Abstract
Objectives
Secretin stimulation testing (SST) is used to evaluate patients with hypergastrinemia in the diagnosis of Zollinger-Ellison syndrome (ZES). Case series have documented false-positive SST in patients with achlorhydria. This study reviews our experience with SST in hypo- and achlorhydric patients.
Methods
We examined 27 patients with hypo- or achlorhydria based on a predefined basal acid output (BAO) measurement of <5.0mEq/hour who also underwent SST for diagnosis of ZES. We report the frequency of false positive SST results in this setting.
Results
330 patients underwent gastric analysis of which 27 had BAO<5.0mEq/hour and SST conducted. The mean fasting gastrin level was 247±304pg/mL and the mean BAO measurement was 1.6±1.8mEq/hour. Twenty patients were off and 7 were on anti-secretory therapy at time of testing. Four patients had false-positive SSTs; three with gastric atrophy (BAO=0mEq/hr) and one with drug-induced hypochlorhydria (BAO=0.5mEq/hr). These false-positive test results were confirmed by structural and functional imaging studies.
Conclusions
We have identified a 14.8% false-positive rate in SST in patients with hypo- or achlorhydria. Growing literature has identified severe consequences associated with discontinuing anti-secretory treatment for testing; therefore, SST will require interpretation in the setting of gastric acid suppression and needs to be interpreted in this context.
Keywords: Zollinger-Ellison syndrome, Secretin, Achlorhydria, Gastrin, proton-pump inhibitor
Introduction
Zollinger-Ellison Syndrome (ZES) is a condition defined by the presence of a gastrin-secreting neuroendocrine tumor resulting in gastric acid hypersecretion (GAH)1,2; these patients often present with complications of GAH including peptic ulcer disease, dyspepsia, gastroesophageal reflux, abdominal pain, and diarrhea3. The hallmark of this condition is hypergastrinemia in the presence of low gastric pH4,5 (i.e., inappropriate hypergastrinemia). Gastrin is normally secreted by G-cells in the gastric antrum and regulated by feedback inhibition via somatostatin from adjacent D-cells in the presence of a low gastric pH6. Patients with exogenous sources of gastrin are unresponsive to these inhibitory mechanisms leading to unopposed gastrin release and subsequent GAH7,8.
Inappropriate hypergastrinemia is the mainstay of ZES diagnosis. This is generally defined as an elevated serum gastrin in the presence of a gastric pH < 3.0. Gastric pH can be measured during routine upper endoscopy by aspiration of gastric secretions; however, if the gastric pH is > 3.0 and < 6.0, formal testing with gastric analysis to calculate true basal acid output (BAO) is used for further evaluation7. BAO is measured by the passage of a nasogastric tube into the dependent portion of the stomach with aspiration and quantification of gastric juice production over a one-hour period. A BAO of > 15 mEq/hour is highly suggestive of ZES. However, BAO testing is not available at all centers and patients with ZES can have variable levels of acid output7,9.
In an effort to improve the diagnosis of ZES, several provocative tests have been developed including the secretin stimulation test (SST)10-14. The secretin stimulation test is based on the observation that serum gastrin rises in an unopposed fashion in patients with ZES in response to secretin injection15. This is theorized to occur due to a lack of inhibitory somatostatin-releasing D cells adjacent to tumor cells as well as the presence of secretin receptors on the gastrinoma cells themselves16. Various diagnostic cut-off values have been proposed for the determination of a positive test for ZES. In a recent study of 830 patients with ZES, Berna et al (2006) proposed a cut off of ≥120 pg/mL as the new standard criterion for diagnosis with an associated sensitivity and specificity of 94% and 100%,17 respectively. The McGuigan criteria, now less commonly used, proposed an increase of ≥200 pg/mL as a positive result14 although Berna et al found this cutoff to be associated with a sensitivity and specificity of 83% and 100%.
Despite being a well-defined entity, the diagnosis of ZES remains challenging. Patients with ZES may have fluctuating or modestly increased levels of serum gastrin3. Additionally, other conditions can result in elevated serum gastrin concentrations including G-cell hyperplasia and conditions associated with achlorhydria such as chronic atrophic gastritis or pharmacologic therapy with proton pump inhibitors (PPIs)10.
