokyo, Japan) after incubation with and without indicated reagents. cDNAs were synthesized from isolated RNA templates with a High-Capacity cDNA Archive Kit (Applied Biosystems, Tokyo, Japan). Predesigned TaqMan probe sets for CCL11, CCL13, CCL24, and CCL26 were purchased from Applied Biosystems. Each probe has a fluorescent reporter dye (FAM) linked to its 5′-end and a downstream quencher dye (TAMRA) linked to its 3′-end. TaqMan Ribosomal RNA probe, which is labelled with a fluorescent reporter dye (VIC), was used as an internal control. Each reaction consisted of 25 μL containing 2× Universal Master Mix (Applied Biosystems), primers, labelled probes, and 50 ng cDNA. Amplification conditions consisted of 40 cycles of 95 °C for 15 s and 60 °C for 1 min after incubation at 95 °C for 10 min. Amplification and fluorescence measurements were carried out during the elongation step with an ABI PRISM 7700 Sequence Detection System (Applied Biosystems). Data are shown as fold induction of non-stimulated control cells.
Assay of CC chemokine ligand 11 secretion
Concentration of CCL11 protein was determined with a commercially available ELISA kit (R&D Systems) as described previously [12]. Standards and samples were added to 96-well microtitre plate coated with anti-chemokine antibody. After incubation at room temperature for 2h, each well was washed five times with wash buffer. Biotinylated antibody against CCL11 and avidin-horseradish peroxidase conjugate were added to the well. After incubation at room temperature for 1 h, each well was washed seven times with wash buffer. Substrate solution (stabilized hydrogen peroxide and tetramethylbenzidine) was added to each well, and the plate was incubated at room temperature for 30 min. Sulphuric acid was then added to each well, and the absorbance was measured at 450 nm. The limit of detection in the assay for CCL11 was 5 pg/mL.
Construction of CC chemokine ligand 11 promoter-luciferase reporter plasmids
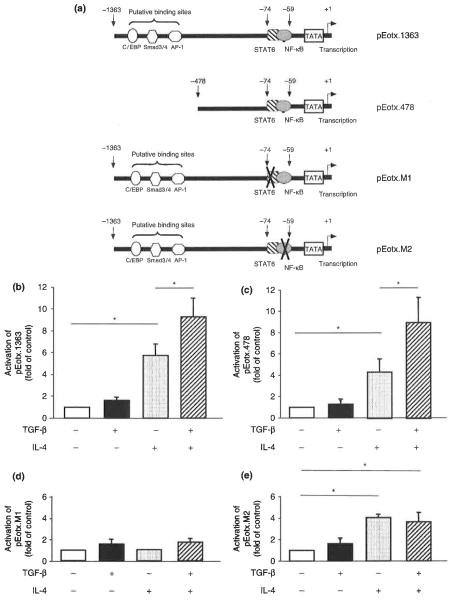
To investigate transcription pathways regulating CCL11 expression in HASM cells, we used CCL11 promoter-reporter plasmids as described previously [13]. A 1363 bp fragment of the promoter region of the CCL11 gene (site −1363 to −1) was amplified by PCR and ligated into the MluI and BglII sites of the luciferase reporter pGL3-Basic vector (Promega Corp., Madison, WI, USA). The construct is referred to as pEotx.1363 (Fig. 4a). pEotx.478 was constructed by deleting lengths of the 5′-end of the CCL11 promoter sequence of pEotx.1363. Construct pEotx.M1 was synthesized by mutating the signal transducers and activators of transcription-6 (STAT6) binding site in pEotx.1363. Construct pEotx.M2 was synthesized by mutating the NF-κB binding site of pEotx.1363.
Fig. 4.
Luciferase reporter assays of CC chemokine ligand 11 (CCL11) promoter activity in human airway smooth muscle cells. Cells were transfected with CCL11 promoter-luciferase reporter plasmids illustrated in (a) (pEotx.1363, 478, M1 or M2) and control vector pRL-TK. Forty-eight hours later, cells were incubated for 20h with or without 10 ng/mL IL-4 and TGF-β. (b) pEotx.1363, (c) pEotx.478, (d) pEotx.M1, and (e) pEotx.M2. Relative luciferase activity was calculated as fold induction compared with control value. The data are presented as the mean±SEM of a total of three independent experiments (*P< 0.05).
