Abstract
The effect of the neuropeptide substance P (SP) on human mast cell (MC) phenotype is poorly understood. In this study, SP effects on human MC expression of the high affinity IgE receptor (FcεRI) were characterized. SP downregulated expression of FcεRI mRNA and protein by approximately 50% and in a concentration dependent manner, the effect was partially mediated by engagement of the neurokinin-1 receptor (NK1R) and resulted in reduced mast cell activation. Sensitization of MC with IgE prior to SP exposure protected MC from SP-mediated FcεRI downregulation. SP release may inhibit MC responses to allergens and these results may have implications in neuroinflammatiion and stress.
Keywords: Stress, Mast cells, IgE, Substance P, Fcε receptor
1. Introduction
Mast cells are storehouses of potent immunomodulatory mediators and play a central role in allergic inflammation and innate immune responses. In allergic disease, mast cells are activated when IgE bound to the Fcε receptor I (FcεRI) on the cell surface is crosslinked by specific antigen, triggering mast cell release of granule-contained mediators by degranulation and de novo synthesis of arachidonic acid metabolites, cytokines and chemokines. Mast cell production of these numerous vasoactive, nociceptive and pro-inflammatory molecules facilitates their interaction with nearby cells and initiates the allergic response (Galli et al., 2005).
In addition to their role in allergic inflammation, mast cells are important in the modulation of neuroimmune responses, particularly in wound repair during colitis (Bulut et al., 2008) and in burns (Kawahira et al., 2008). In the absence of sensory neurons, mast cell-driven skin inflammation is impaired, implying that cutaneous nerves augment mast cell (MC)-driven inflammatory responses by releasing neuropeptides (Siebenhaar et al., 2008). Neuropeptides activate mast cells in an FcεRI-independent manner (Bienenstock et al., 1991) and initiate unique signaling pathways that result in the release of a distinct array of mediators from that triggered by FcεRI-mediated activation. Thus, it appears that mast cells can regulate nerve function and nerve cell-derived neuropeptides can counter-regulate mast cell function. We have previously shown that substance P (SP) activates human mast cell degranulation and chemokine production by a Pertussis-sensitive mechanism (Kulka et al., 2008). In this study, the long-term effect of SP on mast cell expression of FcεRI was characterized.
2. Materials and methods
2.1. Human mast cell culture
LAD2 MC (Kirshenbaum et al., 2003) were cultured in serum free medium (StemPro-34 SFM, Life Technologies, Rockville, MD) supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 50 μg/ml streptomycin and 100 ng/ml stem cell factor (SCF). The cell suspensions were seeded at a density of 105 cells/ml and maintained at 37 °C and 5% CO2. Cells were fed by hemi-depletion and replacement of medium once per week.
Human peripheral blood derived CD34+ cells were cultured in StemPro-34 SFM supplemented with 2 mM L-glutamine, 50 μg/ml streptomycin, 100 IU/ml penicillin, 100 ng/ml SCF, and 100 ng/ml recombinant human IL-6 (PeproTech, Inc., Rocky Hill, NJ). Recombinant human IL-3 (30 ng/ml) was added for the first week. Half of the culture medium was replaced every 7 days. Cultures at 8 to 10 weeks consisted of greater than 99% human mast cells (huMC) (Kirshenbaum et al., 1999).
Unless otherwise stated, experiments were performed in StemPro-34 SFM complete with 10 ng/ml SCF.
2.2. Degranulation assay
Cells were sensitized overnight with 0.5 μg/ml of myeloma IgE (BioDesign International, Kennebunk, ME). Cells were washed, resuspended in buffer and then stimulated with rabbit anti-IgE (Dako, Carpinteria, CA) or other agonists and incubated at 37 °C for 0.5 h. The β-hexosaminidase released into the supernatants and in cell lysates was quantified by hydrolysis of p-nitrophenyl N-acetyl-β-D-glucosamide (Sigma-Aldrich, St Louis, MO) in 0.1 M sodium citrate buffer (pH 4.5) for 90 min at 37 °C. The percentage of β-hexosaminidase release was calculated as a percent of total content. Agonists tested were compound 48/80 (Sigma-Aldrich), SP (Sigma- Aldrich), and SP constructs (Phoenix Pharmaceuticals, Belmont, CA).
In some cases, Laboratory of Allergic Diseases (LAD)2 cells were pretreated with vehicle (0.1% DMSO) or Pertussis toxin (3.5 nM) for 2 h. Cells were then stimulated with SP for 30 min and β-hexosaminidase release was measured. The half maximal inhibitory concentration (IC50) for Pertussis toxin was 0.1 nM. In antagonist studies, mast cells were pretreated with NK1R antagonists spantide I or SP (4–11) at 0.1 μM for 30 min, stimulated with 0.1 μM SP for another 30 min and β-hexosaminidase release was measured.
2.3. Tryptase activity assay
Cells were washed and activated with SP (1 μg/ml) or untreated for 3 h. Cell-free supernatants were treated with soybean trypsin inhibitor (SBTI) for 30 min and tryptase activity was measured using a colorimetric assay as described. Briefly, 50 μL mast cell supernatants were mixed with 100 μL 0.8 mM BAPNA (Nα-Benzoyl-DL-arginine 4-nitroanilide hydrochloride) (Sigma-Aldrich) in TRIS buffer (0.1 M tris/1 M glycerol, pH 7.8) and were incubated for 4 h at 37 °C. The appearance of nitroaniline was measured at 410 nm. Tryptase activity was calculated as the percent of maximum tryptase activity which was defined as the activity present in supernatants from SP (1 μg/ml) stimulated mast cells in the absence of any inhibitor.
2.4. Quantitative (Real-time) PCR analysis
Total RNA was isolated from each preparation using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA). Five micrograms of total cellular RNA was reverse transcribed using the Taqman Reverse Transcription reagents and Random Hexamer primer (Perkin–Elmer Applied Biosystems, Foster City, CA). Gene expression was analyzed using real-time PCR on an ABI7500 SDS system. Fifty ng of cDNA was used in each quantitative PCR assay. Primer sets for PCR amplifications were designed using the Primer Express software (Perkin–Elmer Applied Biosystems; Table 1). All reactions were performed in triplicate for 40 cycles as per the manufacturer's recommendation. Results are expressed as relative mRNA corrected with reference to GAPDH mRNA as an internal control (Vandesompele et al., 2002).
