Abstract
This study was designed to approach two primary questions concerning hematopoietic stem cells (HSC) in mice: what is the concentration of HSC with extensive proliferative potential in marrow, and how long can an HSC continue to function in an intact animal? The assay system was the W/Wv mouse, a mouse with an inherited HSC defect, reflected in a reduction in all myeloid tissue and most particularly in a macrocytic anemia.
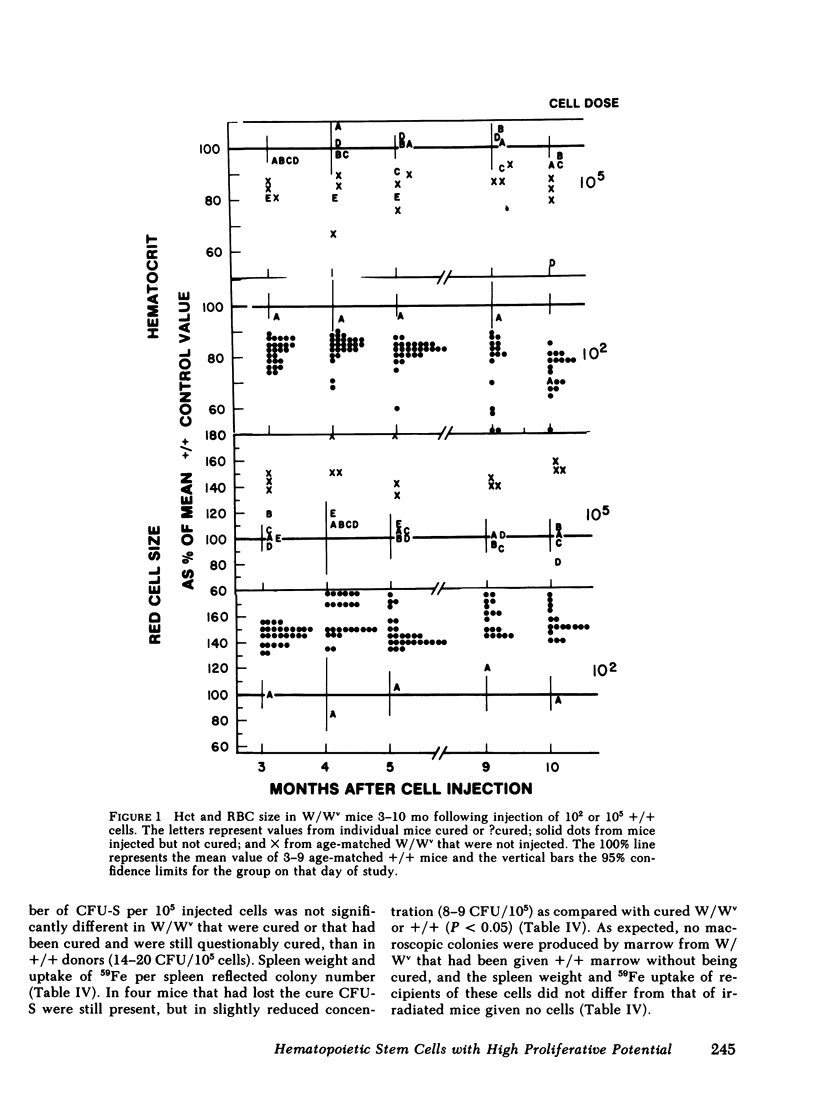
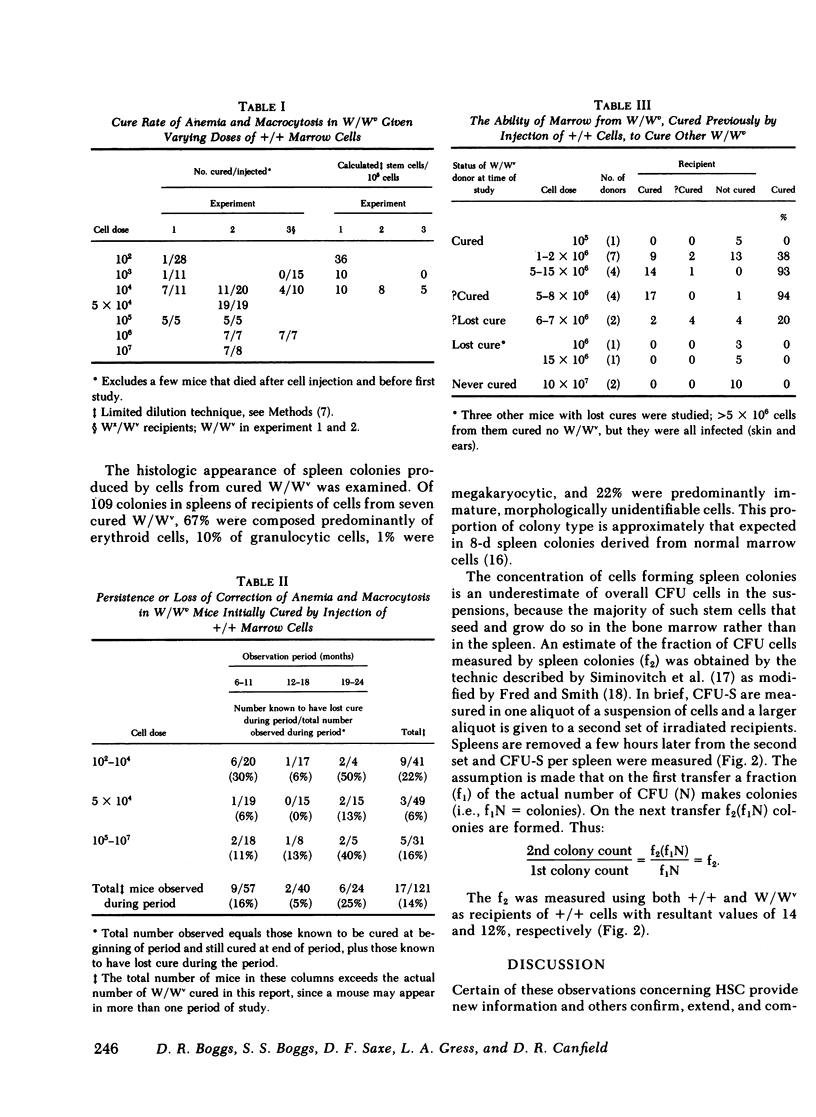
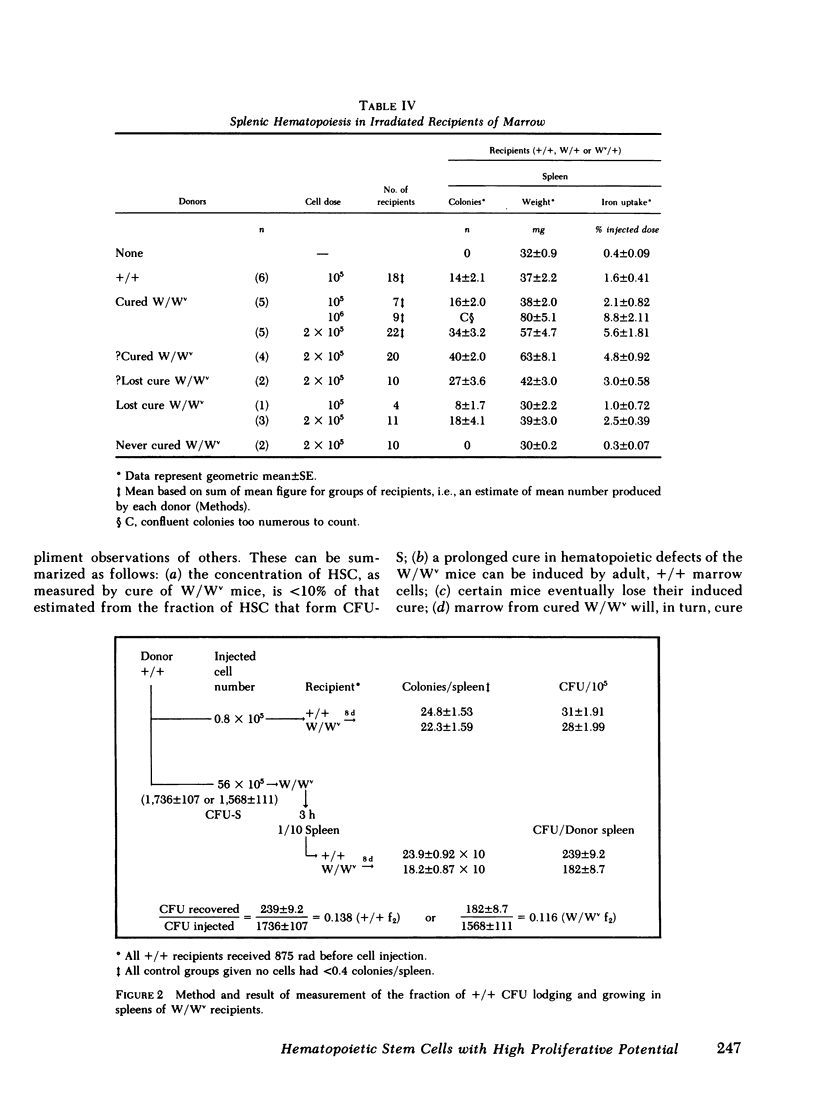
A single chromosomally marked HSC will reconstitute the defective hematopoietic system of the W/Wv. The concentration of HSC in normal littermate (+/+) marrow was assayed by limiting dilution calculation using cure of W/Wv as an end point (correction of anemia and erythrocytes' macrocytosis) and found to be ∼10/105. This is significantly less than spleen colony forming cell (CFU-S) concentration: ∼220/105 in +/+ and ranging from 50 to 270/105 in various other studies. Blood values were studied at selected intervals for as long as 26 mo. Of 24 initially cured mice, which were observed for at least 2 yr, 75% remained cured. However, of all cured mice, 17 lost the cure, returning to a macrocytic anemic state. Cured mice had normal numbers of nucleated and granulocytic cells per humerus and a normal concentration of CFU-S. However, cure of secondary W/Wv recipients by this marrow was inefficient compared with the original +/+ marrow. These studies suggest the CFU-S assay over-estimates extensively proliferating HSC or perhaps does not assay such a cell. A single such HSC can not only cure a W/Wv, but can sustain the cure for 2 yr or more, despite a relative deficit of cells capable of curing other W/Wv. However, the duration of sustained cure may be finite.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam G. Population-dynamical model of cell-density dependent growth regulation and aging of fibroblasts in vitro. J Theor Biol. 1980 May 21;84(2):233–257. doi: 10.1016/s0022-5193(80)80006-3. [DOI] [PubMed] [Google Scholar]
