Abstract
Clinical correlation studies have clearly shown that obesity is associated with breast cancer risk and patient survival. Although several potential mechanisms linking obesity and cancers have been proposed, the detailed molecular mechanism of obesity-mediated breast tumorigenesis has not yet been critically evaluated. In this study, we evaluated the effects of obesity on mammary tumor initiation and progression using mice with genetic and diet-induced obesity bearing mammary tumor xenografts and mouse mammary tumor virusneu transgenic mice that were fed a high-fat diet. We show that obesity promoted mammary tumor growth and development in these animal models. Moreover, the expressions of TNFα, VEGF, IKKβ, and mTOR are upregulated in mammary tumors of obese mice, suggesting that the IKKβ/ mTOR/VEGF signaling pathway is activated by TNFα in the tumors of obese mice. More importantly, inhibitors (rapamycin, bevacizumab, and aspirin) that target members of the pathway suppressed tumorigenesis and prolonged survival more effectively in obese mice than in nonobese mice. Here, we not only identified a specific signaling pathway that contributes to mammary tumorigenesis in obese mice but also a strategy for treating obesity-mediated breast cancer.
Introduction
Obesity is a serious health problem in the United States and is associated with increased risk of diabetes, hypertension, and cardiovascular diseases. Moreover, growing evidence has shown that obesity is a risk factor for multiple types of human cancer (1–4) and known to be correlated with breast cancer tumorigenesis and development (5, 6). Breast cancer is the second-leading cause of cancer-related deaths in women in the United States, and obese breast cancer patients have a higher risk of recurrent disease, higher metastasis and mortality rates, larger tumor masses, and a poorer prognosis than nonobese breast cancer patients (7–12).
Several mechanisms relating to breast cancer prognosis in obese patients have been proposed. For example, estrogen (13, 14), which is expressed at significantly higher levels in obese postmenopausal women than in nonobese postmenopausal women is believed to increase the risks of developing breast cancer (6). The expression of insulin and insulin-like growth factors, which is also higher in obese patients, has also been proposed to play a role in breast cancer cell growth and survival (15). In addition, adipose tissues that secrete adipokines, such as leptin, adiponectin, growth factors, and proinflammatory cytokines, also enhance cancer cell growth (16–18). Particularly, recent studies have indicated that expression of the inflammatory cytokine TNFα is increased in obese patients, thereby suggesting that this cytokine plays a role in obesity-mediated breast cancer development and progression (19, 20). However, the specific signaling pathway linking TNFα and obesity to breast tumorigenesis and the exact mechanisms underlying the connection between obesity and breast cancer are still a matter of debate. Thus, investigating signaling pathways involved in obesitymediated breast cancer, which could lead to the development of targeted therapies that inhibit obesity-related tumor growth, is a significant and timely goal.
Previously, we showed that TNFα/ induces the IKKβ mTOR/VEGF pathway to promote breast cancer progression by enhancing angiogenesis (21). The activated TNFα receptor activates IKKβ, which in turn phosphorylates tuberous sclerosis protein 1 (TSC1) and actives the downstream signaling pathway such as mTOR. Moreover, IKKβ activation, TSC1 phosphorylation, and VEGF expression are correlated with poor clinical outcome in breast cancer patients (21). Because obese patients have higher levels of adipocytes and thus can secrete more TNFα (22), we speculated that this newly identified mechanism could link TNFα/ activated IKKβ/mTOR/VEGF (23) to obesity and therefore contribute to obesity-mediated breast cancer progression.
In this study, we sought to determine the effects of obesity on mammary tumor progression using 2 different mouse models of obesity: mice with genetic and dietinduced obesity (DIO) bearing orthotopic xenograft mammary tumors. Moreover, we also assessed the effects of DIO on tumor initiation in a mouse mammary tumor virus (MMTV)-neu model. Using these models, we clearly showed that obesity promotes both the development and growth of mammary tumors. More importantly, we found that the IKKβ/mTOR/VEGF signaling pathway was highly activated by TNFα in the tumors of obese mice, and inhibition of this pathway with clinically used drugs reduced obesity-mediated tumorigenesis. Thus, our study may provide not only a molecular interpretation for the higher incidence of breast cancer and poor prognosis in obese women but also a new avenue from which to develop therapeutic strategies for obesity-mediated breast cancer.
Materials and Methods
Cell lines
EO771 mouse mammary cancer cells were obtained from Dr. F.M. Sirotnak (Memorial Sloan-Kettering, New York, NY). No further authentication was carried out. Cells were cultured in RPMI-1640 medium supplemented with 10% FBS.
Animal model
We purchased 4-week-old female C57BL/6J wild-type (WT), B6.V-Lepob/J obese (OB), and MMTV-neu mice (10 per group) from The Jackson Laboratory and maintained them under the institutional animal care protocol established by the Department of Veterinary Medicine and Surgery at The University of Texas MD Anderson Cancer Center. The C57BL/6J and MMTV-neu mice were fed either a high-fat diet (HFD; Research Diets, Inc. D12492; ref. 24) or a normal chow diet (Teklad Global Diets, #2919; Supplementary Table S1) starting at 4 weeks of age. DIO was initiated in mice via ad libitum access to high-fat food for at least 8 to 12 weeks as described previously (25) until the experiments were completed. Normal chow diet and HFD mice were injected with 2 × 105 EO771 mammary adenocarcinoma cells at age 14 weeks. The OB mice, which spontaneously develop obesity even on normal chow diet (26, 27), were fed only the normal chow diet and injected with 2 × 105 EO771 mammary adenocarcinoma cells at age 14 weeks. Mice were removed from the experiment when tumor size reached more than 1.5 cm diameters in accordance with the Institutional Animal Care and Use Committee guidelines. The incidence of spontaneous tumor growth in MMTV-neu mice was noted when tumors growth was larger than 5 mm in diameter in the mammary gland. MMTV-neu develops mammary tumors spontaneously at about age 6 to 12 months (28, 29).
For the mammary tumor model, 2 × 105 EO771 cells were injected into the mammary fat pads of 14-week-old C57BL/6J or OB mice. After the tumors reached 3 to 5 mm in diameter, the mice were administered aspirin at 120 mg/kg/d (Sigma) via subcutaneous injection (30); rapamycin (LC Labs) at 5 mg/kg twice per week via intraperitoneal injection (31); and bevacizumab (Avastin, purchased from the Department of Pharmacy at MD Anderson Cancer Center) at 10 mg/kg once per week via intravenous injection (32).
Immunofluorescence assay
Mammary tumor tissue was harvested and embedded in Tissue-Tek OCT compound (Sakura Finetek) for frozen embedded and sectioned. The tumor samples were stained separately with anti-TNFα and anti-VEGF (IHC World); phosphorylated IKKβ (S176/180) [pIKKβ (S176/ 180)], phosphorylated S6 (S240/244) [pS6 (S240/244), (an indicator for mTOR activity)]; phosphorylated VEGF receptor (S996) [pIKKβ (S996)]; the previous 3 antibodies from Cell Signaling Technology); CD31 and CD45 antibody (BD Biosciences); Ki67 antibody as an indicator of cancer cell proliferation (Abcam). Nuclei were stained with 4’,6-diamidino-2-phenylindole blue and fluorescence intensity was analyzed using AxioVision software version 4.4 (Carl Zeiss).