Table 1.
Sequences of oligonucleotides used for quantitative PCR.
| Gene | Forward primer | Reverse primer | Probe (FAM/TAMRA) |
|---|---|---|---|
| FcεRIα | tgtggcagctggactatgagtct | acttctcacgcggagcttttat | gcccctcaacatta |
| FcεRIβ | acgggattaccatcctgatcat | aaatttctggcaactgtggatc | aagaagagcttggcctata |
| FcεRIγ | ctggatgccatcctgtttctgta | cctttcgcacttggatcttca | cctcaccctcctctact |
| β-actin | ctggccgggacctgact | gcagccgtggccatctc | caccaccacggccga |
2.5. Flow cytometric analysis
Cells were untreated or treated with stimulants (SP and vasoactive intestinal peptide (VIP)) for 24 h unless otherwise specified. In cases where cells were stimulated via FcεRI (Fig. 1A), cells were sensitized with 0.5 μg/ml human myeloma IgE for 16 h, then treated with anti-IgE (Dako) for an additional 24 h. Cells were washed and resuspended at 5×105 cells/ml in PBS/0.1% BSA and incubated with anti-FcεRI-PE (eBiosciences, San Diego, CA) or anti-IgE-FITC (Invitrogen, Carlsbad, CA) or appropriate isotype control antibody (BD Biosciences, Mississauga, ON) for 30 min at 4 °C. Cells were washed twice and in the cases where the primary antibody was unconjugated, anti-rabbit-PE (BD Biosciences) or anti-mouse-PE (BD Biosciences) was added for 30 min at 4 °C. Cells were washed twice, resuspended in PBS/0.1% BSA and analyzed on a FACSArray or FACSAria (BD Biosciences). FcεRI expression was calculated as a percentage of FcεRI expression of untreated cells (as determined by mean fluorescence intensity (MFI)). Percent inhibition of treated samples was determined by using the following formula:
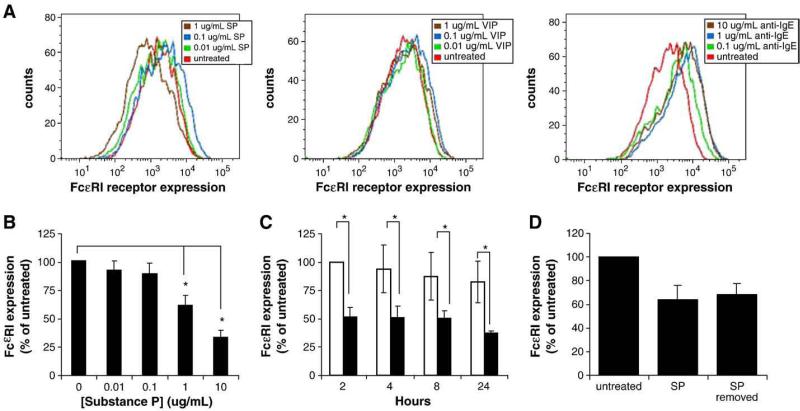
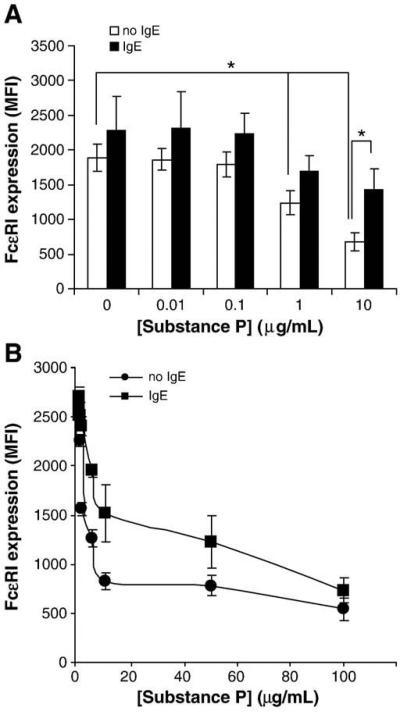
Fig. 1.
Substance P downregulates FcεRI surface expression by mast cells. A, Primary cultured CD34+-derived mast cells were treated with SP (SP; 0.01, 0.1 and 1 μg/ml), VIP (VIP; 0.01, 0.1 and 1 μg/ml) or IgE/anti-IgE (0.1, 1 and 10 μg/ml anti-IgE) for 24 h and FcεRI expression was measured by flow cytometry. B, LAD2 mast cells were treated with SP for 24 h and FcεRI expression was analyzed by flow cytometry. (*=P value<0.01) C, LAD2 mast cells were treated with PBS (white bars) or 10 μg/ml SP (black bars) for times indicated and FcεRI expression was measured by flow cytometry. D, LAD2 mast cells were treated with 1 μg/ml SP for 30 min, washed with fresh media and then incubated in complete media (10 ng/ml SCF) lacking SP. FcεRI expression was measured by flow cytometry. All results are representative of three separate experiments. *=P value<0.05.
To measure cell surface IgE binding, LAD2 mast cells were incubated for 24 h with SP, sensitized with IgE (0.5 μg/ml) for 30 min, incubated with anti-IgE-FITC (BD Biosciences, 5 μg/ml) for 30 min on ice and analyzed by flow cytometry. Maximum IgE binding was determined as the MFI of cells sensitized with 0.5 μg/ml IgE for 30 min.
2.6. ELISA for CysLT production
LAD2 cells were washed with media, suspended at 1×106 cells per well, and incubated for 24 h with SP. Cells were then sensitized with IgE (0.5 μg/ml) for 30 min, and stimulated with anti-IgE for 3 h. Cell-free supernatants were analyzed for CysLT production by using a commercial ELISA kit (Cayman Chemical, Ann Arbor, MI). In some cases, LAD2 mast cells were incubated for 24 h with SP (10 μg/ml), sensitized with IgE for 30 min, and then stimulated with anti-IgE for 3 h. Cell-free supernatants were analyzed for CysLT production by ELISA as above.