The now ubiquitous use of PPIs has provided gastroenterologists with another notable challenge in the diagnosis of ZES. As described by Corleto et al (2001), effective control of gastric acid secretion may mask ZES symptoms and potentially delay diagnosis allowing an underlying gastrinoma to grow unchecked18. More directly applicable to this study, testing patients while on PPIs can affect serum gastrin levels as well as SST response19; however, stopping anti-secretory therapy in these patients can lead to severe rebound GAH with potentially fatal consequences20.
To date, there have been a number of case reports and series describing false-positive rises in gastrin after secretin injection in patients who are achlorhydric from atrophic gastritis or proton pump inhibitor therapy19,21-24. However, no clinical studies have evaluated the frequency at which false-positive results occur. In the era of PPI use and with a growing literature citing the hazards of PPI cessation in patients suspected of having ZES, the answer to this question has become increasingly significant.
We performed a retrospective descriptive analysis examining the frequency of false-positive SST in patients who were hypo- or achlorhydric at the time of testing. We present our observed rate of false-positive tests in this subgroup of patients and offer recommendations on how to proceed in the diagnosis of ZES in this growing class of patients.
Materials and Methods
This study was reviewed and approved by the Institutional Review Board at the Hospital of the University of Pennsylvania. All patients who underwent both gastric analysis with BAO calculation and SST at this institution between January 1994 and September 2009 were identified and reviewed. Patients were referred for evaluation of ZES because of an elevated fasting gastrin level.
BAO was calculated after aspiration of gastric contents via nasogastric tube placed into the dependent portion of the stomach after an overnight fast, as previously described7. For this study, we predefined hypochlorhydria as a calculated BAO of < 5 mEq per hour and achlorhydria as a calculated BAO of 0 mEq per hour.
All patients with hypochlorhydria and achlorhydria in the setting of an intact stomach were included in the study population while those with prior gastric acidreducing surgery were excluded. Clinical and demographic data, BAO measurements, and SST results were collected for evaluation.
Fasting serum gastrin determinations were performed by a company contracted with the Hospital of the University of Pennsylvania, Associated Regional and University Pathologists, Inc. (ARUP, Salt Lake City, Utah). The gastrin serum assay is a double-antibody radioimmunoassay kit manufactured by Diagnostic Products Corporation (Los Angeles, CA). This assay assesses only biologically active gastrin with no known cross reactivity with NH2 terminal or glycine-extended fragments. Intra- and interassay coefficients of variability are 5% and 7% at the upper reference limit. The detection limit is approximately 4.5 pg/mL and the upper limit of normal for this assay is 100 pg/mL.
SST was performed at our institution as previously described by Frucht et al10. Prior to 2001, biologically-derived porcine secretin (formerly marketed by Ferring Pharmaceuticals, Tarrytown, NY. No longer available in the United States) was used and administered at a dose of 2 CU/kg per standard protocol. After 2001, a synthetic secretin produced by ChiRhoClin Inc. (Silver Spring, MD) replaced the biologically-derived peptide and was administered at an equivalent dose of 0.4 μg/kg. The performance of this agent in the diagnosis of ZES was examined by our principal investigator at the time of crossover25. Blood draws for serum gastrin determination were obtained immediately before intravenous secretin administration and then 1, 2, 5, 10, 15 and 30 min later. The primary end-point for a positive diagnosis of ZES was an increase in serum gastrin concentration by ≥120 pg/mL, as defined by Berna et al17. Secondary end-points consisted of a 50% increase in serum gastrin concentration and ≥200 pg/mL increase in serum gastrin concentration as defined by McGuigan et al14.
All patients with positive SST underwent radiographic and functional imaging testing with CT scan and/or octreotide scanning to further evaluate for the presence of gastrinoma. Patients with positive SST but negative structural and functional imaging and documented hypo- or achlorhydria were considered false-positive for SST.
Statistical analysis was performed using summary statistics in Stata® software (StataCorp LP, College Station, Texas).
