Abstract
Currently there are three targeted therapies approved for the treatment of colorectal cancers. These include the epidermal growth factor receptor (EGFR) inhibitors, cetuximab and panitumumab, and the multikinase inhibitor regorafenib. It is important to understand and recognize the common presentations of cutaneous toxicity that result from these agents to effectively manage symptoms and prevent premature discontinuation of anticancer treatment.
Key Words: Cetuximab, panitumumab, regorafenib
Currently there are three targeted therapies approved for the treatment of colorectal cancers. These include the epidermal growth factor receptor (EGFR) inhibitors, cetuximab and panitumumab, and the multikinase inhibitor regorafenib. It is important to understand and recognize the common presentations of cutaneous toxicity that result from these agents to effectively manage symptoms and prevent premature discontinuation of anticancer treatment.
EGFR inhibitors
Cetuximab and panitumumab are intravenous monoclonal antibody EGFR inhibitors. Cetuximab was first FDA-approved in 2004 for metastatic colorectal carcinoma and in 2012, it was approved as first-line treatment of KRAS mutant-negative, EGFR-expressing metastatic colorectal cancer. Panitumumab was first FDA-approved in 2006 for the treatment of EGFR-expressing metastatic colorectal cancer.
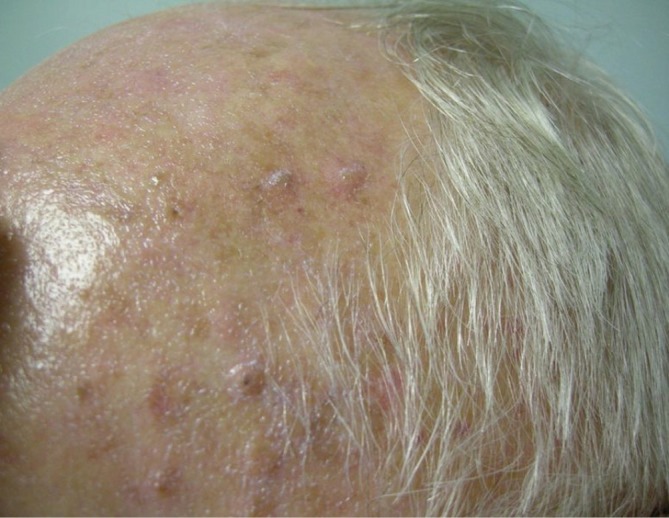
The most common cutaneous toxicity resulting from treatment with EGFR inhibitors is the development of an acneiform eruption. This consists of follicular sterile pustules and papules usually involving the face, scalp, and upper trunk (Figures 1,2,3). Secondary infections are commonly observed, but must be confirmed by bacterial culture. Histopathology shows folliculitis with collections of neutrophils within the follicles and lymphocytes surrounding the follicles. It is essential to understand that this eruption resembles acne (hence the term “acneiform”), but is actually not acne. This eruption lacks comedones and does not respond to topical retinoids, both of which are cornerstones of traditional acne vulgaris. The basic differential diagnosis for the acneiform eruption induced by EGFR inhibitors includes steroid induced acne and infectious folliculitis caused by bacteria or yeast. Positive correlations between the development of acneiform eruptions and clinical outcomes have been observed so it is important to treat through these reactions and reserve discontinuation of medication as a last resort.
Figure 1.
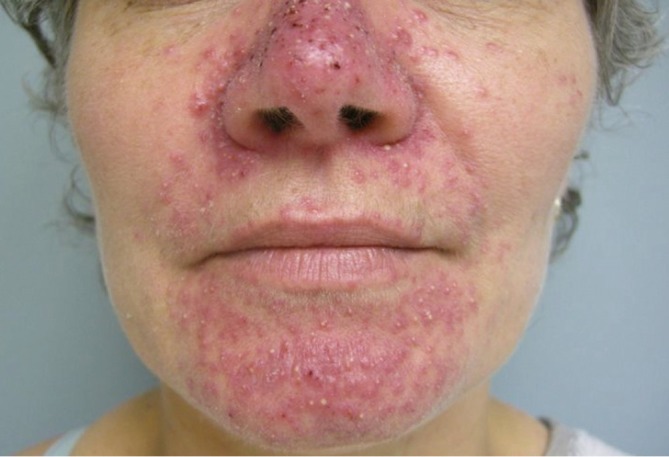
Acneiform rash affecting the face during EGFR inhibitor treatment
Figure 2.
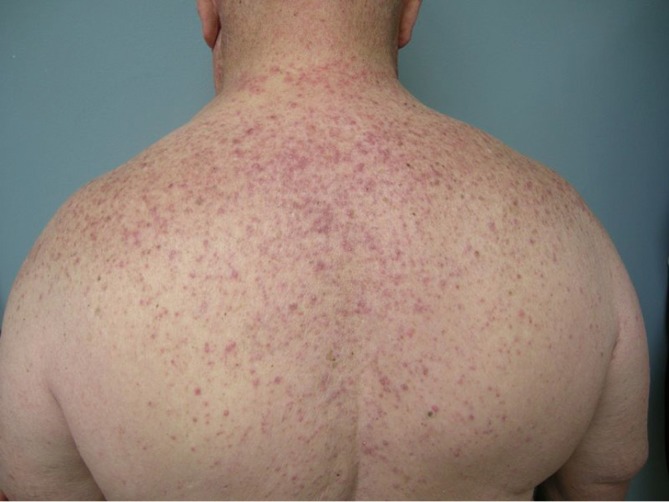
Acneiform rash affecting the back during EGFR inhibitor treatment
Figure 3.
