Abstract
Background
Exercise improves quality of life (QOL) in cancer survivors, although characteristics of efficacious exercise interventions for this population have not been identified.
Purpose
The present meta-analysis examines the efficacy of exercise interventions in improving QOL in cancer survivors, as well as features that may moderate such effects.
Method
Studies were identified and coded, and QOL effect sizes were calculated and analyzed for trends.
Results
Overall, exercise interventions increased QOL, but this tendency depended to some extent on exercise and patient features. Although several features were associated with effect sizes, models revealed that interventions were particularly successful if they targeted more intense aerobic exercise and addressed women. These tendencies emerged over longer periods of time and were more prominent in studies with higher methodological quality.
Conclusion
Appropriately designed exercise interventions enhance QOL for cancer survivors and this pattern is especially evident for women. Limitations are discussed.
Keywords: Cancer, Oncology, Exercise, Behavioral interventions, Quality of life, Meta-analysis, Exercise interventions, Cancer survivors
Introduction
At least 9.8 million people in the USA live with cancer, and the lifetime probability of developing cancer is 38–45% [1, 2]. Improvements in cancer diagnosis and treatment have led to an increased life expectancy for those diagnosed, although cancer and its treatment carry serious physical and psychological consequences that can dramatically decrease quality of life (QOL; see 3]. QOL in this context refers to physical, emotional, and social well-being [4], and research indicates that QOL can be affected negatively by cancer and its treatment [5].
Fortunately, lifestyle changes such as exercise may decrease physical and psychological issues associated with cancer, thus improving QOL [6–11]. However, many estimates of exercise among cancer survivors indicate that spontaneous adoption of exercise post-diagnosis is not routine [12–17]. Survivors of chronic diseases such as cancer may have difficulty changing physical activity behaviors because symptoms and treatment can make exercise more difficult [15]. Despite the added difficulties that these individuals face with regard to changing their exercise behaviors, with appropriate help from interventions or healthcare professionals, they could potentially modify their lifestyles with some success.
Mixed results have been reported for the efficacy of exercise interventions in changing QOL of cancer survivors, and these interventions include a wide variety of design and other methodological characteristics [18–21]. A review of the literature notes that interventions vary on a wide variety of dimensions, including number and length of sessions, theory-driven content, exercise leader training and structure, and inclusion of different exercise modalities such as aerobic, resistance, and flexibility [22]. Participants in the exercise interventions also vary in a number of ways, including cancer diagnosis, age, gender, and treatment. These differences may account for discrepancies in findings concerning the efficacy of these exercise interventions, and meta-analytic evidence concerning the moderating effects of these variables on efficacy of interventions may greatly benefit the field, but no substantial empirical reviews reporting moderating effects of these interventions have been undertaken.
Prior systematic reviews examined exercise interventions and QOL among cancer survivors and documented that although they tend to improve QOL [22–26], the effects are variable. Three of these reviews included meta-analytic assessments of intervention efficacy in improving QOL [22–24] and focused primarily on the size and variability of exercise effects on QOL without considering potentially important features of the interventions that may moderate efficacy. Knowledge of what types of interventions are most efficacious, and for whom, is valuable to the professional organizations that set standards, such as the American College of Sports Medicine [7], among other parties. One prior meta-analysis also restricted main outcome analyses to breast cancer patients [25], which limits its generalizability. Another meta-analysis reported on QOL descriptively, but only reported meta-analytic results for fatigue outcomes [26]. The third meta-analysis did not restrict its sample to particular diagnoses of cancer or to controlled comparisons, and included moderator analyses, including variables such oncology characteristics, supervision, presence of fitness test, and funding status [22]. Yet, this analysis did not consider potentially important moderating intervention characteristics, such as number and length of intervention sessions, type and intensity of exercise, training of interventionists, or study quality; moreover, numerous new trials have since appeared. In sum, a comprehensive meta-analysis that explores moderators of intervention efficacy in this context is timely.
The current study is a meta-analytic assessment of the efficacy of exercise interventions for improving QOL in cancer survivors, including potentially important moderators of efficacy, such as number and duration of intervention sessions, training of interventionists, type, intensity, and length of exercise, study design, and supervision of sessions. Although exercise interventions may affect a number of outcomes in this population, such as fatigue and aerobic fitness [27, 28], the present study focuses solely on the QOL outcome and moderation of this outcome due to the complexities of the moderator analyses undertaken. The analysis is inclusive of all relevant studies across the literature, provided QOL was included as an outcome measure. Interventions including both controlled comparisons and one-group pre-test post-test comparisons are analyzed to determine efficacy of exercise interventions in improving QOL in this population, and moderators of efficacy, providing a more comprehensive examination of the patterns that emerge in these types of studies than analyses including controlled comparisons alone.
Method
Search Strategy
PsycINFO, PubMed, Cochrane Library, OregonPDF in Health and Performance, CINAHL Plus, Dissertations Abstracts, and SPORTSdiscus were searched to locate pertinent studies through February 2010. The search terms were [cancer OR malign*] AND [diagnos*OR post-diagnos* OR survivor* OR patient OR treatment OR recover* OR “with cancer”] AND [intervention OR randomized OR controlled OR effect* OR trial OR program* OR study AND lifestyle OR physical activity OR exercise OR “weight training” OR “resistance training” OR rehabilitat*]. Reference sections of pertinent review articles and meta-analyses were also manually searched [15, 17, 22–26, 29–31], as were reference sections of included studies.
Selection Criteria
To qualify, studies must have evaluated an intervention designed to affect exercise behavior in adult human cancer survivors, included a measure of QOL, and provided adequate statistical information to calculate effect sizes. Studies must have provided an appropriate comparison for QOL values post-intervention, either through a randomized control group or pre-tests. Separate analyses were performed on studies with controlled comparisons and those that only offered one-group post- vs. pre-test comparisons; quality of study was coded and included in analyses as a control variable (described below). No language restrictions were applied. Preliminary abstract screening yielded 405 potentially appropriate studies that were screened in full (see Fig. 1); of these, 327 studies were excluded due to (a) lack of QOL measure, (b) inappropriate population (e.g., pediatric cancer survivors), (c) inadequate statistical information and inability to obtain such information from study authors, (d) lack of control group of pre-test comparison measures, (e) self-selection to intervention or control condition, or (f) inclusion of data published in another study included in the present meta-analysis.
Fig. 1.
Flow diagram of study selection
Consistent with meta-analytic convention, each intervention was treated as an individual study during analysis [32, 33], including cases when an article provided information regarding multiple interventions or when statistical summaries grouped results separately for gender, location, or targeted group. The control groups in some studies were not “true” controls, in that participants were given some exercise instruction or content, and in these cases, the control group was considered a separate intervention group in the present meta-analyses; such cases were analyzed as one-group pre-post comparisons. The final sample included 91 interventions from 78 studies [18–21, 34–106]. The total number of participants in intervention groups in the final sample at the first follow-up was 3,629. Table 1 lists these studies.
Table 1.
