Abstract
The problem of targeted delivery of antisense and siRNA oligonucleotides can be resolved into two distinct aspects. The first concerns devising ligand-oligonucleotide or ligand-carrier moieties that bind with high selectivity to receptors on the cell type of interest and that are efficiently internalized by endocytosis. The second concerns releasing oligonucleotides from pharmacologically inert endomembrane compartments so that they can access RNA in the cytosol or nucleus. In this review we will address both of these aspects. Thus we present information on three important receptor families, the integrins, the receptor tyrosine kinases, and the G protein-coupled receptors in terms of their suitability for targeted delivery of oligonucleotides. This includes discussion of receptor abundance, internalization and trafficking pathways, and the availability of suitable high affinity ligands. We also consider the process of oligonucleotide uptake and intracellular trafficking and discuss approaches to modulating these processes in a pharmacologically productive manner. Hopefully the basic information presented in this review will be of value to investigators involved in designing delivery approaches for oligonucleotides.
Keywords: integrins, receptor tyrosine kinases, G protein-coupled receptors, trafficking Receptors, Endocytosis, and Trafficking: the Biological Basis of Targeted Delivery of Antisense and siRNA Oligonucleotides
Introduction
Currently there is immense interest in the potential therapeutic applications of both single and double stranded oligonucleotides. Multiple clinical trials of siRNA, classical antisense oligonucleotides and splice switching oligonucleotides are underway (Bennett and Swayze, 2010; Burnett and Rossi, 2012) and research at the level of cellular and animal studies is increasingly active. However, oligonucleotide-based therapies face two formidable unsolved problems.
The first entails controlling the selectivity of action so as to avoid unintended side effects. Part of this issue involves off-target effects at the molecular level (Singh et al., 2011) while another aspect concerns effects mediated through the innate immune system (Sioud, 2009). However, yet another important aspect involves the delivery of the oligonucleotide to the tissue of interest. While in some cases the oligonucleotide may target an RNA that is uniquely expressed in a certain tissue or disease state, in many other instances the RNA target may be widely distributed and its perturbation may produce undesirable effects. Thus there have been many attempts to deliver oligonucleotides in a selective manner, for example, to tumor cells rather than normal cells or to sites of inflammation rather than normal tissue, but thus far these attempts have been only partially successful (Juliano et al., 2009; Nguyen and Szoka, 2012).
The second key unsolved problem involves the access of oligonucleotides to their sites of action in the cytosol or nucleus. These relatively large and highly polar molecules do not cross lipid bilayer membranes. Thus, under most circumstances, they are accumulated in cells by endocytosis and much of the accumulated material remains entrapped in endomembrane compartments and is thus pharmacologically inert (Juliano et al., 2012a). There have been many attempts to address this problem using polymeric or lipid-based delivery systems, but once again success has been limited.
The intent of this review is to inform investigators interested in oligonucleotide-based therapeutics about the possibilities and limitations involved in targeted delivery. First, we will discuss the internalization and intracellular trafficking of oligonucleotides. Second, we will discuss several cell surface receptor families that may have the potential to support selective delivery to particular cells or tissues, with emphasis on integrins, receptor tyrosine kinases (RTKs) and G Protein-Coupled Receptors (GPCRs), as well as several specific receptors in heart and liver. Finally we will analyze how receptor targeting and intracellular trafficking can be manipulated to enhance the pharmacological effects of oligonucleotides. This review reflects our opinion that oligonucleotide-based therapeutics will benefit greatly from a biologically sophisticated approach to receptor targeting and intracellular trafficking. It should be noted that the review does not provide a survey of recent publications on oligonucleotide delivery; rather it focuses on the underlying biology of receptors, endocytosis and trafficking.
Endocytosis and Trafficking Pathways
Antisense and siRNA oligonucleotides, either in unmodified form, as conjugates, or associated with most nanocarriers, usually enter cells via endocytosis (Juliano et al., 2012a). However, this broad statement fails to reveal the complexities inherent in the existence of diverse pathways of internalization and intracellular trafficking that are based upon intricate and highly selective protein machinery.
Endocytosis Mechanisms
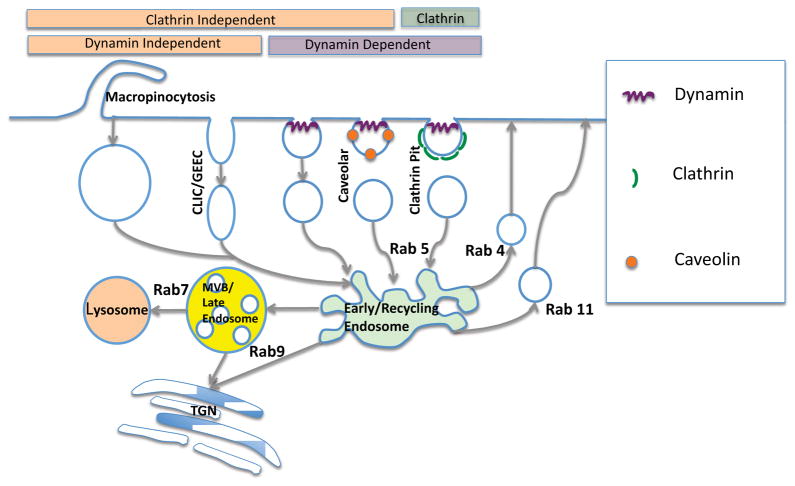
It is clear that there exist multiple pathways of endocytosis in addition to the well-known clathrin coated pit pathway (Doherty and McMahon, 2009; Howes et al., 2010). A simplified depiction of some of these pathways is shown in Figure 1. During classical clathrin-mediated endocytosis, cell surface receptors and their bound ligands associate with adapter proteins such as AP-2, and with a host of accessory factors, that drive the receptors into specialized membrane areas subtended by a meshwork of clathrin triskelions. The clathrin network, along with specialized BAR domain proteins such as SNX9 and amphiphysin that sense and promote membrane curvature, contribute to invagination of the clathrin bounded membrane area. This is quickly followed by pinching off of a clathrin-coated vesicle through the action of the dynamin GTPase (Doherty and McMahon, 2009). The coated endosome then uncoats under the influence of auxilin and hsc70 and the uncoated vesicle is then ready to begin its intracellular journey. Some of the receptors and ligands internalized via the clathrin pathway include the LDL receptor, transferrin receptor, and many agonist liganded GPCRs (Wolfe and Trejo, 2007)
Figure 1. Routes of Endocytosis.
This depicts several of the common pathways of endocytosis including the clathrin-coated pit/vesicle pathway, the caveolar pathway, macropinocytosis, and clathrin/caveolin-independent pathways. Key proteins including clathrin, caveolin, dynamin and various Rab GTPases are shown.
The caveolar pathway is also of great interest (Lajoie and Nabi, 2010). Many cells display small plasma membrane invaginations that are rich in cholesterol and sphingolipids and that contain caveolin1, a 21kD protein that inserts a hydrophobic loop into the membrane. Additionally, the cavins are coat proteins that help stabilize caveolae. Although there is still some controversy as to whether caveolae generate independent intracellular vesicles or whether they remain as tubular structures linked to the plasma membrane, substantial evidence suggests that caveolae generate vesicles that contribute to intracellular membrane traffic. Generally caveolae are smaller (<100 nanometers) than other types of endocytotic vesicles. The caveolar pathway is known to internalize Cholera toxin, SV40 virus, and GPI-linked membrane proteins; however, some of these moieties are also internalized by other pathways. Dynamin is likely involved in the disjunction of caveolae, but the evidence is not as clear as for clathrin-coated vesicles. Many additional proteins have been observed to be associated with caveolae; in particular these structures are rich in signal transduction proteins (Sorkin and von Zastrow, 2009).
Several clathrin- and caveolin-independent pathways have also been delineated to various extents. An interesting internalization mechanism is the CLIC/GEEC pathway that may be particularly important for fluid phase endocytosis (Doherty and McMahon, 2009). The acronym is for Clathrin and Dynamin Independent Carriers (CLIC)/GPI-AP Enriched Early Endosomal Compartments (GEEC). This pathway gives rise to tubular endosomes that are rich in GPI-linked proteins and that typically incorporate large volumes of extracellular fluid. Thus they can be visualized using fluid phase markers such as dextrans. As the name implies, dynamin is not necessary for the formation of these vesicles; rather membrane disjunction may involve GRAF1, a GTPase activating protein that has a BAR domain (Howes et al., 2010). Additional clathrin and caveolin independent pathways exist (Doherty and McMahon, 2009) including a dynamin-dependent pathway for internalization of the IL2-receptor and the FCεR1 immunoglobulin receptor, and a dynamin-independent pathway linked to the internalization of MHC class I histocompatibility proteins. The flotillins are membrane-inserted proteins involved in ordering lipid domains and supporting endocytosis. Both Cholera toxin and GPI-linked proteins have been associated with flotillin-rich membrane microdomains. There is evidence that dynamin is not needed for internalization of cargo via flotillin containing vesicles (Glebov et al., 2006). Macropinocytosis is a process quite different from other forms of endocytosis. It involves cell protrusions pinching off large volumes of extracellular fluid and is thus an important contributor to fluid phase endocytosis (Kerr and Teasdale, 2009). Generation of the relatively large macropinosomes involves the actinomyosin contractile machinery and its typical regulators including the Rac GTPase and PAK family kinases. The actin cytoskeleton plays a key role in most internalization events; however, not all forms of endocytosis require actin. For example, certain arenaviruses enter cells via a pathway that is independent of clathrin, caveolin, dynamin and actin (Kunz, 2009). Surprisingly, we recently observed that phosphorothioate antisense oligonucleotides also enter cells by a clathrin, caveolin, dynamin and actin independent pathway (Alam et al., 2010).
Intracellular Trafficking
Whether in unmodified form, as a chemical conjugate, or associated with a nanocarrier, an oligonucleotide accumulating in a cell by endocytosis enters a complex network of trafficking pathways that can lead to many intracellular destinations. Some of the key subcellular membrane bound compartments include early and recycling endosomes, late endosomes/multi-vesicular bodies, lysosomes, the Golgi apparatus and the endoplasmic reticulum. (see Figure 2). Trafficking is not random, but rather it is a carefully orchestrated process that allows the cell to deliver endogenous and exogenous materials to the most appropriate place. Several pathogens have learned to exploit these pathways; for example certain viruses as well as some bacterial toxins traffic via the so-called retrograde transport process (Pfeffer, 2011) and are thus delivered to the trans-Golgi compartment and thence to cytosol. In many ways this is similar to the goal of oligonucleotide delivery, that is to exit from membrane bound compartments and access the cytosol and nucleus.
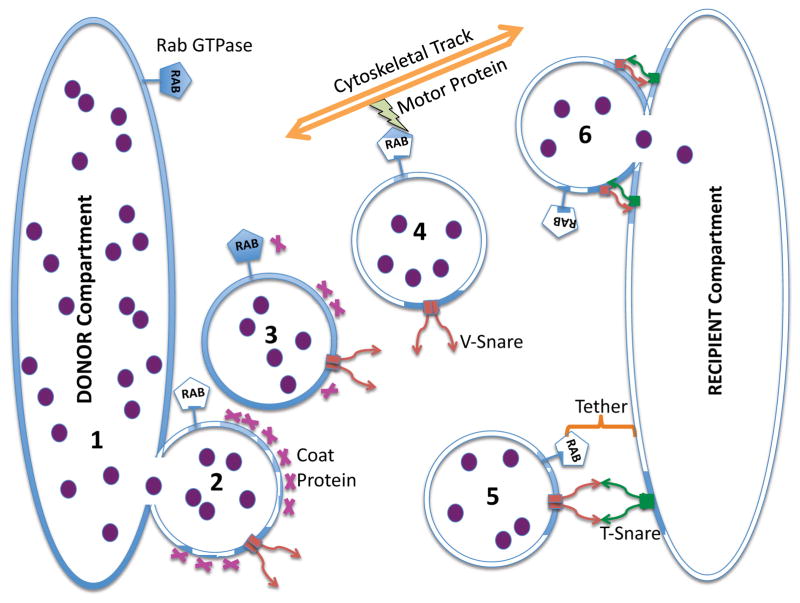
Figure 2. Intracellular Trafficking.