- Altman A., Gilmartin T. D., Katz D. H. Differentiation of murine bone marrow stem cells in vitro: long-term growth promoted by a lymphocyte-derived mediator. Science. 1981 Jan 2;211(4477):65–67. doi: 10.1126/science.6934621. [DOI] [PubMed] [Google Scholar]
- Bell E., Marek L. F., Levinstone D. S., Merrill C., Sher S., Young I. T., Eden M. Loss of division potential in vitro: aging or differentiation? Science. 1978 Dec 15;202(4373):1158–1163. doi: 10.1126/science.725592. [DOI] [PubMed] [Google Scholar]
- Boggs D. R., Boggs S. S. Lack of correlation between splenic and marrow hematopoiesis following irradiation or irradiation and transplantation in mice. Transplantation. 1978 Jun;25(6):328–330. doi: 10.1097/00007890-197806000-00010. [DOI] [PubMed] [Google Scholar]
- Boggs D. R., Marsh J. C., Chervenick P. A., Cartwright G. E., Wintrobe M. M. Factors influencing hematopoietic spleen colony formation in irradiated mice. 3. The effect of repetitive irradiation upon proliferative ability of colony-forming cells. J Exp Med. 1967 Nov 1;126(5):871–880. doi: 10.1084/jem.126.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs D. R., Marsh J. C., Chervenick P. A., Cartwright G. E., Wintrobe M. M. Factors influencing hematopoietic spleen colony formation in irradiated mice. V. Effect of foreign plasma upon colony forming cell kinetics. J Cell Physiol. 1968 Jun;71(3):227–238. doi: 10.1002/jcp.1040710305. [DOI] [PubMed] [Google Scholar]
- Boggs D. R. Normal hematocrits in preadolescent children. Am J Clin Pathol. 1976 Aug;66(2):442–445. doi: 10.1093/ajcp/66.2.442. [DOI] [PubMed] [Google Scholar]
- Boggs S. S., Chervenick P. A., Boggs D. R. The effect of postirradiation bleeding or endotoxin on proliferation and differentiation of hematopoietic stem cells. Blood. 1972 Sep;40(3):375–389. [PubMed] [Google Scholar]
- Breivik H. Haematopoietic stem cell content of murine bone marrow, spleen, and blood. Limiting dilution analysis of diffusion chamber cultures. J Cell Physiol. 1971 Aug;78(1):73–78. doi: 10.1002/jcp.1040780111. [DOI] [PubMed] [Google Scholar]