Characteristics of studies included in the sample
| Study | Population (and number of treatment groups) | Final n in treatment group(s) | QOL measure used in analyses | Post-treatment FUPsa | Analytic designb |
|---|---|---|---|---|---|
| Adamsen et al. (2003) | Cancer patients undergoing chemotherapy (1) | 21 | EORTC QLQ-30 | 1 | Within |
| Adamsen et al. (2009) | Cancer patients undergoing chemotherapy (1) | 118 | EORTC QLQ-30 | 1 | RCT |
| Basen-Enquist et al. (2006) | Post-treatment breast cancer survivors (1) | 28 | SF-36 | 1 | RCT |
| Berglund et al. (1994) | Cancer patients (1) | 89 | 2 Global itemsc | 2 | RCT |
| Borst (2005) | Cancer patients (1) | 62 | FACTIT | 1 | Within |
| Burnham & Wilcox (2002) | Breast, colon, and lung cancer patients (1) | 6 | Quality of Life Indexc | 2 | RCT |
| Cadmus et al. (2009) | Breast cancer patients during/after treatment (2) | 25, 37 | FACT-G | 1 | RCT |
| Campbell et al. (2005) | Scottish breast cancer patients (1) | 12 | FACT-G | 1 | Within |
| Cheema & Gaul (2006) | Breast cancer survivors (1) | 27 | WHOQOL-BREF | 1 | Within |
| Cho et al. (2006) | South Korean breast cancer patients (1) | 28 | QOL Instrumentc | 1 | RCT |
| Courneya, Friedenreich, Quinney et al. (2003) | Colorectal cancer survivors < 3 months post-surgery (1) | 62 | FACT-G | 1 | RCT |
| Courneya, Friedenreich, Sela et al. (2003) | Cancer survivors (1) | 51 | FACT-G | 1 | RCT |
| Courneya et al. (2008) | Anemic cancer patients receiving Darbepoetin Alfa (1) | 26 | FACT-An | 1 | RCT |
| Courneya, Mackey, et al. (2003) | Breast cancer patients who had completed radiation or surgery (1) | 28 | FACT-B | 1 | RCT |
| Courneya et al. (2007) | Breast cancer patients beginning chemotherapy (2) | 74, 75 | FACT-An | 2 | RCT |
| Courneya et al. (2009) | Lymphoma patients (1) | 60 | FACT-An | 1 | RCT |
| Culos-Reed et al. (2006) | Cancer survivors not undergoing treatment (1) | 18 | EORTC QLQ-30 | 1 | Within |
| Culos-Reed et al. (2007) | Prostate cancer patients receiving ADT in next 6 months (1) | 31 | EORTC QLQ-30 | 2 | Within |
| Culos-Reed et al. (2010) | Prostate cancer patients receiving ADT (1) | 40 | EORTC QLQ-30 | 1 | RCT |
| Daley et al. (2007) | Post-treatment breast cancer survivors (2) | 31, 34 | FACT-G | 2 | RCTd |
| Damush et al. (2006) | Post-treatment breast cancer survivors > 50 years old (1) | 30 | CARES-SF | 1 | Within |
| Danhauer et al. (2008) | Ovarian and breast cancer survivors who had surgery over 2 years prior to enrollment (2) | 14, 37 | FACT-G | 2 | Within |
| De Backer et al. (2007) | Dutch cancer patients (1) | 57 | EORTC QLQ-30 | 1 | Within |
| De Backer et al. (2008) | Dutch cancer survivors who had completed surgical or radiotherapy (1) | 30 | EORTC QLQ-30 | 1 | RCT |
| Demark-Wahnefried, Clipp, Lipkus et al. (2007) | Early stage breast and prostate cancer patients (1) | 253 | FACT-G | 1 | RCT |
| Demark-Wahnefried, Clipp, Morey et al. (2007) | Breast and prostate cancer patients > 65 years old (1) | 77 | FACT-B, FACT-P | 2 | RCT |
| Dimeo et al. (2004) | Post-surgery cancer patients (1) | 34 | EORTC QLQ-30 | 1 | RCT |
| Durak & Lilly (1999) | Carcinoma/lymphoma/leukemia patients (1) | 47 | Rotterdam QOL | 2 | Within |
| Earle et al. (1996) | Breast cancer patients receiving adjuvant therapy (1) | 5 | FACT-G | 2 | Within |
| Fillion et al. (2008) | Breast cancer survivors who completed initial treatment < 2 years prior (1) | 44 | SF-36 | 2 | RCT |
| Galavo et al. (2010) | Prostate cancer patients undergoing AST (1) | 29 | SF-36 | 1 | RCT |
| Hall (1998) | Breast cancer survivors (2) | 9, 11 | FACT-G | 1 | RCT |
| Study | Population (and number of treatment groups) | Final n in treatment group(s) | QOL measure used in analyses | Post-treatment FUPsa | Analytic designb |
| Hawkes et al. (2009) | Cancer survivors 6 months post-diagnosis (1) | 20 | FACT-C | 1 | Within |
| Hayes et al. (2004) | Cancer patients undergoing chemotherapy after blood stem cell transplantation (1) | 6 | CARES | 1 | RCT |
| Headley et al. (2004) | Stage IV breast cancer patients (1) | 16 | FACTIT | 1 | Within |
| Heim et al. (2007) | German breast cancer patients (2) | 29, 30 | FACT-G | 2 | Within |
| Herrero et al. (2006) | Breast cancer survivors (1) | 8 | EORTC QLQ-30 | 1 | RCT |
| Holley & Borger (2001) | Cancer patients (1) | 20 | FLIC | 1 | Within |
| Hughs (2004) | Hispanic breast cancer survivors (1) | 29 | SF-36 | 1 | Within |
| Hwang et al. (2009) | Breast cancer patients receiving radiotherapy (1) | 17 | WHOQOL-BREF | 1 | RCT |
| Ives (2009) | Post-treatment breast cancer survivors (2) | 7, 7 | FACT-B | 1 | Within |
| Jarden et al. (2009) | Cancer patients scheduled to undergoing allo-HSCT (1) | 17 | EORTC QLQ-30 | 3 | RCT |
| Kolden et al. (2002) | Breast cancer survivors (1) | 40 | FACT-G | 1 | Within |
| Kramer (1996) | Breast cancer survivors (1) | 13 | QOL Indexb | 1 | Within |
| Lee et al. (2010) | Korean gastric cancer survivors (1) | 21 | FACT-G | 1 | Within |
| Losito et al. (2006) | Cancer patients undergoing treatment (1) | 9 | SF-36 | 1 | Within |
| Lucas (2009) | Post-treatment breast cancer survivors (1) | 19 | FACT-B | 1 | Within |
| May et al. (2009) | Cancer patients < 3 months post-treatment (2) | 65, 66 | EORTC QLQ-30 | 3 | RCT |
| McClure et al. (2010) | Breast cancer patients with lymphedema (1) | 16 | SF-36 | 1 | RCTd |
| McKenzie & Kalda (2003) | Breast cancer patients > 6 months post-treatment (1) | 7 | SF-36 | 1 | RCT |
| McNeely (2007) | Head and neck cancer survivors (1) | 8 | FACT H&N | 1 | RCT |
| Milne et al. (2007) | Breast cancer patients within 24 months of diagnosis (2) | 28, 29 | FACT-B | 3, 1 | RCT/Withine |
| Monga et al. (2007) | Prostate cancer patients undergoing radiotherapy (1) | 11 | FACT-G | 1 | RCT |
| Morey et al. (2009) | Overweight cancer survivors diagnosed > 5 years prior to enrollment (1) | 319 | SF-36 | 1 | RCT |
| Mustian et al. (2009) | Breast and prostate cancer patients undergoing radiation therapy (1) | 19 | FACTIT | 2 | RCT |
| Mutrie et al. (2007) | Breast cancer patients undergoing treatment (1) | 82 | FACT-G | 2 | RCT |
| Ohira et al. (2006) | Breast cancer survivors (1) | 39 | CARES-SF | 1 | Within |
| Oldervoll et al. (2006) | Cancer patients 3–12 month life expectancy (1) | 34 | EORTC QLQ-30 | 1 | Within |
| Rogers et al. (2009) | Breast cancer patients receiving hormonal therapy (1) | 18 | FACT-G | 1 | RCTd |
| Schulz (1998) | Breast cancer patients (1) | 28 | SF-36 | 1 | Within |
| Schwartz (1999) | Breast cancer patients beginning chemotherapy (1) | 27 | QOL Indexc | 1 | Within |
| Segal et al. (2001) | Stage I and II breast cancer patients (2) | 32, 33 | FACT-G | 1 | RCT |
| Segal et al. (1997) | Advanced prostate cancer patients (1) | 7 | SF-36 | 1 | Within |
| Segal et al. (2003) | Prostate cancer patients receiving androgen deprivation therapy (1) | 74 | FACT-P | 1 | RCT |
| Speck et al. (2009) | Breast cancer survivors with or at risk for lymphedema (2) | 54, 59 | SF-36 | 1 | RCT |
| Tang et al. (2009) | Taiwanese Cancer patients (1) | 36 | SF-36 | 2 | RCT |
| Study | Population (and number of treatment groups) | Final n in treatment group(s) | QOL measure used in analyses | Post-treatment FUPsa | Analytic designb |
| Taylor et al. (2006) | Prostate cancer patients (1) | 36 | SF-36 | 1 | RCT |
| Thorsen et al. (2005) | Cancer patients who had received chemotherapy (1) | 59 | EORTC QLQ-30 | 1 | RCT |
| Todd et al. (2008) | Breast cancer patients who had undergone axillary node dissection (1) | 58 | FACT-B | 1 | Within |
| Turner et al. (2004) | Post-treatment breast cancer survivors | 10 | FACT-B | 1 | Within |
| Vallance et al. (2007) | Breast cancer patients (4) | 81, 84, 85, 88 | FACT-B | 1 | Within |
| Vito (2008) | Breast cancer patients being diagnosed or treated at enrollment (1) | 13 | FACT-B | 1 | Within |
| von Gruenigen et al. (2009) | Obese endometrial cancer survivors | 23 | FACT-G | 3 | RCT |
| Wiggins & Simonavice (2009) | Cancer survivors (1) | 10 | FACT-G | 2 | Within |
| Wilson, Jacobsen et al. (2005) | Cancer survivors who had received stem cell transplantation (1) | 13 | SF-36 | 1 | Within |
| Wilson, Taliaferro et al. (2006) | Cancer patients undergoing chemotherapy (1) | 13 | SF-36 | 1 | Within |
| Yang (2008) | Cancer patients undergoing treatment (1) | 11 | SF-36 | 1 | RCT |
CARES Cancer Rehabilitation Evaluation System [131], CARES-SF Cancer Rehabilitation Evaluation System Short Form [132], EORTC QLQ-30 European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire C30 [133], FACT-An Functional Assessment of Cancer Therapy—Anemia [134], FACT-B Functional Assessment of Cancer Therapy—Breast [135], FACT-G Functional Assessment of Cancer Therapy—General [110], FACT H&N Functional Assessment of Cancer Therapy—Head & Neck [136], FACT-P Functional Assessment of Cancer Therapy—Prostate [137], FACTIT Functional Assessment of Chronic Illness Therapy [138], FLIC Functional Living Index [139], SF-36 Short Form-36 [140], Rotterdam QOL[141], WHOQOL-BREF World Health Organization Quality of Life Assessment [142]
Follow-ups