This illustration shows the movement of internalized material (purple circles) from one endomembrane compartment (DONOR)(1) to another (RECIPIENT) via shuttle vesicles. This involves pinching off of the shuttle vesicle with the help of a coat protein (2); uncoating of the vesicle (3); movement through the cytosol on cytoskeletal tracks (4); recognition of the RECIPIENT using Snares and Tethers (5); and lastly fusion of the shuttle vesicle with the RECIPIENT membrane using Snares (6).
During the past few years there has been great progress in understanding the molecular mechanisms of intracellular trafficking (Huotari and Helenius, 2011). In essence, all membrane trafficking involves the same basic steps: (i) a small coated vesicle is pinched off from a larger donor membrane bound compartment; (ii) the vesicle is uncoated permitting the display of tethering and fusion proteins; (iii) the vesicle migrates to its destination along cytoskeletal ‘tracks’ provided by actin- or tubulin- based filaments; (iv) the vesicle uses tethering proteins to recognize the recipient membrane compartment and then utilizes SNARE proteins to engage in fusion and deliver membrane and contents to the target compartment (Angers and Merz, 2011). A simplified diagram of this process is shown in Figure 2. There are numerous variations on this theme, but the overall picture is quite clear.
Important aspects of intracellular trafficking are regulated and controlled by the Rab family of proteins. The Rabs are a large family of small GTPases (over 60 members) (Stenmark, 2009) and are the best known proteins that temporally and spatially regulate membrane trafficking and signaling (Schwartz et al., 2007). They are molecular switches, cycling between active GTP-bound and inactive GDP-bound forms. Similar to other small GTPases, their activity is regulated by Rab-specific GEFs (Guanine nucleotide Exchange Factors), GAPs (GTPase Activating Proteins) and GDIs (GDP Dissociation Inhibitors) (Grosshans et al., 2006). Activated Rab GTPases act as molecular scaffolds to regulate membrane trafficking including vesicle budding, cytoskeletal transport, and targeted docking and fusion. Hence, they represent attractive targets for genetic manipulation aimed at unraveling mechanism of membrane trafficking. For example, mutants of the Rab GTPases (Rab7 N125I, Rab5 Q79L, Rab5 S34N) cause dramatic changes in both endosome membrane fusion and morphology (Feng et al., 1995; Stenmark et al., 1994). Thus dominant negative or constitutively activated versions of Rab proteins can potentially be used as molecular tools to probe the intracellular trafficking of oligonucleotides. Additionally, since different Rabs are preferentially associated with different endomembrane compartments, they can serve as marker proteins. This aspect has already been exploited in probing the trafficking of oligonucleotides (Ming et al., 2010).
In our estimation, a fuller understanding of the intracellular trafficking patterns of various types of oligonucleotides (and of the delivery agents used for these molecules) will greatly enhance the possibility of rational and productive development of oligonucleotides as therapeutic agents. Thus, we believe, an investment in basic research on trafficking will ultimately pay off in terms of drug development.
Receptor Families for Oligonucleotide Delivery
Here we discuss aspects of several families of cell surface receptors that seem to have substantial potential in terms of supporting selective delivery of antisense or siRNA. We will describe their basic structural and functional characteristics, their expression levels and distribution, their modes of internalization, trafficking and recycling, and the availability of specific, high affinity ligands. Ideally, one would like to be able to utilize a receptor that is expressed in only one tissue or disease state, that is abundant, that internalizes rapidly and extensively, and for which selective high affinity ligands are readily available. Obviously, all existing receptors fall short of this ideal. However, there are many instances where individual receptors have at least some of these ideal characteristics.
The Integrins
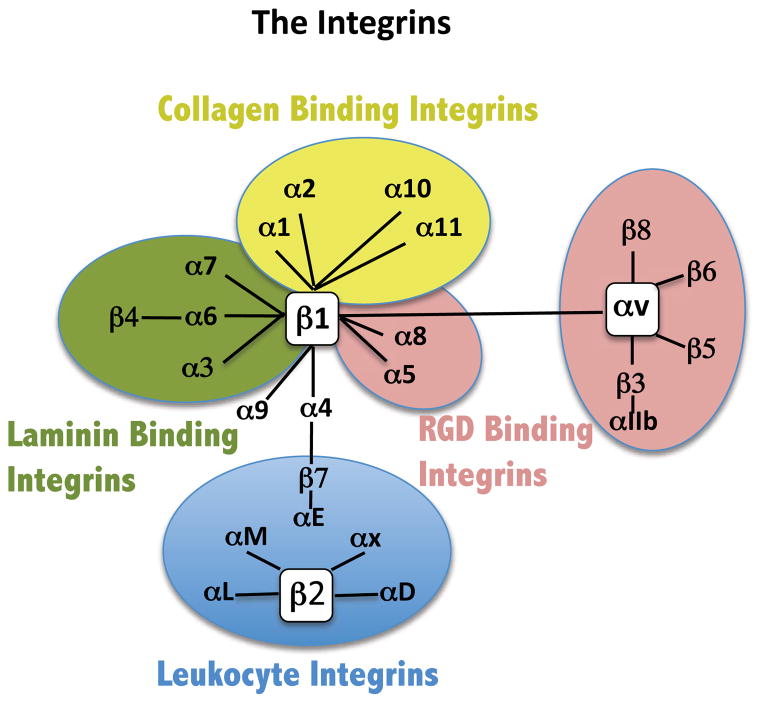
Members of the integrin family of heterodimeric cell surface receptors play multiple critical roles in development and in the adult organism (Hynes, 2002a; Juliano, 2002; Schwartz, 2010). Because of their ubiquity and relatively abundant expression integrins have been a favorite mediator for selective delivery of both oligonucleotides and small molecules (Juliano et al., 2011). In mammals the integrin family is comprised of 18 alpha subunits and 8 beta subunits that form 24 individual heterodimers (Margadant et al., 2011). Each subunit has a large extracellular domain, a single transmembrane segment, and a relatively short cytoplasmic tail. The normal ligands for integrins are primarily various large extracellular matrix (ECM) proteins such as fibronectin, laminins and collagens (Hynes, 2002a) (see Figure 3), but the discovery that the short peptide sequence arg-gly-asp (RGD) could bind to certain integrins (Pierschbacher and Ruoslahti, 1984) has led to the development of many small molecule ligands, as further discussed below. The cytoplasmic tails of integrins associate with proteins such as talin, filamin and kindlin that can then link to actin filaments. Thus integrins provide a mechanochemical linkage between the ECM and the cytoskeleton; during cell adhesion integrins and their multiple associated proteins cluster to form a variety of organized but dynamic structures including focal contacts, fibrillar adhesions, podosomes and others (Margadant et al., 2011; Schwartz, 2010).
Figure 3. The Integrins.
This is a depiction of the subunit organization of the integrin family of cell adhesion receptors. The binding propensities of the various integrins are color-coded. Diagram adapted from (Margadant, C., H.N. Monsuur, J.C. Norman, and A. Sonnenberg. 2011. Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol. 23:607-614).
Integrin Signaling
In addition to their structural role, integrins are highly involved in signal transduction processes. Integrin signaling has often been divided into ‘inside out signaling’ and ‘outside in signaling’. The former refers to the fact that integrins can exist in activated, inactive or intermediate states, in response to external cues. The solution of the crystal structure of the external domain of the αvβ3 integrin (Xiong et al., 2001), followed by further progress on structure and function, has been very helpful in understanding integrin activation. Thus, in the inactive state the integrin external domain is thought to be in a bent configuration while the cytoplasmic tails are closely juxtaposed by charge-charge interaction. In the active form the external domain is extended and the tails separate. The association of the cytosolic protein talin with the beta subunit tail is the key final step in integrin activation (Kim et al., 2011; Margadant et al., 2011). However, a multiplicity of other proteins, particularly the small GTPase Rap1, are involved in the regulation of ‘inside out signaling’. Perhaps the most dramatic example of this process concerns the unique platelet integrin αIIbβ3 whose affinity for its physiological ligand fibrinogen is vastly increased in response to signaling molecules released at wound sites, leading to platelet aggregation and thrombus formation.
So-called ‘outside in signaling’ (from extracellular space to cytosol) involving integrins is a very complex story that we can only briefly touch upon. Integrins can both signal directly and also strongly influence signaling pathways primarily mediated through other receptors (Streuli and Akhtar, 2009). Thus one aspect involves integrins forming complexes with receptor tyrosine kinase (RTKs) that then affect the ability of the RTK to become activated by its normal ligand (Cabodi et al., 2004). Integrin engagement with ECM can also modulate the classic Erk/MAP Kinase pathway downstream from RTK activation (Juliano et al., 2004). This involves regulation at the level of the Raf-1 kinase (Edin and Juliano, 2005; Lin et al., 1997) and also effects on the trafficking of activated Erk to the nucleus (Aplin et al., 2001). (see Figure 4).
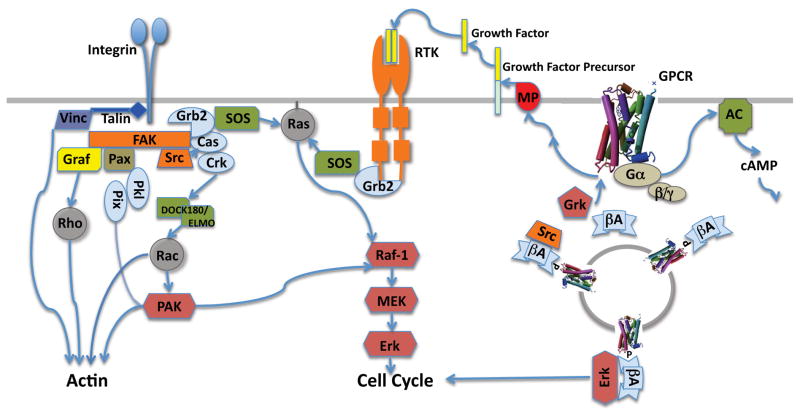
Figure 4. Signaling by Integrins, RTKs and GPCRs.
This illustrates highly simplified versions of the downstream signaling pathways regulated by these three families of receptors as well as some of the interconnections between pathways. Cytoskeletal proteins-Talin, Vinculin (Vinc), Paxilin (Pax); Adaptor proteins-Grb2, Cas, Crk, Pkl, Pix, beta-arrestin (βA); Small GTPases-Ras, Rac, Rho; Heterotrimeric GTPase (Gα/βγ) Tyrosine kinases-Focal Adhesion Kinase (FAK), c-Src, RTK; Serine-threonine kinases-PAK, Raf-1, MEK (dual specificity), Erk, Grk; Guanine nucleotide exchange factors-SOS,DOCK180/ELMO; Metalloprotease (MP).