Statistical analysis
Data are shown as the mean ± SD of 10 individual mice or treatments. Statistical significance between groups was analyzed using a Student t test or one-way or 2-way ANOVA. Survival was analyzed using a Kaplan-Meier plot, and MMTV-neu mice tumor incidence was measured via a log-rank test (Mantel-Cox test; refs. 33, 34). A P value of less than 0.05 was considered statistically significant and is henceforth indicated by an asterisk.
Results
Genetic obesity and DIO promote mammary tumorigenesis and increase mortality
Although clinical evidence indicates that obesity is one of the major risk factors for breast cancer (35) and despite the fact that researchers have proposed several mechanisms by which obesity and tumor progression are linked, limited information is available about the causal relationships between the molecular mechanisms and obesitymediated breast cancer. To further explore the underlying mechanisms of obesity-mediated breast cancer, we first used 2 kinds of obesity mouse models, DIO and genetic obesity, to establish mammary tumor xenograft in these mice and further investigated the effects of obesity tumor progression.
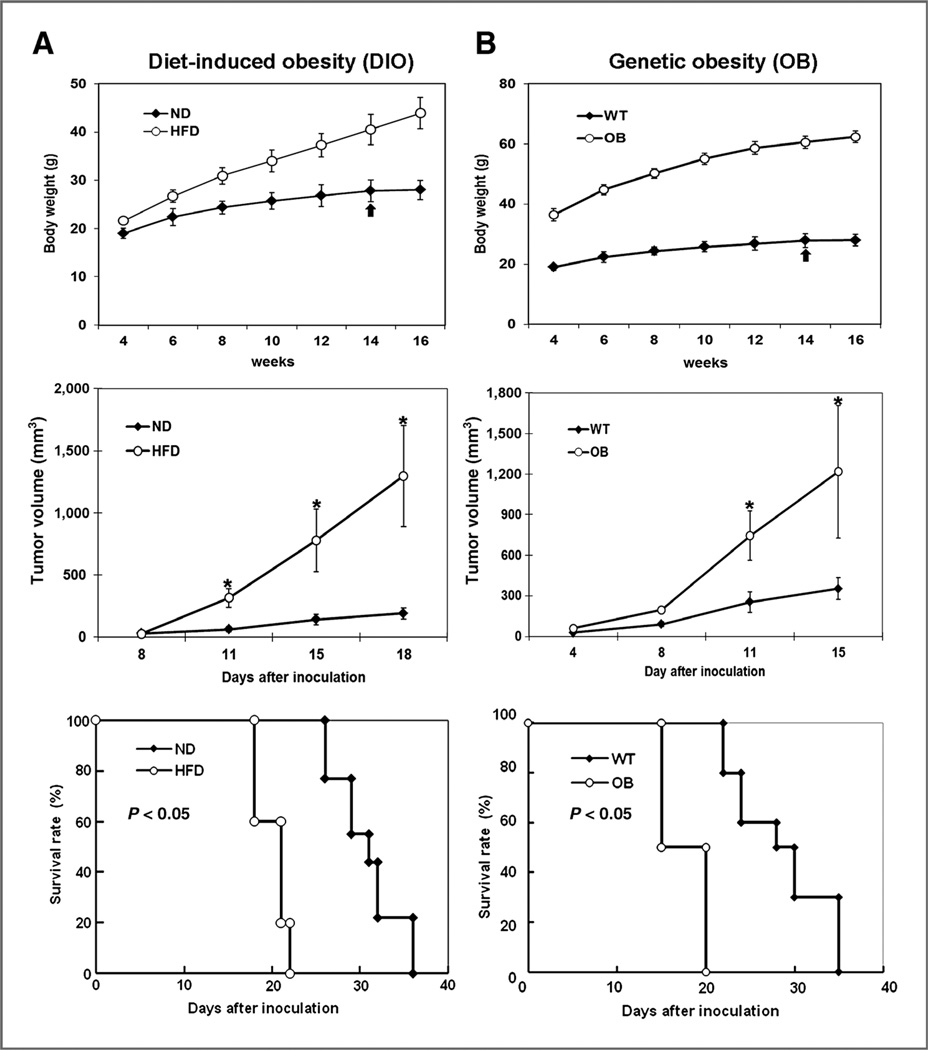
We monitored the body weight of mice in these 2 models first and confirmed that these mice weighed more than control mice (Fig. 1A and B, top panels). We then established mammary tumors in these mice and observed significantly higher tumor growth in obese mice (HFD/ DIO and OB)than in lean mice (normal chow diet and WT) in both models (Fig. 1A and B, middle panels). Moreover, the survival rates of obese mice in both models were significantly lower than those of the control mice (Fig. 1A and B, bottom panels). Similar results were observed in the 4T1 mouse mammary tumor models (Supplementary Fig. S1A and S1B). Together, these results indicated that obesity promoted mammary tumor growth in vivo and reduced median survival and that these animal models are feasible for studying the underlying mechanisms of obesity-mediated tumor progression.
Figure 1.
Genetic and dietary obesity promotes mammary tumorigenesis and increases mice mortality. A, C57BL/6J female mice that were fed a HFD or normal diet. B, genetic obese B6. V-Lepob/J (OB) mice that were fed normal chow diet. Top, the body weights of genetic obese and diet-induced obese mice as well as control mice were monitored. Middle, in both HFD-induced obese mice and genetic obese mice, 2 × 105 EO771 mammary adenocarcinoma cells were injected into the mammary fat pads at the time indicated by the arrow. Tumor growth was monitored twice a week. Bottom, survival rate of C57BL/6J female mice that were fed HFD or normal chow diet; survival rate of genetic obese B6.V-Lepob/J (OB) mice or control mice. Mice were removed from the experiment when tumor size reached above 1.5 cm diameter in accordance with the Institutional Animal Care and Use Committee guidelines. The data represent the mean ±SD (10 mice per group). ND, normal chow diet. *, P < 0.05.