Some randomized controlled trials (RCTs) had no true control group (e.g., studies where individuals were randomized to receive either exercise intervention or exercise + meditation intervention). As such, these studies were treated as within subjects comparisons with two intervention groups in analyses, though they received PEDro scores that were consistent with randomization
Generic, study-generated scale
No data were available for these studies to calculate within subjects experimental and control group ES for the combined meta-regression
This study had a delayed treatment control group that began receiving the intervention immediately after the experimental group had completed the intervention. Therefore, the experimental group was compared to the control group during the intervention and immediately post-intervention, and was treated as a pre–post study for the two follow-ups during which the control group was receiving the intervention (three total post-intervention follow-ups). The control group was treated as a pre–post study for the follow-up immediately after the intervention was administered to this group (one total post-intervention follow-up)
Data Extraction
Information was drawn from all studies by two coders who exhibited high inter-rater reliability (for the variables included in the analyses, Cohen’s κ = 0.97 for categorical variables; r = 0.95 for continuous variables); they were assisted by a native speaker in the case of one non-English report. The following moderator variables pertaining to intervention content were coded: (a) length of intervention (in weeks), (b) length of intervention sessions (min), (c) number of intervention sessions, (d) supervised vs. unsupervised exercise content, (e) training of intervention facilitators (e.g., exercise physiologist), (f) targeted aerobic metabolic equivalents of task (METs; an indicator of the level of physical exertion or exercise intensity), (g) targeted resistance METs, (h) inclusion of flexibility exercises, (i) use (or non-use) of theory in development of intervention content, and (k) interval of follow-up. The following moderator variables pertaining to participants were also coded: (a) age, (b) cancer type, and (c) gender. Study quality was also coded using the PEDro Scale [107], a modified version of the Delphi list [108]. The 10-item PEDro scale has been widely used to rate the quality of randomized controlled trials [109; www.pedro.fhs.usyd.edu.au) and assesses study characteristics such as random and concealed allocation of participants to study groups, blinding of assessors, and reporting of outcome measures.
Effect Sizes and Analyses
Statistical information was extracted from the studies in order to calculate effect sizes for the main outcome variable, QOL. In four cases, authors were contacted to request the necessary information to calculate an effect size; three provided it. In the few studies that provided multiple QOL scales and included the Functional Assessment of Cancer Therapy [4, 110] scale, this scale was used in effect size calculations, as QOL in this population is most often measured using this scale. In the absence of Functional Assessment of Cancer Therapy scores, other QOL scales were used, including non-standardized QOL scales (e.g., 18). Table 1 details the studies’ QOL measures.
Because outcomes are continuous, effect sizes for each intervention were calculated as standardized mean differences [111, 112]. They were calculated for first available post-intervention follow-up, which was either immediately post-intervention or shortly after; if present, effect sizes were also calculated for any delayed, last follow-up. For two-group comparisons, the d indicates the difference between the mean QOL values of the control and intervention groups, divided by the pooled standard deviation [113]. For one-group pre–post comparisons, d indicates the difference between the mean values of the pre-test and post-test, divided by the pre-test standard deviation [112]. The sign of effect sizes was set so that positive values indicated that intervention participants had improved QOL relative to baseline or to the control group. Only three studies reported difference scores and the correlation between observations; therefore no estimate of the correlation was used in calculating pre–post effect sizes. Instead, we used an estimator of the standardized mean difference and its variance that makes them equivalent [114]. All effect sizes were corrected for the bias that results from small sample sizes [113]. In addition, ds from two-group designs were corrected for baseline differences.
Analyses were performed using macros for SPSS and Stata [33]. Weighted mean effect sizes were computed under both fixed- and random-effects meta-analytic assumptions; homogeneity analyses (Q and I2) followed fixed-effects assumptions [115]. Moderator models followed fixed-effects assumptions. Sensitivity analyses were undertaken in cases when outliers were suspected to impact the main results. Publication bias was analyzed through three different strategies: trim and fill [116], Begg’s strategy [117], and Egger’s test [118]. In the first set of moderator analyses, moderators were assessed separately in bivariate analyses. In the second set of moderator analyses, coded features were also analyzed as possible moderators in a comprehensive meta-regression, as a large enough sample of effect sizes permitted moderator testing for change effects in all interventions and for those with a control group, a technique used in the literature to achieve adequate power for this type of meta-regression model [119]. Study dimensions that related significantly to effect size variability in bivariate analyses were entered into a model with simultaneous inclusion of all predictors, controlling for inter-correlations among the maintained study dimensions. Such models permit a determination of the extent to which variation may be uniquely attributed to surviving study dimensions. Dimensions that retained significance and exhibited stable coefficients were retained. I2 was used to determine whether the effect sizes were homogeneous after application of the model in question. Values of I2 range from 0% to 100%, where significantly non-zero values imply the absence of homogeneity (i.e., greater variability than would be expected by sampling error alone, see 115]. In subsequent analyses, moderators were explored in this manner separately among higher quality (PEDro scores < 6) and lower quality studies.
Results
Characteristics of Studies
Studies appeared recently, between 1994 and 2010 (M = 2006) and were in English except for one in German [84]. Ninety percent of the studies were from peer-reviewed journal articles, and the remaining 10% were dissertations or theses. The mean length of intervention was 13.5 weeks (SD = 11.1), the mean length of intervention session was 51.1 min (SD = 30.6), and the mean number of sessions per intervention was 22.8 (SD = 22.0). The mean level of targeted aerobic METs was 4.2 (SD = 2.2), and the mean level of targeted resistance METs was 2.5 (SD = 2.2). Thirty-six percent of interventions included used trained intervention leaders; 56% of the interventions featured supervised exercise sessions. Only 19% of the studies reported the use of theory in intervention development. The mean participant age was 55.0 years (SD = 6.8). Approximately 54% of the studies featured breast cancer survivors, 8% featured prostate cancer survivors, 2% featured colorectal cancer survivors, 1% each featured endometrial, head–neck, lymphoma, and ovarian cancer survivors, and the remainder (32%) featured survivors of mixed diagnosis. There were no significant differences among study characteristics between published and unpublished studies, although unpublished studies had marginally smaller sample sizes overall (p = 0.07).
Overall Intervention Effects
Exercise interventions had a positive and significant effect on QOL among all intervention groups and in controlled comparisons. In studies that reported a delayed follow-up assessment, effect sizes remained positive and significant. As depicted in Table 2, effect sizes remain consistently positive, regardless of design and using both fixed- and random-effects assumptions at the first available and delayed post-intervention follow-up. Examining distribution anomalies for controlled comparisons, trim-and-fill identified 15 studies were necessary to add to normalize the effect size distribution and Begg’s test (z = 3.35, p < 0.001) and Egger’s test (t = 2.99, p < 0.01) indicated the bias was significant. For pre–post comparisons, 23 studies were necessary to add to correction bias and Begg’s test (z = 3.12, p < 0.001) and Egger’s test (t = 4.10, p < 0.001) indicated the bias was significant. For both types of effect sizes, trim-and-fill suggested that both fixed-effects mean effect sizes remained significant after imputing potentially missing effect sizes. The random-effects means indicated significance except for controlled comparisons. Omitting unpublished research from these calculations left the amount of bias the same. In the observed effect sizes, I2 statistics indicated there is more variability in effect sizes than sampling error alone would predict (Table 2), which implies that the means inadequately model trends in the studies and that more complex models are needed.
Table 2.