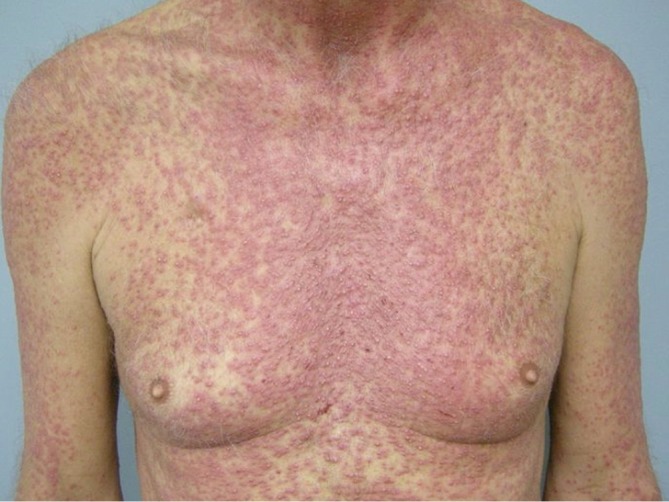
Acneiform rash affecting the chest during EGFR inhibitor treatment
The Common Terminology Criteria for Adverse Events established by the National Cancer Institute defines the severity of acneiform rash from grades one through five as shown in Table 1.
Table 1. Common Terminology Criteria for Adverse Events-Acneiform rash.
| Reaction | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Rash acneiform | Papules/pustules covering <10% BSA; +/- pruritis or tenderness | Papules/pustules covering 10-30% BSA; psychosocial impact & limiting instrumental activities of daily living | Papules/pustules covering >30% BSA; limiting self care activities of daily living; associated with local superinfections with oral antibiotics indicated | Papules/pustules covering any percentage BSA; extensive superinfection with intravenous antibiotics indicated; life-threatening consequences | Death |
Reproduced from the Common Terminology Criteria for Adverse Events version 4.0. (http://www.cancer.gov) (1)
Studies have been conducted to explore the pathogenesis of the acneiform eruption caused by EGFR inhibitors. The EGF receptor is present in keratinocytes in the basal and suprabasal layers of the epidermis and the outer layers of hair follicles. Stimulation of the EGFR pathway promotes keratinocyte survival and proliferation. Han et al. found increased expression of cytokines such as interleukin-1 alpha, tumor necrosis factor-alpha, and interferon-gamma in acneiform lesions of patients when EGFR was inhibited by cetuximab. These increased cytokines may lead to inflammation in the dermis. This inflammation is characterized by neutrophilic inflammatory infiltrates followed by follicular proliferation and plugging that causes the papulopustular eruption. The mechanism is distinct from the etiology of acne vulgaris, where inflammation follows comedone formation. This explains why topical steroids are an effective treatment for the severe papulopustular eruption caused by cetuximab but may worsen acne vulgaris.
Most patients receiving cetuximab or panitumumab (up to 90%) will develop the acneiform eruption within the first two weeks of therapy (2,3). Tol et al. reported a phase III study comparing toxicity of adding cetuximab to a combination treatment with capecitabine, oxaliplatin and bevacizumab (4). In the cohort not taking cetuximab only 7 of 197 experienced an acneiform skin rash. In the cetuximab group 156 of 197 developed an acneiform skin rash with fifty of these patients categorized as grades 3 or 4 severity. Patients on panitumumab have an increased incidence of acneiform eruptions but similar clinical findings when compared to the cutaneous toxicities induced by cetuximab. Douillard et al. reported results of a phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone (5). In the 545 patients treated with FOLFOX4 alone only ten developed skin toxicity. Of patients treated with panitumumab plus FOLFOX4 182 of 539 developed skin toxicity.
Perez-Soler and Saltz were the first to report the association of acneiform rash due to EGFR inhibitors as a surrogate marker for efficacy in 2005 (6). This association only holds true for the acneiform rash due to EGFR inhibitors. Other forms of EGFR inhibitor cutaneous toxicity such as paronychia, hair and nail changes, and xerosis discussed later are not considered markers for efficacy. Multiple studies suggest that a positive correlation exists between occurrence of an acneiform rash and both the cancer’s response to the EGFR-targeted therapy and patient survival. Since cutaneous toxicity may be associated with improved clinical outcomes, it is important to avoid stopping EGFR inhibitor treatments for cutaneous toxicities and, instead, treat through eruptions. To better counsel patients about the risks of the cutaneous toxicities of EGFR inhibitors, Jatoi et al. evaluated whether any patients have died from rashes caused by EGFR inhibitors (7). After reviewing 117 trials including 8,998 cancer patients where the rate of rash development was greater than 50%, they concluded that there were no reported rash-related deaths.