Transient transfections and luciferase assay
HASM cells at passage 2 were seeded onto six-well plates and allowed to grow to 50% confluence. Cells were transfected with 1 μg of each reporter plasmid and 10 ng of the control renilla-luciferase vector pRL-TK (Promega, Tokyo, Japan) with 5 μL Lipofectamine 2000 (Promega) and grown in medium without gentamicin for 24 h. The medium was changed to medium containing gentamicin. After 24 h, cells were incubated with the indicated cytokines for 20 h. Cells were washed twice with Ca2+- and Mg2+-free phosphate-buffered saline, solubilized by incubation in 500 μL lysis buffer for 20 min, transferred to microtubes, and centrifuged to pellet cellular debris. Supernatants were stored at −80 °C until luciferase activity was measured with the Dual-Luciferase Assay System (Promega) and a luminometer (Gene-Light 55. Microtech Nichion, Chiba, Japan). Firefly luciferase activity of the reporter plasmid was normalized to renilla-luciferase activity and expressed as fold induction of control.
Statistical analysis
Data were expressed as mean±SEM. Statistical differences were determined by ANOVA with Fisher’s PLSD. Data were analysed with Stat View IV (Abacus Concepts Inc., Berkeley, CA, USA).
Results
Regulation of expression of CC chemokine receptor 3 ligand mRNAs in human airway smooth muscle cells
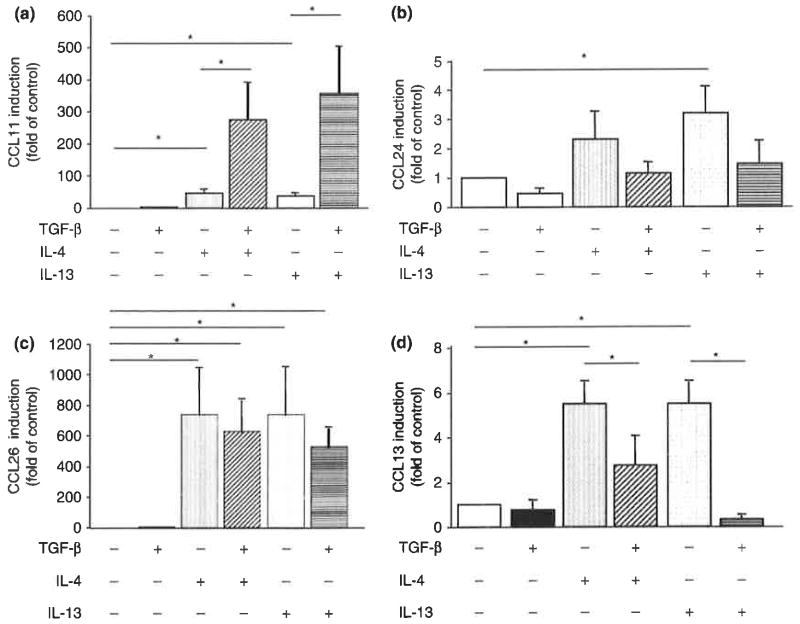
We first analysed the regulation of CCR3 ligand mRNAs in HASM cells. Real-time PCR showed that the Th2 cytokines H-4 and IL-13 significantly increased the expression of mRNA for CCL11 and CCL26 at 24 h after stimulation (Fig. 1). Expression of CCL13 mRNA was moderately increased by IL-4 or IL-13. CCL24 mRNA tended to be slightly increased in response to IL-13. TGF-β did not directly stimulate the expression of any of the chemokines. However, it enhanced the expression of CCL11 raRNA stimulated by IL-4 or IL-13. This cooperative activity of TGF-β and Th2 cytokines was not observed for CCL13, CCL24, and CCL26. TGF-β suppressed the expression of CCL13 mRNA stimulated by IL-4 or IL-13.
Fig. 1.
Regulation of CCR3 ligand mRNA expression in human airway smooth muscle (HASM) cells, (a) CC chemokine ligand 11 (CCL11)/eotaxin, (b) CCL24/eotaxin-2, (c) CCL26/eotaxin-3, and (d) CCL13/monocyte chemoattractant protein-4. RNA was extracted from cells stimulated with 10ng/mL IL-4, IL-13, and/or TGF-β for 24 h and subjected to real-time PCR. Levels of mRNA were calculated as fold induction compared with non-stimulated control cells. The data are presented as the mean±SEM of three independent experiments (*P<0.05).