- Chertkov J. L., Lemeneva L. N., Mendelevitch O. A. Evaluation of factors effective in the determination of the transplanted fraction of colony-forming cells. Folia Biol (Praha) 1972;18(4):277–283. [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R. Decreased neutrophils and megakaryocytes in anemic mice of genotype W/W. J Cell Physiol. 1969 Feb;73(1):25–30. doi: 10.1002/jcp.1040730104. [DOI] [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R., Marsh J. C., Cartwright G. E., Wintrobe M. M. Quantitative studies of blood and bone marrow neutrophils in normal mice. Am J Physiol. 1968 Aug;215(2):353–360. doi: 10.1152/ajplegacy.1968.215.2.353. [DOI] [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R. Spleen colonies produced by cells obtained from colonies grown in vitro. Proc Soc Exp Biol Med. 1971 Dec;138(3):967–970. doi: 10.3181/00379727-138-36028. [DOI] [PubMed] [Google Scholar]
- DeGowin R. L., Miller S. H., Grund F. M. Studies of recovery of erythropoiesis after irradiation as a function of the cellularity of the endocolonized spleen. J Lab Clin Med. 1971 Feb;77(2):219–227. [PubMed] [Google Scholar]
- Fred S. S., Smith W. W. Induced changes in transplantability of hemopoietic colony forming cells. Proc Soc Exp Biol Med. 1968 Jun;128(2):364–366. doi: 10.3181/00379727-128-33015. [DOI] [PubMed] [Google Scholar]
- Gordon M. Y., Gordon-Smith E. C. Lymphocytes and haemopoiesis. Br J Haematol. 1981 Feb;47(2):163–169. doi: 10.1111/j.1365-2141.1981.tb02776.x. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Goldstein S. Retesting the commitment theory of cellular aging. Science. 1980 Jan 11;207(4427):191–193. doi: 10.1126/science.7350654. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Astle C. M., DeLaittre J. A. Processing by the thymus is not required for cells that cure and populate W/WV recipients. Blood. 1979 Nov;54(5):1152–1157. [PubMed] [Google Scholar]
- Harrison D. E., Astle C. M., Delaittre J. A. Loss of proliferative capacity in immunohemopoietic stem cells caused by serial transplantation rather than aging. J Exp Med. 1978 May 1;147(5):1526–1531. doi: 10.1084/jem.147.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E. Normal production of erythrocytes by mouse marrow continuous for 73 months. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3184–3188. doi: 10.1073/pnas.70.11.