In addition to the physical effects of EGFR inhibitors, several researchers addressed the psychological and emotional effects of cutaneous toxicity. Romito et al. studied the psychological effect of the cutaneous skin rash in eighty advanced colorectal cancer patients treated with cetuximab (8). Forty-one percent reported psychological distress caused by the rash. When questioned about how the rash affected the willingness of patients to go out into public, 22% “very much” avoided going out and 25% “somewhat” avoided going out. In addition to the cosmetic effects, a significant psychological and quality of life effect from these eruptions results from physical symptoms of burning, stinging, and itching (9). It is, therefore, clear that treating the cutaneous toxicities of EGFR inhibitors not only allows patients to continue on potentially life saving oncology treatments but also can greatly improve their quality of life.
Several authors have reviewed treatments of the cutaneous toxicity associated with EGFR inhibitor receptors. Jatoi et al. conducted a randomized, double-blinded placebo controlled study with 65 patients comparing tetracycline 500 mg orally twice per day for 28 days versus placebo (10). Monitoring was done for the four weeks of treatment and an additional four weeks with primary objective to compare incidence of grade 2 or worse rash between the groups. This study found that oral tetracycline did not significantly lessen rash incidence or severity in patients taking EGFR inhibitors. Scope et al. conducted a randomized double-blind controlled trial of oral minocycline for cetuximab induced acneiform eruption published in 2007 (11). Of 48 patients enrolled, half were randomly assigned to minocycline and the other half to placebo for 8 weeks of treatment. Total facial lesion counts were significantly lower for patients receiving treatment rather than placebo at week one through four. At week four patients in the minocycline treatment group had a lower frequency of moderate to severe rash than patients receiving placebo and at week eight there were diminished total facial lesion counts. No patients treated with minocycline had to discontinue cetuximab treatment due to acneiform eruption but four patients in the placebo group had to interrupt treatment because of grade 3 skin rash. Topical tazarotene use was also studied. Tazarotene was not helpful in controlling the acneiform rash and caused significant irritation, supporting the observation that this condition does not respond like traditional acne vulgaris.
De Noronha et al. reviewed the management of cutaneous side effects during erlotinib and cetuximab treatment in lung and colorectal cancer patients (12). They presented a treatment algorithm to help manage these patients. Upon initiation of treatment with the EGFR inhibitor they started patients on daily sunscreen, mild skin cleanser, and moisturizing cream. In patients who developed mild acneiform eruptions they began topical antibiotics plus topical benzoyl peroxide. For patients who developed grade 2 or 3 cutaneous reactions they started oral doxycycline or minocycline at a dose of 100 mg/day. In one case that was not responsive to oral antibiotics they initiated oral low dose isotretinoin. Antihistamines were recommended when patients experienced pruritis. In the nineteen cases described by these authors none had to stop EGFR inhibitor treatment because of cutaneous side effects, all but one patient showed improvement on oral antibiotics, and 42% experienced a complete response.
The skin toxicity evaluation protocol with panitumumab (STEPP) study conducted by Lacouture et al. was a randomized trial evaluating pre-emptive versus reactive treatment with doxycycline for patients receiving panitumumab (13). All patients started a standard regimen of daily skin moisturizer, sunscreen, and topical steroid at the onset of chemotherapy. Forty-eight patients also received pre-emptive treatment with doxycycline 100 mg twice per day, while forty-seven received doxycycline only after skin toxicity developed. The incidence of grade 2 skin toxicities during the six-week treatment period was 29% for the pre-emptive treatment group and 62% for the reactive treatment group. The pre-emptive treatment with doxycycline was well tolerated and patients in this group reported less impairment of quality of life.
Requena et al. reported three cases of severe acneiform eruptions induced by EGFR inhibitors that were successfully treated to the point of complete response with oral isotretinoin (14). In data pending publication, we have also had success with over a dozen patients using oral isotretinoin to successfully treat cases of severe acneiform eruptions caused by EGFR inhibitors (15).
Other cutaneous toxicities can be observed during treatment with EGFR inhibitors. Patients may develop xerosis and painful fissuring (Figure 4). As described by Han et al. EGFR inhibitor use leads to abnormal differentiation of keratinocytes with decreased levels of filaggrin and loricrin (16). These are both components of the outer skin layer known as the stratum corneum and play a role in the retention of moisture. Decreased levels of these proteins may explain the xerosis observed in the cutaneous EGFR induced drug rashes. Rodríguez-Murphy et al. studied a group of forty-three patients treated with cetuximab and observed xerosis in less than a quarter of patients after a mean delay of 40 days (17). Three patients in this group developed painful fissures on the hands and feet. Xerosis is actually much more common though and likely the follow-up in this study was not adequate for assessment. In 2009, Osio et al. reported a study describing the cutaneous side-effects in sixteen patients on long-term treatment with epidermal growth factor receptor inhibitors with the range of follow-up from 6 to 27 months and mean treatment 10 months and found xerosis present in all patients (18). All patients should be counseled on dry skin care prevention with lukewarm showers or baths, minimal soap usage (primarily axilla, groin, and feet), and thick emollient usage daily. Fissures are best treated with super glue for immediate closure.
Figure 4.