Cooperative up-regulation of CCL11 with IL-4/IL-13 and TGF-β was concordant with the previous reports in fibroblasts and HASM cells reported by Wenzel et al. [14] and Zuyderduyn et al. [15]. There was some difference between our current data and previous reports concerning regulation of CCL26 with TGF-β. These discrepancies may depend on the dose of TGF-β and the system of experiments. We have preliminarily analysed the adequate dose of TGF-β to stimulate CCL11 expression in HASM cells. We needed > 1 ng/mL of TGF-β to activate CCL11 expression in cooperation with IL-4 or IL-13 (data not shown). Some reporters have used 10 ng/mL of TGF-β to investigate the activation of PI-3 kinase in HASM cells [16]. Taken together, we chose 10 ng/mL for dose of TGF-β to investigate the effects of TGF-β and its mechanisms in HASM cells in the following experiments.
CC chemokine ligand 11 secretion by human airway smooth muscle cells
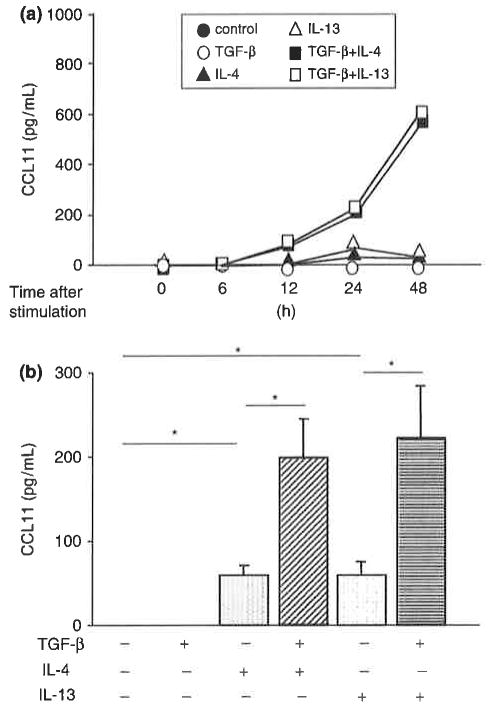
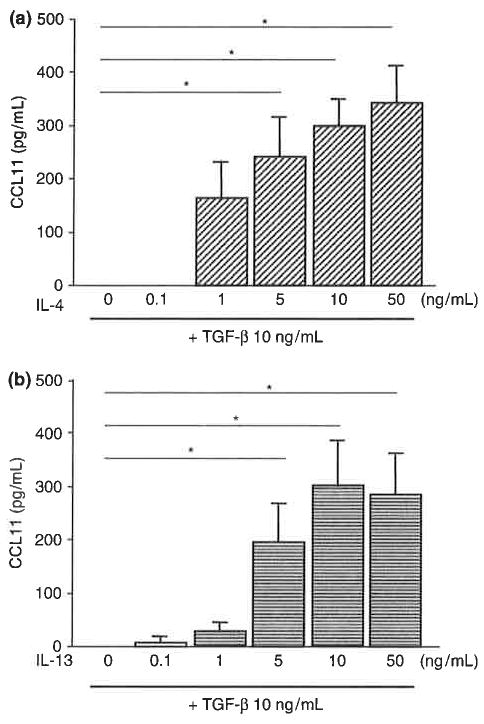
We then examined the kinetics of CCL11 protein expression and secretion. TGF-β plus IL-4 or IL-13 stimulated secretion of CCL11 protein into the culture medium in a time-dependent manner (Fig. 2a). IL-4 or IL-13 also stimulated the secretion of CCL11 protein into the culture medium at 24 h after stimulation (Fig. 2b). TGF-β alone did not stimulate the secretion of CCL11 but enhanced the effect of IL-4 or IL-13. These results are consistent with those observed with CCL 11 mRNA. Cooperative stimulation of CCL11 protein expression by TGF-β and IL-4 or IL-13 was dependent on the concentration of IL-4 or IL-13 (Fig. 3). Maximum induction of CCL11 protein expression was observed in response to 10–50 ng/mL IL-4 or IL-13.