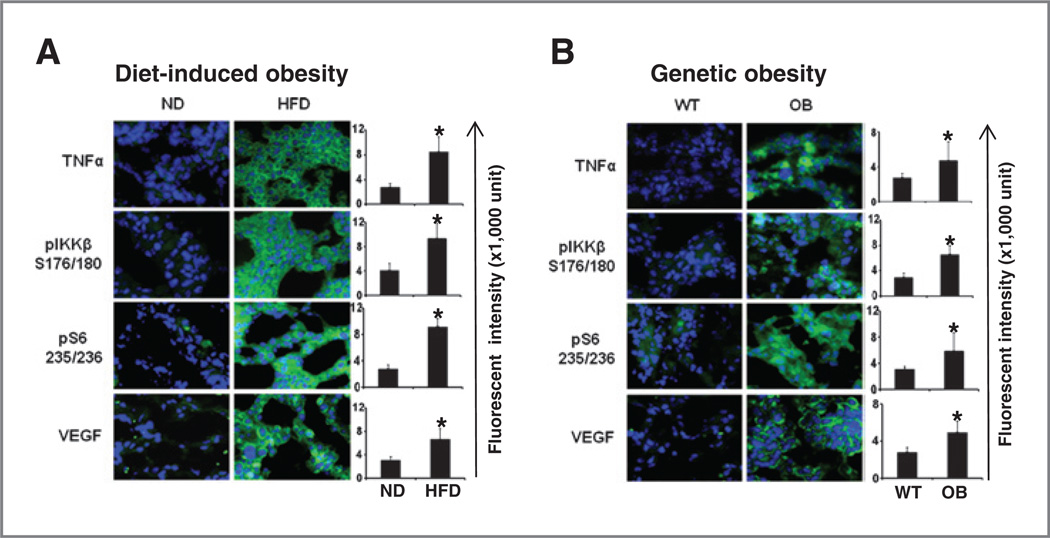
In a previous study, we showed that the IKKβ/ mTOR/VEGF pathway was more highly activated by TNFα in the livers of obese mice that had been fed an HFD than in the livers of normal-weight mice that had been fed a normal chow diet (23). Moreover, TNFα expression was recently shown to induce liver inflammation and promote tumorigenesis in mice with DIO and genetic obesity (36). TNFα, the first adipocytokine identified in adipose tissue, is known to contribute to inflammation-mediated tumor progression (21, 37, 38). To determine whether the IKKβ/mTOR/VEGF signaling pathway is also involved in obesity-mediated mammary tumorigenesis, we harvested mammary tumors from these mice and subjected them to immunofluorescence staining. Immunofluorescence staining (Fig. 2A and B) showed that TNFα and VEGF protein levels in tumors were significantly higher in mice with DIO and genetic obesity than in control (lean) mice. We also determined via ELISA that circulating β (Supplementary Fig. S2A and S2B) and VEGF (Supplementary Fig. S2C and S2D) levels in the blood were higher in mice with DIO and genetic obesity than in control (lean) mice. Moreover, we observed higher levels of phosphorylated IKKβ and phosphorylated S6, an indicator for mTOR activity, in obese mice than in control mice (Fig. 2A and B), indicating that the IKKβ/mTOR/VEGF pathway was indeed activated in the tumor cells of obese mice. Together, these results suggested that TNFα plays an important role in obesity-mediated mammary tumorigenesis by activating the IKKβ/mTOR/VEGF pathway.
Figure 2.
Increased TNFα/IKKβ/ mTOR/VEGF in mammary tumor of genetic and dietary obesity mice. Representative images of mmunofluorescence staining of mammary tumors with TNFα, VEGF, phosphorylated IKKβ (S176/180) [pIKKβ (S176/180)], and phosphorylated S6 (S240/244) [pS6 (S240/244)] antibodies (right). A, mammary tumor in C57BL/6J female mice that were fed a HFD or normal diet. B, mammary tumor in genetic obese B6.V-Lepob/J (OB) mice that were fed normal chow diet. The relative fluorescence intensity from the images is shown on the right. The data represent the mean ± SD. ND, normal chow diet. *, P < 0.05.
Obesity mediates mammary tumorigenesis by increasing angiogenesis and tumor cell proliferation
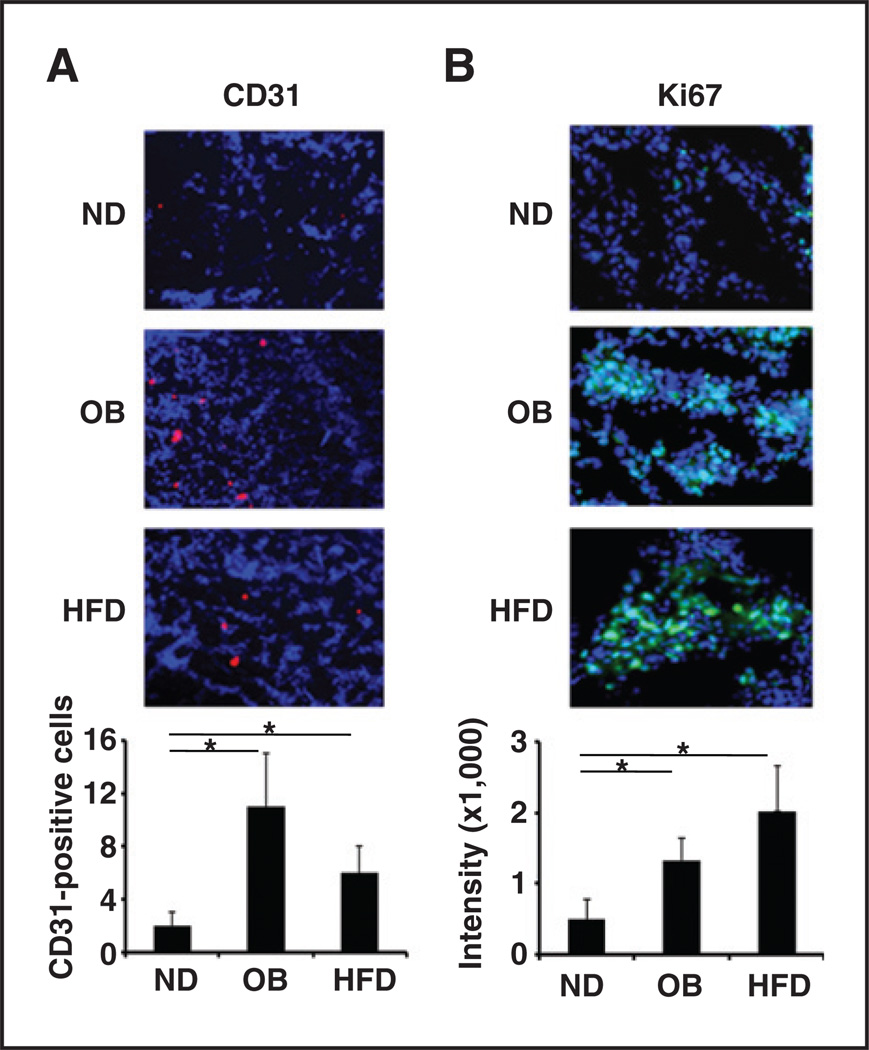
Our previous findings indicated that enhanced angio-genesis plays a central role in TNFα-induced breast tumor development by activating the IKKβ/mTOR/VEGF pathway. This activation resulted in VEGF expression in multiple tumor types and correlated with poor clinical outcome in breast cancer patients (21). To characterize the role of TNFα-mediated VEGF expression in our obesitymediated mammary tumor models, we further examined in vivo angiogenesis phenomena or cell proliferation via immunofluorescence staining of tumor tissue using CD31 antibody (a marker for endothelial cells). The vascular density of mammary tumors in obese mice (OB and HFD/DIO) was higher than that of mammary tumors in control (lean) mice (Fig. 3A). Likewise, using Ki67 as an indicator of cancer cell proliferation, we also found that the proportion of proliferating cancer cells was higher in mammary tumors in OB and HFD/DIO mice than in the tumors of control (lean) mice fed normal chow diet (Fig. 3B). These results suggested that obesity plays a role in promoting mammary tumorigenesis, at least in part, by enhancing tumor angiogenesis, which is in line with our previous studies that showed TNFα produced by adipose tissues increases of tumor angiogenesis (21, 36).