Central tendencies and variability in the impact of exercise interventions on quality of life
| Effect size comparison | k | M weeks after intervention (SD) | Weighted mean d+ (95% CI)
|
Homogeneity of effect sizes I2 (95% CI)a | |
|---|---|---|---|---|---|
| Fixed-effects assumptions | Random-effects assumptions | ||||
| Immediate FUP | |||||
| All intervention groups | 81 | 1.33 (3.67) | 0.24 (0.20, 0.28) | 0.34 (0.25, 0.43) | 69 (60.88, 75.18) |
| All intervention groups in RCTs | 43 | 0.19 (0.85) | 0.19 (0.14, 0.23) | 0.30 (0.18, 0.42) | 72 (62.38, 79.49) |
| All control groups (in RCTs) | 42 | 0.19 (0.86) | 0.12 (0.072, 0.16) | 0.13 (0.0072, 0.26) | 71 (60.75, 78.88) |
| Intervention vs. control (in RCTs controlling for baseline differences) | 53 | 0.47 (2.44) | 0.16 (0.10, 0.21) | 0.24 (0.12, 0.35) | 66 (54.47, 74.42) |
| Delayed FUP | |||||
| All intervention groups | 21 | 18.76 (21.39) | 0.35 (0.27, 0.44) | 0.42 (0.23, 0.61) | 76 (63.96, 84.39) |
| All intervention groups in RCTs | 8 | 18.00 (8.94) | 0.21 (0.11, 0.32) | 0.21 (−0.049, 0.47) | 52 (0, 78.59) |
| All control groups (in RCTs) | 8 | 18.00 (8.94) | 0.13 (0.028, 0.23) | 0.11 (−0.16, 0.39) | 22 (0, 64.04) |
| Intervention vs. control (controlling for baseline differences) | 10 | 17.60 (7.93) | 0.15 (0.017, 0.28) | 0.20 (−0.058, 0.46) | 36 (0, 69.60) |
Weighted mean effect sizes (d+) are positive for differences that favor the intervention group (higher quality of life) relative to the comparison (a control group or a pre-test baseline assessment)
CI confidence interval, FUP follow-up assessment, RCT randomized controlled trial
Values significantly higher than 0 imply the rejection of the hypothesis of homogeneity (i.e., there is more variability in effect sizes than expected by sampling error alone)
Bivariate Moderator Analyses
First examined were the controlled comparisons for the first available post-exercise intervention follow-up. Intervention efficacy increased as (a) the sample size decreased (β = −0.32, p < 0.01); (b) the length of intervention in weeks decreased (β = −0.20, p = 0.02); (c) exercise was supervised (β = −0.26, p < 0.01); (d) the intervention was administered to breast cancer patients (β = 0.36, p < 0.01); (e) percentage of breast cancer patients increased (β = 0.22, p < 0.01), and (f) percentage of breast cancer patients increased (β = 0.22, p < 0.01). Targeted aerobic activity intensity was also a significant predictor of QOL improvements as a quadratic trend (β = 0.25, p = 0.03). Study quality and minutes per intervention session were not significant predictors of intervention efficacy, and percentage of women was only a marginally significant predictor. Results from pre–post comparisons among all interventions generally supported these patterns, although in these analyses supervision and type of cancer did not moderate intervention effects. Number of intervention sessions, targeted resistance METs, training of facilitators, and inclusion of flexibility content were not significant moderators in either analysis. Sensitivity analyses detected outliers on length of intervention and minutes per session, but excluding such outliers left the pattern of results intact. Table A1 in the electronic supplementary materials summarizes these results.
Bivariate moderator analyses stratified by study quality revealed that several moderators related to effect sizes in different patterns depending on study quality. Sample size, percentage of women, and percentage of breast cancer survivors were more strongly associated with QOL outcomes among lower quality studies than higher quality studies. In addition, length had a significantly larger relation in lower quality studies than for higher quality studies. The quadratic trend of aerobic METs was significant for higher but not lower quality studies. Number of intervention sessions, targeted resistance METs, training of facilitators, and inclusion of flexibility content were once again not significantly related to QOL outcomes. Table A2 in the electronic supplementary materials details these results.
Combined Moderator Analyses
When all predictors were entered simultaneously (Table 3), intervention efficacy increased as (a) aerobic METS increased, in a quadratic trend that grew more pronounced with increasing length; (b) percentage of women increased; and (c) study quality (PEDro score) decreased; the latter trend narrowly missed statistical significance. Other moderators were non-significant when these variables were controlled and therefore were trimmed. This model explained 20.45% of the variance in QOL effect sizes. Examining these trends among studies that scored 6 or higher on the PEDro scale (Table 3), (a) the gender pattern remained intact (β = 0.38, p < 0.001); (b) the length × linear trend of METs interaction remained significant (β = 0.35, p = 0.028); (c) the quadratic trend for METs remained significant (β = 0.59, p < 0.001); but (c) the quadratic trend did not interact with length (β = 0.039, p = 0.80). With the quadratic term’s interaction with length omitted, this model explained 28.31% of the variance in QOL effect sizes and had a significantly better model fit than the same moderators in the studies with PEDro values less than 6 (I2 statistics = 58% vs. 64%, respectively). Figure 2 shows the quadratic pattern for aerobic METs emerges markedly in longer- but not shorter-duration studies, holding percentage of females constant at its sample mean (79% female).
Table 3.
Quality of life as a function of intervention characteristics, for all type of cancers and all interventions (pre- and post-intervention groups) and for all types of cancers and all interventions with high methodological quality (pre- and post-intervention groups) at first follow-up
| Study dimension and levela | All intervention groupsb
|
Higher quality studiesc
|
|||||
|---|---|---|---|---|---|---|---|
| Adjustedd d+ (95% CI) | β | p | Adjustedd d+ (95% CI) | β | p | ||
| Quadratic trend for aerobic METs × lengthe | 0.18 | 0.025 | 0.039 | 0.80 | |||
| Shorter (8 weeks) | 1 MET | 0.32 (0.11, 0.53) | 0.41 (−0.13, 0.96) | ||||
| 4 METs | 0.29 (0.23, 0.35) | 0.15 (0.064, 0.24) | |||||
| 8 METs | 0.24 (−0.014, 0.48) | 0.72 (0.14, 1.31) | |||||
| Longer (26 weeks) | 1 MET | 0.23 (−0.12, 0.58) | 0.21 (−0.64, 1.069) | ||||
| 4 METs | 0.22 (0.17, 0.28) | 0.16 (0.010, 0.22) | |||||
| 8 METs | 1.46 (0.90, 2.03) | 1.40 (0.50, 2.29) | |||||
| Percentage of women | 0.15 | 0.023 | 0.38 | 0.0035 | |||
| 0% | 0.18 (0.062, 0.29) | 0.10 (−0.00084, 0.20) | |||||
| 100% | 0.33 (0.27, 0.39) | 0.328 (0.21, 0.34) | |||||
| Study quality | −0.13 | 0.053 | |||||
| 1 | 0.34 (0.27, 0.41) | NA | |||||
| 9 | 0.24 (0.18, 0.31) | ||||||
Each model in a weighted least-squares multiple regression, with study dimensions simultaneously entered as independent variables and the inverse variance as the weights. Positive ESs imply greater quality of life for the intervention group relative to the comparison group. High and low values for moderator category reflect maximum and minimum in sample.
CI confidence interval, d+ effect size, NA not applicable
Levels represent discrete observed categories
k = 81 post- vs. pre-test comparisons. This model explains 20.45% of the variance but is not correctly specified, I2 (77) = 64 (95% CI = 54.25, 72.07)
k = 41 post- vs. pre-test comparisons with methodological quality above 5 on the PEDro scale; this model explains 28.31% of the variance but is not correctly specified, I2 (43) = 58 (95% CI = 39.38, 71.31)
Adjusted (statistically controlling) for the inclusion of other study dimensions at their mean levels
The value of 8 weeks was the mean of the studies below the median and 26 weeks was the mean of the studies above the median for length; this model controls for the linear effects of aerobic METs and length, the patterns for which are not presented because they are less interpretable in the face of higher-order terms
Fig. 2.