Integrins can also signal directly via activation of tyrosine kinases. The discovery that cell adhesion or integrin clustering could activate tyrosine phosphorylation via focal adhesion kinase (FAK) was a key event in understanding the function of integrins (Kornberg et al., 1991; Schaller et al., 1992). FAK autophosphorylation subsequent to integrin engagement leads to the recruitment of several SH2 and SH3 domain proteins, particularly c-Src (Parsons, 2003). Recruitment of Src leads to additional tyrosine phosphorylations with several consequences including recruitment of the SH2-adaptor Grb2 in complex with SOS, a GEF for Ras, leading to activation of the Ras-MAP Kinase cascade. Further, SH3 domains mediate binding of GRAF and ASAP1, which are GAPs for the Rho and Arf 1/6 small GTPases, leading to effects on the actin cytoskeleton. Additionally the adaptor protein Cas and its partner Crk recruit the DOCK180/ELMO complex which acts as a GEF for the Rac GTPase and thus to further effects on the cytoskeleton. Finally, the adaptor protein paxilin binds to a sequence in the c-terminal of FAK and serves both as a structural protein linking to actin, and as an adaptor for the PKL-PIX-PAK complex thus bringing PAK kinase to the focal adhesion where it can regulate LIM-kinase and myosin light chain kinase and also influence the Erk pathway via Raf-1. Increasingly, the integrin-FAK connection is seen as a key mediator of the activity of Rho-family GTPases and their cytokeletal functions (Huveneers and Danen, 2009). Thus FAK serves as the hub of a multi-protein signal transduction complex that influences cell cycle, cytoskeletal organization, and cell motility. (See Figure 4 for a visualization of Integrin, RTK and GPCR signaling).
Yet another modality of integrin signaling involves the activation of the growth factor TGF-β by certain integrins, particularly αvβ6, αvβ8, αvβ5 (Munger and Sheppard, 2011). TGF-β is released from cells as a latent multi-protein complex that includes an inhibitory pro-peptide termed LAP; integrin binding to LAP then releases functional TGF-β, which Mcan Mthen Minitiate MitsM signalingM cascade. Interestingly one of the consequences of TGF-β activity is up-regulation of certain integrins, thus creating a very complex feedback loop.
Integrin Trafficking and Recycling
It has been known for many years that integrins are internalized and usually recycle to the cell surface (Bretscher, 1992; Sczekan and Juliano, 1990). Recently, these processes and their implications have been explored in more detail and the critical role of Rab GTPases identified (Caswell et al., 2009; Margadant et al., 2011). At one time it was thought that integrin recycling allowed transport of integrins from the rear of the cell to newly forming focal adhesions at the leading edge; now, however, it is thought that the recycling is more localized, at least in non-dividing cells. It has also become clear that there are reciprocal effects on recycling between integrins and other receptors, with, for example, integrin engagement affecting RTK trafficking and vice versa (Caswell et al., 2008; Wickstrom and Fassler, 2011). Internalization of certain integrins via clathrin-coated vesicles is clearly an important aspect of focal adhesion disassembly and integrin trafficking. The cytoplasmic tails of integrin beta subunits contain NXXY motifs that associate with clathrin adaptors such as AP2, while adaptors such as Dab and Numb, which contain phosphotyrosine binding domains, can interact with NPXY motifs in certain beta tails (Margadant et al., 2011). However, certain integrins are also known to be internalized by clathrin-independent mechanisms. For example, αvβ3 and α5β1 have been reported to internalize via lipid raft or caveolar mechanisms, as has α2β1 (Karjalainen et al., 2008), while macropinocytosis has also been observed in internalization of integrins during cell migration (Gu et al., 2011). (see Figure 5).
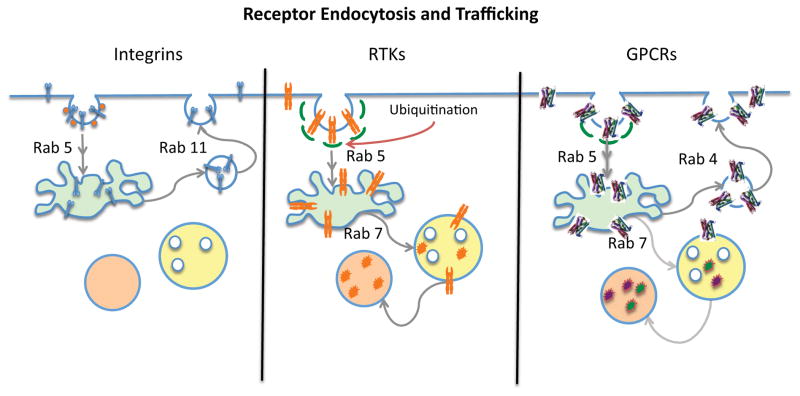
Figure 5.
Endocytosis and Trafficking of Receptors. The left panel illustrates the α5β1 integrin being internalized via a caveolar pathway to Rab 5 positive early endosomes and then returning to the cell surface via Rab 11 positive recycling vesicles. The middle panel illustrates EGFR being internalized in clathrin coated vesicles, entering Rab 5 positive early endsomes and then, because of previous ubiquitination, being destined for Rab 7 positive late endosomes and then lysosomes where the receptor is degraded. The right panel illustrates a GPCR being internalized in clathrin coated vesicles and then returning from early endosomes to the cell surface via Rab 4 positive recycling vesicles. A portion of the GPCR may also traffic to lysosomes. The fate of the ligand is not illustrated in these diagrams.
While in some cases integrins can be ubiquitinated and thus targeted for lysosomal degradation (Lobert et al., 2010), for the most part un-liganded internalized integrins recycle back to the cell surface. Thus after entry into Rab5 positive early endosomes integrins can recycle to the plasma membrane via a Rab4 associated ‘short loop’ or via a ‘long loop’ involving the Rab11 positive perinuclear recycling compartment. For example, several studies have found that αvβ3 primarily recycles via the Rab 4 pathway while α5β1 primarily uses the Rab11 pathway (Caswell et al., 2009). Recent evidence has indicated that certain Rab proteins can associate with integrin cytoplasmic tails either directly (Rab 21 with α2 and α5)(Mai et al., 2011) or via multiprotein complexes. The process of integrin trafficking clearly plays a key role in the regulation of cell motility; thus the appropriate type and amount of integrin needs to be delivered to the advancing front of the cell and, as discussed above, integrins are key regulators of Rho-family GTPases and thus essential to control of the cytoskeleton.
Differential Expression of Integrins
Since they are structural as well as signaling proteins integrins are usually expressed at relatively high levels. For example, the αIIbβ3 integrin is present at several tens of thousand of copies per platelet (Savage et al., 1990), while αvβ3 is found in several tumor cell lines at levels of ~1–3 x105 copies per cell (Mulgrew et al., 2006), or, in other terms, the αvβ3 integrin is present in a tumor cell line at 15000 fmol/mg membrane protein (Szabo et al., 2012). These are relatively high numbers for receptor expression. More important than absolute numbers is the differential expression of various integrins in individual tissues or under differing physiological conditions. While most tissues/cell types express several different integrins, there are some notable examples of specificity. Thus αIIbβ3 is exclusively expressed in platelets (or their precursors) while the β2 family of integrins is expressed only in leukocytes. There are a number of examples of altered expression of integrins in cancer, although the literature on this topic is complex and often confusing (Desgrosellier and Cheresh, 2010; Guo and Giancotti, 2004; Moschos et al., 2007). One clear finding is the increased expression of the αvβ3 integrin during tumor angiogenesis (Avraamides et al., 2008); this has led to enormous interest in using selective ligands for αvβ3 to image or treat the tumor vasculature (Niu and Chen, 2011). Increased expression of particular integrins has been associated with progression or poor prognosis in certain cancers; for example, αvβ6 in several carcinomas, α6β4 in breast cancer, and αvβ3 in melanoma and glioblastoma (Desgrosellier and Cheresh, 2010). On the other hand α2β1 may function as a tumor suppressor, while α5β1 can paradoxically inhibit transformation although it is sometimes over-expressed in tumors. Unfortunately, there is not a systemic body of information concerning integrin expression at the protein level in tissues or in tumors. However, if one makes some assumptions about the correlation between mRNA and protein levels, there is abundant information available in the various gene expression databases such as those from EMBL-EBI or NCBI. For example, this entry provides data on expression profiling of the human beta 2 integrin in a variety of tissues and disease states. (http://www.ebi.ac.uk/gxa/gene/ENSG00000160255#list.pagenum=1). Similar data are available for virtually all integrin subunits and may at least provide initial guidance as to differential expression in various cell types.
Integrin Ligands
One of the virtues of integrins as potential mediators of selective oligonucleotide delivery is the existence of a variety of highly selective integrin ligands, as well as detailed information about how the ligands affect integrin structure and function (Shimaoka and Springer, 2003). As mentioned above the endogenous ligands for integrins are all large proteins, either from the ECM, or in the case of β2 integrins intracellular adhesion molecules of the ICAM family (Humphries et al., 2006). However, the last decade has seen the development of a wealth of small molecule ligands particularly for αIIbβ3, αvβ3 and α4β1. Integrin ligands bind in the cleft between the α and β subunits and virtually all small molecules are ligand mimetic competitive inhibitors. Interestingly however, some antagonists can partially induce the activated (extended) conformation of the integrin (Shimaoka and Springer, 2003); thus responses can be complex. A vast amount of information on small molecule ligands of integrins is available in the Binding DB; for example, this entry lists ligands for the integrin α4β1 (http://www.bindingdb.org/bind/ByPubMed_server.jsp?target=Fibronectin%20receptor%20beta/Integrin%20alpha-4) while entries for many other integrins are also found at this web site.
The most highly developed group of integrin ligands are the small molecule inhibitors of the platelet αIIbβ3. Epifibatide and Tirofiban have been in the clinic for almost two decades and have been beneficial in several acute coronary syndromes; however, their use has declined somewhat with the advent of orally available anti-platelet drugs that affect the P2Y12 receptor (Kristensen et al., 2012). A variety of analogs of Tirofiban have been developed but are not in clinical use (Millard et al., 2011).
The α4β1 integrin plays a key role in leukocyte endothelial cell interaction and has thus been of interest in terms of modulating inflammatory processes (Jackson, 2002). Several small molecule antagonists of α4β1 are in clinical trials but there have been issues concerning their effectiveness and potential toxicities (Millard et al., 2011).
Perhaps the most complex and interesting situation concerning specific integrin ligands involves inhibitors of αvβ3. The apparent key role of this protein in tumor angiogenesis as well as its over-expression in several refractory cancers such as glioblastoma provoked rapid development of both monoclonal and small molecule inhibitors. The most advanced of the latter is Cilengitide a cyclic pentapeptide containing the RGD sequence that is a highly selective inhibitor of the avb3 and avb5 integrins (Desgrosellier and Cheresh, 2010; Millard et al., 2011). The rationale for the development of this drug is that multiple observations in cell culture and in animal tumor models have shown that blocking αvβ3 in various ways reduces angiogenesis. However, there has always been controversy in this area since genetic ablation of av in mice does not completely block angiogenesis (Hynes, 2002b). Cilengitide has been in advanced clinical trials but results have been somewhat disappointing (Millard et al., 2011). Recently an interesting possible explanation has emerged from studies of integrin effects on trafficking. The vascular endothelial growth factor (VEGF) receptor VEGFR2 (FLK1) is central to the angiogenic process (Zhu and Witte, 1999). Interestingly when αvβ3 is functional, VEGF induced recycling of FLK1 is limited and the receptor is degraded in lysosomes. However, when αvβ3 is inhibited, a Rab4 mediated rapid recycling pathway carries FLK1 back to the plasma membrane where it can be re-stimulated by VEGF leading to increased angiogenesis (Caswell et al., 2009). Thus, cilengitide, as well as other αvβ3 inhibitors, may be caught in a push-pull situation where they both block and promote tumor angiogenesis. On a brighter note, cyclic peptide antagonists of avb3 have shown great promise for the targeting of imaging agents to tumors (Chen, 2006). Similar molecules have also been explored for oligonucleotide delivery in the form of peptide-oligonucleotide conjugates (Alam et al., 2008; Alam et al., 2011) or as part of various lipid or polymer based nanoparticles (Juliano et al., 2011), but potential toxicities have not been fully explored.