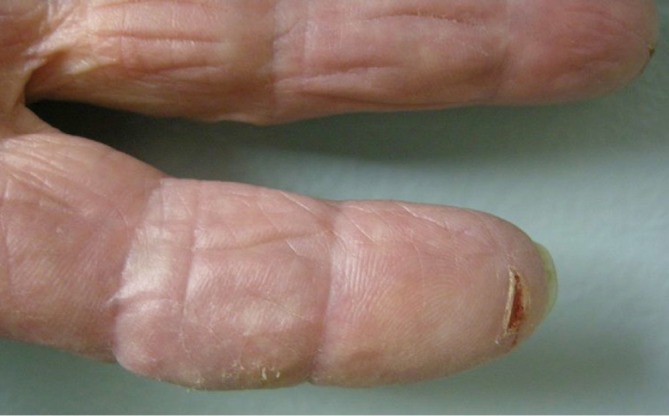
Fissure on finger developed during EGFR inhibitor treatment
Patients on EGFR inhibitors may develop nailfold changes after two or more months of treatment. These most commonly include nailfold inflammation (paronychia) and periungual pyogenic granuloma-like lesions (Figure 5). As a secondary processes resulting from nail matrix inflammation, the nails can become dystrophic or the nail plates may lift from the nail beds (onycholysis). Trauma is not required to precede the changes but is likely an aggravating factor. Both fingernails and toenails can be affected and the first digits are most commonly affected. The affected digits are painful and morbidity may be high due to impaired functionality limiting activities of daily living. Rodríguez-Murphy et al. studied a group of forty-three patients treated with cetuximab and found that two developed paronychia (17).
Figure 5.
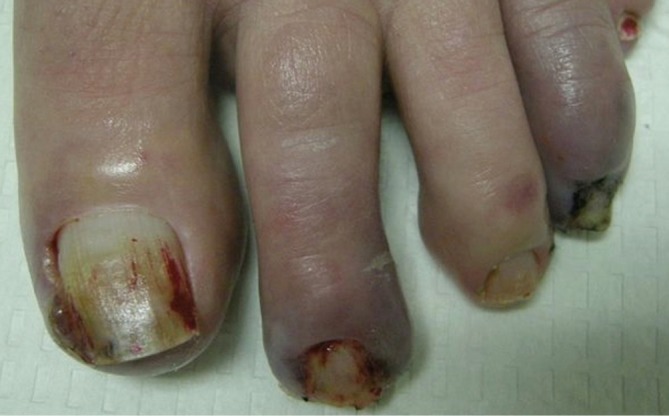
Paronychia with periungual pyogenic granuloma-like lesions associated with EGFR inhibitor treatment
Although paronychia is often sterile, lesions may become superinfected so culturing the lesion is recommended for appropriate antimicrobial selection and treatment. Lacouture et al. described a retrospective study of 152 patients treated with cetuximab in which 27 cases of paronychia developed for an incidence of 17.7% (19). Forty-two culture swabs were performed and all cultures grew some organisms. Nosocomial colonization with coagulase-negative gram-positive bacteria was found in 31% and Staphylococcus aureus infection was found in 23%. Recommendations for minimizing periungual trauma include comfortable shoes, keeping nails trimmed but avoiding aggressive manicuring, and wearing gloves for protection while cleaning and doing housework. Topical corticosteroids and anti-inflammatory doses of tetracyclines may help decrease periungual inflammation while antimicrobial soaks such as dilute bleach in water or dilute white vinegar in water can prevent superinfection.
The periungual pyogenic granuloma-like lesions clinically appear as friable vascular tissue overgrowth and commonly bleed. Local trauma may precede development of the lesions or aggravate them leading to increased symptoms of bleeding. Santiago et al. studied fourteen patients on EGFR inhibitors cetuximib or erlotinib and observed that five patients developed periungual pyogenic granulomas and four of these patients also had paronychia (20). The pyogenic granulomas occurred an average of eight weeks after beginning treatment. Medical intervention may be necessary to eliminate excessive granulation tissue and treatment options include electrocautery, silver nitrate, and nail avulsion.
Abnormalities of the hair can develop in patients taking EGFR inhibitors. Patients may experience hypertrichosis or increased hair growth. Specifically, increased hair growth of the eyebrows and eyelashes (trichomegaly) may occur (Figure 6). Patients can also develop scalp alopecia, which may be scarring or nonscarring.
Figure 6.