Figure 3.
Increased tumor angiogenesis and tumor cell proliferation in mammary tumors in obese mice. Representative images of immunofluorescence staining of mammary tumor tissues:CD31 antibody as an indicator of tumor vascular density (red; A) and Ki67 antibody as an indicator of tumor cell proliferation (green; B). The relative fluorescence intensity from the images is shown below. The data represent the mean ± SD. ND, normal chow diet. *, P < 0.05.
Blocking the IKKβ/mTOR/VEGF pathway inhibits obesity-mediated tumorigenesis
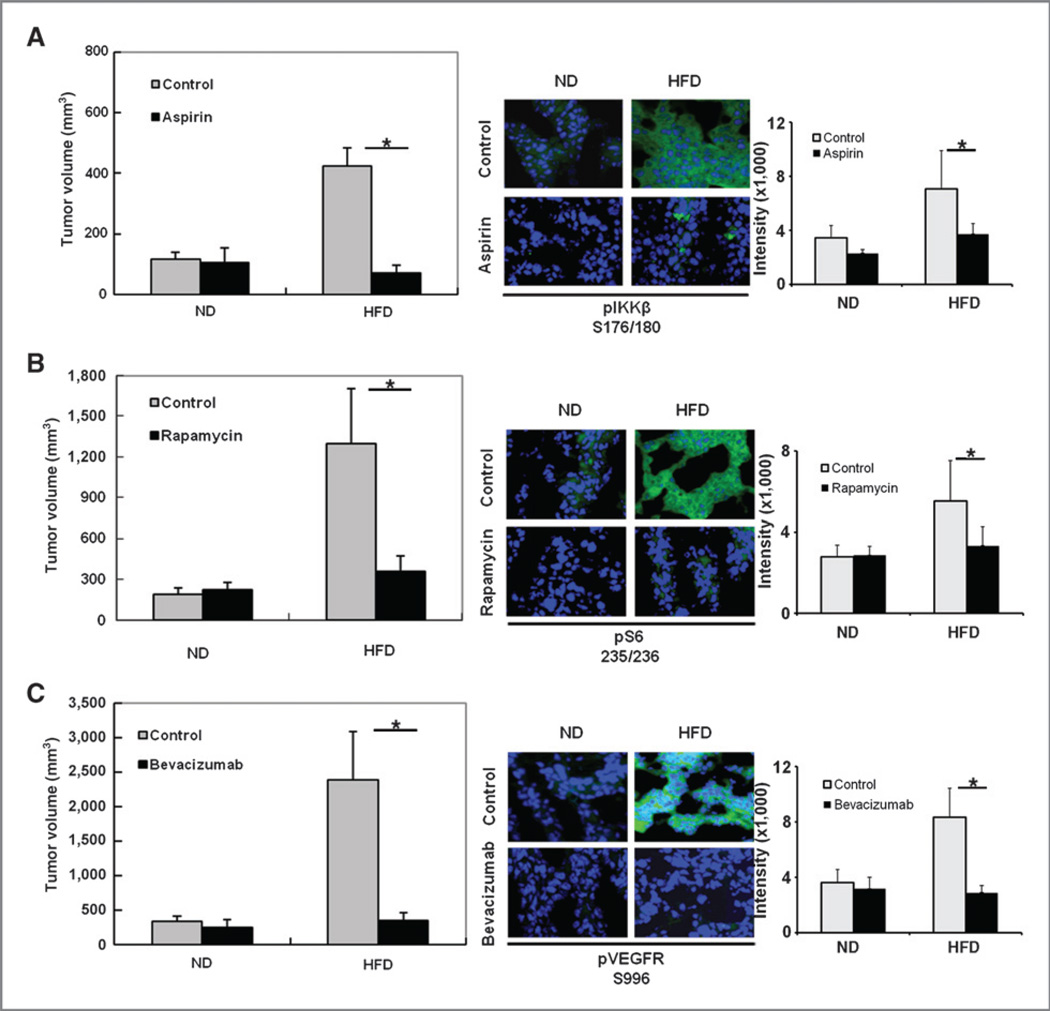
The data shown here suggest that the IKKβ/mTOR/ VEGF pathway may play an important role in mammary tumor progression in obese mice. Thus, targeting this pathway may lead to new intervention strategies for obesity-mediated breast cancer. To determine whether inhibition of the IKKβ/mTOR/VEGF signaling pathway reduces the development of mammary tumor in DIO mice, we first established mammary tumors in obese and control mice as described above and then administered inhibitors or antagonists of this specific pathway to obese and control (lean) mice after the tumor volume reached 3 to 5 mm in diameter. Interestingly, after treatment with aspirin (an IKKβ inhibitor), rapamycin (an mTOR inhibitor), or bevacizumab (a VEGF antagonist) for 2 weeks, a significant reduction in mammary tumor volume was observed in obese mice but not in control (lean) mice (left panels, Fig. 4A–C).
Figure 4.
Inhibitors of the IKKβ/mTOR/VEGF pathway block obesity-mediated tumorigenesis. HFD-induced obesity mice and control (lean) mice with mammary tumors were prepared as described in Fig. 1 and then treated with or without aspirin (A), rapamycin (B), or bevacizumab (C). Tumor-bearing mice were administered aspirin at 120 mg/kg/d by subcutaneous injection; rapamycin at 5 mg/kg twice per week by intraperitoneal injection; and bevacizumab at 10 mg/kg once per week by intravenous injection (10 mice/treatment group). Tumor volume was measured after 2 weeks of drug treatment (left). Treatment with these drugs significantly reduced tumor volume in the HFD group but not in the normal chow diet (control/lean) group. Representative images of immunofluorescence staining of tumor tissues stained with indicated antibodies (middle). The relative fluorescence intensity from the images is shown on the right. The data represent the mean ± SD (10 mice per group). ND, normal chow diet. *, P < 0.05.
To validate that these drugs indeed blocked the specific pathway we proposed, tumor samples from the mice treated or untreated with the drugs were subjected to immunostaining with specific antibodies against the IKKβ/mTOR/VEGF pathway. The activity of IKKβ, mTOR, and VEGFR, as indicated by their phosphorylation status, was reduced in tumor-bearing obese mice that were treated with aspirin, rapamycin, and bevacizumab, respectively (Fig. 4, middle and right panels). These results supported our hypothesis that blocking the IKKβ/mTOR/VEGF pathway could inhibit obesity-mediated breast tumorigenesis.