Integrins offer substantial promise as mediators of selective oligonucleotide delivery. Members of this receptor family are usually expressed at high levels, they traffic actively between the cell surface and endosomal compartments and their trafficking pathways are relatively well understood, in some cases they are differentially expressed in particular tissues or pathological conditions, and a wealth of high affinity peptide and peptidomimetic ligands exist. Despite these advantages there are some concerns about utilizing integrins for targeting. The primary issue is that integrins play key roles both in cell structure and in signal transduction. Thus the use of integrin ligands to deliver oligonucleotides runs the risk of compromising essential cellular functions; this may be particularly relevant when multiple copies of the integrin ligand are used, as in the case with most nanocarrier systems. The example of Cilengitide, discussed above, provides a warning that use of ligands that perturb integrins can have unexpected negative consequences.
Receptor Tyrosine Kinases
Agents that inhibit receptor tyrosine kinases (RTKs), both monoclonals and small molecules, have become important tools in cancer therapy (Ciardiello and Tortora, 2008; Gschwind et al., 2004). This is because RTKs, also known as growth factor receptors, play an absolutely key role in signal transduction processes that regulate cell proliferation. Additionally, many malignancies are driven by mutations in RTKs or by their altered expression. In humans the RTK family is comprised of 58 members grouped into multiple subfamilies. Each RTK has a large extracellular domain, a single transmembrane segment and a cytosolic region that includes a tyrosine kinase domain. The extracellular domains of RTKs are quite varied, perhaps reflecting the diversity of their ligands; however, there is substantial conservation of the basic organization of the kinase domains. All RTKs are activated by essentially the same basic mechanism (Hynes and Lane, 2005; Lemmon and Schlessinger, 2010); binding of the specific growth factor causes dimerization (or oligomerization) leading to activation of tyrosine kinase activity and cell signaling. Many growth factors are themselves dimeric and in some cases, such as Trk A, the nerve growth factor receptor, the dimeric ligand simply bridges between two receptors. However, in other cases binding of the ligand induces conformational change in the receptor and dimerization takes place purely via receptor domain interactions, for example, in the epidermal growth factor receptor family (EGFRs).
RTK Signaling
Ligand binding and dimerization of RTKs leads to autophosphorylation of their cytosolic regions, as well as tyrosine phosphorylation of bound partner proteins (Koch and Claesson-Welsh, 2012; Lemmon and Schlessinger, 2010). There are complex relationships between autophosphorylation patterns and progressive activation of kinase catalytic activity, with the details varying between different RTKs. The net result is the creation of multiple phosphorylated tyrosines that can then serve as docking sites for proteins containing SH2 or PTB domains. In many cases the phosphotyrosine binding proteins are, or are bound to, enzymes involved in further steps of signal transduction. Thus the initial activation of the RTK leads to the creation of a multi-protein signal transduction hub (see Figure 4). The classic example of this is the relationship between the EGFR and signaling through the mitogenic Erk/MAP kinase pathway (Chang and Karin, 2001). Thus upon autophosphorylation of the EGFR cytoplasmic domain the SH2 domain adapter protein Grb2 binds to phosphotyrosine residues, particularly Y-1068 in the tail region (Batzer et al., 1994). Grb2 is constitutively associated with SOS, a GEF for the Ras small GTPase. This activates Ras to its GTP loaded form whereupon it can bind to and activate Raf-1, the upstream kinase in the MAP kinase cascade, and recruit it to the plasma membrane. This is followed by Raf activation of MEK, a dual specificity kinase, MEK activation of the Erk MAP Kinase, and finally migration of Erk to the nucleus where it phosphorylates and activates ETS family transcription factors that contribute to cell cycle control and proliferation. While this classic example of RTK signaling nicely illustrates ideas about multi-protein complexes and the triggering of pathways, we now know that the reality is far more complex. Rather than triggering simple linear pathways, RTK activation modulates complex signaling networks (Lazzara and Lauffenburger, 2009; Rangamani and Iyengar, 2008) that include multiple branch points and negative and positive feedback loops.
RTK Trafficking and Recycling
The intracellular trafficking of RTKs is a key aspect of their function (Parachoniak and Park, 2012). As in the case of signaling transduction, the trafficking of RTKs often involves multiple interacting processes rather than simple linear pathways (Zwang and Yarden, 2009). The EGFR is one of the better-studied RTKs in terms of trafficking and can serve as an example for other receptors (see Figure 5). Thus the cytosolic tail of liganded EGFR interacts with adapter proteins such as AP-2 to cluster the receptor in clathrin-coated pits. After scission of the coated vesicles from the membrane by dynamin and release of the clathrin coat, the liganded EGFR enters early endosomes and is then subsequently trafficked to late endosomes/multi-vesicular bodies and then to lysosomes, where both ligand and receptor are degraded thus terminating signaling (Lemmon and Schlessinger, 2010). However, in some circumstances EGFR (as well as other RTKs) can enter cells via a non-clathrin pathway and/or recycle back to the plasma membrane. Ubiquitination of the EGFR by the c-Cbl E3 ubiquitin ligase is a key aspect of targeting the receptor for degradation via recognition by the ESCRT complexes in multivesicular bodies followed by sorting to lysosomes (Sorkin and Goh, 2008). It should be noted, however, that EGFR remains liganded and activated throughout much of the trafficking process and is thus capable of signaling. Interestingly, internalization of EGFR is essential for signaling to one of its downstream pathways (that involving the AKT kinase) but not for signaling in the Erk/MAP kinase pathway (Goh et al., 2010). Other instances of interplay between the signaling and trafficking pathways of RTKs abound in the literature (Miaczynska and Bar-Sagi, 2010; Sorkin and von Zastrow, 2009). For example, c-Met, the receptor for hepatic growth factor, must traffic to a perinuclear compartment in order to activate STAT3, but this localization is not needed for activation of the MAP kinase pathway (Kermorgant and Parker, 2008). As compared to the integrin family of receptors, RTKs tend to recycle to a lesser degree and be more likely to traffic all the way to the lysosome; however there are many variations on this theme for different members of the RTK family.
Differential Expression of RTKs
The most prominent example of RTK expression playing a role in disease is of course the over-expression of HER2 in certain forms of breast cancer (Shepard et al., 2008). However, differential expression of several RTKs is seen in different tissues or in various disease states. For example, members of the Trk family of RTKs are primarily expressed in neuronal or neuroepithelial tissues and may have a role in neuroblastoma (Brodeur et al., 2009). VEGFR2 is primarily expressed in vascular endothelial cells, although other members of this RTK subfamily may be expressed elsewhere (Koch and Claesson-Welsh, 2012). Over-expression of the Mer and Axl RTKs in certain cancers has recently become of therapeutic interest (Verma et al., 2011). The number of RTKs per cell can vary widely. For example, in one study the EGFR was present in various cell lines in amounts ranging from 103 to 106 copies per cell (Imai et al., 1982). In another study the VEGFR2 was present at about 0.5–1.0 × 105 copies per vascular endothelial cell (Napione et al., 2012). Thus RTKs are often expressed at levels of thousands to hundreds of thousand of copies per cell, a potentially useful range in terms of using these receptors for targeted delivery. Unfortunately there is no systematic information concerning RTK expression at the protein level; thus the NCBI and EMBL-EBI gene expression resources are valuable for obtaining initial insights into possible levels of expression.
RTK Ligands
The endogenous ligands for RTKs are all relatively large polypeptides and thus are not ideal for use in delivery approaches. However, there are a variety of high affinity monoclonal reagents for the external domains of RTKs (Hynes and Lane, 2005; Krause and Van Etten, 2005). These can be used themselves as targeting reagents, or converted to Fab fragments, or reconfigured as scFv reagents (Tohidkia et al., 2012) from phage display; there are a number of examples of delivery of drugs or nucleic acids with RTK targeted nanoparticles using antibody based approaches (Hwang et al., 2012; Kirpotin et al., 2006; Liu et al., 2011). However, in contrast to the situation with integrins or with GPCRs, there are few small peptide or small molecule ligands for the external binding sites of RTKs. Thus, for the most part, ligands suitable for direct conjugation with oligonucleotides are not available for this family of receptors.
Like integrins, RTKs are often expressed at reasonably high levels and thus may offer useful vehicles for targeted delivery of oligonucleotides. Liganded or antibody clustered RTKs are primarily internalized via the ‘classic’ clathrin pit pathway and are trafficked through low pH endosomal compartments and thence to lysosomes. Thus RTKs provide an effective means of delivery to these compartments; this may offer important opportunities for pharmacological enhancement, for example by using pH sensitive delivery agents. However, unlike some integrins that rapidly recycle, the cell surface display of RTKs needs to be restored by synthesis of new molecules. Thus delivery strategies that involve multiple dosing may not be appropriate if RTKs are used for targeted delivery. Perhaps even more than the case of integrins, RTKs are involved in critical signaling pathways. Thus delivery approaches that either over-stimulate or inhibit those pathways could be very detrimental to the cell.
GPCRs
The G protein-coupled receptors (GPCRs) comprise the largest receptor family in the human genome (Armbruster and Roth, 2005) with about 800 members of which about half are odorant or taste receptors while the rest control a multiplicity of physiological functions in the CNS, the cardiovascular system, the GI tract and elsewhere. While GPCRs vary greatly in primary sequence they share a common topology based on seven transmembrane helices and are thus sometimes called heptahelical receptors. Broadly speaking, there are three types of ligands for GPCRs. First, small molecule ligands that bind near the extracellular face in clefts between the helices; an example would be the beta-adrenergic receptors that are targets for antihypertensives. Second, polypeptide ligands that bind to the GPCR extracellular loops; pituitary hormones such as ACTH would be examples of this type. Finally, in the protease activated receptor (PAR) subfamily (Soh et al., 2010) an external protease such as thrombin cleaves the extracellular N-terminus of the GPCR to release a latent peptide agonist.
GPCR Signaling and Trafficking
In their inactive state GPCRs firmly associate with heterotrimeric G proteins while upon ligand binding a conformational change occurs in the receptor that results in the exchange of GTP for GDP on the G protein α subunit and release of both the Gα subunit and the βγ complex (Cabrera-Vera et al., 2003). The G protein subunits can then interact with a variety of downstream effectors (Gilman, 1995). For various members of the heterotrimeric G protein family the effectors would include adenylate cyclase, phospholipase Cβ and various ion channels. The GTP loading and activity of the Gα subunit is negatively regulated by members of the RGS protein family (Kimple et al., 2011). (see Figure 4).
The signaling activity of GPCRs is highly interconnected with their endocytosis and intracellular trafficking (Drake et al., 2006; Hanyaloglu and von Zastrow, 2008). (see Figure 5). Internalization of GPCRs can desensitize the receptor thus reducing signaling via ‘classical’ second messengers such as cyclic AMP, but can also result in the creation of new signaling processes with different downstream effectors. Internalization of agonist activated GPCRs begins with the phosphorylation of their cytoplasmic tails by a family of GPCR Kinases (GRKs). This results in the recruitment of an adaptor protein termed β-arrestin that in turn can interact with the AP-2 adaptor thus recruiting clathrin and segregating the GPCR into coated pits. Thus for many GPCRs the primary route of internalization of agonist-activated receptors is via the clathrin-dependent endocytotic pathway. However, it should be noted that GPCRs have also been observed in association with lipid rafts/caveolae. Importantly, GRK action and β-arrestin binding inactivate the GPCR before internalization is fully manifested; thus desensitization involves more than simple removal of the receptor from the cell surface. Subsequent to trafficking to Rab5 positive early endosomes, the internalized GPCR can undergo two distinct fates. One possibility is return to the plasma membrane via Rab4- or Rab11-positive recycling endosomes. However, GPCRs can also be sorted to multivesicular bodies and thence to lysosomes where they are degraded; this process depends on receptor ubiquitination by E3 ligases. The degradation of GPCRs is a slow process compared to the initial desensitization, but it has the effect of a gradual reduction of available receptors in the cell.