2.7. Statistical analysis
Each experiment was performed at least 3 separate times and in quadruplicate and values displayed represent mean±standard error of the mean. P values were determined by Student's t test (between groups) or one-way ANOVA (comparing more than two groups).
3. Results
3.1. Substance P downregulated human mast cell surface FcεRI expression
SP activates LAD2 mast cells and primary cultured mast cells (differentiated from CD34+ peripheral blood stem cells) to degranulate and produce cytokines and chemokines (Kulka et al., 2008). To determine whether SP had long-term effects on human mast cell IgE-mediated activation, we treated primary cultured human mast cells with SP for 24 h and measured FcεRI expression by flow cytometry. SP dose dependently downregulated FcεRI expression compared to untreated mast cells (Fig. 1A). To determine whether this effect was specific to SP or whether it was a common phenomenon also triggered by other neuropeptides, we treated human mast cells with VIP, a neuropeptide that similarly activates G protein receptors on human mast cells. VIP had no effect on FcεRI expression, even at the highest concentration of 1 μg/ml. IgE sensitization (not shown) or a combination of IgE sensitization and activation with anti-IgE increased FcεRI expression (Fig 1A). Annexin-V and propidium iodide staining showed no effect on mast cell viability after 24 h treatment with SP, VIP or IgE/anti-IgE (data not shown).
To further explore the effects of SP on FcεRI expression, LAD2 human mast cells were treated with SP for 24 h and FcεRI expression was measured by flow cytometry (Fig 1B). At a concentration of 1 μg/ml, SP downregulated FcεRI expression by approximately 30%, and the highest concentration tested, 10 μg/ml, reduced expression by nearly 70%. The effect was observed relatively rapidly, as mast cells treated with 10 μg/ml SP for only 2 h showed a 50% reduction in FcεRI protein expression compared to untreated cells (Fig. 1C).
To determine whether SP-mediated downregulation of FcεRI requires prolonged exposure to SP, we treated LAD2 cells with SP for 30 min, then washed with fresh media and incubated the cells overnight in complete media lacking SP. As shown in Fig. 1D, the short stimulation of LAD2 with SP for 30 min was sufficient to downregulate FcεRI expression. Therefore, a prolonged period of SP treatment is not required for downregulation of FcεRI.
3.2. Substance P downregulated human mast cell FcεRI gene expression
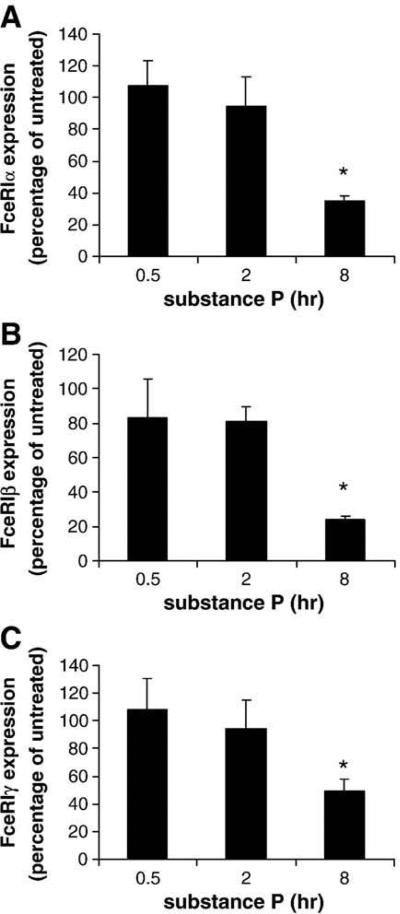
To determine whether SP-mediated downregulation of FcεRI expression was dependent upon changes in gene transcription, expression of FcεRI α, β and γ subunit mRNA was analyzed by real-time PCR (Fig. 2). After at least 8 h of incubation, SP significantly inhibited the expression of FcεRIα (Fig. 2A), β (Fig. 2B) and γ (Fig. 2C) subunits when compared to the untreated control.
Fig. 2.
Substance P decreases transcript-level expression of FcεRI α, β and γ by mast cells. LAD2 mast cells were treated with 1 μg/ml SP or PBS for times indicated. FcεRI α (A), β (B) and γ (C) subunit expression by SP-treated cells is presented as percentage of FcεRI expression by untreated cells (PBS) at each time point. (n=3, P<0.01).