TNFα-mediated signaling pathway promotes the development of mammary tumors in mice
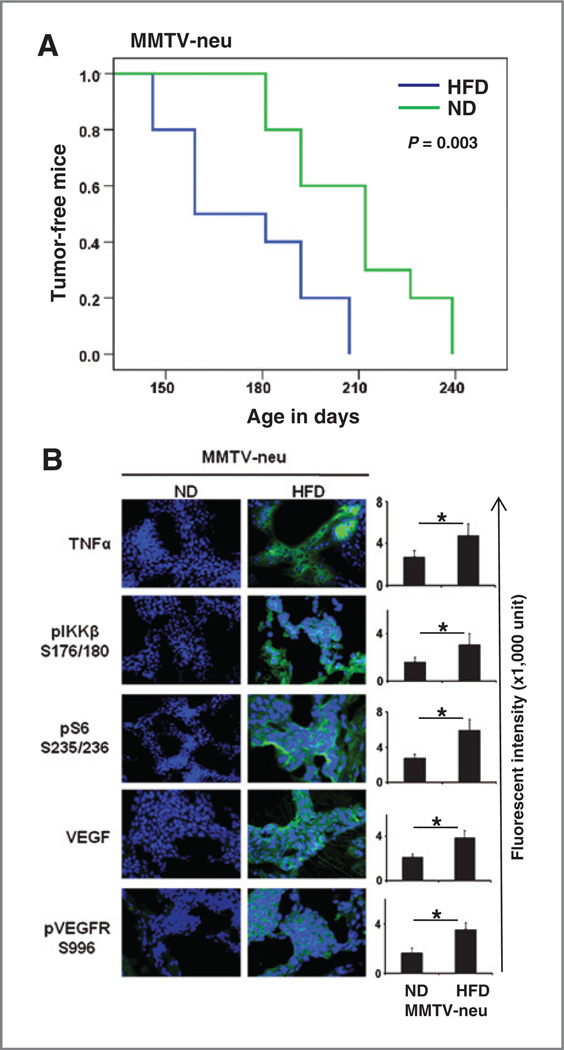
The data above strongly support the notion that the TNFα-induced signaling pathway plays a critical role in mammary tumor cell proliferation in obese mice. Next, we sought to determine whether the TNFα-mediated signaling pathway also promotes mammary tumor initiation in obese mice.To address this question, we used MMTV-neu transgenic mice, which develop mammary tumors spontaneously at about age 6 to 12 months (28, 29). After the weaning stage, one group of MMTV-neu transgenic mice was fed an HFD, and the other group was fed a normal chow diet. We monitored tumor development and found that spontaneous development of mammary tumors was accelerated in the MMTV-neu transgenic mice group fed the HFD, compared with the group that was fed a normal chow diet (Fig. 5A), suggesting that obesity also promotes tumor initiation. Mice exhibited tumor formation from day 146 to day 207 (median = 159 days) and from day 181 to day 239 (median = 212 days) in the HFD and normal chow diet groups, respectively (P = 0.003). The expression of TNFα and VEGF, as well as phosphorylated IKKβ and phosphorylated S6, was significantly higher in mammary tumors of the HFD group than in the tumors of the normal chow diet group, thereby suggesting that the IKKβ/ mTOR/VEGF pathway is also activated in the spontaneously developing mammary tumors of MMTV-neu trans-genic mice with HFD (Fig. 5B).
Figure 5.
TNFα-mediated signaling pathway promotes mammary tumor development. A, spontaneous mammary tumor development in MMTV-neu mice fed with either a HFD or normal diet. Each group contained 10 mice. The incidence of spontaneous tumor growth in MMTV-neu mice was based on tumors larger than 5 mm in diameter in the mammary gland. B, representative images of immunofluorescence staining of tumor tissues with the indicated antibodies. The relative fluorescence intensity from the images is shown on the right. The data represent the mean ± SD, (10 mice per group). ND, normal chow diet. *, P < 0.05.
Inhibition of the TNFα-mediated signaling pathway prevents the development of mammary tumor in mice
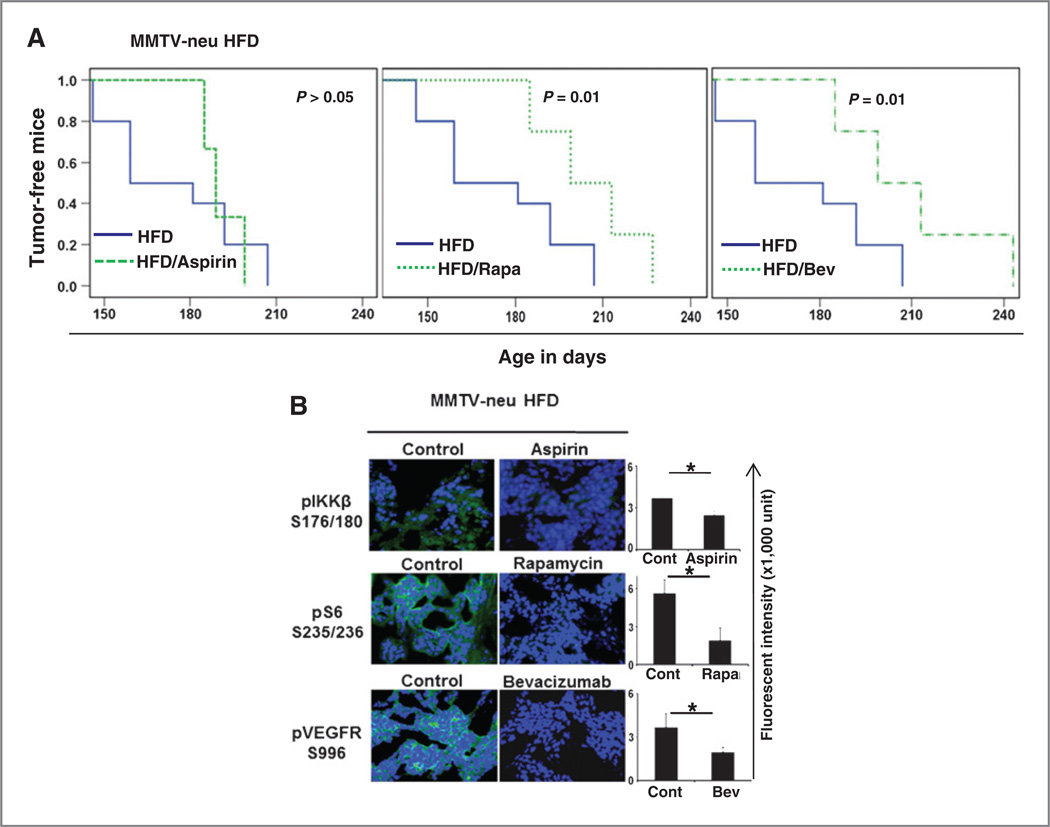
In a parallel experiment, after the weaning stage, the MMTV-neu transgenic mice were fed an HFD but treated with aspirin, rapamycin, or bevacizumab. We found that these IKKβ/mTOR/VEGF pathway inhibitors delayed mammary tumors development tin MMTV-neu transgenic mice that were fed an HFD. The mice in the HFD, HFD aspirin-treated, HFD rapamycin-treated, and HFD bevacizumab-treated groups developed tumors from days 146 to 207, days 185 to 199, days 185 to 227, and days 185 to 243, respectively. The median time for tumor formation in both rapamycin- and bevacizumab-treated obese mice was 199 days, and the median time for tumor formation in the HFD group without drug treatment was 159 days (Fig. 6A). In addition, immunofluorescence staining of tumor tissues also showed that phosphorylation of the IKKβ/mTOR/VEGF signaling pathway was decreased in mammary tumors of the obese MMTV-neu mice that were treated with these drugs (Fig. 6B). Taken together, these results suggested that obesity promotes breast tumor initiation, at least in part, by the activation of the IKKβ/ mTOR/VEGF pathway.