The formation of the GPCR/β-arrestin complex on the cytosolic face of endosomes has important signaling consequences. In essence, the GPCR and β-arrestin form a G protein-independent signaling complex, with β-arrestin serving a key role as a scaffold protein. Thus β-arrestin can associate with several key mitogenic signaling molecules such as the c-Src tyrosine kinase and members of the MAP kinase cascade, including Erk itself, thereby projecting GPCR signaling into these pathways (Shukla et al., 2011). In effect, the endosome-localized GPCR/β-arrestin complex forms a ‘signalosome’ that is biochemically distinct and topographically separated from GPCR/G-protein complexes at the cell surface. However, there is data suggesting that some of the ‘classic’ signaling effectors such as adenylate cyclase may also function from an endosomal location, but with different kinetics (Calebiro et al., 2010). Thus the story of role of β-arrestin and GPCR internalization in signaling has grown increasingly complex and is only touched on here.
An additional important aspect of GPCR signaling concerns ‘cross-talk’ or ‘transactivation’ between GPCRs and RTKs. Agonist stimulation of certain GPCRs can lead to parallel activation of RTK signaling pathways; this can involve several distinct mechanisms (Natarajan and Berk, 2006). First, GPCR signaling can cause the activation of proteases that cleave the precursor forms of growth factors leading to release of active factor and RTK stimulation. Second, GPCR activation can lead to generation of reactive oxygen species that can affect RTK signaling. Third, GPCRs and their adaptor proteins can associate with RTKs and their adaptors to create specialized signaling domains in the membrane leading to enhanced activity (Pyne and Pyne, 2011). Finally, as discussed above the GPCR/arrestin complex can directly signal to elements downstream or RTK activation such as the MAP kinase cascade. Receptor transactivation is a rapidly expanding area of research and additional mechanisms may come to light.
Differential Expression of GPCRs
Many GPCRs have distinct distributions in tissues or have altered expression levels in disease states. Perhaps the most elegant example is rhodopsin, the photon sensitive GPCR that is uniquely expressed in the retina. A number of GPCRs including several orphan receptors with unknown ligands have been reported to be overexpressed in various cancers (Li et al., 2005). For example, overexpression of the gastrin releasing peptide receptor GRPR has been implicated in a number of cancers (Cornelio et al., 2007) and radiolabeled GRPR ligands have been used for tumor imaging (Garrison et al., 2007). We have also used a peptide ligand for GRPR to deliver oligonucleotides to GRPR-positive tumor cells (Ming et al., 2010). In another disease context, variations in the expression of β and α adrenergic receptors and of angiotensin receptors have been observed in various stages of heart disease (Salazar et al., 2007). Because of their longstanding pharmacological importance and the existence of convenient radioligand binding assays, there is more information readily available on the abundance of GPCRs at the protein level than for other receptor families. The expression of GPCRs varies widely between receptor types and between tissues and cell types, but in general is lower than expression levels of integrins or RTKs. For example, rat cardiac myocytes were found to have about 2×105 beta adrenergic receptors per cell (Post et al., 1995) a relatively large number, while erythrocytes had about 103 copies per cell (Levitzki et al., 1974). P2Y1 receptors were expressed in rat brain at a level of about 50 fmol per mg protein (Houston et al., 2006) and beta receptor in human iris at about 100 fmol per mg (Wax and Molinoff, 1987); these numbers might be compared to 15000 fmol/mg for the αvβ3 integrin in tumor cells (Szabo et al., 2012). As a broad generalization, GPCRs tend to be present at 103 to 104 copies per cell as compared to roughly 105 per cell for integrins.
Ligands for GPCRs
Molecules that act on GPCRs account for approximately 40% of all clinically utilized drugs; further, GPCRs may comprise about 28% of the ‘druggable genome’ and are subject of continuing active drug discovery research (Filmore, 2004; Hopkins and Groom, 2002). Thus there exists a huge stockpile of GPCR ligands, including both those used clinically and molecules that were abandoned during the development process. While the majority are small organic molecules there are also a large number of peptide ligands for various GPCRs. There is a vast amount of information on GPCRs and their ligands in the International Union of Basic and Clinical Pharmacology (IUPHAR) data base (http://www.iuphar-db.org/index.jsp).
As with integrins and RTKs, delivery strategies utilizing GPCRs must be concerned about compromising critical downstream signaling pathways. Additionally GPCRs present some unique opportunities as well as challenges for drug delivery. On the positive side, the signaling and trafficking pathways for GPCRs are relatively well understood. Further, there is a plethora of selective high affinity ligands available for possible conjugation directly to oligonucleotides or to nanocarriers. On the negative side GPCRs are usually not very abundant and thus their ability to convey large amounts of material to the cell interior may be limited. A more subtle issue concerns the small molecule nature of most GPCR ligands. Obviously the original ligand will need to be modified with a reactive group in order to be chemically coupled to the oligonucleotide or carrier. For small molecules it can be difficult to add such groups without compromising the affinity or selectivity of the original ligand. In our experience, in terms of direct conjugation to oligonucleotides, we have had both successes (Nakagawa et al., 2010) and failures. For a more extended discussion of this issue see the following reference (Juliano et al., 2012b).
Tissue Specific Receptors- the Heart
In addition to discussion of broad families of receptors, it is worth noting some individual receptors that have restricted tissue distributions and thus might serve as useful vehicles for tissue selective oligonucleotide delivery. Thus in the section below we examine a receptor in the heart and several liver receptors.
A targetable receptor in the heart?
The urotensin receptor (UT), formerly GPR14, is a G-protein coupled receptor that is expressed in cardiovascular, renal, central nervous system, and endocrine tissue (Yoshimoto et al., 2004). Within the cardiovascular system the UT is widely distributed and is present in cardiac myocytes, vascular smooth muscle cells and endothelial cells (Ames et al., 1999). Activation of the UT induces contraction of smooth muscle cells and may be a driver of cardiovascular disease. Indeed, there is an up-regulation of UT expression in many pathologies of the cardiovascular system including heart failure (Douglas et al., 2002; Zhu et al., 2006), atherosclerosis (Bousette et al., 2004), and the receptor expression is proportional to the severity of infarct during myocardial infarction (Tzanidis et al., 2003). The increase in receptor density in the pathologic tissue makes the urotensin receptor an intriguing target for directed delivery in cardiovascular disease.
Urotesin II (UII), the first UT ligand discovered, is an endogenously expressed hendecapeptide (Ross et al., 2010) and it was further determined that the seven C-terminal amino acids were key to binding to UT. The UII-related peptide (URP), Ala-Cys-Phe-Trp-Lys-Tyr-Cys-Val, is an octapeptide which contains the seven C-terminal amino acids of UII and has a high potency (EC50 = 4.8 nM) and high affinity (Kd = 170 pM) for the UT. It has been suggested that URP acts as an endogenous functional agonist (Sugo and Mori, 2008). The relatively small size and high receptor specificity makes URP a potential conjugation factor for targeted drug delivery to tissue expressing the urotensin receptor. However, since activation of the UT is involved in the progression of cardiovascular disease an agonist conjugate may prove to be counterproductive to therapy. Other peptides and non-peptide small molecules have been synthesized as antagonists to the UT receptor (Zhu et al., 2006) that may serve as both as inhibitors of UT second messenger signaling as well as mediators of drug delivery. The urotensin receptor is rapidly internalized upon ligand binding with a halftime of 5.6 minutes and is rapidly recycled back to the membrane, within 60 minutes of ligand removal (Giebing et al., 2005). However, dependency of the urotensin receptor on arrestin for internalization is not clear (Giebing et al., 2005; Proulx et al., 2005). Upon internalization the urotensin receptor co-localizes with markers of early endosomes (EEA1) but not with markers of late endosomes or lysosomes (Rab9 and LAMP-1), indicating that the receptor is recycled back to the cellular membrane early in the trafficking process. These data suggest that, in addition to up-regulated expression in cardiovascular disease and high ligand affinity, the UT is readily accessible for ligand binding due to rapid trafficking and recycling – making the UT potentially an ideal candidate for targeted drug delivery.
Tissue specific receptors- Liver and Kidney
Antisense, and to some extent siRNA, oligonucleotides are distributed to the liver after intravenous administration without delivery systems (Juliano et al., 2009), which provides opportunity for using therapeutic oligonucleotides to treat liver diseases. The challenge for this method is to target specific intrahepatic cell types for effective and safe therapy, as the cell types play different roles in pathogenesis of liver diseases (Poelstra K, 2012). Utilization of membrane receptors that are selectively expressed in different cell types in the liver will provide opportunity for cell specific drug targeting. Thus, we will introduce several major receptors in the liver that have been used for targeted delivery of drugs, especially therapeutic oligonucleotides.
The asialoglycoprotein receptor
The asialoglycoprotein receptor (ASGPR) is a C-type lectin receptor expressed on the sinusoidal membrane of the hepatocytes (Zelensky and Gready, 2005). Its primary role is to remove N-acetylgalactosamine (GalNAc) and galactose (Gal)-containing glycoproteins by mediating the capture, endocytosis, and lysosomal degradation of the substrates (Stockert, 1995). ASGPR targeting can be employed for drug delivery to hepatocytes by coupling galactose residues or lactose moieties to drug or their carriers (Poelstra et al., 2012). ASGPR is a high-capacity but low affinity receptor and multivalent galacotose ligands are often utilized to enhance the binding affinity of monosaccharides to the receptor (Khorev et al., 2008). Thus, incorporation of multiple ligands in nanoparticles can achieve higher specificity and efficiency for ASGPR targeting. For example, multiple GalNAc ligands and PEGs were conjugated to a membrane-active polymer, which was also linked to a molecule of siRNA (Rozema et al., 2007). When the so-called “PolyConjugates” are delivered into the hepatocytes by ASGPR-mediated endocytosis, the polymer is activated in the acidic environment of endosomes, and then triggers the endosomal release of the conjugates, which is followed by gene silence of the target genes (Rozema et al., 2007). Therapeutic outcome has been demonstrated when using this conjugate to deliver apoB siRNA to the liver by intravenous injection (Rozema et al., 2007). This study highlights the importance of multivalent ligands and endosomal-release functionality in effective siRNA delivery.
Lipoprotein receptors
Hepatocytes highly express lipoprotein receptors, including low-density lipoprotein (LDL) and high-density lipoprotein (HDL) receptor, for transport of cholesterol (Brown and Goldstein, 1985). They have been utilized to deliver oligonucleotides into hepatocytes by conjugating oligonucleotides with cholesterol and then complexing the conjugate with the lipoproteins. A recent study indicated that cholesterol-siRNA conjugates preassembled with HDL or LDL further improved liver uptake and RNAi activity compared to the “free” cholesterol-siRNA conjugate (Wolfrum et al., 2007). Knocking out the HDL or LDL receptors reversed the improvement of hepatic uptake of the conjugate-lipoprotein complexes, suggesting that cholesterol-siRNA generates in vivo RNAi by forming lipoprotein particles that are internalized by receptor-mediated endocytosis (Wolfrum et al., 2007). Interestingly, this targeted delivery system does not include any endosomal-release functionality. In the endocytosis of lipoprotein particles, cholesterol is released from lysosome and is available to the cell for new membrane synthesis (Brown and Goldstein, 1985). It is still unknown whether siRNA-cholesterol conjugates can be released from lysosome by this mechanism. However, it may indicate that choosing receptor-mediated endocytosis pathways that end with cargo release in the cytosol may provide effective delivery systems for transporting oligonucleotides to their pharmacological sites in the cytosol and nucleus.