3.3. Inhibition of mast cell degranulation or protease activity does not affect Substance P downregulation of FcεRI expression
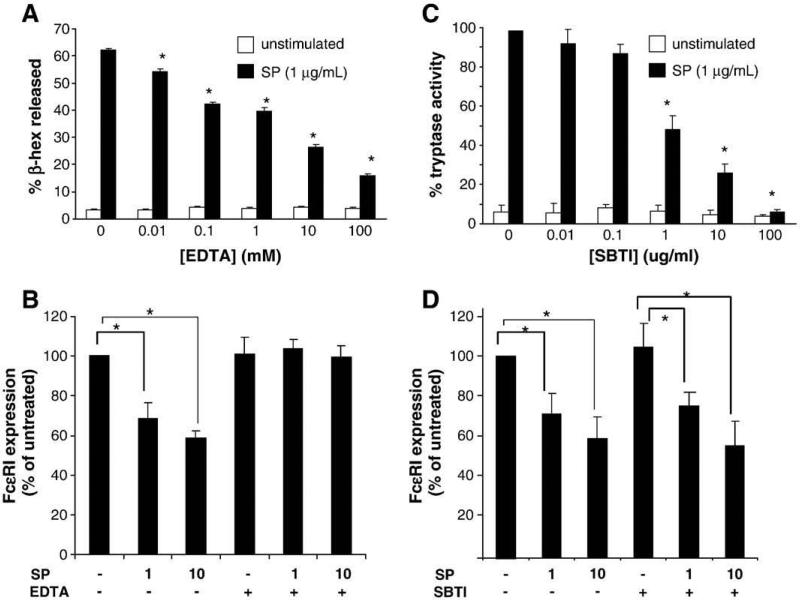
Since SP degranulates human mast cells (Kulka et al., 2008), the contribution of degranulation to the SP effect on FcεRI expression was evaluated. Mast cell degranulation is dependent upon calcium flux, and some investigators have used EDTA to block mast cell degranulation in experimental systems. EDTA blocked SP-induced degranulation by LAD2 in a concentration-dependent manner (Fig. 3A). Pretreatment of mast cells with EDTA and then activation with SP for 24 h blocked the SP-mediated downregulation of FcεRI (Fig. 3B). To further test the hypothesis that degranulation, and subsequent release of mast cell proteases, might be necessary for the SP effect on FcεRI expression, LAD2 were pretreated with the broad spectrum protease inhibitor, soybean trypsin inhibitor (SBTI), activated with SP, and FcεRI expression was analyzed by flow cytometry. SBTI was an effective inhibitor of mast cell tryptase activity in our system, and was able to block the activity of tryptase released by SP activation (Fig. 3C), confirming other reports (Fukuoka and Schwartz, 2004). Others have shown that SBTI is also an effective inhibitor of mast cell chymase (Alshurafa et al., 2004; He and Zheng, 2004; Muramatsu et al., 2000). However, SBTI did not affect SP-mediated downregulation of FcεRI suggesting that it was not mediated by the enzymatic activity of released tryptase or chymase. SBTI did not affect LAD2 cell degranulation in response to SP (data not shown).
Fig. 3.
Substance P decreases FcεRI surface expression on mast cells through a degranulation-dependent but trypsin-independent mechanism. (A) Cells were pretreated with EDTA for 30 min before activation with SP and β-hexosaminidase release was measured. (n=3, P<0.01 when compared to SP alone control) (B), Cells were treated with 50 mM EDTA for 5 min prior to treatment with 1 or 10 μg/ml SP. (C), Cells were untreated or treated with SP with or without STBI for 24 h and supernatants were assayed for tryptase activity. (n=3, P<0.01 when compared to SP alone control) (D), Cells were treated with 100 μg/ml SBTI for five minutes prior to treatment with 1 or 10 μg/ml SP. LAD2 were treated with SP for 24 h and FcεRI surface expression was measured by flow cytometry. (n=3, P<0.01).
3.4. Pertussis toxin sensitivity of the downregulation of FcεRI expression by Substance P
SP activation of human mast cell degranulation and cytokine and chemokine production is sensitive to Pertussis toxin, a G(i/o) inhibitor. To determine if SP-mediated downregulation of FcεRI expression was similarly dependent upon G(i/o) protein activation, we pretreated human mast cells with toxin prior to treatment with SP and evaluated FcεRI expression by flow cytometry. Pertussis toxin selectively blocked SP-mediated downregulation of FcεRI (Fig. 4).
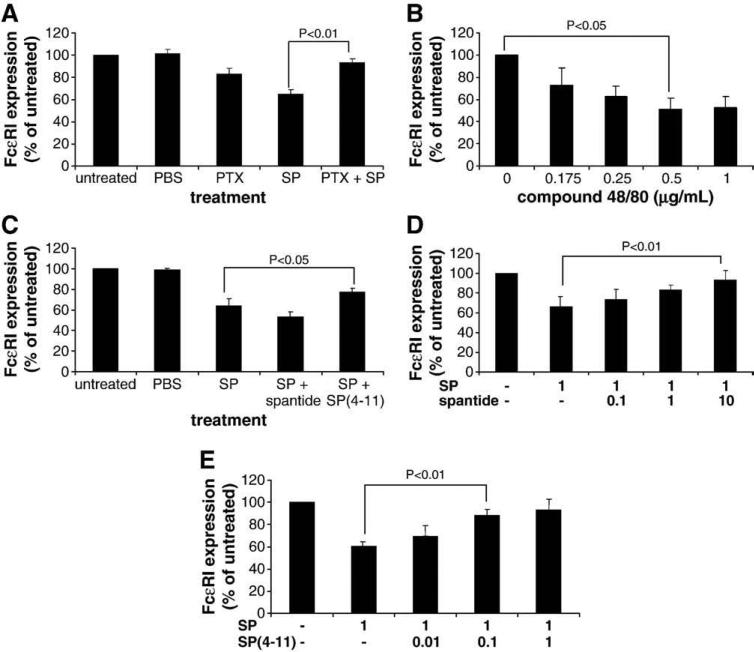
Fig. 4.
Substance P-mediated downregulation of FcεRI expression is Pertussis toxin-sensitive and partially blocked by a neurokinin receptor antagonist. (A), Cells were pretreated with Pertussis toxin for 2 h before activation with 1 μg/ml SP for 24 h and FcεRI expression was measured by flow cytometry. (n=3, P<0.01) (B), Cells were treated with compound 48/80 for 24 h and FcεRI expression was measured by flow cytometry. (C), Cells were pretreated with spantide I or SP(4–11) (0.1 μM) for 30 min before activation with 1 μg/ml SP for 24 h and FcεRI expression was measured by flow cytometry. (n=3, P<0.05) (D), Cells were pretreated with spantide I (μM) for 30 min before activation with 1 μg/ml SP for 24 h and FcεRI expression was measured by flow cytometry. (n=3, P<0.01) (E), Cells were pretreated with SP(4–11) (μM) for 30 min before activation with 1 μg/ml SP for 24 h and FcεRI expression was measured by flow cytometry. (n=3, P<0.01).
Since compound 48/80 directly activates G proteins and activates mast cell degranulation, LAD2 cells were treated with compound 48/80 and FcεRI expression was measured. Similar to SP, compound 48/80 downregulated FcεRI expression (Fig. 4B).