Figure 6.
Inhibition of TNFα-mediated signaling pathway prevents mammary tumor development. A, HFD-fed MMTV-neu mice were treated with drugs that inhibit the IKKβ/mTOR/VEGF pathway and spontaneous mammary tumor development was monitored. Mice were administered aspirin at 120 mg/kg/d by subcutaneous injection; rapamycin at 5 mg/kg twice per week by intraperitoneal injection; and bevacizumab at 10 mg/kg once per week by intravenous injection. Each group contained 10 mice. The incidence of spontaneous tumor growth in MMTV-neu mice was based on tumors larger than 5 mm in diameter in the mammary gland. B, representative images of immunofluorescence staining of tumor tissues with the indicated antibodies. The relative fluorescence intensity from the images is shown on the right. The data represent the mean ± SD (10 mice per group); *, P < 0.05.
Discussion
In this study, we showed a novel molecular mechanism that provides a link between obesity and breast cancer in mouse models.We found that the expression of TNFα and VEGF, as well as the activation of IKKβ and mTOR, is specifically upregulated in tumors in obese mice. More importantly, multiple clinical drugs and preclinical agents that are already available to target the IKKβ / mTOR/VEGF signaling pathway have been shown to effectively reduce obesity-mediated mammary tumor growth and tumor development.
The epidemiology data have shown that obesity primarily increases breast cancer risk after menopause and affects ER-positive breast cancer (2). Our results suggest that the IKKβ/mTOR/VEGF signaling pathway plays a role in obesity-mediated tumor progression in both ER- positive and ER-negative mammary tumors, as evident from our estrogen receptor (ER)-positive EO771 mouse model and MMTV-neu transgenic mouse model, which spontaneously develop ER-negative mammary tumors (39). Thus, the IKKβ/mTOR/VEGF pathway might contribute to the ER pathway–dependent development and progression of breast cancer and should be further studied in human patient samples in future. However, we cannot exclude the possibility that factors or pathways other than those identified in this study are also involved in the obesity-mediated mammary tumors. For example, Morris and colleagues found that macrophages forming crown-like structures can release TNFα to further activate NF-κB transcription factor and increase aromatase levels in the mammary glands of obese mice and in obese breast cancer patients (40, 41). A monoclonal antibody (mAb) called infliximab, which targets TNFα, is approved for several autoimmune diseases. Although we have not included this in our assay to show a direct role of TNFα in our model as it has yet to be approved for breast cancer treatment, it would be of interest to determine the effect of infliximab in the future.
The identification of specific signaling pathways is important for the clinical application of therapeutic targets in obesity-mediated breast cancer development. In this study,we focused on the TNFα/IKKβ/mTOR/VEGF signaling pathway, which can be specifically targeted by multiple clinical drugs. Aspirin is an anti-inflammatory drug, and high-dose aspirin treatment can reverse obesity-induced insulin resistance through targeted inhibition of IKKβ (30). Rapamycin and its analogs such as ever-olimus (RAD-001) are immunosuppressant drugs used in organ transplantation but have also been used as anticancer drugs because they suppress cancer cell proliferation by blocking the mTOR signaling pathway (42). Bevacizumab, an anti-VEGF mAb approved by the U.S. Food and Drug Administration in 2004, has shown anticancer activity when used both alone and in combination with other drugs in several cancer types (43). We examined the effects of each of these drugs on the major targets of the IKKβ/mTOR/VEGF pathway (Supplementary Fig. S3). Interestingly, we found that when obese mice with mammary tumors were treated with these drugs, all of the specific target molecules in the IKKβ/mTOR/VEGF signaling pathway were inhibited. This is somewhat expected as aspirin, a phytochemical derivative with typically broad activity, has recently been shown to also activate AMPK, which influences the mTOR activity (44, 45). Moreover, other studies have also reported the activation of IKKβ by VEGF via the Flk-1/Cbl/Akt signaling pathway. Therefore, IKKβ can be downregulated once VEGF is blocked by bevacizumab (46). Although pathways other than the one we proposed may play a role, our results provide a rationale for targeting the IKKβ/mTOR/ VEGF pathway in obesity-mediated mammary tumor development.
Because aspirin did not significantly inhibit obesity-mediated mammary tumor incidence compared with rapamycin and bevacizumab in the MMTV-neu mouse model (Fig. 6A), we further examined the effect of these drugs on inflammatory infiltration (CD45, leukocytes common antigen) induced by TNFα proinflammatory cytokine that is linked to tumor insurgence and tumor progression. We found that inflammatory infiltration was more obvious in mice fed an HFD than those fed a normal chow diet and can be decreased by aspirin, rapamycin, or bevacizumab treatment (Supplementary Fig. S4A), suggesting that inflammation is one key point linking these factors. It is possible that the Her2/neu-mediated signaling pathway can overcome the inhibition of the IKKβ/ mTOR/VEGF signaling pathway in the MMTV-neu model (47) and would be of interest to further investigate the detailed molecular mechanisms of each drug treatment in the future.
Recent preclinical and clinical reports have shown that aspirin can be used for both cancer therapy and cancer prevention (48, 49). Furthermore, epidemiologic evidence shows that aspirin can be used for adjuvant therapy in cancer patients (50). However, in our MMTV-neu obesity mouse model, aspirin did not show inhibitory effects of mammary tumor initiation compared with rapamycin and bevacizumab (Fig. 6A). Thus, in this particular mouse model (Her2-positive breast cancer), aspirin may not be a good cancer prevention drug, but may be useful for cancer prevention for other types of breast cancers (ER-positive or triple-negative breast cancers). Although clinical trials show that aspirin can reduce tumor incidence by 10% in all cancer types, we believe the benefit-to-risk ratio must be defined in different cancer types (51, 52) as side effects, including gastrointestinal bleeding, from long-term aspirin treatment have been reported (48, 53, 54).