Receptors on hepatic stellate cells
Hepatic stellate cells (HSCs) play a key role in pathogenesis of liver fibrosis (Hernandez-Gea and Friedman, 2011). Thus selectively targeting anti-fibrotic agents to hepatic stellate cells may provide effective and safe treatment for liver fibrosis. Mannose-6-phosphate/insulin growth factor type 2 receptor (M6P/IGF2R) is a primary target for HSC targeting, since its expression is increased when HSCs are activated during fibrosis (Saperstein et al., 1994). M6P/IGF2R targeting can be achieved by coupling M6P moiety to drug or their carriers (Beljaars et al., 1999). In one example, M6P-PEG was conjugated to the sense strands of siRNAs via disulfide linkage, and the result siRNA conjugates silenced reporter gene expression by 40% in a rat HSC cell line (Zhu and Mahato, 2010). HSCs are depositories of 50–80% of vitamin A (retinol) in the whole body and thus have an outstanding capability of transporting vitamin A, most likely through receptor-mediated endocytosis of retinol binding protein (Senoo et al., 2010). Thus, vitamin A has also been formulated into liposomes for siRNA delivery to HSCs (Sato et al., 2008). After systemic administration, the siRNAs targeting heat shock protein were delivered to HSCs via retinol-binding proteins, leading to the cure of liver fibrosis in rats (Sato et al., 2008). In spite of the outstanding RNAi activity in vivo, the receptor has not been identified and thus endocytosis mechanisms for this delivery system are unclear.
Kidney specific receptors
Compared to the liver or tumors, less work has been done on targeting membrane receptors in the kidney for drug and gene delivery (Dolman et al., 2010; Shimizu et al., 2010). Most of the successful cases utilized lysozyme as a delivery carrier and targeted megalin, a receptor in the apical membrane of PTECs mediating endocytosis of small proteins such as lysozyme that are filtered in renal glomeruli (Dolman et al., 2010). Therapeutic siRNAs have been applied to treat kidney diseases in animals, however, most of the studies have utilized endogenous uptake of naked olignucleotides in renal tubular cells (Molitoris et al., 2009; Vaishnaw et al., 2010; van de Water et al., 2006; Zheng et al., 2008). Two-photon microscopy in live rats demonstrated that fluorescent labeled siRNAs mainly accumulate in the renal proximal tubules after quick cellular entry from the apical membrane (Molitoris et al., 2009). This study supported the notion that siRNAs enter renal proximal tubular cells by receptor-mediated endocytosis. However, the receptor has not been identified.
Conclusion: Manipulation of Oligonucleotide Targeting and Trafficking for Enhanced Pharmacological Effects
To summarize, there are two important aspects to the effective targeting of therapeutic oligonucleotides. The first is to select a receptor/ligand combination that will afford substantial oligonucleotide accumulation in the cell type of interest while still maintaining a high degree of selectivity. The second is to overcome the trapping of oligonucleotides in pharmacologically inert endomembrane compartments.
In this review we have focused on integrins, ‘classic’ RTKs such as the EGFR, and typical GPCRs as potential receptors for oligonucleotide delivery. Obviously there are many other receptor families that could serve in this manner including lectin-like receptors such as the selectins, cadherin family cell-cell adhesion receptors, the semaphorins, plexins and neuropilins so prominent in the CNS, the atypical Eph subfamily of tyrosine kinases and their transmembrane ephrin ligands, the TGF-β/activin family of receptors, the Wnt/Frizzled signaling pathway, and finally the multiple Ig-domain receptors that are so important in regulation of the immune system. However, in many of these cases there are few ligands available that have the appropriate size and chemical characteristics to permit facile coupling to oligonucleotides or their delivery moieties. Likewise we have not discussed the folate-folate receptor system; this topic has been frequently reviewed and the advantages and disadvantages of this approach are well known (Garcia-Bennett et al., 2011; Low and Kularatne, 2009). With the abundance of information on various receptor families and their ligands, as well as an ever-increasing repertory of clever coupling approaches such as ‘click chemistry’ (Juliano et al., 2012b; Yamada et al., 2011), designing targeted oligonucleotide conjugates or carriers should be the easier of the two problems addressed in this review.
A greater challenge is the release on oligonucleotides from non-productive membrane bound compartments without causing serious harm to the cell. There has been a huge amount of work on what are essentially physical approaches to enhancing oligonucleotide delivery. Thus membrane destabilizing cationic lipids, as well as polymers that produce ‘proton sponge’ effects, are widely used manifestations of the physical approach to oligonucleotide delivery. There are numerous excellent reviews on these topics (Nguyen and Szoka, 2012; Schroeder et al., 2010), including consideration of their toxicities (Akhtar, 2010), and we will not discuss them further. Rather we will focus on biological and chemical approaches to manipulating cellular endomembrane compartments. There is a vast amount of information on this topic that appears in the cell biology literature, but until recently it had not penetrated the drug delivery literature to any great degree.
A powerful strategy is to use plasmid or viral vectors to express mutant versions of proteins that play key roles in intracellular trafficking so as to modulate these processes. For example, constitutively activated Rab proteins have been used to enhance specific aspects of trafficking pathways, while conversely, dominant negative Rabs cause pathway blockade (Mainou and Dermody, 2012; Stenmark et al., 1994). This type of approach is just beginning to be applied in the oligonucleotide field; for example we have used dominant negative versions of dynamin and caveolin to interdict early steps in uptake of oligonucleotides or of oligonucleotide/nanocarrier complexes (Alam et al., 2010; Ming et al., 2011)
Although molecular approaches to trafficking pathway manipulation are very powerful investigative tools, they obviously cannot be used in a therapeutic context. For pharmacologically significant manipulation of oligonucleotide trafficking one needs to use small molecules. A number of inhibitors of uptake or trafficking have been known for many years (von Kleist and Haucke, 2011). Many of these work essentially by physical mechanisms and are thus rather non-specific. Some examples would include inhibition of clathrin-mediated endocytosis by K+ depletion or hyperosmolarity, inhibition of calveolar/lipid raft-mediated processes by extraction/sequestration of cholesterol with cyclodextrins or filipin, and lysosomotropic drugs like chloroquine that disrupt low pH compartments such as lysosomes essentially by a protein sponge effect. Most of these physical agents act only at high concentrations, disrupt many cellular activities, and are quite toxic
More recently a few agents have emerged that manipulate endocytosis and trafficking by acting on key proteins involved in these processes; thus these agents have true drug-like properties and could conceivably be used in therapeutic contexts. Perhaps the most widely known are dynasore and the dynoles, which inhibit the GTPase activity of dynamin and thus its function in vesicle budding. The ‘pitstops’ are compounds that bind to the globular terminal domain of clathrin and immobilize coated pits. Since both pitstops and dynasore/dynole inhibit endocytosis, they obviously cannot be used to enhance oligonucleotide action, but they can be useful in exploring uptake mechanisms. Proceeding further into the cell, there are several sets of compounds that affect the function/stability of endosomes or the Golgi apparatus. The best known is brefeldin A that inhibits GBF1, BIG1 and BIG2 that are GEFs for the Arf1 GTPase that is essential for Golgi trafficking. Golgicide is a more specific compound that selectively blocks GBF1 but not the other Arf1 GEFs. Both brefeldin A and golgicide cause dispersion of the Golgi network. A compound termed A5 is thought to block trans-Golgi to endosome traffic by affecting the AP1 adaptor protein. High throughput screening for agents that inhibit the actions of bacterial and plant toxins led to the Retro group of compounds. These act by blocking the so-called retrograde trafficking pathway from endosomes to the trans-Golgi used by some toxins and viruses. Thus there are now a variety of modulators of endocytosis and trafficking that can be explored in the context of oligonucleotide delivery (Duncan et al., 2007; Saenz et al., 2009; Stechmann et al., 2010; von Kleist and Haucke, 2011).
References
- Akhtar S. Cationic nanosystems for the delivery of small interfering ribonucleic acid therapeutics: a focus on toxicogenomics. Expert Opin Drug Metab Toxicol. 2010;6:1347–1362. doi: 10.1517/17425255.2010.518611. [DOI] [PubMed] [Google Scholar]
- Alam MR, Dixit V, Kang H, Li ZB, Chen X, Trejo J, Fisher M, Juliano RL. Intracellular delivery of an anionic antisense oligonucleotide via receptormediated endocytosis. Nucleic Acids Res. 2008;36:2764–2776. doi: 10.1093/nar/gkn115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MR, Ming X, Dixit V, Fisher M, Chen X, Juliano RL. The biological effect of an antisense oligonucleotide depends on its route of endocytosis and trafficking. Oligonucleotides. 2010;20:103–109. doi: 10.1089/oli.2009.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MR, Ming X, Fisher M, Lackey JG, Rajeev KG, Manoharan M, Juliano RL. Multivalent cyclic RGD conjugates for targeted delivery of small interfering RNA. Bioconjug Chem. 2011;22:1673–1681. doi: 10.1021/bc200235q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, Romanic AM, Louden CS, Foley JJ, Sauermelch CF, Coatney RW, Ao Z, Disa J, Holmes SD, Stadel JM, Martin JD, Liu WS, Glover GI, Wilson S, McNulty DE, Ellis CE, Elshourbagy NA, Shabon U, Trill JJ, Hay DW, Ohlstein EH, Bergsma DJ, Douglas SA. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- Angers CG, Merz AJ. New links between vesicle coats and Rab-mediated vesicle targeting. Semin Cell Dev Biol. 2011;22:18–26. doi: 10.1016/j.semcdb.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin AE, Stewart SA, Assoian RK, Juliano RL. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J Cell Biol. 2001;153:273–282. doi: 10.1083/jcb.153.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Roth BL. Mining the receptorome. J Biol Chem. 2005;280:5129–5132. doi: 10.1074/jbc.R400030200. [DOI] [PubMed] [Google Scholar]
- Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batzer AG, Rotin D, Urena JM, Skolnik EY, Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol. 1994;14:5192–5201. doi: 10.1128/mcb.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beljaars L, Molema G, Weert B, Bonnema H, Olinga P, Groothuis GM, Meijer DK, Poelstra K. Albumin modified with mannose 6-phosphate: A potential carrier for selective delivery of antifibrotic drugs to rat and human hepatic stellate cells. Hepatology. 1999;29:1486–1493. doi: 10.1002/hep.510290526. [DOI] [PubMed] [Google Scholar]
- Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 2010;50:259–293. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- Bousette N, Patel L, Douglas SA, Ohlstein EH, Giaid A. Increased expression of urotensin II and its cognate receptor GPR14 in atherosclerotic lesions of the human aorta. Atherosclerosis. 2004;176:117–123. doi: 10.1016/j.atherosclerosis.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Bretscher MS. Circulating integrins: alpha 5 beta 1, alpha 6 beta 4 and Mac-1, but not alpha 3 beta 1, alpha 4 beta 1 or LFA-1. EMBO J. 1992;11:405–410. doi: 10.1002/j.1460-2075.1992.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur GM, Minturn JE, Ho R, Simpson AM, Iyer R, Varela CR, Light JE, Kolla V, Evans AE. Trk receptor expression and inhibition in neuroblastomas. Clin Cancer Res. 2009;15:3244–3250. doi: 10.1158/1078-0432.CCR-08-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. The receptor model for transport of cholesterol in plasma. Ann N Y Acad Sci. 1985;454:178–182. doi: 10.1111/j.1749-6632.1985.tb11856.x. [DOI] [PubMed] [Google Scholar]