3.5. Substance P reduction of FcεRI expression was blocked by neurokinin receptor antagonists
SP binds and activates the neurokinin receptor NK1R. To determine if the effect of SP on FcεRI expression was NK1R-dependent, mast cells were pretreated with two peptide antagonists of NK1R, SP(4–11) [D-Pro4 D-Trp7.9] and spantide I, treated with SP for 24 h and FcεRI expression was analyzed by flow cytometry. Although spantide I did not have any effect on the SP-mediated downregulation of FcεRI (Fig. 4C), mast cells pretreated with SP(4–11) and SP expressed significantly greater levels of FcεRI than mast cells that were treated with SP alone (P<0.05), indicating that SP(4–11) blocked SP-mediated downregulation of FcεRI expression. Since SP(4–11) blocked the SP effect and spantide I did not, different concentrations of both antagonists were tested to determine whether potency was concentration dependent. Spantide I blocked the SP-mediated downregulation of FcεRI expression at a dose of 10 μM (Fig. 4C) whereas SP(4–11) was much more potent and blocked the SP-mediated downregulation of FcεRI expression at a concentration of 0.1 μM but not 0.01 μM (Fig. 4E).
3.6. Substance P inhibition of mast cell activation via FcεRI
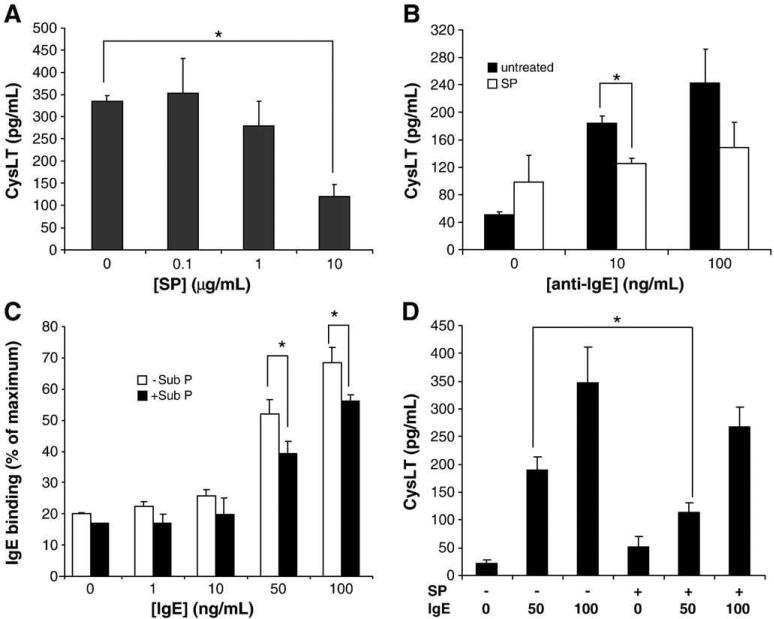
Since SP downregulated FcεRI protein and mRNA expression by approximately 40–60% (see Fig. 1 and 2), we attempted to test whether reduced FcεRI expression resulted in a decreased sensitivity to IgE-mediated activation. Obviously, there are technical difficulties in answering this question since both IgE/anti-IgE and SP activate mast cell degranulation, and once mast cells have been treated with SP, they will have degranulated and they no longer respond to SP. However, we have made the observation that SP, unlike IgE/anti-IgE, does not activate mast cells to produce cysteinyl leukotrienes (CysLT), an important pro-inflammatory mediator and thus used CysLT as a marker of the response to IgE crosslinking.
LAD2 cells treated with SP for 24 h, sensitized with IgE/anti-IgE for 1 h, and then activated with anti-IgE produced significantly less CysLT than untreated cells activated with IgE/anti-IgE (Fig. 5A). Although it was apparent at both high and low concentrations of anti-IgE, the inhibitory effect of SP on CysLT production was only statistically significant when LAD2 were stimulated with 10 μg/ml anti-IgE (Fig. 5B).
Fig. 5.
Substance P inhibits FcεRI-mediated production of CysLT. (A) LAD2 mast cells were incubated for 24 h with SP, sensitized with IgE (0.5 μg/ml) for 30 min, and then stimulated with anti-IgE for 3 h. Cell free supernatants were analyzed for CysLT production by ELISA (n=3, P<0.01). (B) LAD2 mast cells were incubated with SP (10 μg/ml) and analyzed for CysLT production by ELISA (n=3, P<0.01). (C) LAD2 mast cells were incubated with SP, sensitized with IgE , incubated with anti-IgE-FITC and analyzed by flow cytometry. Maximum IgE binding was determined as the MFI of cells sensitized with 0.5 μg/ml IgE for 30 min (n=5, P<0.01). (D) LAD2 mast cells were incubated with SP (10 μg/ml), sensitized with IgE, and stimulated with anti-IgE for 3 h. CysLT production was measured by ELISA (n=3, P<0.01).
If SP-mediated downregulation of FcεRI was solely responsible for the decrease in CysLT production observed in Fig. 5, then under-sensitizing LAD2 with IgE would likewise cause a decrease in CysLT production. To test this hypothesis, SP-treated and untreated LAD2 were sensitized with different concentrations of IgE (1-100 ng/ml for 30 min) and the amount of bound IgE was evaluated by flow cytometry and an anti-IgE FITC antibody (Fig. 5C). As expected, the SP-treated LAD2 bound less IgE than the untreated controls. SP-treated mast cells sensitized with 100 ng/ml IgE bound the same amount of IgE as untreated LAD2 sensitized with 50 ng/ml of IgE. SP significantly inhibited CysLT production by LAD2 sensitized with 50 ng/ml IgE but SP did not significantly affect CysLT production by LAD2 sensitized with 100 ng/ml IgE (Fig. 5D). This data suggested that IgE had a protective effect on SP-mediated downregulation of FcεRI.