Fig. 2.
CC chemokine ligand 11 (CCL11) protein secretion into the culture medium of human airway smooth muscle cells, (a) Time course of CCL11 secretion, and (b) CCL11 secretion at 24h after stimulation. Medium was collected from cells stimulated with 10ng/mL IL-4, IL-13, and/or TGF-β for the indicated times and subjected to ELISA. The data are presented as the mean±SEM of each three independent experiments (*P<0.05).
Fig. 3.
CC chemokine ligand 11 (CCL11) protein secretion into the culture medium of human airway smooth muscle cells. Concentration-dependent secretion of CCL11 in response to IL-4 (a) orIL-13 (b) in the presence of TGF-β. Medium was collected from cells stimulated with the indicated concentrations of IL-4 or IL-13 combined with 10 ng/mL TGF-β for 24 h and subjected to ELISA. The data are presented as the mean±SEM of three independent experiments (*P<0.05).
CC chemokine ligand 11 promoter assay
We examined the mechanisms of regulation of CCL11 transcription by TGF-β and IL-4. IL-4 activated the CCL11 promoter reporter pEotx.1363 (Fig. 4b). TGF-β alone did not activate this promoter but enhanced the effect of IL-4. We then examined the mechanisms of regulation of CCL11 transcription by IL-4 and TGF-β. Activation of the CCL11 promoter by TGF-β and IL-4 was preserved in pEotx.478 (Fig. 4c). IL-4- and IL-4 plus TGF-β-induced activation of the promoter was lost in pEotx.M1 that lacks a STAT6 site (Fig. 4d). IL-4 stimulated activation of pEotx.M2, which lacks a NF-κB site, to a level similar to that of pEotx.1363, but cooperative activation of the promoter by TGF-β and IL-4 was not observed (Fig. 4e).
Cooperative induction of CC chemokine ligand 11 expression is inhibited by nuclear factor-κB inhibitor and glucocorticoid
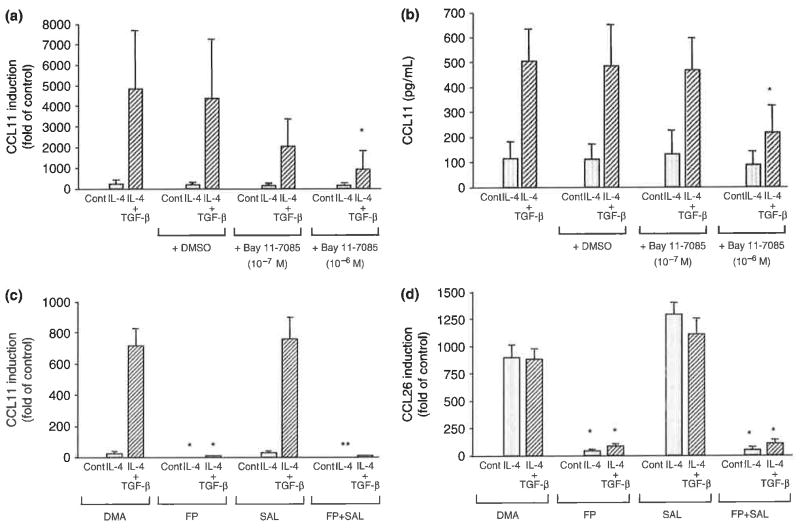
An inhibitor of NF-κB, BAY 11-7085 (10−6M), inhibited the expression of CCL11 mRNA (Fig. 5a) or protein secretion (Fig. 5b) stimulated by IL-4 plus TGF-β. However, it did not inhibit the effect of IL-4 alone. The diluent dimethyl sulphoxide had no effect. Its expression seemed to be moderately inhibited with 10−7M of BAY 11-7085; however, its effect was not statistically significant. Non-specific effects were observed in experiments using high-dose BAY 11-7085 (10−5M), suggesting that it maybe due to cytotoxic effects (data not shown). These results indicate that NF-κB is involved in the cooperative induction of CCL11 expression by DL-4 plus TGF-β. Pretreatment with the glucocorticoid fluticasone propionate inhibited the expression of CCL11 mRNA stimulated by IL-4 or IL-4 plus TGF-β (Fig. 5c). The expression of CCL26 mRNA was also significantly inhibited by fluticasone propionate (Fig. 5d). The β2-agonist salmeterol had no effect on the expression of CCL11 mRNA but it moderately increased the expression of CCL26 mRNA. This up-regulation was inhibited by fluticasone propionate.