Results
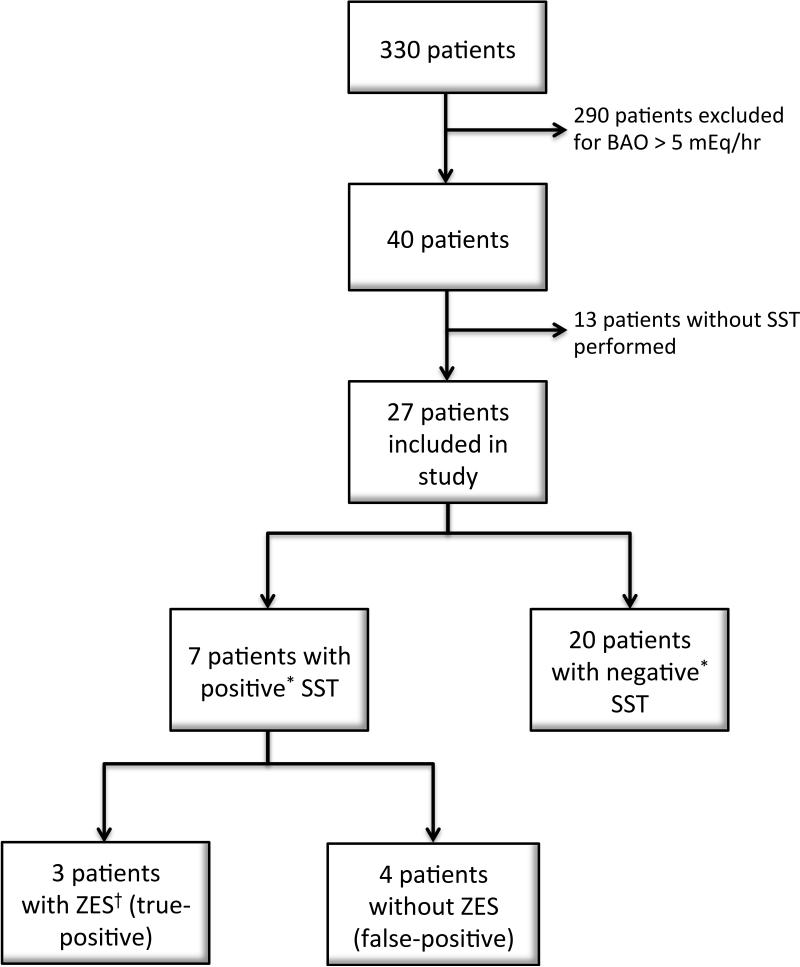
A total of 330 patients underwent gastric analysis with BAO calculation at our institution between January 1, 1994 and December 31, 2009. A total of 40 patients had BAO values of < 5 mEq/hour and were reviewed for inclusion in the study cohort. Of these, 27 patients met the inclusion criteria of no prior surgery and SST completed and were included in the final analysis (see Figure 1). The clinical and demographic information for these patients is presented in Table 1. The mean age of these patients was 47.9 years (±15.4, range 17-74). Nineteen patients were female (70%) and 8 were male. The mean BAO was 1.63 mEq/hour (±1.75 mEq/hour, range 0-4.9 mEq/hour). The mean basal serum gastrin level at the time of SST testing was 318 pg/mL (±413, range 6 -1670 pg/mL). Twenty patients were hypo- or achlorhydric due to chronic atrophic gastritis while seven patients (26%) had decreased acid production due to anti-secretory therapy including omeprazole, lansoprazole, esomeprazole, and rabeprazole at the time of testing. These patients were kept on anti-secretory therapy due to severe symptoms of GAH.
Figure 1. Patient flow chart of all patients undergoing Basal Acid Output and Secretin Stimulation Testing.
Abbreviations: SST – Secretin Stimulation Test; ZES – Zollinger-Ellison Syndrome
*Positive SST defined as an increase in serum gastrin concentration by ≥120 pg/mL positive as discussed in Materials and Methods section.
† Patients determined to have ZES based on positive SST, symptoms of gastric acid hypersecretion, and evidence of gastrinoma on cross-sectional imaging as discussed in Materials and Methods.
Table 1.