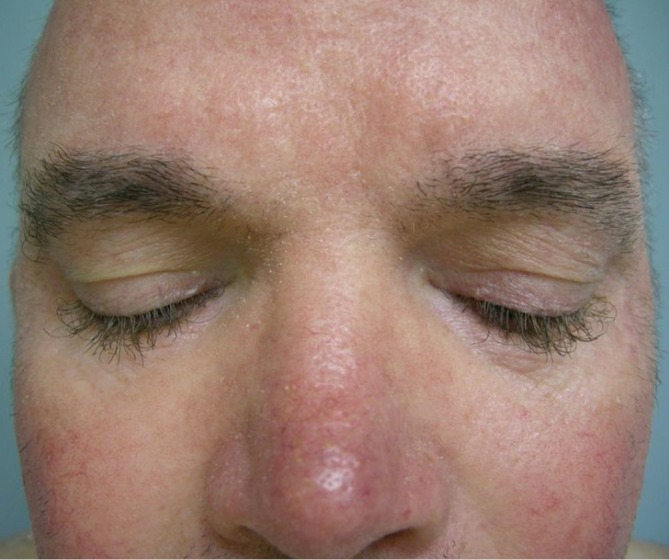
Trichomegaly during EGFR inhibitor treatment
Cutaneous superinfections can complicate the cutaneous toxicities affecting patients treated with EGFR inhibitors (Figure 7). Several studies have been conducted to explore the microbiology of these infections. Amitay-Laish et al. studied 29 patients on EGFR inhibitors cetuximab or erlotinib and found that 24 patients had a papulopustular reaction (21). They divided this cohort into two groups based on when they developed the papulopustular eruption. The early phase group contained seventeen patients and had a median onset at 8 days. The late phase group had a median onset at 200 days and contained seven patients. Staphylococcus aureus was found in 7 of 13 early phase patients and in all 7 late phase patients. The high incidence of staphylococcal infection demonstrates the importance of bacterial cultures in the assessment and treatment of EFGR inhibitor eruptions. This study also emphasizes the importance of seeking a pathogenic microbial cause when patients who were stable on the EGFR inhibitors develop a late onset papulopustular reaction. Eilers et al. studied 221 patients treated with EGFR inhibitors and found that 84 showed evidence of infection at the sites of the cutaneous toxicity (22). Cultures revealed that fifty were positive for Staphylococcus aureus and twelve were positive for methicillin-resistant Staphylococcus aureus. Other less common infections included herpes simplex, herpes zoster, and dermatophytes. Occasionally uncommon pathogens can be identified in patients taking EGFR inhibitors, as demonstrated by Bark et al. who reported a case of disseminated cutaneous Mycobacterium chelonae in a patient with head and neck cancer on salvage chemotherapy with cetuximab (23). This reinforces the value of bacterial cultures to determine specific pathogenic agents in cases that are not responding to typical treatment regimens.
Figure 7.
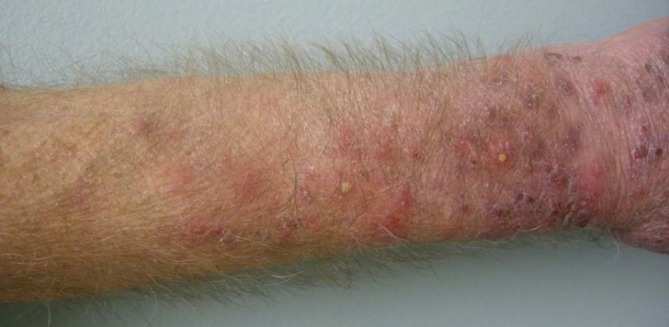
Cutaneous bacterial superinfection during EGFR inhibitor treatment
Our treatment algorithm begins with examining patients in clinic either before or soon after starting EGFR inhibitors but preceding the development of significant cutaneous toxicity. Patients are instructed to use sunscreen and dry skin care is reviewed with an emphasis placed on using emollients such as Vaseline or thick body creams regularly. Topical steroids can be prescribed such as triamcinolone 0.1% cream or ointment for use once to twice per day as needed for itchy scaly red rashes. Oral antibiotics are started when chemotherapy is initiated, prior to rash development, to prevent or minimize the acneiform eruption. The usual treatment consists of minocycline 50 or 100 mg twice per day and side effects such as headache, dizziness, hypersensitivity reaction, and drug-induced rash are reviewed. Patients are instructed to stop the medication immediately if a new rash or other side effect develops. Another option for treatment is doxycycline 50 or 100 mg twice per day and side effects of gastrointestinal upset and photosensitivity are reviewed. Patients are instructed to take the pills with food and a full glass of water to limit the gastrointestinal side effects. When lesions persist or worsen despite treatment with oral antibiotics and topical steroids it may helpful to rule out superinfection. When infection is absent in the setting of a difficult to manage acneiform eruption, the practitioner should start oral isotretinoin. If the practitioner is uncomfortable prescribing or managing treatment with oral isotretinoin, referral to a dermatologist with knowledge of EGFR inhibitor induced cutaneous toxicities may be beneficial for the initiation of treatment.
Regorafenib
The newest targeted therapy approved for the treatment of colorectal cancers is the multikinase-inhibitor regorafenib. Regorafenib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptor 2, platelet-derived growth factor receptor-β, fibroblast growth factor receptor 1, C-KIT, RET, and B-RAF (24). Regorafenib is currently being studied for use in gastrointestinal stromal tumors (GIST), colorectal carcinoma, and renal cell carcinoma. While similar to older multikinase inhibitors such as sorafenib and sunitinib, regorafenib also has structurally and biologically unique properties allowing for its use when tumors become resistant to these older agents. Phase III clinical trials of regorafenib in gastrointestinal stromal tumors that developed resistance to imatinib and sunitinib have shown increased medial progression free survival compared to placebo (25). Similarly, in colorectal cancer median overall survival was 6.4 months in the regorafenib group versus 5 months in the placebo group (26). We will review the side effects of similar multikinase-inhibitors, sorafenib and sunitinib, and present what is known to date to occur from Regorafenib. Sorafenib targets B-RAF, VEGF-2, C-KIT, fetal liver TK(Flt)-3, and PDGFR. It is associated with hand-foot skin reaction (HFSR) and splinter hemorrhages as well as a seborrhea-like facial rash and a follicular rash on the trunk and extremities. Sunitinib targets VEGF-2, C-KIT, Flt-3, and PDGFR. It is associated with HFSR and splinter hemorrhages plus hair depigmentation, skin discoloration, and neutrophilic dermatoses.