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman S., Botnick L. E., Hannon E. C., Vigneulle R. M. Proliferative capacity of murine hematopoietic stem cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):490–494. doi: 10.1073/pnas.75.1.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson G. S., Bradley T. R. Properties of haematopoietic stem cells surviving 5-fluorouracil treatment: evidence for a pre-CFU-S cell? Nature. 1979 Oct 4;281(5730):381–382. doi: 10.1038/281381a0. [DOI] [PubMed] [Google Scholar]
- Holliday R., Huschtscha L. I., Kirkwood T. B. Cellular aging: further evidence for the commitment theory. Science. 1981 Sep 25;213(4515):1505–1508. doi: 10.1126/science.7280670. [DOI] [PubMed] [Google Scholar]
- Holliday R., Huschtscha L. I., Tarrant G. M., Kirkwood T. B. Testing the commitment theory of cellular aging. Science. 1977 Oct 28;198(4315):366–372. doi: 10.1126/science.910134. [DOI] [PubMed] [Google Scholar]
- Kretchmar A. L., Conover W. R. Colony-forming cells in the spleen. Determination of the fraction transplanted. Transplantation. 1969 Nov;8(5):576–581. doi: 10.1097/00007890-196911000-00004. [DOI] [PubMed] [Google Scholar]
- Leith A. Detection of limited life span in small clones. J Theor Biol. 1978 Apr 6;71(3):453–464. doi: 10.1016/0022-5193(78)90172-8. [DOI] [PubMed] [Google Scholar]
- Lord B. I., Hendry J. H. Observations on the settling and recoverability of transplanted hemopoietic colony-forming units in the mouse spleen. Blood. 1973 Mar;41(3):409–415. [PubMed] [Google Scholar]
- MCCULLOCH E. A., SIMINOVITCH L., TILL J. E. SPLEEN-COLONY FORMATION IN ANEMIC MICE OF GENOTYPE WW. Science. 1964 May 15;144(3620):844–846. doi: 10.1126/science.144.3620.844. [DOI] [PubMed] [Google Scholar]
- Martin G. M. Cellular aging--clonal senescence. A review (Part I). Am J Pathol. 1977 Nov;89(2):484–512. [PMC free article] [PubMed] [Google Scholar]
- Matioli G., Vogel H., Niewisch H. The dilution factor of intravenously injected hemopoietic stem cells. J Cell Physiol. 1968 Dec;72(3):229–234. doi: 10.1002/jcp.1040720310. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Johnson G. R., Mandel T. E. Colony formation in agar by multipotential hemopoietic cells. J Cell Physiol. 1979 Feb;98(2):401–420. doi: 10.1002/jcp.1040980216. [DOI] [PubMed] [Google Scholar]