3.7. Substance P-mediated downregulation of FcεRI was abrogated by the presence of soluble IgE
In the next set of experiments, we determined whether mast cell sensitization with human myeloma IgE would influence the effect of SP on FcεRI expression. We hypothesized that SP would not decrease expression of FcεRI of sensitized mast cells since membrane FcεRI expression is stabilized in the presence of soluble IgE (Yamaguchi et al., 1999). LAD2 cells were either sensitized with human myeloma IgE (1 μg/ml) for 24 h or left untreated and then stimulated with SP for an additional 24 h. Sensitized mast cells stimulated with SP still showed a decline in FcεRI expression, though it was reduced in magnitude (Fig. 6A). Further analysis showed that in the presence of IgE, the IC50 of SP was 22.5 μg/ml (Fig. 6B) whereas the IC50 of SP in the absence of IgE was 5.2 μg/ml (Fig. 6B). These data suggest that the presence of IgE increases the amount of SP required to downregulate FcεRI by 50% but does not prevent FcεRI downregulation.
Fig. 6.
IgE sensitization abrogates the decrease in FcεRI expression caused by Substance P. (A) LAD2 mast cells were incubated overnight with or without 0.5 μg/ml IgE. Following incubation, cells were treated with SP for 24 h and FcεRI surface expression was measured by flow cytometry. (n=3, P<0.01). (B) LAD2 mast cells were incubated overnight with 0.5 μg/ml IgE and then stimulated with different concentrations of SP and FcεRI surface expression was measured by flow cytometry (n=3).
4. Discussion
Although the ability of SP to induce acute mast cell activation has been known for some time, our study is the first to demonstrate that SP has profound effects on the IgE receptor signaling pathway.
SP is a member of the tachykinin family of neuropeptides which bind three neurokinin receptors (NK1R, NK2R and NK3R), although SP preferentially binds NK1R (O'Connor et al., 2004). While SP has been described as a neuronal peptide, studies in rodents have demonstrated that macrophages, eosinophils, lymphocytes and dendritic cells may also produce SP (Lambrecht, 2001; Lambrecht et al., 1999; Weinstock et al., 1988). In the spinal cord, SP participates in neurotransmission of pain and modulates autonomic reflexes (O'Connor et al., 2004). In the periphery, SP is localized in the primary sensory neurons and neurons intrinsic to the gastrointestinal, respiratory and genitourinary tracts (Montero Mora et al., 2004). Centrally and peripherally released SP is involved in stress-induced bladder damage and inhibition of NK1R in mouse models of bladder damage prevents stress-induced inflammation (Ercan et al., 2006), a process thought to be mediated by mast cells. The SP content of human airways is increased in asthma suggesting that SP may be involved in asthma (Nieber et al., 1993; Nieber et al., 1992). SP-induced release of inflammatory mediators such as histamine may potentiate tissue injury, and stimulate further leukocyte recruitment, thereby amplifying the inflammatory response (Groneberg et al., 2004).
We demonstrate here that exposure of the human mast cell line LAD2 to concentrations of SP as low as 1 μM led to clear down-regulation of mast cell expression of the high affinity Fc receptor for IgE. SP reduced IgE receptors within 30 mins and the effect was blocked by treatment with EDTA. Since EDTA also inhibited SP-induced degranulation, this suggested that the two processes might be related. EDTA is a non-specific inhibitor and blocks any process requiring divalent cations and although we have not eliminated the possibility that changes in FcεRI expression may be due to the process of degranulation, some of our data suggests that this is not the case. Crosslinking of IgE, which also induced degranulation, did not lead to the downregulation of IgE receptors. In addition, the potent protease inhibitor, SBTI, completely blocked the proteolytic activity of tryptase but failed to alter the downregulation of IgE receptors. While this suggests that released tryptase is not mediating the effect, it leaves open the possibility that other, SBTI-insensitive, mast cell proteases, that are released by SP but not IgE crosslinking mediate the effect. Spantide I, which has blocking activity against at least two NK receptors, prevented the downregulation of IgE receptors. We have previously demonstrated that spantide I fails to inhibit SP-mediated degranulation in mast cells (Kulka et al., 2008) and therefore these data further support the argument that SP effects on FcεRI expression are not dependent upon degranulation. The SP-mediated decrease in IgE receptor expression was of a magnitude that was functionally relevant as mast cells pretreated with SP demonstrated reduced ability to release CysLT in response to IgE crosslinking.
The signaling mechanism by which SP achieves the reduction of IgE receptors is unknown. SP is a short-lived peptide and it is likely that the majority of the added SP is degraded by mast cell-derived proteases shortly after addition to culture. However, it is clear that SP activation of mast cells, even within the first few minutes, initiates signaling pathways that ultimately lead to a profound change in mast cell phenotype. Since only brief exposure to SP was necessary, we presume that these effects do not require the continued presence of the agonist. Studies with the G protein inhibitor, pertussis toxin, suggest that the process is pertussis toxin-sensitive, in agreement with the possibility that it is mediated by one of the known receptors for SP. Furthermore, compound 48/80, which directly activates G proteins in mast cells (Mousli et al., 1990b), also downregulated FcεRI expression suggesting that this process is dependent upon G protein signaling pathways. It is puzzling, however, that VIP, which also activates mast cells via G proteins (Kulka et al., 2008), did not similarly downregulate FcεRI expression. It is possible that SP activates a unique G protein pathway that is distinct from that of VIP and that is NK receptor- but not VIP receptor-mediated. For example, VIP binds both VPAC1 and VPAC2 which have very distinct signaling pathways (Langer and Robberecht, 2007). Our earlier studies have shown that human mast cells express only VPAC2 (Kulka et al., 2008) which couples to pertussis-insensitive Gαs and Gα16 and requires the presence of free Gβγ subunits to elevate intracellular Ca2+ (Langer et al., 2005). However, it is believed that compound 48/80 and SP may directly bind the Gαi proteins, through direct interaction with the C terminus (Mousli et al., 1990a) rendering them sensitive to pertussis inhibition. These signaling differences may explain why SP and compound 48/80 downregulated FcεRI expression while VIP did not.