Fig. 5.
Effects of drugs on the expression of CC chemokine ligand 11 (CCL11) mRNA (a and c), CCL11 protein (b), or CCL26 mRNA (d) induced by IL-4 or IL-4 plus TGF-β in human airway smooth muscle cells. Cells were stimulated with IL-4 or IL-4 plus TGF-β (10 ng/mL each) with or without pre-incubation with the indicated drugs, (a and b) Effect of the nuclear factor-κB inhibitor Bay 11-7085, and (c and d) effects of the glucocorticoid fluticasone propionate (FP) and long-acting β2-agonist salmeterol (SAL) (10−7M each). The data are presented as the mean±SEM of three independent experiments [*P< 0.05 compared with the cells with same stimulation and treated with diluent dimethyl sulphoxide or dimethyl acetamide (DMA) as indicated].
Discussion
In this report, we show that TGF-β enhanced CCL11 expression induced by Th2 cytokine IL-4 or IL-13, but it did not enhance the expression of CCL24, CCL26, and CCL13 stimulated with IL-4 or IL-13. These results indicate that effects of TGF-β may be specific for CCL11 among CCR3 ligands. STAT6 appears to play a pivotal role in the stimulation of transcription of CCL11 in HASM cells in response to IL-4. TGF-β alone showed no ability to activate transcription of CCL11. However, NF-κB may be important in the cooperative activation of CCL11 transcription by TGF-β and IL-4.
Activation of the CCL11 promoter by IL-4 was lost in pEotx.M1, in which the binding site for STAT6 was mutated. We also confirmed that IL-4 stimulated nuclear translocation of STAT6 and its binding to the CCL11 promoter region in HASM cells [11]. Other investigators have reported that IL-4 activates STAT6 in HASM cells [17] and have shown STAT6-dependent CCL11 expression in HASM cells [18]. These results are consistent with reports of studies in airway epithelial cells [13] and indicate that STAT6 plays a pivotal role in the regulation of CCL11 transcription in HASM cells.
TGF-β binds to its type II receptor and subsequently phosphorylates Smad proteins after activation of type I receptor activin receptor-like kinase (ALK)-5 [19]. Smad heteromultimers, such as Smad3/4, translocate to the nucleus and bind to the promoter region of target genes. Two consensus sequences for Smad binding have been reported. One consists of the sequence GTCTAGAC, called the Smad-binding element (SBE) [20]. The second consists of the sequence CAGACA called the CAGA box [21]. The SBE sequence was not found in the CCL11 promoter region that we sequenced, but the CAGA box comprises bases −1170 to −1165 of the CCL11 promoter. TGF-β is also known to activate CCAAT/enhancer-binding protein (C/EBP) or activator protein-1 (AP-1). A putative-binding site for C/EBP has been reported in the CCL11 promoter region at bases −1278 to −1270, and a putative-binding site for AP-1 has been reported at bases −1076 to −1082 [22, 23]. We suspected these putative-binding sites for Smad, C/EBP, and AP-1 were responsible for the activation of the CCL11 promoter by TGF-β. However, the cooperative activity of IL-4 and TGF-β was preserved in pEotx.478, in which these putative-binding sites were deleted. Results with pEotx.M2 indicate that the binding site for NF-κB may be important for the activation of CCL11 transcription by IL-4 plus TGF-β. Pretreatment with an NF-κB inhibitor significantly inhibited the cooperative induction of CCL11 mRNA and protein expression by the combination of IL-4 and TGF-β. Some reports have shown the activation of NF-κB by TGF-β; however, it is not clear whether activation of NF-κB by TGF-β is sufficient for activation of CCL11 transcription because TGF-β alone did not stimulate CCL11 expression in our experiments. Further study is necessary to clarify these mechanisms. Nonetheless, NF-κB may be important in the cooperative activation of CCL11 expression by IL-4 and TGF-β in HASM cells. The glucocorticoid fluticasone propionate also inhibited the cooperative stimulation of CCL11 mRNA expression by TGF-β and IL-4 in the present study. Additional studies are necessary to elucidate the mechanisms involved, but inhibition of NF-κB would be likewise candidate for the mechanism of glucocorticoid’s effects in this study [24].