Demographic and clinical data of all patients who underwent BAO and SST testing
| Age | 47.9 |
|---|---|
| Female (n[%]) | 19 (70%) |
| Basal Gastrin Level (mean mEq/hr ± SD) | 318 ± 431 |
| Basal Acid Output (mean mEq/hr ± SD) | 1.6 ± 1.8 |
| On PPI at time of SST (n[%]) | 7 (26%) |
| History of Acid-Limiting Surgery (n) | 0 |
The results of all patients with positive SSTs are presented in Table 2. Of twenty-seven patients, 7 patients had positive SSTs with results as follows: 3 of these patients had achlorhydria due to pharmacologic therapy with positive SST and a confirmed diagnosis of gastrinoma on imaging. These patients were true-positive patients with ZES. Two patients were noted to have a BAO < 5 mEq/hour and positive SST with an increase in gastrin after secretin injection of > 200 pg/mL. The first was noted to have a BAO of 0.5 mEq/hour and was hypochlorhydric due to pharmacologic therapy with rabeprazole dosed at 20 mg twice daily. The second was noted to have a BAO of 0 mEq/hour and was achlorhydric due to atrophic gastritis. These patients had no evidence of gastrinoma on subsequent testing and were considered false-positives by SST. Two additional patients were captured as positive for SST when an increase in serum gastrin after secretin injection of > 120 pg/mL was used as the diagnostic criteria. Both of these patients were achlorhydric with BAOs of 0 mEq/hour due to atrophic gastritis and were diagnosed as having false-positive SST after a negative imaging work up for ZES. In total, in our small cohort, 4 patients out of 27 (14.8%, 95% CI 4.74-37.38%) were identified with a BAO < 5 mEq/hour and false-positive SST.
Table 2.
Clinical data of all patients with positive secretin stimulation testing
| Patient No | Age | Gender | On PPI at time of SST | BAO (mEq/hr) | Basal Gastrin Level (pg/mL) | Delta (pg/mL) | Cross-sectional imaging for ZES | Interpretation |
|---|---|---|---|---|---|---|---|---|
| 1 | 47 | F | N | 0 | 1049 | 131 | Negative | Atrophy |
| 2 | 37 | F | N | 0 | 191 | 213 | Negative | Atrophy |
| 3 | 51 | F | N | 0 | 1025 | 170 | Negative | Atrophy |
| 4 | 31 | M | Y | 0.5 | 1670 | 1300 | Negative | Drug-induced |
| 5 | 69 | F | Y | 1.3 | 513 | 1897 | Positive | ZES |
| 6 | 27 | F | Y | 0.4 | 877 | 341 | Positive | ZES |
| 7 | 24 | F | Y | 1.2 | 205 | 154 | Positive | ZES |
Discussion
Despite the evolution of provocation testing, ZES remains a difficult syndrome to diagnose. The introduction of the secretin stimulation test has aided clinicians in the diagnosis of ZES due to its high sensitivity and specificity; however, there are clinical circumstances under which a false-positive test result can occur. Several case series have reported false-positive SST results in patients who are hypo- or achlorhydric because of atrophic gastritis or proton pump inhibitor use19,21-23.
This study aimed to quantify the frequency of false-positive SST results in one cohort of patients at a single institution to provide an assessment of how important it may or may not be to ensure SST is only done in combination with a measurement of acid secretory capability. In our cohort of patients with hypo- or achlorhydria, approximately 15% have false-positive SST results.