- Micklem H. S., Anderson N., Ross E. Limited potential of circulating haemopoietic stem cells. Nature. 1975 Jul 3;256(5512):41–43. doi: 10.1038/256041a0. [DOI] [PubMed] [Google Scholar]
- Monette F. C., Stockel J. B. Immunological evidence for murine hematopoietic stem cell subpopulations differing in self-renewal capacity. Stem Cells. 1981;1(1):38–52. [PubMed] [Google Scholar]
- Ogden D. A., Mickliem H. S. The fate of serially transplanted bone marrow cell populations from young and old donors. Transplantation. 1976 Sep;22(3):287–293. doi: 10.1097/00007890-197609000-00010. [DOI] [PubMed] [Google Scholar]
- Prothero J. Control of stem cell proliferation: a density-dependent committment model. J Theor Biol. 1980 Jun 21;84(4):725–736. doi: 10.1016/s0022-5193(80)80030-0. [DOI] [PubMed] [Google Scholar]
- Quesenberry P., Levitt L. Hematopoietic stem cells (second of three parts). N Engl J Med. 1979 Oct 11;301(15):819–823. doi: 10.1056/NEJM197910113011505. [DOI] [PubMed] [Google Scholar]
- Rosendaal M., Hodgson G. S., Bradley T. R. Organization of haemopoietic stem cells: the generation-age hypothesis. Cell Tissue Kinet. 1979 Jan;12(1):17–29. doi: 10.1111/j.1365-2184.1979.tb00110.x. [DOI] [PubMed] [Google Scholar]
- SIMINOVITCH L., MCCULLOCH E. A., TILL J. E. THE DISTRIBUTION OF COLONY-FORMING CELLS AMONG SPLEEN COLONIES. J Cell Physiol. 1963 Dec;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- Shadduck R. K., Rickard K. A., Howard D. E., Stohlman F., Jr The effect of preirradiation of recipient mice on the proliferation of transplanted hemopoietic stem cells. Blood. 1971 Mar;37(3):330–339. [PubMed] [Google Scholar]
- Sharkis S. J., Cahill R., Ahmed A., Jedrzejczak W. W., Sell K. W. Genetic requirements for bone marrow transplantation for stem-cell-defective W/Wv mice. Transplant Proc. 1979 Mar;11(1):511–516. [PubMed] [Google Scholar]
- Sharkis S. J., Spivak J. L., Ahmed A., Misiti J., Stuart R. K., Wiktor-Jedrzejczak W., Sell K. W., Sensenbrenner L. L. Regulation of hematopoiesis: helper and suppressor influences of the thymus. Blood. 1980 Mar;55(3):524–527. [PubMed] [Google Scholar]