- Burnett JC, Rossi JJ. RNA-based therapeutics: current progress and future prospects. Chem Biol. 2012;19:60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabodi S, Moro L, Bergatto E, Boeri Erba E, Di Stefano P, Turco E, Tarone G, Defilippi P. Integrin regulation of epidermal growth factor (EGF) receptor and of EGF-dependent responses. Biochem Soc Trans. 2004;32:438–442. doi: 10.1042/BST0320438. [DOI] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Insights into G protein structure, function, and regulation. Endocr Rev. 2003;24:765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- Calebiro D, V, Nikolaev O, Persani L, Lohse MJ. Signaling by internalized G-protein-coupled receptors. Trends Pharmacol Sci. 2010;31:221–228. doi: 10.1016/j.tips.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D, Norman JC. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol. 2008;183:143–155. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chen X. Multimodality imaging of tumor integrin alphavbeta3 expression. Mini Rev Med Chem. 2006;6:227–234. doi: 10.2174/138955706775475975. [DOI] [PubMed] [Google Scholar]
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- Cornelio DB, Roesler R, Schwartsmann G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann Oncol. 2007;18:1457–1466. doi: 10.1093/annonc/mdm058. [DOI] [PubMed] [Google Scholar]
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Dolman ME, Harmsen S, Storm G, Hennink WE, Kok RJ. Drug targeting to the kidney: Advances in the active targeting of therapeutics to proximal tubular cells. Adv Drug Deliv Rev. 2010;62:1344–1357. doi: 10.1016/j.addr.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Douglas SA, Tayara L, Ohlstein EH, Halawa N, Giaid A. Congestive heart failure and expression of myocardial urotensin II. Lancet. 2002;359:1990–1997. doi: 10.1016/S0140-6736(02)08831-1. [DOI] [PubMed] [Google Scholar]
- Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res. 2006;99:570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- Duncan MC, Ho DG, Huang J, Jung ME, Payne GS. Composite synthetic lethal identification of membrane traffic inhibitors. Proc Natl Acad Sci U S A. 2007;104:6235–6240. doi: 10.1073/pnas.0607773104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin ML, Juliano RL. Raf-1 serine 338 phosphorylation plays a key role in adhesion-dependent activation of extracellular signal-regulated kinase by epidermal growth factor. Mol Cell Biol. 2005;25:4466–4475. doi: 10.1128/MCB.25.11.4466-4475.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Press B, Wandinger-Ness A. Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmore D. Its a GPCR world. Modern Drug Discivery. 2004;7:24–28. [Google Scholar]
- Garcia-Bennett A, Nees M, Fadeel B. In search of the Holy Grail: Folatetargeted nanoparticles for cancer therapy. Biochem Pharmacol. 2011;81:976–984. doi: 10.1016/j.bcp.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Garrison JC, Rold TL, Sieckman GL, Figueroa SD, Volkert WA, Jurisson SS, Hoffman TJ. In vivo evaluation and small-animal PET/CT of a prostate cancer mouse model using 64Cu bombesin analogs: side-by-side comparison of the CB-TE2A and DOTA chelation systems. J Nucl Med. 2007;48:1327–1337. doi: 10.2967/jnumed.107.039487. [DOI] [PubMed] [Google Scholar]
- Giebing G, Tolle M, Jurgensen J, Eichhorst J, Furkert J, Beyermann M, Neuschafer-Rube F, Rosenthal W, Zidek W, van der Giet M, Oksche A. Arrestin-independent internalization and recycling of the urotensin receptor contribute to long-lasting urotensin II-mediated vasoconstriction. Circulation research. 2005;97:707–715. doi: 10.1161/01.RES.0000184670.58688.9F. [DOI] [PubMed] [Google Scholar]
- Gilman AG. Nobel Lecture. G proteins and regulation of adenylyl cyclase. Biosci Rep. 1995;15:65–97. doi: 10.1007/BF01200143. [DOI] [PubMed] [Google Scholar]
- Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrinindependent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- Goh LK, Huang F, Kim W, Gygi S, Sorkin A. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J Cell Biol. 2010;189:871–883. doi: 10.1083/jcb.201001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- Gu Z, Noss EH, Hsu VW, Brenner MB. Integrins traffic rapidly via circular dorsal ruffles and macropinocytosis during stimulated cell migration. J Cell Biol. 2011;193:61–70. doi: 10.1083/jcb.201007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- Houston D, Ohno M, Nicholas RA, Jacobson KA, Harden TK. [32P]2- iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate ([32P]MRS2500), a novel radioligand for quantification of native P2Y1 receptors. Br J Pharmacol. 2006;147:459–467. doi: 10.1038/sj.bjp.0706453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes MT, Mayor S, Parton RG. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Curr Opin Cell Biol. 2010;22:519–527. doi: 10.1016/j.ceb.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers S, Danen EH. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- Hwang JY, Park J, Kang BJ, Lubow DJ, Chu D, Farkas DL, Shung KK, Medina-Kauwe LK. Multimodality imaging in vivo for preclinical assessment of tumor-targeted doxorubicin nanoparticles. PLoS One. 2012;7:e34463. doi: 10.1371/journal.pone.0034463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002a;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Hynes RO. A reevaluation of integrins as regulators of angiogenesis. Nat Med. 2002b;8:918–921. doi: 10.1038/nm0902-918. [DOI] [PubMed] [Google Scholar]
- Imai Y, Leung CK, Friesen HG, Shiu RP. Epidermal growth factor receptors and effect of epidermal growth factor on growth of human breast cancer cells in long-term tissue culture. Cancer Res. 1982;42:4394–4398. [PubMed] [Google Scholar]
- Jackson DY. Alpha 4 integrin antagonists. Curr Pharm Des. 2002;8:1229–1253. doi: 10.2174/1381612023394737. [DOI] [PubMed] [Google Scholar]
- Juliano R, Bauman J, Kang H, Ming X. Biological barriers to therapy with antisense and siRNA oligonucleotides. Mol Pharm. 2009;6:686–695. doi: 10.1021/mp900093r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Ming X, Nakagawa O. Cellular uptake and intracellular trafficking of antisense and siRNA oligonucleotides. Bioconjug Chem. 2012a;23:147–157. doi: 10.1021/bc200377d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano RL, Ming X, Nakagawa O. The chemistry and biology of oligonucleotide conjugates. Acc Chem Res. 2012a;45:1067–1076. doi: 10.1021/ar2002123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano RL, Ming X, Nakagawa O, Xu R, Yoo H. Integrin targeted delivery of gene therapeutics. Theranostics. 2011;1:211–219. doi: 10.7150/thno/v01p0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano RL, Reddig P, Alahari S, Edin M, Howe A, Aplin A. Integrin regulation of cell signalling and motility. Biochem Soc Trans. 2004;32:443–446. doi: 10.1042/BST0320443. [DOI] [PubMed] [Google Scholar]
- Karjalainen M, Kakkonen E, Upla P, Paloranta H, Kankaanpaa P, Liberali P, Renkema GH, Hyypia T, Heino J, Marjomaki V. A Raft-derived, Pak1- regulated entry participates in alpha2beta1 integrin-dependent sorting to caveosomes. Mol Biol Cell. 2008;19:2857–2869. doi: 10.1091/mbc.E07-10-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermorgant S, Parker PJ. Receptor trafficking controls weak signal delivery: a strategy used by c-Met for STAT3 nuclear accumulation. J Cell Biol. 2008;182:855–863. doi: 10.1083/jcb.200806076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- Khorev O, Stokmaier D, Schwardt O, Cutting B, Ernst B. Trivalent, Gal/GalNAc-containing ligands designed for the asialoglycoprotein receptor. Bioorg Med Chem. 2008;16:5216–5231. doi: 10.1016/j.bmc.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Kim C, Ye F, Ginsberg MH. Regulation of integrin activation. Annu Rev Cell Dev Biol. 2011;27:321–345. doi: 10.1146/annurev-cellbio-100109-104104. [DOI] [PubMed] [Google Scholar]
- Kimple AJ, Bosch DE, Giguere PM, Siderovski DP. Regulators of Gprotein signaling and their Galpha substrates: promises and challenges in their use as drug discovery targets. Pharmacol Rev. 2011;63:728–749. doi: 10.1124/pr.110.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2012;2:a006502. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg LJ, Earp HS, Turner CE, Prockop C, Juliano RL. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991;88:8392– 8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- Kristensen SD, Grove EL, Hvas AM. P2Y12 inhibitors in acute coronary syndromes: How do we choose the best drug for our patients? Thromb Haemost. 2012;108:203–205. doi: 10.1160/TH12-06-0430. [DOI] [PubMed] [Google Scholar]
- Kunz S. Receptor binding and cell entry of Old World arenaviruses reveal novel aspects of virus-host interaction. Virology. 2009;387:245–249. doi: 10.1016/j.virol.2009.02.042. [DOI] [PubMed] [Google Scholar]
- Lajoie P, I, Nabi R. Lipid rafts, caveolae, and their endocytosis. Int Rev Cell Mol Biol. 2010;282:135–163. doi: 10.1016/S1937-6448(10)82003-9. [DOI] [PubMed] [Google Scholar]
- Lazzara MJ, Lauffenburger DA. Quantitative modeling perspectives on the ErbB system of cell regulatory processes. Exp Cell Res. 2009;315:717–725. doi: 10.1016/j.yexcr.2008.10.033. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A, Atlas D, Steer ML. The binding characteristics and number of beta-adrenergic receptors on the turkey erythrocyte. Proc Natl Acad Sci U S A. 1974;71:2773–2776. doi: 10.1073/pnas.71.7.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Huang S, Peng SB. Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. Int J Oncol. 2005;27:1329–1339. [PubMed] [Google Scholar]
- Lin TH, Chen Q, Howe A, Juliano RL. Cell anchorage permits efficient signal transduction between ras and its downstream kinases. J Biol Chem. 1997;272:8849–8852. [PubMed] [Google Scholar]
- Liu X, Wang Y, Hnatowich DJ. A nanoparticle for tumor targeted delivery of oligomers. Methods Mol Biol. 2011;764:91–105. doi: 10.1007/978-1-61779-188-8_6. [DOI] [PubMed] [Google Scholar]
- Lobert VH, Brech A, Pedersen NM, Wesche J, Oppelt A, Malerod L, Stenmark H. Ubiquitination of alpha 5 beta 1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev Cell. 2010;19:148–159. doi: 10.1016/j.devcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Low PS, Kularatne SA. Folate-targeted therapeutic and imaging agents for cancer. Curr Opin Chem Biol. 2009;13:256–262. doi: 10.1016/j.cbpa.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Mai A, Veltel S, Pellinen T, Padzik A, Coffey E, Marjomaki V, Ivaska J. Competitive binding of Rab21 and p120RasGAP to integrins regulates receptor traffic and migration. J Cell Biol. 2011;194:291–306. doi: 10.1083/jcb.201012126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainou BA, Dermody TS. Transport to late endosomes is required for efficient reovirus infection. J Virol. 2012;86:8346–8358. doi: 10.1128/JVI.00100-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant C, Monsuur HN, Norman JC, Sonnenberg A. Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol. 2011;23:607–614. doi: 10.1016/j.ceb.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Bar-Sagi D. Signaling endosomes: seeing is believing. Curr Opin Cell Biol. 2010;22:535–540. doi: 10.1016/j.ceb.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard M, Odde S, Neamati N. Integrin targeted therapeutics. Theranostics. 2011;1:154–188. doi: 10.7150/thno/v01p0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Alam MR, Fisher M, Yan Y, Chen X, Juliano RL. Intracellular delivery of an antisense oligonucleotide via endocytosis of a G proteincoupled receptor. Nucleic Acids Res. 2010;38:6567–6576. doi: 10.1093/nar/gkq534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Sato K, Juliano RL. Unconventional internalization mechanisms underlying functional delivery of antisense oligonucleotides via cationic lipoplexes and polyplexes. J Control Release. 2011;153:83–92. doi: 10.1016/j.jconrel.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ, Thompson JD, Kalinski H, Skaliter R, Erlich S, Feinstein E. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol. 2009;20:1754–1764. doi: 10.1681/ASN.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschos SJ, Drogowski LM, Reppert SL, Kirkwood JM. Integrins and cancer. Oncology (Williston Park) 2007;21:13–20. [PubMed] [Google Scholar]