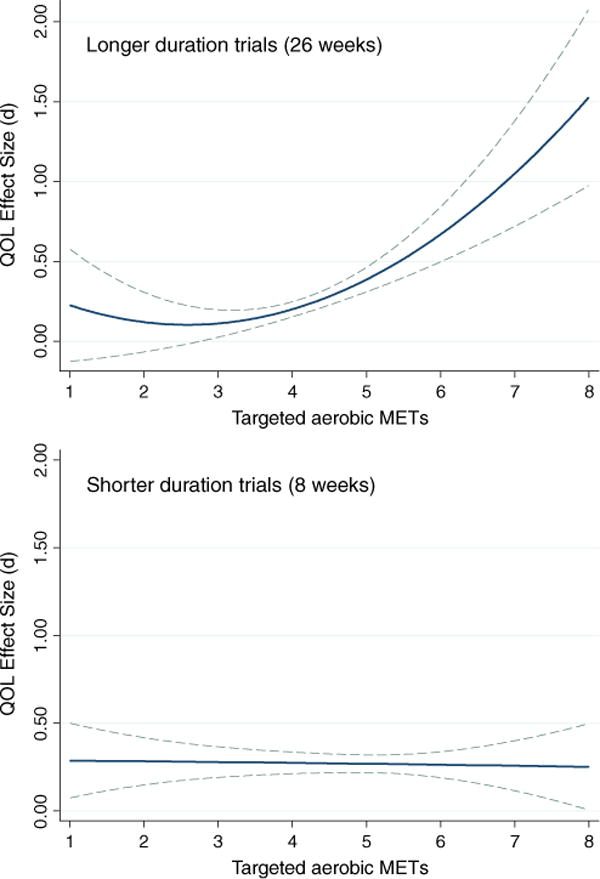
Quadratic trends of targeted aerobic METs on improvements in QOL for cancer survivors for longer (top panel) or shorter (bottom panel) duration exercise interventions; the top panel assumes 26 weeks and the bottom panel 8 weeks of training; both control the percentage of women in the sample at the mean. Error (95% CI) is noted with dashed lines
Non-significant Moderators and Sensitivity Analyses
In two-group and one-group pre–post comparisons, no significant moderating effects were found for targeted resistance METs, targeted aerobic METs, length of exercise session, or age. In two-group comparisons, as length of intervention (in weeks) increased, QOL effect sizes decreased. Yet, sensitivity analyses identified an outlier: One study lasted 52 weeks, whereas the mean number of weeks excluding this outlier was 13.16 (SD = 7.40). The effect size for this outlier study was non-significant (d = −0.005, 95% CI = −0.18, 0.17). In this intervention [51], intensive intervention content was delivered only in the early weeks, and in later weeks, minimal intervention content was delivered. When this outlier study was excluded from analyses, length of intervention was no longer significant. In one-group pre–post comparisons, length of intervention in weeks was positively and significantly associated with QOL effect size. Once again sensitivity analyses identified an outlier: one study lasted only a week, and had a moderate negative effect size (d = −0.41, 95% CI = −0.82, 0.17). When this study [85] was excluded from analyses, length of intervention was no longer significant in one-group pre–post comparisons.
Discussion
The present meta-analysis evaluated the efficacy of exercise interventions on QOL outcomes among cancer survivors. Overall, results showed that cancer patients of various diagnoses who participated in exercise interventions subsequently reported higher QOL in the studies’ first follow-up assessments, an effect that was significant in relation to simultaneous control groups and in relation to their baseline levels of QOL. Of great import, these effects were still intact on assessments usually taken months later (Table 2), a finding not explored in previous meta-analyses, which did not examine extended follow-up [24–26]. The main effect sizes for first available follow-up were similar in direction and magnitude—positive and medium—to overall effect sizes reported in previously published meta-analyses examining the efficacy of exercise interventions in improving quality of life in cancer patients [24–26]. These effects compare favorably to other health promotion literatures [120].
A burgeoning sample of available interventions made it feasible to examine what features of the interventions offer the most benefit for quality of life, something that one prior meta-analysis [22] had done only to a limited extent. Across bivariate and combined analyses in both high- and low-quality studies, targeted aerobic METs emerged as an important predictor of intervention efficacy. Low amounts of aerobic activity were associated with little or no QOL change, but in studies of longer duration, larger amounts of aerobic activity were associated with substantial QOL change (Fig. 2). Thus, aerobic exercises like moderate intensity bicycling (six METs) were associated with greater QOL increases than lower intensity aerobic exercises like walking (four METs; see 121 for a detailed listing of physical activities and corresponding METs), especially in trials of long duration. The physiological and psychological benefits of aerobic activity, including its impact on QOL, may not appear in just a short period with moderate METs, but rather may take considerable time with relatively high METs to emerge consistently. Thus, to see an impact, an intervention would need to facilitate extended participation in exercise, either through longer intervention duration, or maintain greater fidelity to the exercise routine once the intervention ends, or both. Indeed, our analyses support this assertion: at first available post-intervention follow-up, QOL change was most likely to appear in studies of longer duration whereas higher targeted METs had little impact for studies of shorter duration (Fig. 2). This pattern provides some evidence that the advantage of interventions with lower targeted METs among interventions targeting one to three METs may be illusory or due to non-exercise factors such as social support, and may disappear at longer intervals. Although QOL might improve following this pattern as METs further increased, few interventions in this analysis exceeded six targeted aerobic METs, meaning inferences beyond that value are tenuous and further research is necessary to determine whether these effects hold at higher METs. Additionally, studies that included more women tended to produce greater improvements in QOL. This tendency for the interventions to work better for women than men parallels findings of a recent meta-synthesis of health promotion meta-analyses [120]. Of note, in the current meta-analysis, the impact of targeted aerobic METs and percentage of women was more marked in higher quality intervention studies.
A prior meta-analysis reported that supervised exercise was marginally linked to QOL improvement [22]. The current work found mixed results concerning its role in intervention efficacy, such that it was significant in bivariate analyses among controlled but not pre–post comparisons, and was not significant when entered simultaneously with other moderators in pre–post comparisons of all interventions. These patterns suggest that supervision is not directly linked to improvement. Cancer type was previously found not to be a significant moderator [22]; our bivariate analyses showed a relation, but it did not remain significant when entered simultaneously with other predictors like percentage of women, which is highly correlated. Thus, type of cancer appears to be less directly connected to QOL changes than other variables.
Several other variables related on a bivariate basis, including sample size, length of intervention in weeks, and percentage of breast cancer patients. Yet, when they were entered simultaneously with aerobic METS and sample gender, these dimensions did not retain significance, suggesting they are less directly related to QOL improvements. Number of intervention sessions, minutes per intervention session, targeted resistance METs, training of facilitators, and inclusion of flexibility content did not explain variation. Note that the mean value for targeted resistance METs was quite low (2.5), which limits conclusions concerning this potential moderator, as interventions may not have targeted high enough resistance METs to yield an effect.
The current research has several important implications. First, exercise interventions appear to be a generally efficacious way to improve QOL among cancer patients of various diagnoses, supporting previous research concerning the impact of physical activity on improving cancer survivors’ QOL [6]. It also supports the current recommendations of cancer patient providers concerning exercise in cancer patients, which encourage physically active cancer patients to continue previously established exercise habits and sedentary cancer patients to adopt a moderate program of exercise [7, 8].
Taken together, these results support the development of interventions focusing on levels of aerobic METs of moderate intensity in the range of five to six METs, although do not identify particular types of cancer survivors for whom more moderate intensity aerobic exercise will be most beneficial. In addition, there is limited evidence that supervised exercise sessions and intervention length play a more distal role in moderating efficacy; these moderators did not remain significant in combined analyses. There is also evidence that these types of interventions are more efficacious for women, which highlights not only the benefit of these types of interventions for this group, but also the need for development and refinement of interventions for men. No evidence suggests that number or length of sessions, resistance METS, training of facilitators or flexibility content moderate exercise-induced QOL improvements.
Limitations
There are several limitations to the present research. First, the present meta-analysis did not examine adherence or contamination within the exercise intervention protocol, as many of the studies did not report such measures. Yet, the current work’s primary aim was to identify interventions that were efficacious in increasing QOL of cancer survivors, rather than to identify whether exercise per se affected QOL. Therefore, adherence to the exercise intervention protocol can be seen as a function of the intervention itself, and failing to control for adherence therefore does not affect the validity of the meta-analysis.
An additional limitation concerns the search strategy. Seven large research databases were searched for relevant studies, but no unpublished literature was obtained other than dissertations and theses, which comprised 10% of the sample. Additionally, the analysis included published studies that yielded non-significant or negative effect sizes. Publication bias was also explored using three statistical techniques, and bias was present to some extent. Yet, the fact that the aerobic METs and gender effects patterns were more marked in higher than lower quality studies (Table 3) suggests that aerobic exercise genuinely impacts QOL especially for female cancer survivors.
Another limitation concerns the study population and potential interactions with moderator variables. It is possible that recruitment into trials was selective, such that high-functioning survivors were more likely to elect or be eligible to participate. These high-functioning survivors may tolerate, and benefit from, more intense exercise than would their low-functioning counterparts. Measures of functioning among survivors recruited into studies, or proxy measures of functioning such as time since treatment, are not routinely reported across the literature, making it difficult to evaluate whether high- and low-functioning survivors benefit from different interventions targeting different intensities of aerobic activity. Similarly, although the results suggest that exercise interventions work best for women, there were too few male samples to evaluate whether the aerobic intensity relates to QOL improvements in the same pattern as that shown for women. Until more studies with males and with identifiably lower functioning patients are available, the results of this meta-analysis should be interpreted with caution when generalizing the current results to these target groups.