It is not entirely clear which receptor(s) are responsible for SP-induced mast cell responses. However, we have previously demonstrated that LAD2 mast cells express several NK and VIP receptors (Kulka et al., 2008). However, pretreatment of human mast cells with an NK receptor antagonists, spantide I and SP(4–11), blocked SP-induced downregulation of FcεRI supporting the suggestion that this SP effect is mediated by a NK receptor. There are three neurokinin/tachykinin receptors, NK1R, NK2R, and NK3R, with SP having the highest affinity for NK1R (Tuluc et al., 2009). Since SP is able to bind NK2R with lower affinity (Kang et al., 2004), it is possible that NK2R may also play a role in downregulating expression of FcεRI. Certainly, the role of NK1R in this study remains speculative. Spantide I abrogated SP-induced changes in FcεRI expression at concentrations much higher than the binding constant for the NK1R and SP(4–11) abrogated the SP effect at 1 μg/ml. Recently, it has been shown that Mas-related gene (Mrg) receptors can act through G proteins and activate human mast cell degranulation (Tatemoto et al., 2006). Since SP can potentially bind and activate MrgX2, it is possible that Mrg receptors may be involved in SP-mediated downregulation of FcεRI expression. Further investigation will be required to identify the specific receptors that mediate this potentially important effect of SP. Unlike FcεRI, SP likely does not require tyrosine kinases for initiation of degranulation but initiates degranulation by activating Gα subunits and Gβγ subunits, like many other activators of G protein coupled receptors (GPCRs) (Mousli et al., 1990a,b). Ultimately, both FcεRI crosslinking and SP activate phosphoinositol-3 kinase (PI3K) targeting to the membrane via Grb2-associated binding protein 2 (Gab2)19. However, unlike FcεRI crosslinking, SP likely acts like other activators of GPCRs which utilize the PLCβ and the p110γ subunit isoform of PI3K (Kuehn and Gilfillan, 2007). Therefore, it is plausible that these different signaling events (or pathways not yet described) account for the downregulation of FcεRI — a phenomenon not observed in response to crosslinking of FcεRI itself.
Studies to determine the influence of occupancy of the IgE receptors with IgE on their downregulation by SP indicate that sensitized mast cells are relatively resistant to the influence of SP on FcεRI receptor numbers. This suggests, but does not prove, that occupied receptors may be relatively resistant to the process by which SP leads to their disappearance. Studies by several groups of investigators have demonstrated that binding of IgE protects FcεRI receptors from an internalization mechanism that regulates receptor numbers. By this mechanism, the number of receptors on the mast cells reflects the levels of IgE in the environment of the mast cell. It is not surprising therefore that IgE might protect high affinity receptors from SP-mediated downregulation. This raises the interesting possibility that SP can downregulate mast cell IgE receptor levels by a physiological process that utilizes the established mechanisms of internalization. Although some receptors are recycled during this process, it is believed that a large proportion of the receptors are destroyed after internalization.
Mast cells are important regulators of neurogenic inflammation during stress responses and in various inflammatory diseases. Activation of mast cells has been known for some time to stimulate nerves by a variety of mechanisms. It has also been clear that activation of sensory nerves to release neuropeptides such as SP can lead to local inflammatory responses mediated by activation of mast cell degranulation. The present study demonstrates that SP release by sensory nerves may also influence the response of mast cells by altering the expression of the high affinity IgE receptor. The cross talk between nerves and mast cells may be more regulated than we previously believed, and activation of sensory nerves may alter the response of various tissues to specific antigens.
Older published studies have shown that pretreatment with SP along with antigen in human subjects reduces the subsequent skin test response to antigen (Patterson et al., 1999). It is possible that the effect described in the present report may underlie the clinical observations with SP used therapeutically along with antigen exposure. It is increasingly clear that an improved understanding of the activation and response to neuropeptides of mast cells may better elucidate our understanding of diseases in which mast cells and nerves interact. In particular, since responses of mast cells to SP and some other neuropeptides are most observed in cutaneous mast cells, these findings may have relevance to diseases of the skin.
Acknowledgements
The authors would like to thank Cecilia Sheen for her assistance in the culture of the human mast cells and Ms. Barb Mitchell for her assistance in the preparation of this manuscript.
This work was funded by the Ernest S. Bazely Trust to Northwestern Memorial Hospital and Northwestern University, a grant from the Bryan and Christina Cressey Foundation and an interest section award from the American Academy of Allergy, Asthma and Immunology.
References
- Alshurafa HN, Stenton GR, Wallace JL, Hollenberg MD, Befus AD, Vliagoftis H. A protease activated receptor-2 (PAR-2) activating peptide, tc- LI GRL O-NH2, induces protease release from mast cells: role in TNF degradation. BMC Pharmacol. 2004;4:12. doi: 10.1186/1471-2210-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J, MacQueen G, Sestini P, Marshall JS, Stead RH, Perdue MH. Mast cell/nerve interactions in vitro and in vivo. Am. Rev. Respir. Dis. 1991;143:S55–S58. doi: 10.1164/ajrccm/143.3_Pt_2.S55. [DOI] [PubMed] [Google Scholar]
- Bulut K, Felderbauer P, Deters S, Hoeck K, Schmidt-Choudhury A, Schmidt WE, Hoffmann P. Sensory neuropeptides and epithelial cell restitution: the relevance of SP-and CGRP-stimul mast cells. Int. J. Colorectal. Dis. 2008;23:535–541. doi: 10.1007/s00384-008-0447-7. [DOI] [PubMed] [Google Scholar]