HFSR can occur with regorafenib, and has long been a known side effect of multikinase inhibitors such as sorafenib and sunitinib. HFSR from multikinase inhibitors is a unique cutaneous toxicity pattern that should be distinguished from acral erythema (also known as hand foot syndrome and palmoplantar dysthesthesia) seen with classic cytotoxic chemotherapy. Patients with HFSR from multikinase inhibitors experience acral pain and dysesthesia, but usually to a lesser extent and with less edema than when caused by chemotherapy agents such as 5-flourouracil, doxorubicin, and cytosine arabinoside. The most characteristic feature of HFSR is the development of palmar and plantar hyperkeratotic plaques (Figures 8,9). These occur most often over areas of friction. During treatment with sorafenib and sunitinib, high grade hand-foot skin reactions have been reported to occur in up to 9% of cases resulting in impaired functionality from blisters and ulceration (27). Nardone et al. found these drug induced hand-foot skin reactions negatively impacted the patients’ health-related quality of life scores (28).
Figure 8.
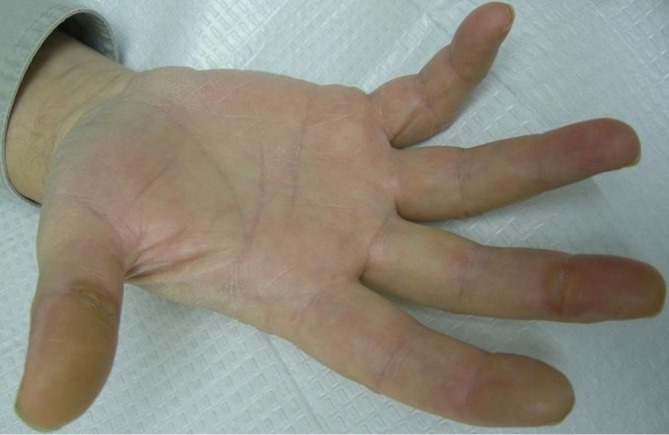
Hyperkeratotic plaques on areas of friction from regorafenib
Figure 9.
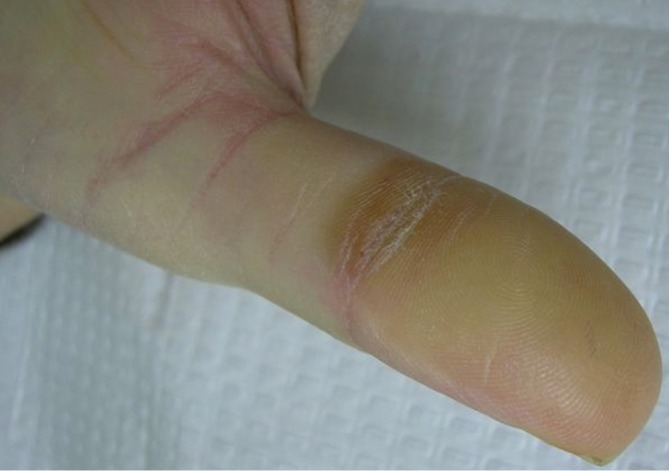
Hyperkeratotic plaque on thumb from regorafenib
Hand and foot skin reactions are known to occur in patients receiving regorafenib for the treatment of metastatic adenocarcinoma of the colon. Often several weeks after starting the medication painful blistering plaques or rash develop on the feet and tender thickened plaques may develop on fingertips. This rash may affect activities of daily living because of the blistering, thickening, and discomfort that is frequently most severe at pressure points such as balls of the feet and fingertips. In clinical trials treating gastrointestinal stromal tumors with regorafenib, Demetri et al. reported that 20% of patients (26 of 132) developed a hand-foot skin reaction (25). In clinical trials treating colorectal cancer with regorafenib, Grothey et al. observed grade three or higher hand-foot skin reactions in 17% of patients (83 of 500) (26).
Management of the HFSR can be challenging but the basic principles include minimizing friction and trauma with comfortable well fitting shoes and protective gloves. Topical corticosteroids can minimize inflammation and thickened hyperkeratotic plaques on the hands and feet can be softened with the use of keratolytic creams such as urea or lactic acid. Dose reduction of the regorafenib is another option for reducing the bothersome side effects. Unlike with the acneiform eruption seen with EFGR inhibitors, there is no known correlation of the HFSR rash or any other cutaneous toxicity from regorafenib to efficacy of the medication.
A seborrheic dermatitis-like rash may occur while taking multikinase inhibitors, including regorafenib (Figure 10). The seborrhea-like facial rash can typically be controlled with topical medications. Low potency corticosteroids such as hydrocortisone 2.5% cream or ketoconazole cream may be beneficial.
Figure 10.