A related limiting factor is lack of detail about the exercise interventions and their samples [122, 123]. It is possible that a consideration of such aspects as wellness or social support, which has been shown to play a role in cancer outcomes [124], and many of the moderating variables could be correlated with social support or other behavioral change tactics, including minutes per session, number of sessions, and length of intervention. Yet, the analyses could not control for social support, as studies did not report the necessary descriptive information for such analyses, such as whether participants in unsupervised interventions engaged in exercise alone or with friends. As stated previously, functioning level of cancer survivors may be another important factor we cannot characterize, similar to any potential benefits of exercise interventions that focus on resistance exercise. Additionally, there may be other intervention characteristics, such as tone of intervention delivery, setting, and cohesion of participants that could affect outcome but are not reported, thus limiting our ability to predict variations in efficacy. These unreported characteristics represent a restriction of range and likely contributed to relatively poor model fit in moderator analyses. In the future, more emphasis should be placed on identifying and publishing characteristics of interventions whether they are efficacious or not, so that this knowledge may be used in the development of novel exercise interventions.
This meta-analysis also highlights the lack of theoretically driven interventions in this domain. The majority of the interventions did not explicitly state whether they were theoretically informed, and as such moderator analyses examining the differential efficacy of theory-based interventions were not possible. Of the few studies that did mention theory, most did not explain how the theory contributed to intervention development, so inclusion of theory-based intervention content could not be evaluated. Interventions that draw on empirically validated theory may be more efficacious in changing behavior than those that do not [125–127], and as such, future exercise interventions for cancer survivors may benefit from the use of theory in their development.
Future Directions
Future meta-analyses should examine outcomes in addition to those in this study. Possibilities include fatigue, depression, body mass index, and aerobic capacity. Several recent systematic literature reviews and meta-analyses have, in fact, addressed the efficacy of exercise interventions in reducing fatigue in cancer survivors, although these analyses had strict selection criteria and therefore may not represent the body of literature well enough [29, 30], or were broader reviews but failed to explore plausible moderators of intervention efficacy [31].
It may be of great interest to clinicians and cancer survivors alike to determine whether exercise interventions would provide additional benefits to the currently increasing length or rate of post-cancer diagnosis survival. Although preliminary evidence suggests that exercise increases life expectancy after cancer diagnosis [128, 129], no studies in the current meta-analysis examined this variable of interest. One potential reason for this absence is that available resources have limited researchers’ ability to retain participants for observation in studies spanning a time period long enough to measure post-diagnosis survival in a meaningful way. The lack of life expectancy outcome measures in this literature highlights the need for additional funding devoted to designing and implementing studies that could capture this outcome reliably. Additionally, absent resources to examine actual survival, researchers could measure surrogate markers of survival, such as maximum VO2[15, 130], the gold-standard measure of cardiorespiratory physical fitness. Of note, to date, published studies do not consistently report such statistics. Future studies should incorporate such measures.
Related, there is a lack of consistency across the literature in terms of what outcomes are reported. A large number of studies that involve exercise interventions did not assess QOL, examining instead biological measures such as maximum VO2 or timed distance tests [28] or other psychological outcomes such as fatigue or depression [27]. Better standardizing the outcomes of interest would permit more thorough comparisons of intervention impact across the literature. We recommend researchers in this field measure and report a wide and consistent variety of psychological measures, including QOL, fatigue, and depression, as well as measures of health fitness including cardiovascular physical fitness, muscle strength and endurance, body composition, and flexibility. In addition, we recommend that future research incorporate extended follow-up measures of these outcomes in addition to immediately post-intervention outcome evaluations. Future meta-analyses could examine the extent to which improvements on these latter outcomes are related to QOL, among other patterns.
The present study also validates recommendations to develop interventions designed to improve QOL by increasing exercise levels in cancer survivors, and highlights intervention characteristics that may be important to explore further in improving QOL. Future intervention development may benefit from the conclusions of this meta-analysis. Specifically, this analysis supports further exploring optimal levels of targeted aerobic METs and provides limited support for further exploring the role of intervention length and supervised exercise sessions. The development of future interventions designed to ascertain optimal levels of aerobic and resistance METs, intervention length, and supervision is necessary. Though the analysis demonstrates the efficacy of such interventions in improving QOL in female cancer survivors, and thus supports the development of future interventions in this population, it also highlights the lack of efficacious interventions of this type for male cancer survivors. As such, the development and refinement of exercise interventions for male cancer survivors is encouraged. Since the present meta-analysis demonstrates the importance of exercise, and exercise interventions, in improving QOL of cancer survivors, future research should attempt to refine the development of such interventions, guided by recommendations from research synthesis, in order to identify optimal ways to increase exercise behavior in this population.
Supplementary Material
Acknowledgments
This research was supported by University of Connecticut Research Foundation Grant 433527 to Blair T. Johnson and Linda B. Pescatello and facilitated by NIH grants F31MH080626 to Rebecca A. Ferrer and R01-MH58563 to Blair T. Johnson. We thank Michelle R. Warren for her feedback on prior versions of this manuscript.
Footnotes
Conflict of Interest Statement The authors have no conflict of interest to disclose.
Contributor Information
Rebecca A. Ferrer, Email: ferrerra@mail.nih.gov, Behavioral Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, 6130 Executive Blvd., MSC 7326, Room 4089A, Rockville, MD 20852, USA.
Tania B. Huedo-Medina, Department of Psychology and Center for Health, Intervention, and Prevention, University of Connecticut, Storrs, CT, USA
Blair T. Johnson, Department of Psychology and Center for Health, Intervention, and Prevention, University of Connecticut, Storrs, CT, USA
Stacey Ryan, Department of Kinesiology and Center for Health, Intervention, and Prevention, University of Connecticut, Storrs, CT, USA
Linda S. Pescatello, Department of Kinesiology and Center for Health, Intervention, and Prevention, University of Connecticut, Storrs, CT, USA
References
- 1.Jemal A, Clegg LX, Ward E. Annual report to the nation on the status of cancer, 1875–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun, MJ Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.de Jong N, Courtens AM, Au-Saad HH, Schouten, HC Fatigue in patients with breast cancer receiving adjuvant chemotherapy: A review of the literature. Cancer Nurs. 2002;25:283–297. doi: 10.1097/00002820-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Cella DF, Tulsky DS. Quality of life in cancer: Definition, purpose, and method of measurement. Cancer Invest. 2009;11:327–336. doi: 10.3109/07357909309024860. [DOI] [PubMed] [Google Scholar]
- 5.Victorson D, Barocas J, Song J, Cella D. Reliability across studies from the functional assessment of cancer therapy-general (FACT-G) Qual Life Res. 2008;17:1137–1146. doi: 10.1007/s11136-008-9398-2. [DOI] [PubMed] [Google Scholar]
- 6.Demark-Wahnefried W, Clipp EC, McBride C. Design of FRESH START: A randomized trial of exercise and diet among cancer survivors. Med Sci Sports Exerc. 2003;35:415–424. doi: 10.1249/01.MSS.0000053704.28156.0F. [DOI] [PubMed] [Google Scholar]
- 7.Thomspon WR, Gordon NF, Pescatello LS, editors. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 9. Baltimore, MD: Lippincott Williams & Wilkins; [Google Scholar]