- Ercan F, Akici A, Ersoy Y, Hurdag C, Erin N. Inhibition of substance P activity prevents stress-induced bladder damage. Regul. Pept. 2006;133:82–89. doi: 10.1016/j.regpep.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Fukuoka Y, Schwartz LB. Human beta-tryptase: detection and characterization of the active monomer and prevention of tetramer reconstitution by protease inhibitors. Biochemistry. 2004;43:10757–10764. doi: 10.1021/bi049486c. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Quarcoo D, Frossard N, Fischer A. Neurogenic mechanisms in bronchial inflammatory diseases. Allergy. 2004;59:1139–1152. doi: 10.1111/j.1398-9995.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- He SH, Zheng J. Stimulation of mucin secretion from human bronchial epithelial cells by mast cell chymase. Acta Pharmacol. Sin. 2004;25:827–832. [PubMed] [Google Scholar]
- Kang HS, Trzaska KA, Corcoran K, Chang VT, Rameshwar P. Neurokinin receptors: relevance to the emerging immune system. Arch. Immunol Ther. Exp. Warsz. 2004;52:338–347. [PubMed] [Google Scholar]
- Kawahira K, Sumiyoshi M, Sakanaka M, Kimura Y. Effects of ginsenoside Rb1 at low doses on histamine, substance P, and monocyte chemoattractant protein 1 in the burn wound areas during the process of acute burn wound repair. J. Ethnopharmacol. 2008;117:278–284. doi: 10.1016/j.jep.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenit or cell populati on that is CD34 (+), c-kit (+), and expresses aminopeptidase N CD13. Blood. 1999;94:2333–2342. [PubMed] [Google Scholar]
- Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metcalfe DD. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcεRI or FcgammaRI. Leukoc. Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcεRI-mediated mast cell activation. Immunol. Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2007 doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123:398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN. Immunologists getting nervous: neuropeptides, dendritic cells and T cell activation. Respir. Res. 2001;2:133–138. doi: 10.1186/rr49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN, Germonpre PR, Everaert EG, Carro-Muino I, De Veerman M, de Felipe C, Hunt SP, Thielemans K, Joos GF, Pauwels RA. Endogenously produced substance P contributes to lymphocyte proliferation induced by dendritic cells and direct TCR ligation. Eur. J Immunol. 1999;29:3815–3825. doi: 10.1002/(SICI)1521-4141(199912)29:12<3815::AID-IMMU3815>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Langer I, Robberecht P. Molecular mechanisms involved in vasoactive intestinal peptide receptor activation and regulation: current knowledge, similarities to and differences from the A family of G-protein-coupled receptors. Biochem. Soc. Trans. 2007;35:724–728. doi: 10.1042/BST0350724. [DOI] [PubMed] [Google Scholar]
- Langer I, Langlet C, Robberecht P. Effect of inactivating mutations on phosphorylation and internalization of the human VPAC2 receptor. J. Mol. Endocrinol. 2005;34:405–414. doi: 10.1677/jme.1.01717. [DOI] [PubMed] [Google Scholar]
- Montero Mora P, Gonzalez Perez Mdel C, Almeida Arvizu V, Matta Campos JJ. [Autoimmune urticaria. Treatment with methotrexate]. Rev. Alerg. Mex. 2004;51:167–172. [PubMed] [Google Scholar]
- Mousli M, Bronner C, Bockaert J, Rouot B, Landry Y. Interaction of substance P, compound 48/80 and mastoparan with the alpha- subunit C-terminus of G protein. Immunol. Lett. 1990a;25:355–357. doi: 10.1016/0165-2478(90)90207-7. [DOI] [PubMed] [Google Scholar]
- Mousli M, Bronner C, Landry Y, Bockaert J, Rouot B. Direct activation of GTP-binding regulatory proteins (G-proteins) by substance P and compound 48/80. FEBS Lett. 1990b;259:260–262. doi: 10.1016/0014-5793(90)80023-c. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Katada J, Hattori M, Hayashi I, Majima M. Chymase mediates mast cell-induced angiogenesis in hamster sponge granulomas. Eur. J. Pharmacol. 2000;402:181–191. doi: 10.1016/s0014-2999(00)00350-2. [DOI] [PubMed] [Google Scholar]
- Nieber K, Baumgarten CR, Rathsack R, Furkert J, Oehme P, Kunkel G. Substance P and beta-endorphin-like immunoreactivity in lavage fluids of subjects with and without allergic asthma. J. Allergy Clin. Immunol. 1992;90:646–652. doi: 10.1016/0091-6749(92)90138-r. [DOI] [PubMed] [Google Scholar]
- Nieber K, Baumgarten C, Rathsack R, Furkert J, Laake E, Muller S, Kunkel G. Effect of azelastine on substance P content in bronchoalveolar and nasal lavage fluids of patients with allergic asthma. Clin. Exp. Allergy. 1993;23:69–71. doi: 10.1111/j.1365-2222.1993.tb02486.x. [DOI] [PubMed] [Google Scholar]
- O'Connor TM, O'Connell J, O'Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J. Cell. Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- Patterson R, Harris KE, Grammer LC, Greenberger PA, Ditto AM, Shaughnessy MA. Potential effect of the administration of substance P and allergen therapy on immunoglobulin E-mediated allergic reactions in human subjects. J. Lab. Clin. Med. 1999;133:189–199. doi: 10.1016/s0022-2143(99)90012-4. [DOI] [PubMed] [Google Scholar]
- Siebenhaar F, Magerl M, Peters EM, Hendrix S, Metz M, Maurer M. Mast cell-driven skin inflammation is impaired in the absence of sensory nerves. J. Allergy Clin. Immunol. 2008;121:955–961. doi: 10.1016/j.jaci.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, Ogasawara H, Edamura K, Takagi H, Iwamura H, Noguchi M, Naito T. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun. 2006;349:1322–1328. doi: 10.1016/j.bbrc.2006.08.177. [DOI] [PubMed] [Google Scholar]
- Tuluc F, Lai JP, Kilpatrick LE, Evans DL, Douglas SD. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol. 2009;30:271–276. doi: 10.1016/j.it.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock JV, Blum A, Walder J, Walder R. Eosinophils from granulomas in murine schistosomiasis mansoni produce substance P. J. Immunol. 1988;141:961–966. [PubMed] [Google Scholar]
- Yamaguchi M, Sayama K, Yano K, Lantz CS, Noben-Trauth N, Ra C, Costa JJ, Galli SJ. IgE enhances Fcε receptor I expression and IgE-dependent release of histamine and lipid mediators from human umbilical cord blood-derived mast cells: synergistic effect of I L-4 and IgE on human mast cell Fcepsilon receptor I expression and mediator release. J. Immunol. (Baltimore, Md.: 1950) 1999;162:5455–5465. [PubMed] [Google Scholar]