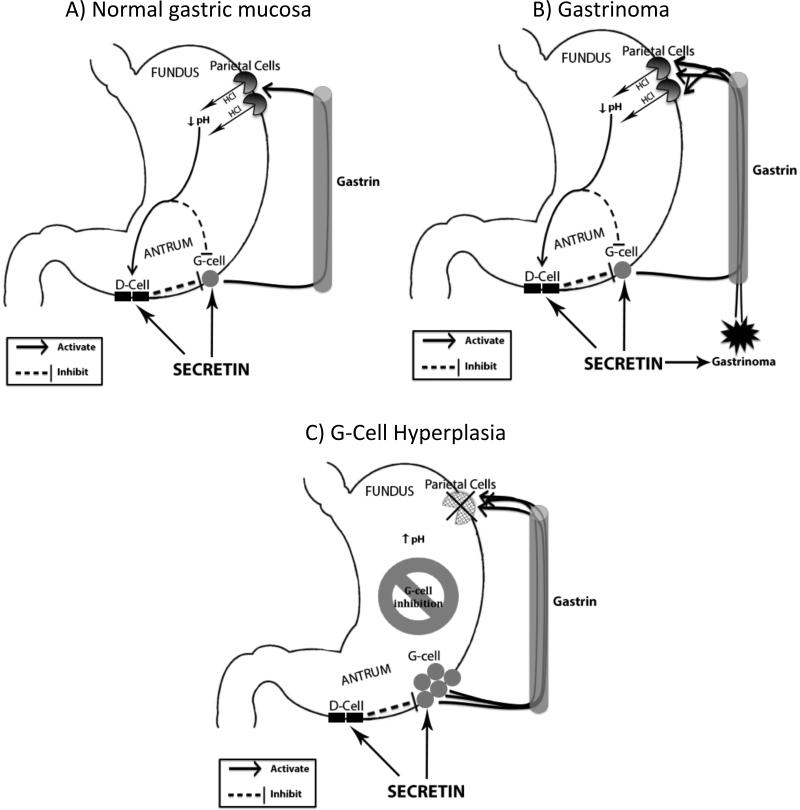
The mechanism by which patients with hypo- or achlorhydria develop false positive SST results is unclear. One proposed mechanism by Goldman et al suggests that patients with hypo- and achlorhydria may develop a decrease in D-cell density with a relative paucity of the potent inhibitor, somatostatin19. We support an alternative mechanism proposed by others22,26-28 and shown in Figure 2. Patients with hypo- or achlorhydria may develop a relative G-cell hyperplasia from decreased acid secretion and lack of inhibitory signaling. G-cells have been shown to release gastrin in response to secretin29 with serum levels shown to be a function of antral G-cell mass30. Thus, we believe G-cell hyperplasia likely functions in a similar fashion as a gastrinoma in response to secretin stimulation resulting in a positive rise in gastrin and false-positive SST.
Figure 2. Theorized response of serum gastrin level to secretin injection.
A) Secretin injection leads to a serum gastrin level increase that is normally attenuated by D-cell inhibition. This leads to a negative SST. B) Serum gastrin level rises in response to secretin injection due to exogenous release from a gastrinoma that is not subject to D-cell inhibition. C) A proposed mechanism for a rise in serum gastrin level in patients with achlorhydria after secretin injection. A relative G-cell hyperplasia from decreased acid secretion and lack of inhibitory signaling leads to an exaggerated response to secretin injection similar to that of a gastrinoma.
This study has significant clinical implications. Proton pump inhibitor (PPI) therapy is currently one of the most commonly prescribed classes of medications worldwide with more than 20 million prescriptions written annually. Additionally, patients with clinical conditions that trigger the suspicion of ZES are frequently placed on PPI therapy before SST can be completed as part of the evaluation. Atrophic gastritis can result from autoimmune conditions such as pernicious anemia or from chronic gastritis secondary to long-standing Helicobacter Pylori infection. These conditions frequently go undetected because of their insidious and relatively asymptomatic nature. Thus, patients on PPI therapy or with atrophic gastritis may present for SST testing because of documented hypergastrinemia with unknown underlying hypo- or achlorhydria.
In patients on PPI therapy, it is recommended to withhold medication and recheck fasting serum gastrin before proceeding with SST testing. However, with a developing literature describing the hazards of gastric acid rebound in ZES patients taken off these medications, an understanding of the potential limitations of SST in the setting of active PPI use is necessary. A novel biomarker, pancreastatin, has recently been shown to rise in the setting of neuroendocrine tumors independent of PPI use and may become an option in these patients that would preclude the need for PPI cessation; however, its use in the setting of gastrinoma requires further testing31,32. For now, to improve diagnostic accuracy in the setting of PPI use, it is our recommendation that patients who are hypo- or achlorhydric who present for evaluation with SST have their results interpreted within the context of their gastric acid status. If ZES is strongly suspected in patients with sporadic disease who may benefit from exploration and cure, retesting off PPI therapy is only advised after a careful wean under controlled circumstances33 and preferably together with gastric acid analysis in centers with experience.