- 8.Doyle C, Kushi LH, Byers T, Nutrition, Physical Activity, and Cancer Survivorship Committee Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56:323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 9.Rock CL, Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: A review of the evidence. J Clin Oncol. 2002;20:3302–3316. doi: 10.1200/JCO.2002.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20:1128–1143. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 11.Freedland SJ, Aronson WJ, Kane CJ. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: A report by the Shared Equal Access Regional Cancer Hospital database stuffy group. J Clin Oncol. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 12.Pinto BM, Truzo JJ, Reiss P, Shiu S. Exercising participation after diagnosis of breast cancer: Trends and effects on mood and quality of life. Psycho-Oncol. 2002;11:389–400. doi: 10.1002/pon.594. [DOI] [PubMed] [Google Scholar]
- 13.Pinto B, Maruyama N, Clark M. Motivation to modify lifestyle risk behaviors in women treated for breast cancer. Mayo Clin Proc. 2002;77:122–129. doi: 10.4065/77.2.122. [DOI] [PubMed] [Google Scholar]
- 14.Blanchard CM, Baker F, Denniston MM. Do adults change their lifestyle behaviors after cancer diagnosis? Am J Health Beh. 2003;27:246–256. doi: 10.5993/ajhb.27.3.6. [DOI] [PubMed] [Google Scholar]
- 15.Irwin ML. Physical activity interventions for cancer survivors. Br J Sports Med. 2008;43:32–38. doi: 10.1136/bjsm.2008.053843. [DOI] [PubMed] [Google Scholar]
- 16.Gross G, Ott C, Lindsey A, Twiss JJ, Waltman N. Postmenopausal breast cancer survivors at risk for osteoporosis: Physical activity, vigor, and vitality. Oncol Nurs Forum. 2002;29:1295–1300. doi: 10.1188/02.ONF.1295-1300. [DOI] [PubMed] [Google Scholar]
- 17.Blanchard CM, Courneya KS, Stein K, American Cancer Society Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26:2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 18.Basen-Engquist K, Taylor CLC, Rosenblum C, et al. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Educ Couns. 2006;64:225–234. doi: 10.1016/j.pec.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Berglund G, Bolund C, Gustafsson U-L, Sjoden P-L. A randomized study of a rehabilitation program for cancer patients: The “Starting Again” group. Acta Oncol. 1994;32:15–21. doi: 10.3109/02841869309083879. [DOI] [PubMed] [Google Scholar]
- 20.Hayes S, Davies PSW, Parker T, Bashford J, Newman B. Quality of life changes following peripheral blood stem cell transplantation and participation in a mixed-type moderate-intensity exercise program. Bone Marrow Transplant. 2004;33:553–558. doi: 10.1038/sj.bmt.1704378. [DOI] [PubMed] [Google Scholar]
- 21.Ohira T, Schmitz K, Ahmed R, Yee, D Effects of weight training on quality of life in recent breast cancer survivors: The weight training for breast cancer survivors (WTBS) study. Cancer. 2006;106:2076–2083. doi: 10.1002/cncr.21829. [DOI] [PubMed] [Google Scholar]
- 22.Conn VS, Hafdahl AR, Porock DC, McDaniel R, Nielsen PJ. A meta-analysis of exercise interventions among people treated for cancer. Support Care Cancer. 2006;14:699–712. doi: 10.1007/s00520-005-0905-5. [DOI] [PubMed] [Google Scholar]
- 23.Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005;16:3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- 24.Liu RDKS, Chinapaw MJM, Huijgens PC, van Mechelen W. Physical exercise interventions in haematological cancer patients, feasible to conduct but effectiveness to be established: A systematic literature review. Cancer Treat Rev. 2009;35:185–193. doi: 10.1016/j.ctrv.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 25.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. Can Med Assoc J. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cramp DF. Exercise for the management of cancer-related fatigue in adults (Review) The Cochrane Library. 2008;4:1–37. doi: 10.1002/14651858.CD006145.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Carlson LE, Smith D, Russell J, Fibich C, Whittaker T. Individualized exercise program for the treatment of severe fatigue in patients after allogenic hematopoietic stem-cell transplant: A pilot study. Bone Marrow Transplant. 1006;37:945–954. doi: 10.1038/sj.bmt.1705343. [DOI] [PubMed] [Google Scholar]
- 28.Dimeo F, Fetscher S, Lange W, Mertelsmann R, Keul J. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high dose chemotherapy. Blood. 1997;9:3390–3394. [PubMed] [Google Scholar]
- 29.Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psycho. 2007;26:660–667. doi: 10.1037/0278-6133.26.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lofti-Jam K, Carey M, Jefford M, Schofield P, Charleson C, Aranda S. Nonpharmalogical strategies for managing common chemotherapy adverse side effects: A systematic review. Journal of Clinical Oncology. 2008;26:5618–5629. doi: 10.1200/JCO.2007.15.9053. [DOI] [PubMed] [Google Scholar]
- 31.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: A systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008;134:700–741. doi: 10.1037/a0012825. [DOI] [PubMed] [Google Scholar]
- 32.Johnson BT, Boynton MH. Cumulating evidence about the social animal: Meta-analysis in social-personality psychology. Pers Soc Psych Com. 2008;2:817–841. [Google Scholar]
- 33.Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 34.Adamsen L, Midtgaard J, Rorth M, et al. Feasibility, physical capacity, and health benefits of a multidimensional exercise program for cancer patients undergoing chemotherapy. Support Care Cancer. 2003;11:707–16. doi: 10.1007/s00520-003-0504-2. [DOI] [PubMed] [Google Scholar]
- 35.Adamsen L, Quist M, Andersen C, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: Randomized controlled trial. BMJ. 2009;339:b3410. doi: 10.1136/bmj.b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borst JM. An exercise program for cancer patients: Physical and emotional well-being as indicators of quality of life. Michigan State University; 2005. Unpublished Doctoral Dissertation. [Google Scholar]
- 37.Burnham T, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Med Sci Sports Exerc. 2002;34:1863–1867. doi: 10.1097/00005768-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Cadmus LA, Salovey P, Yu H, Chung G, Kasl S, Irwin ML. Exercise and quality of life during and after treatment for breast cancer: Results of two randomized controlled trials. Psycho-Oncol. 2009;18:343–352. doi: 10.1002/pon.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell A, Mutrie N, White F, McGuire F, Kearney N. A pilot study of a supervised group exercise programme as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. Eur J Oncol Nurs. 2005;9:56–63. doi: 10.1016/j.ejon.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Cheema BSB, Gaul CA. Full-body exercise training improves fitness and quality of life in survivors of breast cancer. J Strength Cond Res. 2006;20:14–21. doi: 10.1519/R-17335.1. [DOI] [PubMed] [Google Scholar]
- 41.Cho O-H, Yoo Y-S, Kim N-C. Efficacy of a comprehensive group rehabilitation for women with early breast cancer in South Korea. Nurs Health Sci. 2006;8:140–146. doi: 10.1111/j.1442-2018.2006.00271.x. [DOI] [PubMed] [Google Scholar]
- 42.Courneya KS, Friedenreich C, Quinney H, Fields A, Jones L, Fairey A. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care. 2003;12:347–357. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 43.Courneya KS, Friedenreich C, Sela R, Quinney H, Rhodes R, Handman M. The group psychotherapy and home-based physical exercise (group-hope) trial in cancer survivors: Physical fitness and quality of life outcomes. Psycho-Oncol. 2003;12:357–374. doi: 10.1002/pon.658. [DOI] [PubMed] [Google Scholar]
- 44.Courneya KS, Jones LW, Peddle CJ, et al. Effects of aerobic exercise training in anemic cancer patients receiving Darbepoetin Alfa: A randomized controlled trial. Oncol. 2008;13:1012–1020. doi: 10.1634/theoncologist.2008-0017. [DOI] [PubMed] [Google Scholar]
- 45.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in post-menopausal breast cancer survivors: Cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21:1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 46.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 47.Courneya KS, Sellar CM, Stevinson C, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27:4605–4612. doi: 10.1200/JCO.2008.20.0634. [DOI] [PubMed] [Google Scholar]
- 48.Culos-Reed SN, Carlson L, Daroux L, Hately-Aldous S. A pilot study of yoga for breast cancer survivors: Physical and psychological benefits. Psycho-Oncol. 2006;15:891–897. doi: 10.1002/pon.1021. [DOI] [PubMed] [Google Scholar]
- 49.Culos-Reed SN, Robinson J, Lau H, O’Connor K, Keats M. Benefits of a Physical Activity Intervention for Men With Prostate Cancer. J Sport Exerc Psychol. 2007;29:118–127. doi: 10.1123/jsep.29.1.118. [DOI] [PubMed] [Google Scholar]
- 50.Culos-Reed SN, Robinson JW, Lau H, et al. Physical activity for men receiving androgen deprivation therapy for prostate cancer: Benefits from a 16 week intervention. Support Cancer Care. 2010;18:591–599. doi: 10.1007/s00520-009-0694-3. [DOI] [PubMed] [Google Scholar]
- 51.Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007;25:1713–1721. doi: 10.1200/JCO.2006.09.5083. [DOI] [PubMed] [Google Scholar]
- 52.Damush TM, Perkins A, Miller K. The implementation of an oncologist referred, exercise self-management program for older breast cancer survivors. Psycho-Oncol. 2006;15:884–890. doi: 10.1002/pon.1020. [DOI] [PubMed] [Google Scholar]