In addition to CCL11, fluticasone propionate significantly inhibited the expression of CCL26 mRNA stimulated by IL-4 or IL-4 plus TGF-β. These results are consistent with reports of the inhibitory effect of glucocorticoids on the expression of CCL11 in airway epithelial cells [25] and support evidence of a potent anti-inflammatory effect of inhaled corticosteroids in patients with asthma. ASM cell-derived CCR3 ligands such as CCL11 and CCL26 may contribute to the accumulation of inflammatory cells and to airway remodelling. Interaction of mast cells and HASM cells may play a role as mentioned above. Joubert et al. [26] reported that ASM cells also express CCR3 and migrate in response to CCL11. Their data also indicate a contribution of CCR3 ligands to airway remodelling. The role of inhaled corticosteroids in the prevention of airway remodelling in asthma is not well established. However, inhibition of the expression of CCR3 ligands by glucocorticoids might slow the progress of ASM cell hyperplasia. β2-agonists have been reported to inhibit the expression of cytokines and chemokines in ASM cells [27, 28]. It has also been reported that TNF-α-induced CCL11 expression is inhibited by β2-agonists in ASM cells [29, 30]. In our experiments, salmeterol did not inhibit the expression of CCL11 stimulated by IL-4 or IL-4 plus TGF-β. These discrepancies may be due to the differences in stimulation. Salmeterol slightly enhanced the expression of CCL26 mRNA stimulated by IL-4, but salmeterol did not diminish the profound inhibitory effect of fluticasone propionate on expression of CCL11.
IL-13 seemed to enhance the expression of mRNA for CCL24 in HASM cells. But its expression was very modest. We analysed the levels of protein release of CCL24 in the supernatants of HASM cells using ELISA; however, the levels of protein production were under the detection limit (data not shown). Taken together, we think that regulation of CCL24 mRNA by IL-13 or TGF-β may not be relevant.
We need further studies to clarify the mechanisms of down-regulation of CCL13 expression with TGF-β in HASM cells. However, we hypothesize that TGF-β may inhibit the transcription of CCL13 gene. Hein et al. [31] reported the sequence of CCL13 promoter region. Its proximal region contains GAS motif, which is consensus-binding site for STATs. Activation of CCL13 transcription with IL-4 or IL-13 may depend on the binding of STAT6 to the GAS motif. The promoter region of CCL13 is also reported to consist of binding site for nuclear factor Yin Yang 1 (YY1). YY1 is known to activate transcription of CCL13 [32]. Transcription factor Smad, which transducts the TGF-β’s signal, is possible to inhibit the binding of YY1 to the CCL13 promoter and subsequently down-regulates CCL13 transcription, because investigators have reported the interaction between YY1 and Smad [33].
In conclusion, we provide evidence that TGF-β may synergize with the Th2 cytokines IL-4 and IL-13 in activating the expression of CCL11, a chemokine that attracts mast cells, eosinophils, and basophils, in ASM cells. This effect of TGF-β that appears to be mediated by NF-κB suggests another potential role for this cytokine in allergic airways inflammation. Both TGF-β and CCL11 are found to be elevated in asthma, and future studies will be required to determine if TGF-β plays a role in the expression of CCL11 in vivo [34–36].
Acknowledgments
The authors would like to thank Dr T. Hirano and Dr T. Kasama for their excellent assistance. This work was supported by Environmental Restoration and Conservation Agency and GlaxoSmithKline.