- Sharkis S. J., Wiktor-Jedrzejczak W., Ahmed A., Santos G. W., McKee A., Sell K. W. Antitheta-sensitive regulatory cell (TSRC) and hematopoiesis: regulation of differentiation of transplanted stem cells in W/Wv anemic and normal mice. Blood. 1978 Oct;52(4):802–817. [PubMed] [Google Scholar]
- TILL J. E., MCCULLOCH E. A., SIMINOVITCH L. A STOCHASTIC MODEL OF STEM CELL PROLIFERATION, BASED ON THE GROWTH OF SPLEEN COLONY-FORMING CELLS. Proc Natl Acad Sci U S A. 1964 Jan;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till J. E., McCulloch E. A. The 'f-factor' of the spleen-colony assay for hemopoietic stem cells. Ser Haematol. 1972;5(2):15–21. [PubMed] [Google Scholar]
- Trentin J., Wolf N., Cheng V., Fahlberg W., Weiss D., Bonhag R. Antibody production by mice repopulated with limited numbers of clones of lymphoid cell precursors. J Immunol. 1967 Jun;98(6):1326–1337. [PubMed] [Google Scholar]
- Vos O., Luiten F., Erkens-Versluis M. E. Restoration of hemopoiesis by CFU-S from different backgrounds in the mouse. Blut. 1981 Jul;43(1):33–40. doi: 10.1007/BF00319929. [DOI] [PubMed] [Google Scholar]
- Walford R. L. Immunology and aging Philip Levine Award. Am J Clin Pathol. 1980 Sep;74(3):247–253. doi: 10.1093/ajcp/74.3.247. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Sharkie S., Ahmed A., Sell K. W., Santos G. W. Theta-sensitive cell and erythropoiesis: identification of a defect in W/Wv anemic mice. Science. 1977 Apr 15;196(4287):313–315. doi: 10.1126/science.322288. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Sharkis S. J., Ahmed A., Sensenbrenner L. L., Sell K. W. Engraftment of bone marrow transplants in W anemic mice measured by electronic determination of the red blood cell size profile. Exp Hematol. 1979 Sep;7(8):416–424. [PubMed] [Google Scholar]
- Worton R. G., McCulloch E. A., Till J. E. Physical separation of hemopoietic stem cells differing in their capacity for self-renewal. J Exp Med. 1969 Jul 1;130(1):91–103. doi: 10.1084/jem.130.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Till J. E., Siminovitch L., McCulloch E. A. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968 Mar 1;127(3):455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]