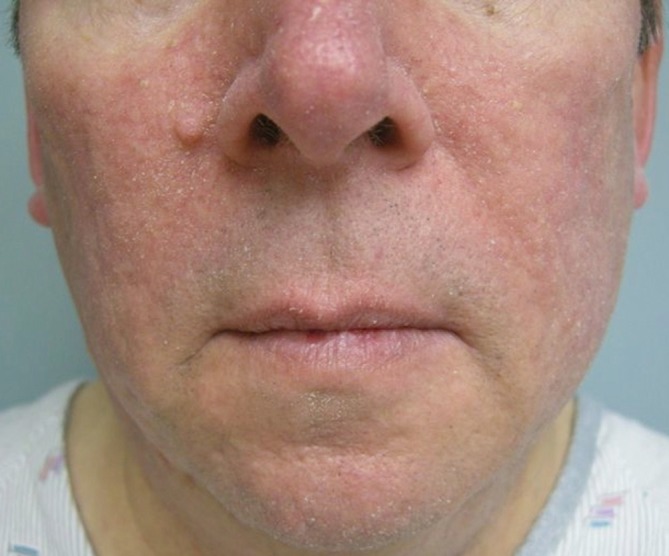
Seborrheic dermatitis-like rash developed during regorafenib treatment
A follicular rash may develop during treatment with multikinase inhibitors as described by Lopez et al. (29). Clinically this manifests as skin colored to erythematous follicular keratotic papules (Figure 11). Histopathology shows prominent follicular hyperplasia. Topical corticosteroids or topical keratolytics may be helpful for symptomatic control.
Figure 11.
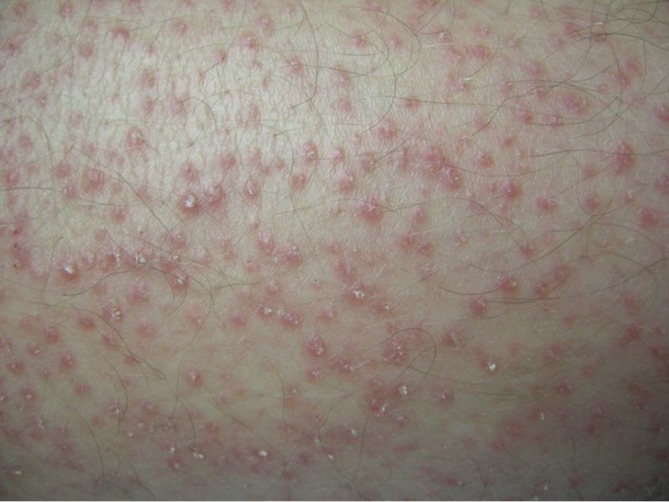
Follicular keratotic papules associated with multikinase-inhibitor treatment
Cutaneous squamous cell carcinoma and the inflammation of actinic keratoses were reported to be associated with sorafenib in 2009 by Dubauskas et al. (30). In 131 patients treated with sorafenib for metastatic renal cell carcinoma, seven cases of cutaneous squamous cell carcinoma and two cases of keratoacanthoma type squamous cell carcinoma were reported. In 2013, Breaker et al. reported an association with skin cancer and the use of sorafenib and sunitinib for renal cell carcinoma (31). Of 69 patients treated with multikinase inhibitors, five patients on sorafenib and two patients on sunitinib developed skin cancers, of which five lesions were squamous cell carcinomas and three lesions were basal cell carcinomas. The median treatment durations before identification of the skin cancer was longer than one year. Figure 12 shows a squamous cell carcinoma that developed during treatment with a multikinase-inhibitor. The BRAF inhibitor vemurafenib is used in the treatment of metastatic melanoma. Vemurafenib also triggers the development of squamous cell carcinomas possibly through the activation of wild-type RAF in sun-damaged keratinocytes. Long-term follow-up of regorafenib treatment will be necessary to determine if similar cutaneous skin cancer risks exist.
Figure 12.
Development of squamous cell carcinoma during treatment with vemurafenib (BRAF-inhibitor)
These findings highlight how familiarity with the characteristic skin reactions observed in classes or families of targeted chemotherapeutics may help predict what reactions to expect from new agents. Knowledge of the presentation and treatment of the cutaneous toxicities caused by targeted therapies approved for the treatment of colorectal cancers is extremely important for the practicing oncologist and dermatologist. Successful treatment improves patients’ quality of life while undergoing these therapies. It addition, by minimizing the cutaneous side effects patients experience these life-saving treatments can be continued at the proper doses and durations to allow for the most effective treatment of their cancers.
Acknowledgements
Disclosure: Dr. Urban has no conflicts of interest or financial interests to report. Dr. Anadkat has received honoraria for consulting and/or speaking in the past from ImClone, Bristol-Myers Squibb, Astra-Zeneca, Eisai, and Therakos.
References
- 1.Common Terminology Criteria for Adverse Events version 4.0. Available online: http://www.cancer.gov [DOI] [PubMed]
- 2.Erbitux® (cetuximab) [package insert]. Branchburg, NJ and Princeton, NJ: ImClone LLC, 2010.
- 3.Panitumumab (Vectibix) [package insert]. Thousand Oaks, CA: Amgen Inc, 2010.