Acknowledgments
Grant funding support provided by: NIH T32-DK007740
Footnotes
There are no conflicts of interest reported by any investigators involved in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zollinger RM, Ellison EH. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg. 1955;142(4):709–723. discussion, 724-708. [PMC free article] [PubMed] [Google Scholar]
- 2.Gregory RA, Tracy HJ, French JM, et al. Extraction of a gastrin-like substance from a pancreatic tumour in a case of Zollinger-Ellison syndrome. Lancet. 1960;1(7133):1045–1048. doi: 10.1016/s0140-6736(60)90932-6. [DOI] [PubMed] [Google Scholar]
- 3.Jensen RT, Gardner JD, Raufman JP, et al. Zollinger-Ellison syndrome: current concepts and management. Ann Intern Med. 1983;98(1):59–75. doi: 10.7326/0003-4819-98-1-59. [DOI] [PubMed] [Google Scholar]
- 4.McGuigan JE, Trudeau WL. Immunochemical measurement of elevated levels of gastrin in the serum of patients with pancreatic tumors of the Zollinger-Ellison variety. N Engl J Med. 1968;278(24):1308–1313. doi: 10.1056/NEJM196806132782402. [DOI] [PubMed] [Google Scholar]
- 5.Isenberg JI, Walsh JH, Grossman MI. Zollinger-Ellison syndrome. Gastroenterology. 1973;65(1):140–165. [PubMed] [Google Scholar]
- 6.Wolfe MM, Reel GM, McGuigan JE. Inhibition of gastrin release by secretin is mediated by somatostatin in cultured rat antral mucosa. J Clin Invest. 1983;72(5):1586–1593. doi: 10.1172/JCI111117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metz DC, Starr JA. A retrospective study of the usefulness of acid secretory testing. Aliment Pharmacol Ther. 2000;14(1):103–111. doi: 10.1046/j.1365-2036.2000.00676.x. [DOI] [PubMed] [Google Scholar]
- 8.Maton P, Dayal Y. Clinical implications of hypergastrinemia. In: Zakim D, Dannenberg A, editors. Peptic Ulcer Disease and Other Acid-Related Disorders. Academic Research Associates, Inc.; Armonk, NY: 1991. p. 213. [Google Scholar]
- 9.Klein K. Gastric secretory testing. In: Drossman D, editor. Manual of Gastroenterologic Procedures. Raven Press; New York, NY: 1993. p. 61. [Google Scholar]
- 10.Frucht H, Howard JM, Slaff JI, et al. Secretin and calcium provocative tests in the Zollinger-Ellison syndrome. A prospective study. Ann Intern Med. 1989;111(9):713–722. doi: 10.7326/0003-4819-111-9-713. [DOI] [PubMed] [Google Scholar]
- 11.Hansky J, Soveny C, Korman MG. Effect of secretin on serum gastrin as measured by immunoassay. Gastroenterology. 1971;61(1):62–68. [PubMed] [Google Scholar]
- 12.Isenberg JI, Walsh JH, Passaro E, Jr., et al. Unusual effect of secretin on serum gastrin, serum calcium, and gastric acid secretion in a patient with suspected Zollinger-Ellison syndrome. Gastroenterology. 1972;62(4):626–631. [PubMed] [Google Scholar]
- 13.Kolts BE, Herbst CA, McGuigan JE. Calcium and secretin-stimulated gastrin release in the Zollinger-Ellison syndrome. Ann Intern Med. 1974;81(6):758–762. doi: 10.7326/0003-4819-81-6-758. [DOI] [PubMed] [Google Scholar]
- 14.McGuigan JE, Wolfe MM. Secretin injection test in the diagnosis of gastrinoma. Gastroenterology. 1980;79(6):1324–1331. [PubMed] [Google Scholar]
- 15.Imamura M, Adachi H, Takahashi K, et al. Gastrin release from gastrinoma cells stimulated with secretin. Dig Dis Sci. 1982;27(12):1130–1136. doi: 10.1007/BF01391453. [DOI] [PubMed] [Google Scholar]
- 16.Long SH, Berna MJ, Thill M, et al. Secretin-receptor and secretin-receptor-variant expression in gastrinomas: correlation with clinical and tumoral features and secretin and calcium provocative test results. J Clin Endocrinol Metab. 2007;92(11):4394–4402. doi: 10.1210/jc.2007-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berna MJ, Hoffmann KM, Long SH, et al. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature. evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features. Medicine (Baltimore) 2006;85(6):331–364. doi: 10.1097/MD.0b013e31802b518c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corleto VD, Annibale B, Gibril F, et al. Does the widespread use of proton pump inhibitors mask, complicate and/or delay the diagnosis of Zollinger-Ellison syndrome? Aliment Pharmacol Ther. 2001;15(10):1555–1561. doi: 10.1046/j.1365-2036.2001.01085.x. [DOI] [PubMed] [Google Scholar]