References
- 1.Elias J, Lee C, Zheng T, Ma B, Homer R, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003;111:291–7. doi: 10.1172/JCI17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panettieri R. Jr Asthma persistence versus progression: does airway smooth muscle function predict irreversible airflow obstruction? Allergy Asthma Proc. 2009;30:103–8. doi: 10.2500/aap.2009.30.3202. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron C, Al-Ramli W, Hamid Q. Remodeling in asthma. Proc Am Thorac Soc. 2009;6:301–5. doi: 10.1513/pats.200808-089RM. [DOI] [PubMed] [Google Scholar]
- 4.Black J, Roth M. Intrinsic asthma: is it intrinsic to the smooth muscle? Clin Exp Allergy. 2009;39:962–5. doi: 10.1111/j.1365-2222.2009.03270.x. [DOI] [PubMed] [Google Scholar]
- 5.Ghaffar O, Hamid Q, Renzi P, et al. Constitutive and cytokine-stimulated expression of eotaxin by human airway smooth muscle cells. Am J Respir Crit Care Med. 1999;159:1933–42. doi: 10.1164/ajrccm.159.6.9805039. [DOI] [PubMed] [Google Scholar]
- 6.Jose PJ, Griffiths-Johnson DA, Collins PD, et al. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflamations. J Exp Med. 1994;179:881–7. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stellate C, Collins P, Ponath P, et al. Production of the novel C-C chemokine MCP-4 by airway cells and comparison of its biological activity to other C-C chemokines. J Clin Invest. 1997;99:926–36. doi: 10.1172/JCI119257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ying S, Meng Q, Zeibecoglou K, et al. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (intrinsic) asthmatics. J Immunol. 1999;163:6321–9. [PubMed] [Google Scholar]
- 9.Brightling C, Bradding P, Symon F, Holgate S, Wardlaw A, Pavord I. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 10.Ochi H, Hirani W, Yuan Q, Friend D, Austen K, Boyce J. T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J Exp Med. 1999;190:267–80. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odaka M, Matsukura S, Kuga H, et al. Differential regulation of chemokine expression by Th1 and Th2 cytokines and mechanisms of eotaxin/CCL-11 expression in human airway smooth muscle cells. Int Arch Allergy Immunol. 2007;143 (Suppl 1):84–8. doi: 10.1159/000101412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsukura S, Kokubu F, Kurokawa M, et al. Molecular mechanisms of repression of eotaxin expression with fluticasone propionate in airway epithelial cells. Int Arch Allergy Immunol. 2004;134:S12–20. doi: 10.1159/000077787. [DOI] [PubMed] [Google Scholar]
- 13.Matsukura S, Stellate C, Plitt J, et al. Activation of eotaxin gene transcription by NF-kappa B and STAT6 in human airway epithelial cells. J Immunol. 1999;163:6876–83. [PubMed] [Google Scholar]
- 14.Wenzel S, Trudeau J, Barnes S, et al. TGF-beta and IL-13 synergistically increase eotaxin-1 production in human airway fibroblasts. J Immunol. 2002;169:4613–9. doi: 10.4049/jimmunol.169.8.4613. [DOI] [PubMed] [Google Scholar]
- 15.Zuyderduyn S, Hiemstra P, Rabe K. TGF-beta differentially regulates TH2 cytokine-induced eotaxin and eotaxin-3 release by human airway smooth muscle cells. J Allergy Clin Immunol. 2004;114:791–8. doi: 10.1016/j.jaci.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 16.Krymskaya V, Hoffman R, Eszterhas A, Ciocca V, Panettieri R. TGF-beta 1 modulates EGF-stimulated phosphatidylinositol 3-kinase activity in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 1997;273 (Part 1):L1220–7. doi: 10.1152/ajplung.1997.273.6.L1220. [DOI] [PubMed] [Google Scholar]
- 17.Hirst S, Hallsworth M, Peng Q, Lee T. Selective induction of eotaxin release by interleukin-13 or interleukin-4 in human airway smooth muscle cells is synergistic with interleukin-1beta and is mediated by the interleukin-4 receptor alpha-chain. Am J Respir Crit Care Med. 2002;165:1161–71. doi: 10.1164/ajrccm.165.8.2107158. [DOI] [PubMed] [Google Scholar]
- 18.Peng Q, Matsuda T, Hirst S. Signaling pathways regulating interleukin-13-stimulated chemokine release from airway smooth muscle. Am J Respir Crit Care Med. 2004;169:596–603. doi: 10.1164/rccm.200307-888OC. [DOI] [PubMed] [Google Scholar]