- 4.Tol J, Koopman M, Rodenburg CJ, et al. A randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicity. Ann Oncol 2008;19:734-8 [DOI] [PubMed] [Google Scholar]
- 5.Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697-705 [DOI] [PubMed] [Google Scholar]
- 6.Peréz-Soler R, Saltz L.Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol 2005;23:5235-46 [DOI] [PubMed] [Google Scholar]
- 7.Jatoi A, Nguyen PL. Do patients die from rashes from epidermal growth factor receptor inhibitors? A systematic review to help counsel patients about holding therapy. Oncologist 2008;13:1201-4 [DOI] [PubMed] [Google Scholar]
- 8.Romito F, Giuliani F, Cormio C, et al. Psychological effects of cetuximab-induced cutaneous rash in advanced colorectal cancer patients. Support Care Cancer 2010;18:329-34 [DOI] [PubMed] [Google Scholar]
- 9.Wu PA, Balagula Y, Lacouture ME, et al. Prophylaxis and treatment of dermatologic adverse events from epidermal growth factor receptor inhibitors. Curr Opin Oncol 2011;23:343-51 [DOI] [PubMed] [Google Scholar]
- 10.Jatoi A, Dakhil SR, Sloan JA, et al. Prophylactic tetracycline does not diminish the severity of epidermal growth factor receptor (EGFR) inhibitor-induced rash: results from the North Central Cancer Treatment Group (Supplementary N03CB). Support Care Cancer 2011;19:1601-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scope A, Agero AL, Dusza SW, et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol 2007;25:5390-6 [DOI] [PubMed] [Google Scholar]
- 12.de Noronha e Menezes NM , Lima R, Moreira A, et al. Description and management of cutaneous side effects during erlotinib and cetuximab treatment in lung and colorectal cancer patients: a prospective and descriptive study of 19 patients. Eur J Dermatol 2009;19:248-51 [DOI] [PubMed] [Google Scholar]
- 13.Lacouture ME, Mitchell EP, Piperdi B, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-Emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:1351-7 [DOI] [PubMed] [Google Scholar]
- 14.Requena C, Llombart B, Sanmartín O.Acneiform eruptions induced by epidermal growth factor receptor inhibitors: treatment with oral isotretinoin. Cutis 2012;90:77-80 [PubMed] [Google Scholar]
- 15.Chiang H, Anadkat MJ. Isotretinoin for high-grade or refractory epidermal growth factor receptor inhibitor-related acneiform papulopustular eruptions. JAAD 2013. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Han SS, Lee M, Park GH, et al. Investigation of papulopustular eruptions caused by cetuximab treatment shows altered differentiation markers and increases in inflammatory cytokines. Br J Dermatol 2010;162:371-9 [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Murphy E, Villanueva-Herraiz S, Ortega-García MP, et al. Cutaneous toxicity associated with cetuximab treatment in metastatic colorectal cancer. Farm Hosp 2011;35:114-20 [DOI] [PubMed] [Google Scholar]
- 18.Osio A, Mateus C, Soria JC, et al. Cutaneous side-effects in patients on long-term treatment with epidermal growth factor receptor inhibitors. Br J Dermatol 2009;161:515-21 [DOI] [PubMed] [Google Scholar]
- 19.Lacouture ME, Anadkat MJ, Bensadoun RJ, et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer 2011;19:1079-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santiago F, Gonçalo M, Reis JP, et al. Adverse cutaneous reactions to epidermal growth factor receptor inhibitors: a study of 14 patients. An Bras Dermatol 2011;86:483-90 [DOI] [PubMed] [Google Scholar]
- 21.Amitay-Laish I, David M, Stemmer SM. Staphylococcus coagulase-positive skin inflammation associated with epidermal growth factor receptor-targeted therapy: an early and a late phase of papulopustular eruptions. Oncologist 2010;15:1002-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eilers RE, Jr, Gandhi M, Patel JD, et al. Dermatologic infections in cancer patients treated with epidermal growth factor receptor inhibitor therapy. J Natl Cancer Inst 2010;102:47-53 [DOI] [PubMed] [Google Scholar]
- 23.Bark CM, Traboulsi RS, Honda K, et al. Disseminated Mycobacterium chelonae infection in a patient receiving an epidermal growth factor receptor inhibitor for advanced head and neck cancer. J Clin Microbiol 2012;50:194-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245-55 [DOI] [PubMed] [Google Scholar]
- 25.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12 [DOI] [PubMed] [Google Scholar]
- 27.Gomez P, Lacouture ME. Clinical presentation and management of hand-foot skin reaction associated with sorafenib in combination with cytotoxic chemotherapy: experience in breast cancer. Oncologist 2011;16:1508-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nardone B, Hensley JR, Kulik L, et al. The effect of hand-foot skin reaction associated with the multikinase inhibitors sorafenib and sunitinib on health-related quality of life. J Drugs Dermatol 2012;11:e61-5 [PubMed] [Google Scholar]
- 29.Lopez V, Pinazo I, Marti N, et al. Follicular hyperplasia on the face subsequent to therapy with sorafenib. A new skin side effect. J Eur Acad Dermatol Venereol 2009;23:959-60 [DOI] [PubMed] [Google Scholar]
- 30.Dubauskas Z, Kunishige J, Prieto VG, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer 2009;7:20-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breaker K, Naam M, La Rosa FG, et al. Skin Cancer Associated With the Use of Sorafenib and Sunitinib for Renal Cell Carcinoma. Dermatol Surg 2013;39:981-7 [DOI] [PubMed] [Google Scholar]