With regard to the effect of angiogenesis on obesity, we did not find significant body weight loss in the aspirin/ rapamycin/bevacizumab treatment group compared with the obese control group. However, antiangiogenesis approaches have been proposed as a method of limiting obesity, and treatment of obese mice with antiangiogenesis inhibitors has been shown to reduce obesity. Although the pathophysiologic connection between angiogenesis and adipogenesis is well recognized, so far, there is limited data from preclinical and clinical studies to address the weight loss research that focused on treating obesity with angiogenesis inhibitors. In addition, some studies have shown different results with different types of antiangiogenic inhibitors. For instance, vatalanib, a VEGFR TKI, showed antitumor activity and changes in body weight in a murine renal cell carcinoma model (55). In contrast, in db/db C57BL/6 mice, a 2-week treatment with an anti-VEGF mAb did not result in a significant change in their body weight (56).In the clinic, much less is known about the effect of VEGF TKI on body weight as only severe weight loss is reported according to the Common Toxicity Criteria Adverse Event criteria. Important adverse events of angiogenesis inhibitors are anorexia, nausea, stomatitis, and diarrhea, and all these adverse events can cause weight loss. Thus, it is not clear for the actual effects of antiangiogenic inhibitors to cause weight loss. Moreover, edema and hypothyroidism, which are also side effects of angiogenesis inhibitors, can contribute to weight gain (57). Therefore, how antiangiogenesis treatment affects obesity and what kinds of antiangiogenic inhibitors work remain unclear.
TNFα-activated IKKβ/mTOR/VEGF signaling pathway has also been shown to play an important role in the development of human esophageal cancer (37). In addition, obese mice with PanO2 pancreatic cancer xenografts responded to the inhibitors targeting this pathway (unpublished data). Taken together, these findings suggest that this pathway could potentially serve as a therapeutic target for other obesity-related human cancers.
Supplementary Material
Acknowledgments
The authors thank Dr. F.M. Sirotnak for providing the EO771 mammary adenocarcinoma cell line, Dr. Jennifer L. Hsu for critical reading of the manuscript, and Dr. Tamara K. Locke and Ms. Markeda L. Wade at Scientific Publications at MD Anderson Cancer Center for editing our manuscript.
In Memoriam
We acknowledge Mrs. Serena Lin-Guo for her courageous fight against breast cancer.
Grant Support
This work was supported by the Breast Cancer Research Foundation (G.N. Hortobagyi and M.-C. Hung), the Breast Cancer SPORE grant (G.N. Hortobagyi and M.-C. Hung), the National Breast Cancer Foundation (M.-C. Hung), Patel Memorial Breast Cancer Research Fund (M.-C. Hung), Sister Institution Fund of China Medical University and Hospital and MD Anderson Cancer Center (M.-C. Hung), Cancer Research Center of Excellence (DOH101-TD-C-111-005, Taiwan; M.-C. Hung), Private University grant (NSC99-2632-B-039-001-MY3, Taiwan; M.-C. Hung), Program for Stem Cell and Regenerative Medicine Frontier Research (NSC100-2321-B-039-002, Taiwan; M.C. Hung), International Research-Intensive Centers of Excellence in Taiwan (NSC101-2911-I-002-303, Taiwan; M.C. Hung), and the NIH of Health through MD Anderson’s Cancer Center Support Grant (CA16672).
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: C.-T. Chen, M.-C. Hung
Development of methodology: C.-T. Chen, Y. Du, H.-P. Kuo
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): C.-T. Chen, Y. Du, H.-P. Kuo, M.-C. Hung
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): C.-T. Chen, Y. Du, G.N. Hortobagyi, M.-C. Hung
Writing, review, and/or revision of the manuscript: C.-T. Chen, H. Yamaguchi, G.N. Hortobagyi, M.-C. Hung
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): C.-T. Chen, J.-M. Hsu, G.N. Hortobagyi
Study supervision: C.-T. Chen, J.-M. Hsu, M.-C. Hung
References
- 1.Basen-Engquist K, Chang M. Obesity and cancer risk: recent review and evidence. Curr Oncol Rep. 2011;13:71–76. doi: 10.1007/s11912-010-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 3.Carmichael AR, Bates T. Obesity and breast cancer: a review of the literature. Breast. 2004;13:85–92. doi: 10.1016/j.breast.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Ronco AL, De Stefani E, Stoll M. Hormonal and metabolic modulation through nutrition: towards a primary prevention of breast cancer. Breast. 2010;19:322–332. doi: 10.1016/j.breast.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Kuhl H. Breast cancer risk in the WHI study: the problem of obesity. Maturitas. 2005;51:83–97. doi: 10.1016/j.maturitas.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Beral V, Bull D, Doll R, Key T, Peto R, Reeves G. Breast cancer hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer, 108,411 women without breast cancer Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997;350:1047–1059. [PubMed] [Google Scholar]
- 7.McTiernan A, Irwin M, Vongruenigen V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. J Clin Oncol. 2010;28:4074–4080. doi: 10.1200/JCO.2010.27.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei EK, Wolin KY, Colditz GA. Time course of risk factors in cancer etiology and progression. J Clin Oncol. 2010;28:4052–4057. doi: 10.1200/JCO.2009.26.9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauner D, Janni W, Rack B, Hauner H. The effect of overweight and nutrition on prognosis in breast cancer. Dtsch Arztebl Int. 2011;108:795–801. doi: 10.3238/arztebl.2011.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner LB. A meta-analysis of fat intake, reproduction, and breast cancer risk: an evolutionary perspective. Am J Hum Biol. 2011;23:601–608. doi: 10.1002/ajhb.21176. [DOI] [PubMed] [Google Scholar]
- 11.Carmichael AR. Obesity and prognosis of breast cancer. Obes Rev. 2006;7:333–340. doi: 10.1111/j.1467-789X.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 12.Maruthur NM, Bolen S, Brancati FL, Clark JM. Obesity and mammography: a systematic review and meta-analysis. J Gen Intern Med. 2009;24:665–677. doi: 10.1007/s11606-009-0939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vrieling A, Buck K, Kaaks R, Chang-Claude J. Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: a meta-analysis. Breast Cancer Res Treat. 2010;123:641–649. doi: 10.1007/s10549-010-1116-4. [DOI] [PubMed] [Google Scholar]
- 14.Williams GP. The role of oestrogen in the pathogenesis of obesity, type 2 diabetes, breast cancer and prostate disease. Eur J Cancer Prev. 2010;19:256–271. doi: 10.1097/cej.0b013e328338f7d2. [DOI] [PubMed] [Google Scholar]