- 19.Goldman JA, Blanton WP, Hay DW, et al. False-positive secretin stimulation test for gastrinoma associated with the use of proton pump inhibitor therapy. Clin Gastroenterol Hepatol. 2009;7(5):600–602. doi: 10.1016/j.cgh.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Poitras P, Gingras MH, Rehfeld JF. The Zollinger-Ellison syndrome: dangers and consequences of interrupting antisecretory treatment. Clin Gastroenterol Hepatol. 2012;10(2):199–202. doi: 10.1016/j.cgh.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Brady CE, 3rd, Utts SJ, Dev J. False-positive gastrin rises after secretin injection. J Lab Clin Med. 1985;106(4):461–462. [PubMed] [Google Scholar]
- 22.Feldman M, Schiller LR, Walsh JH, et al. Positive intravenous secretin test in patients with achlorhydria-related hypergastrinemia. Gastroenterology. 1987;93(1):59–62. doi: 10.1016/0016-5085(87)90314-3. [DOI] [PubMed] [Google Scholar]
- 23.Wollmuth RL, Wagonfeld JB. False-positive secretin test. Ann Intern Med. 1978;88(5):718–719. doi: 10.7326/0003-4819-88-5-718_2. [DOI] [PubMed] [Google Scholar]
- 24.Kuiper P, Biemond I, Masclee AA, et al. Diagnostic efficacy of the secretin stimulation test for the Zollinger-Ellison syndrome: an intra-individual comparison using different dosages in patients and controls. Pancreatology. 2010;10(1):14–18. doi: 10.1159/000265936. [DOI] [PubMed] [Google Scholar]
- 25.Metz DC, Buchanan M, Purich E, et al. A randomized controlled crossover study comparing synthetic porcine and human secretins with biologically derived porcine secretin to diagnose Zollinger-Ellison Syndrome. Aliment Pharmacol Ther. 2001;15(5):669–676. doi: 10.1046/j.1365-2036.2001.00976.x. [DOI] [PubMed] [Google Scholar]
- 26.Solcia E, Vassallo G, Sampietro R. Endocrine cells in the antro-pyloric mucosa of the stomach. Z Zellforsch Mikrosk Anat. 1967;81(4):474–486. doi: 10.1007/BF00541009. [DOI] [PubMed] [Google Scholar]
- 27.Walsh JH, Grossman MI. Gastrin (second of two parts). N Engl J Med. 1975;292(26):1377–1384. doi: 10.1056/NEJM197506262922605. [DOI] [PubMed] [Google Scholar]
- 28.Tatsuta M, Itoh T, Okuda S, et al. Effect of fundusectomy on serum and antral gastrin levels in rats. Gastroenterology. 1977;72(1):78–81. [PubMed] [Google Scholar]
- 29.Hattori Y, Imamura M, Tobe T. Gastrin release from antral G cells stimulated with secretin. Am J Gastroenterol. 1992;87(2):195–200. [PubMed] [Google Scholar]
- 30.Brady CE, 3rd, Hyatt JR, Utts SJ. Is the gastrin response to secretin provocation a function of antral G-cell mass? Results in the hypergastrinemia of acid hyposecretion. J Clin Gastroenterol. 1989;11(1):27–32. doi: 10.1097/00004836-198902000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Raines D, Chester M, Diebold AE, et al. A prospective evaluation of the effect of chronic proton pump inhibitor use on plasma biomarker levels in humans. Pancreas. 2012;41(4):508–511. doi: 10.1097/MPA.0b013e318243a0b6. [DOI] [PubMed] [Google Scholar]
- 32.Ito T, Igarashi H, Jensen RT. Serum pancreastatin: the long sought universal, sensitive, specific tumor marker for neuroendocrine tumors? Pancreas. 2012;41(4):505–507. doi: 10.1097/MPA.0b013e318249a92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metz DC. Diagnosis of the Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol. 2012;10(2):126–130. doi: 10.1016/j.cgh.2011.07.012. [DOI] [PubMed] [Google Scholar]