- Mulgrew K, Kinneer K, Yao XT, Ward BK, Damschroder MM, Walsh B, Mao SY, Gao C, Kiener PA, Coats S, Kinch MS, Tice DA. Direct targeting of alphavbeta3 integrin on tumor cells with a monoclonal antibody, Abegrin. Mol Cancer Ther. 2006;5:3122–3129. doi: 10.1158/1535-7163.MCT-06-0356. [DOI] [PubMed] [Google Scholar]
- Munger JS, Sheppard D. Cross talk among TGF-beta signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol. 2011;3:a005017. doi: 10.1101/cshperspect.a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa O, Ming X, Huang L, Juliano RL. Targeted intracellular delivery of antisense oligonucleotides via conjugation with small-molecule ligands. J Am Chem Soc. 2010;132:8848–8849. doi: 10.1021/ja102635c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napione L, Pavan S, Veglio A, Picco A, Boffetta G, Celani A, Seano G, Primo L, Gamba A, Bussolino F. Unraveling the influence of endothelial cell density on VEGF-A signaling. Blood. 2012;119:5599–5607. doi: 10.1182/blood-2011-11-390666. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Berk BC. Crosstalk coregulation mechanisms of G proteincoupled receptors and receptor tyrosine kinases. Methods Mol Biol. 2006;332:51–77. doi: 10.1385/1-59745-048-0:51. [DOI] [PubMed] [Google Scholar]
- Nguyen J, Szoka FC. Nucleic Acid delivery: the missing pieces of the puzzle? Acc Chem Res. 2012;45:1153–1162. doi: 10.1021/ar3000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu G, Chen X. Why integrin as a primary target for imaging and therapy. Theranostics. 2011;1:30–47. doi: 10.7150/thno/v01p0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parachoniak CA, Park M. Dynamics of receptor trafficking in tumorigenicity. Trends Cell Biol. 2012;22:231–240. doi: 10.1016/j.tcb.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Entry at the trans-face of the Golgi. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Poelstra KPJBL. J Controlled Release. 2012;161:188–195. doi: 10.1016/j.jconrel.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Poelstra K, Prakash J, Beljaars L. Drug targeting to the diseased liver. J Control Release. 2012 doi: 10.1016/j.jconrel.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Post SR, Hilal-Dandan R, Urasawa K, Brunton LL, Insel PA. Quantification of signalling components and amplification in the betaadrenergic- receptor-adenylate cyclase pathway in isolated adult rat ventricular myocytes. Biochem J. 1995;311(Pt 1):75–80. doi: 10.1042/bj3110075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx CD, Simaan M, Escher E, Laporte SA, Guillemette G, Leduc R. Involvement of a cytoplasmic-tail serine cluster in urotensin II receptor internalization. The Biochemical journal. 2005;385:115–123. doi: 10.1042/BJ20040807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. Receptor tyrosine kinase-G-protein-coupled receptor signalling platforms: out of the shadow? Trends Pharmacol Sci. 2011;32:443–450. doi: 10.1016/j.tips.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Rangamani P, Iyengar R. Modelling cellular signalling systems. Essays Biochem. 2008;45:83–94. doi: 10.1042/BSE0450083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B, McKendy K, Giaid A. Role of urotensin II in health and disease. American journal of physiology Regulatory, integrative and comparative physiology. 2010;298:R1156–1172. doi: 10.1152/ajpregu.00706.2009. [DOI] [PubMed] [Google Scholar]
- Rozema DB, Lewis DL, Wakefield DH, Wong SC, Klein JJ, Roesch PL, Bertin SL, Reppen TW, Chu Q, Blokhin AV, Hagstrom JE, Wolff JA. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc Natl Acad Sci U S A. 2007;104:12982–12987. doi: 10.1073/pnas.0703778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz JB, Sun WJ, Chang JW, Li J, Bursulaya B, Gray NS, Haslam DB. Golgicide A reveals essential roles for GBF1 in Golgi assembly and function. Nat Chem Biol. 2009;5:157–165. doi: 10.1038/nchembio.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar NC, Chen J, Rockman HA. Cardiac GPCRs: GPCR signaling in healthy and failing hearts. Biochim Biophys Acta. 2007;1768:1006–1018. doi: 10.1016/j.bbamem.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saperstein LA, Jirtle RL, Farouk M, Thompson HJ, Chung KS, Meyers WC. Transforming growth factor-beta 1 and mannose 6-phosphate/insulinlike growth factor-II receptor expression during intrahepatic bile duct hyperplasia and biliary fibrosis in the rat. Hepatology. 1994;19:412–417. [PubMed] [Google Scholar]
- Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, Takimoto R, Takada K, Miyanishi K, Matsunaga T, Takayama T, Niitsu Y. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol. 2008;26:431–442. doi: 10.1038/nbt1396. [DOI] [PubMed] [Google Scholar]
- Savage B, Marzec UM, Chao BH, Harker LA, Maraganore JM, Ruggeri ZM. Binding of the snake venom-derived proteins applaggin and echistatin to the arginine-glycine-aspartic acid recognition site(s) on platelet glycoprotein IIb.IIIa complex inhibits receptor function. J Biol Chem. 1990;265:11766–11772. [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Lipid-based nanotherapeutics for siRNA delivery. J Intern Med. 2010;267:9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. J Cell Sci. 2007;120:3905–3910. doi: 10.1242/jcs.015909. [DOI] [PubMed] [Google Scholar]
- Sczekan MM, Juliano RL. Internalization of the fibronectin receptor is a constitutive process. J Cell Physiol. 1990;142:574–580. doi: 10.1002/jcp.1041420317. [DOI] [PubMed] [Google Scholar]
- Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative--past, present and future. Cell Biol Int. 2010;34:1247–1272. doi: 10.1042/CBI20100321. [DOI] [PubMed] [Google Scholar]
- Shepard HM, Jin P, Slamon DJ, Pirot Z, Maneval DC. Herceptin. Handb Exp Pharmacol. 2008:183–219. doi: 10.1007/978-3-540-73259-4_9. [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Springer TA. Therapeutic antagonists and conformational regulation of integrin function. Nat Rev Drug Discov. 2003;2:703–716. doi: 10.1038/nrd1174. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Hori Y, Kaname S, Yamada K, Nishiyama N, Matsumoto S, Miyata K, Oba M, Yamada A, Kataoka K, Fujita T. siRNA-based therapy ameliorates glomerulonephritis. J Am Soc Nephrol. 2010;21:622–633. doi: 10.1681/ASN.2009030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of beta-arrestindependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36:457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Narang AS, Mahato RI. Subcellular fate and off-target effects of siRNA, shRNA, and miRNA. Pharm Res. 2011;28:2996–3015. doi: 10.1007/s11095-011-0608-1. [DOI] [PubMed] [Google Scholar]
- Sioud M. Deciphering the code of innate immunity recognition of siRNAs. Methods Mol Biol. 2009;487:41–59. doi: 10.1007/978-1-60327-547-7_2. [DOI] [PubMed] [Google Scholar]
- Soh UJ, Dores MR, Chen B, Trejo J. Signal transduction by proteaseactivated receptors. Br J Pharmacol. 2010;160:191–203. doi: 10.1111/j.1476-5381.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2008;314:3093–3106. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stechmann B, Bai SK, Gobbo E, Lopez R, Merer G, Pinchard S, Panigai L, Tenza D, Raposo G, Beaumelle B, Sauvaire D, Gillet D, Johannes L, Barbier J. Inhibition of retrograde transport protects mice from lethal ricin challenge. Cell. 2010;141:231–242. doi: 10.1016/j.cell.2010.01.043. [DOI] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert RJ. The asialoglycoprotein receptor: relationships between structure, function, and expression. Physiol Rev. 1995;75:591–609. doi: 10.1152/physrev.1995.75.3.591. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem J. 2009;418:491–506. doi: 10.1042/BJ20081948. [DOI] [PubMed] [Google Scholar]
- Sugo T, Mori M. Another ligand fishing for G protein-coupled receptor 14. Discovery of urotensin II-related peptide in the rat brain. Peptides. 2008;29:809–812. doi: 10.1016/j.peptides.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Szabo AM, Howell NR, Pellegrini P, Greguric I, Katsifis A. Development and validation of competition binding assays for affinity to the extracellular matrix receptors, alpha(v)beta(3) and alpha(IIb)beta(3) integrin. Anal Biochem. 2012;423:70–77. doi: 10.1016/j.ab.2011.12.046. [DOI] [PubMed] [Google Scholar]
- Tohidkia MR, Barar J, Asadi F, Omidi Y. Molecular considerations for development of phage antibody libraries. J Drug Target. 2012;20:195–208. doi: 10.3109/1061186X.2011.611517. [DOI] [PubMed] [Google Scholar]
- Tzanidis A, Hannan RD, Thomas WG, Onan D, Autelitano DJ, See F, Kelly DJ, Gilbert RE, Krum H. Direct actions of urotensin II on the heart: implications for cardiac fibrosis and hypertrophy. Circulation research. 2003;93:246–253. doi: 10.1161/01.RES.0000084382.64418.BC. [DOI] [PubMed] [Google Scholar]
- Vaishnaw AK, Gollob J, Gamba-Vitalo C, Hutabarat R, Sah D, Meyers R, de Fougerolles T, Maraganore J. A status report on RNAi therapeutics. Silence. 2010;1:14. doi: 10.1186/1758-907X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Water FM, Boerman OC, Wouterse AC, Peters JG, Russel FG, Masereeuw R. Intravenously administered short interfering RNA accumulates in the kidney and selectively suppresses gene function in renal proximal tubules. Drug Metab Dispos. 2006;34:1393–1397. doi: 10.1124/dmd.106.009555. [DOI] [PubMed] [Google Scholar]
- Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Mol Cancer Ther. 2011;10:1763–1773. doi: 10.1158/1535-7163.MCT-11-0116. [DOI] [PubMed] [Google Scholar]
- von Kleist L, Haucke V. At the Crossroads of Chemistry and Cell Biology: Inhibiting Membrane Traffic by Small Molecules. Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01292.x. [DOI] [PubMed] [Google Scholar]
- Wax MB, Molinoff PB. Distribution and properties of beta-adrenergic receptors in human iris-ciliary body. Invest Ophthalmol Vis Sci. 1987;28:420–430. [PubMed] [Google Scholar]
- Wickstrom SA, Fassler R. Regulation of membrane traffic by integrin signaling. Trends Cell Biol. 2011;21:266–273. doi: 10.1016/j.tcb.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8:462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, Zimmermann T, Koteliansky V, Manoharan M, Stoffel M. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Peng CG, Matsuda S, Addepalli H, Jayaprakash KN, Alam MR, Mills K, Maier MA, Charisse K, Sekine M, Manoharan M, Rajeev KG. Versatile site-specific conjugation of small molecules to siRNA using click chemistry. J Org Chem. 2011;76:1198–1211. doi: 10.1021/jo101761g. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Matsushita M, Hirata Y. Role of urotensin II in peripheral tissue as an autocrine/paracrine growth factor. Peptides. 2004;25:1775–1781. doi: 10.1016/j.peptides.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhang X, Feng B, Sun H, Suzuki M, Ichim T, Kubo N, Wong A, Min LR, Budohn ME, Garcia B, Jevnikar AM, Min WP. Gene silencing of complement C5a receptor using siRNA for preventing ischemia/reperfusion injury. Am J Pathol. 2008;173:973–980. doi: 10.2353/ajpath.2008.080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Mahato RI. Targeted delivery of siRNA to hepatocytes and hepatic stellate cells by bioconjugation. Bioconjug Chem. 2010;21:2119–2127. doi: 10.1021/bc100346n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YC, Zhu YZ, Moore PK. The role of urotensin II in cardiovascular and renal physiology and diseases. British journal of pharmacology. 2006;148:884–901. doi: 10.1038/sj.bjp.0706800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Witte L. Inhibition of tumor growth and metastasis by targeting tumor-associated angiogenesis with antagonists to the receptors of vascular endothelial growth factor. Invest New Drugs. 1999;17:195–212. doi: 10.1023/a:1006314501634. [DOI] [PubMed] [Google Scholar]
- Zwang Y, Yarden Y. Systems biology of growth factor-induced receptor endocytosis. Traffic. 2009;10:349–363. doi: 10.1111/j.1600-0854.2008.00870.x. [DOI] [PubMed] [Google Scholar]