- 15.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab. 2006;17:328–336. doi: 10.1016/j.tem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Jarde T, Perrier S, Vasson MP, Caldefie-Chezet F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer. 2011;47:33–43. doi: 10.1016/j.ejca.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Grossmann ME, Ray A, Nkhata KJ, Malakhov DA, Rogozina OP, Dogan S, et al. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29:641–653. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 18.Ray A, Cleary MP. Leptin as a potential therapeutic target for breast cancer prevention and treatment. Expert Opin Ther Targets. 2010;14:443–451. doi: 10.1517/14728221003716466. [DOI] [PubMed] [Google Scholar]
- 19.Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev. 2004;5:153–165. doi: 10.1111/j.1467-789X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 20.Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110:1564–1571. doi: 10.1161/01.CIR.0000142055.53122.FA. [DOI] [PubMed] [Google Scholar]
- 21.Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 22.Maccio A, Madeddu C, Mantovani G. Adipose tissue as target organ in the treatment of hormone-dependent breast cancer: new therapeutic perspectives. Obes Rev. 2009;10:660–670. doi: 10.1111/j.1467-789X.2009.00592.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee DF, Kuo HP, Chen CT, Wei Y, Chou CK, Hung JY, et al. IKKbeta suppression of TSC1 function links the mTOR pathway with insulin resistance. Int J Mol Med. 2008;22:633–638. doi: 10.3892/ijmm_00000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 25.West DB, Waguespack J, McCollister S. Dietary obesity in the mouse: interaction of strain with diet composition. Am J Physiol. 1995;268:R658–R665. doi: 10.1152/ajpregu.1995.268.3.R658. [DOI] [PubMed] [Google Scholar]
- 26.Friedman JM, Leibel RL, Siegel DS, Walsh J, Bahary N. Molecular mapping of the mouse ob mutation. Genomics. 1991;11:1054–1062. doi: 10.1016/0888-7543(91)90032-a. [DOI] [PubMed] [Google Scholar]
- 27.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 28.Liu JC, Deng T, Lehal RS, Kim J, Zacksenhaus E. Identification of tumorsphere- and tumor-initiating cells in HER2/Neu-induced mammary tumors. Cancer Res. 2007;67:8671–8681. doi: 10.1158/0008-5472.CAN-07-1486. [DOI] [PubMed] [Google Scholar]
- 29.Andrechek ER, Muller WJ. Tyrosine kinase signalling in breast cancer: tyrosine kinase-mediated signal transduction in transgenic mouse models of human breast cancer. Breast Cancer Res. 2000;2:211–216. doi: 10.1186/bcr56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 31.Xing D, Orsulic S. A genetically defined mouse ovarian carcinoma model for the molecular characterization of pathway-targeted therapy and tumor resistance. Proc Natl Acad Sci U S A. 2005;102:6936–6941. doi: 10.1073/pnas.0502256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dings RP, Loren M, Heun H, McNiel E, Griffioen AW, Mayo KH, et al. Scheduling of radiation with angiogenesis inhibitors anginex and Avastin improves therapeutic outcome via vessel normalization. Clin Cancer Res. 2007;13:3395–3402. doi: 10.1158/1078-0432.CCR-06-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng X, Xia W, Yang JY, Hsu JL, Lang JY, Chou CK, et al. Activation of murine double minute 2 by Akt in mammary epithelium delays mammary involution and accelerates mammary tumorigenesis. Cancer Res. 2010;70:7684–7689. doi: 10.1158/0008-5472.CAN-09-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dirat B, Bochet L, Escourrou G, Valet P, Muller C. Unraveling the obesity and breast cancer links: a role for cancer-associated adipocytes? Endocr Dev. 2010;19:45–52. doi: 10.1159/000316896. [DOI] [PubMed] [Google Scholar]
- 36.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yen CJ, Izzo JG, Lee DF, Guha S, Wei Y, Wu TT, et al. Bile acid exposure up-regulates tuberous sclerosis complex 1/mammalian target of rapamycin pathway in Barrett’s-associated esophageal adenocarcinoma. Cancer Res. 2008;68:2632–2640. doi: 10.1158/0008-5472.CAN-07-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashkenazi A. Targeting death and decoy receptors of the tumournecrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 39.Cleary MP, Grossmann ME, Ray A. Effect of obesity on breast cancer development. Vet Pathol. 2010;47:202–213. doi: 10.1177/0300985809357753. [DOI] [PubMed] [Google Scholar]
- 40.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Buck E, Eyzaguirre A, Brown E, Petti F, McCormack S, Haley JD, et al. Rapamycin synergizes with the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and breast tumors. Mol Cancer Ther. 2006;5:2676–2684. doi: 10.1158/1535-7163.MCT-06-0166. [DOI] [PubMed] [Google Scholar]
- 43.Banerjee S, Dowsett M, Ashworth A, Martin LA. Mechanisms of disease: angiogenesis and the management of breast cancer. Nat Clin Pract Oncol. 2007;4:536–550. doi: 10.1038/ncponc0905. [DOI] [PubMed] [Google Scholar]
- 44.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw RJ, Cantley LC. Cell biology Ancient sensor for ancient drug. Science. 2012;336:813–814. doi: 10.1126/science.1223140. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Chang J, Li YC, Li YS, Shyy JY, Chien S. Shear stress and VEGF activate IKK via the Flk-1/Cbl/Akt signaling pathway. Am J Physiol Heart Circ Physiol. 2004;286:H685–H692. doi: 10.1152/ajpheart.00237.2003. [DOI] [PubMed] [Google Scholar]
- 47.Zhou BP, Hu MC, Miller SA, Yu Z, Xia W, Lin SY, et al. HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-kap-paB pathway. J Biol Chem. 2000;275:8027–8031. doi: 10.1074/jbc.275.11.8027. [DOI] [PubMed] [Google Scholar]
- 48.Force USPST Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 49.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-termrisk of death due tocancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 50.Langley RE, Burdett S, Tierney JF, Cafferty F, Parmar MK, Venning G. Aspirin and cancer: has aspirin been overlooked as an adjuvant therapy? Br J Cancer. 2011;105:1107–1113. doi: 10.1038/bjc.2011.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9:259–267. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 52.Avivi D, Moshkowitz M, Detering E, Arber N. The role of low-dose aspirin in the prevention of colorectal cancer. Expert Opin Ther Targets. 2012;16(Suppl 1):S51–S62. doi: 10.1517/14728222.2011.647810. [DOI] [PubMed] [Google Scholar]
- 53.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, et al. European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur J Cardiovasc Prev Rehabil. 2007;14(Suppl 2):S1–S113. doi: 10.1097/01.hjr.0000277983.23934.c9. [DOI] [PubMed] [Google Scholar]
- 54.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Atherosclerosis. 2007;194:1–45. doi: 10.1016/j.atherosclerosis.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 55.Drevs J, Hofmann I, Hugenschmidt H, Wittig C, Madjar H, Muller M, et al. Effects of PTK787/ZK 222584, a specific inhibitor of vascular endothelial growth factor receptor tyrosine kinases, on primary tumor, metastasis, vessel density, and blood flow in a murine renal cell carcinoma model. Cancer Res. 2000;60:4819–4824. [PubMed] [Google Scholar]
- 56.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 57.Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur J Cancer. 2006;42:3127–3139. doi: 10.1016/j.ejca.2006.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.