- 19.Rahimi R, Leof E. TGF-beta signaling: a tale of two responses. J Cell Biochem. 2007;102:593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- 20.Zawel L, Dai J, Buckhaults P, et al. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell. 1998;1:611–7. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- 21.Dennler S, Itoh S, Vivien D, Dijke P, Huet S, Gauthier J. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hein H, Schluter C, Kulke R, Christophers E, Schroder J, Bartels J. Genomic organization, sequence, and transcriptional regulation of the human eotaxin gene. Biochem Biophys Res Commun. 1997;237:537–42. doi: 10.1006/bbrc.1997.7169. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Zepeda E, Rothenberg M, Weremowicz S, Sarafi M, Morton C, Luster A. Genomic organization, complete sequence, and chromosomal location of the gene for human eotaxin (SCYA11), an eosinophil-specific CC chemokine. Genomics. 1997;41:471–6. doi: 10.1006/geno.1997.4656. [DOI] [PubMed] [Google Scholar]
- 24.Scheinman R, Gualberto A, Jewell C, Cidlowski J, Baldwin A. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol. 1995;15:943–53. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stellate C, Matsukura S, Fal A, et al. Differential regulation of epithelial-derived C-C chemokine expression by IL-4 and the glucocorticoid budesonide. J Immunol. 1999;163:5624–32. [PubMed] [Google Scholar]
- 26.Joubert P, Lajoie-Kadoch S, Labonte I, et al. CCR3 expression and function in asthmatic airway smooth muscle cells. J Immunol. 2005;175:2702–8. doi: 10.4049/jimmunol.175.4.2702. [DOI] [PubMed] [Google Scholar]
- 27.Pang L, Knox A. Synergistic inhibition by beta(2)-agonists and corticosteroids on tumor necrosis factor-alpha-induced interleukin-8 release from cultured human airway smooth-muscle cells. Am J Respir Cell Mol Biol. 2000;23:79–85. doi: 10.1165/ajrcmb.23.1.3985. [DOI] [PubMed] [Google Scholar]
- 28.Ammit A, Lazaar A, Irani C, et al. Tumor necrosis factor-alpha-induced secretion of RANTES and interleukin-6 from human airway smooth muscle cells: modulation by glucocorticoids and beta-agonists. Am J Respir Cell Mol Biol. 2002;26:465–74. doi: 10.1165/ajrcmb.26.4.4681. [DOI] [PubMed] [Google Scholar]
- 29.Pang L, Knox A. Regulation of TNF-alpha-induced eotaxin release from cultured human airway smooth muscle cells by beta2-agonists and corticosteroids. FASEB J. 2001;15:261–9. doi: 10.1096/fj.00-0103com. [DOI] [PubMed] [Google Scholar]
- 30.Nie M, Knox A, Pang L. beta2-Adrenoceptor agonists, like glucocorticoids, repress eotaxin gene transcription by selective inhibition of histone H4 acetylation. J Immunol. 2005;175:478–86. doi: 10.4049/jimmunol.175.1.478. [DOI] [PubMed] [Google Scholar]
- 31.Hein H, Schlüter C, Kulke R, Christophers E, Schröder J, Bartels J. Genomic organization, sequence analysis and transcriptional regulation of the human MCP-4 chemokine gene (SCYA13) in dermal fibroblasts: a comparison to other eosinophilic beta-chemokines. Biochem Biophys Res Commun. 1999;255:470–6. doi: 10.1006/bbrc.1999.0216. [DOI] [PubMed] [Google Scholar]
- 32.Kalayci O, Birben E, Wu L, et al. Monocyte chemoattractant protein-4 core promoter genetic variants: influence on YY-1 affinity and plasma levels. Am J Respir Cell Mol Biol. 2003;29:750–6. doi: 10.1165/rcmb.2003-0024OC. [DOI] [PubMed] [Google Scholar]
- 33.Kurisaki K, Kurisaki A, Valcourt U, et al. Nuclear factor YY1 inhibits transforming growth factor beta- and bone morphogenetic protein-induced cell differentiation. Mol Cell Biol. 2003;23:4494–510. doi: 10.1128/MCB.23.13.4494-4510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ying S, Robinson DS, Meng Q, et al. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol. 1997;27:3507–16. doi: 10.1002/eji.1830271252. [DOI] [PubMed] [Google Scholar]
- 35.Vignola A, Chanez P, Chiappara G, et al. Transforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitis. Am J Respir Crit Care Med. 1997;156:591–9. doi: 10.1164/ajrccm.156.2.9609066. [DOI] [PubMed] [Google Scholar]
- 36.Minshall E, Leung D, Martin R, et al. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1997;17:326–33. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]