- 53.Danhauer SC, Tooze JA, Farmer DF, et al. Restorative yoga for women with ovarian or breast cancer: Findings from a pilot study. J Soc Integr Oncol. 2008;6:47–58. [PubMed] [Google Scholar]
- 54.De Backer IC, van Breda E, Vreugdenhil A, Nijziel MR, Kester AD, Schep G. High-intensity strength training improves quality of life in cancer survivors. Acta Oncol. 2007;46:1143–1151. doi: 10.1080/02841860701418838. [DOI] [PubMed] [Google Scholar]
- 55.De Backer IC, Vreugdenhil G, Nijziel MR, Kester AD, Schep G. Long-term follow-up after cancer rehabilitation using high-intensity resistance training: Persistent improvement of physical performance and quality of life. Br J Cancer. 2008;99:30–36. doi: 10.1038/sj.bjc.6604433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demark-Wahnefried W, Clipp EC, Lipkus IM, et al. Main outcomes of the FRESH START Trial: A sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25:2709–718. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 57.Demark-Wahnefried W, Clipp EC, Morey MC, et al. Lifestyle intervention development study to improve physical function in elder adults with cancer: Outcomes from Project LEAD. J Clin Oncol. 2006;20:3465–473. doi: 10.1200/JCO.2006.05.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dimeo FC, Thomas F, Raabe-Menssen C, Propper F, Mathias M. Effect of aerobic exercise and relaxation training on fatigue and physical performance of cancer patients after surgery: A randomised controlled trial. Support Care Cancer. 2004;12:774–79. doi: 10.1007/s00520-004-0676-4. [DOI] [PubMed] [Google Scholar]
- 59.Durak EP, Lilly PC. The application of an exercise and wellness program for cancer patients: A preliminary outcomes report. J Strength Cond Res. 1998;12:3–6. [Google Scholar]
- 60.Earle C, Reid B, Johnson D. Exercise may ameliate the effects of adjuvant breast cancer treatment on physical fitness and quality of life. Clin Invest Med. 1996;19:417. [Google Scholar]
- 61.Fillion L, Gagnon P, Leblond F, et al. A brief intervention for fatigue management in breast cancer survivors. Cancer Nurs. 2008;31:145–159. doi: 10.1097/01.NCC.0000305698.97625.95. [DOI] [PubMed] [Google Scholar]
- 62.Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: A randomized controlled trial. J Clin Oncol. 2010;28:340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 63.Hall J. Psychological interventions for exercise and dietary behavior change with breast cancer survivors. University of Houston; 1998. Unpublished doctoral dissertation. [Google Scholar]
- 64.Hawkes Al, Gollschewski S, Lynch BM, Chambers S. A telephone-delivered lifestyle intervention for colorectal cancer survivors ‘CanChange’: A pilot study. Psycho-Oncol. 2009;18:449–455. doi: 10.1002/pon.1527. [DOI] [PubMed] [Google Scholar]
- 65.Headley JA, Ownby KK, John LD. The effects of seated exercise on fatigue and quality of life in women with advanced breast cancer. Oncol Nurs Forum. 2004;31:977–983. doi: 10.1188/04.ONF.977-983. [DOI] [PubMed] [Google Scholar]
- 66.Heim ME, Elsner VD, Malsburg M-L, Niklas A. Randomized controlled trial of a structured training program in breast cancer patients with tumor-related chronic fatigue. Onkologie. 2007;30:429–434. doi: 10.1159/000104097. [DOI] [PubMed] [Google Scholar]
- 67.Herrero F, San Juan AF, Fleck SJ, et al. Combined aerobic and resistance training in breast cancer survivors: A randomized, controlled pilot trial. Int J Sports Med. 2006;27:573–580. doi: 10.1055/s-2005-865848. [DOI] [PubMed] [Google Scholar]
- 68.Holley S, Borger D. Energy for Living with Cancer: Preliminary findings of a cancer rehabilitation group intervention study. Oncol Nurs Forum. 2001;28:1393–1396. [PubMed] [Google Scholar]
- 69.Hughs DC. Physical activity and stress in Hispanic breast cancer survivors. University of Houston; 2004. Unpublished doctoral dissertation. [Google Scholar]
- 70.Hwang JH, Chang HJ, Shim YH, et al. Effects of supervised exercise therapy in patients receiving radiotherapy for breast cancer. Yonsei Med J. 2008;49:443–450. doi: 10.3349/ymj.2008.49.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ives J. A comparison between a combined exercise and recreation therapy intervention and an exercise only intervention in post-treated breast cancer patients. University of North Carolina; 2009. Unpublished master’s thesis. [Google Scholar]
- 72.Jarden M, Baadsgaard MT, Hovgaard DJ, Boesen E, Adamsen L. A randomized trail on the effect of a multimodal intervention on physical capacity, functional percormance, and quality of life in adult patients undergoing allogeneic SCT. Bone Marrow Transplant. 2009;43:725–737. doi: 10.1038/bmt.2009.27. [DOI] [PubMed] [Google Scholar]
- 73.Kolden GG, Strauman TJ, Ward A, et al. A pilot study of group exercise training (GET) for women with primary breast cancer: Feasibility and health benefits. Psycho-Oncol. 2002;11:447–456. doi: 10.1002/pon.591. [DOI] [PubMed] [Google Scholar]
- 74.Kramer MM. The effects of exercise on psychological well-being in women recovering from breast cancer. Arizona State University; 1996. Unpublished doctoral dissertation. [Google Scholar]
- 75.Lee EO, Chae YR, Song R, Eom A, Lam P, Heitkemper M. Feasibility and effects of a Tai Chi self-help education program for Korean gastric cancer survivors. Oncol Nurs Forum. 2010;37:E1–E6. doi: 10.1188/10.ONF.E1-E6. [DOI] [PubMed] [Google Scholar]
- 76.Losito JM, Murphy SO, Thomas ML. The effects of group exercise on fatigue and quality of life during cancer treatment. Oncol Nurs Forum. 2006;33:821–825. doi: 10.1188/06.ONF.821-825. [DOI] [PubMed] [Google Scholar]
- 77.Lucas J. The effect of a combined exercise and recreation therapy program on quality of life in post-treated female breast cancer patients. University of North Carolina at Chapel Hill; 2009. Unpublished master’s thesis. [Google Scholar]
- 78.May AM, Korstjens I, van Weert E, et al. Long-term effects on cancer survivors’ quality of life of physical training versus physical training combined with cognitive-behavioral therapy: Results from a randomized trial. Support Cancer Care. 2009;17:653–663. doi: 10.1007/s00520-008-0519-9. [DOI] [PubMed] [Google Scholar]
- 79.McClure MK, McClure RH, Day R, Brufsky AM. Randomized controlled trial of the breast cancer recovery program for women with breast cancer-related lymphedema. Am J Occup Ther. 2010;64:59–72. doi: 10.5014/ajot.64.1.59. [DOI] [PubMed] [Google Scholar]
- 80.McKenzie DC, Kalda AL. Effect of upper extremity exercise on secondary lymphedema in breast cancer patients: A pilot study. J Clin Oncol. 2006;21:463–466. doi: 10.1200/JCO.2003.04.069. [DOI] [PubMed] [Google Scholar]
- 81.McNeely ML. Exercise rehabilitation for breast and head and neck cancer survivors. University of Alberta; 2007. Unpublished doctoral dissertation. [Google Scholar]
- 82.Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: A randomized controlled trial. Breast Cancer Res Treat. 2007;108:279–288. doi: 10.1007/s10549-007-9602-z. [DOI] [PubMed] [Google Scholar]
- 83.Monga U, Garber SL, Thornby J, et al. Exercise prevents fatigue and improves quality of life in prostate cancer patients undergoing radiotherapy. Arch Phys Med Rehab. 2007;88:1416–1422. doi: 10.1016/j.apmr.2007.08.110. [DOI] [PubMed] [Google Scholar]
- 84.Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: The RENEW: randomized clinical trial. JAMA. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mustian KM, Peppone L, Darling TV, Palesh O, Heckler CE, Morrow GR. A 4-week home-based aerobic and resistance exercise program during radiation therapy: A pilot randomized clinical trial. J Support Oncol. 2009;9:158–167. [PMC free article] [PubMed] [Google Scholar]
- 86.Mutrie N, Campbell AM, Whyte F, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: Pragmatic randomised controlled trial. Br Med J. 2007;334:517–520. doi: 10.1136/bmj.39094.648553.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oldervoll LM, Loge JH, Paltiel H, et al. The effect of a physical exercise program in palliative care: A phase II study. J Pain Symptom Manage. 2006;31:421–430. doi: 10.1016/j.jpainsymman.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 88.Rogers LQ, Hopkins-Price P, Vicari S, et al. Physical activity and health outcomes three months after completing a physical activity behavior change intervention: Persistent and delayed effects. Cancer Epidemiol Biomarkers Prev. 2009;18:1410–1418. doi: 10.1158/1055-9965.EPI-08-1045. [DOI] [PubMed] [Google Scholar]
- 89.Schulz KH. Implementierung und evaluation eines ambulanten bewegungs-therapeutischen rehabilitations-angebotes fur brustk-rebspatientinnen. Psychother Psychosom, Med Psychol. 1998;48:398–407. [PubMed] [Google Scholar]
- 90.Schwartz AL. Fatigue mediates the effects of exercise on quality of life. Qual Life Res. 1999;8:529–538. doi: 10.1023/a:1008978611274. [DOI] [PubMed] [Google Scholar]
- 91.Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: Results of a randomized controlled trial. J Clin Oncol. 2001;19:657–665. doi: 10.1200/JCO.2001.19.3.657. [DOI] [PubMed] [Google Scholar]
- 92.Segal R, Reid B, Johnson D. Progressive resistance exercise training in men with advanced prostate cancer. Clinl Invest Med. 1997;20:S58. [abstract]. [Google Scholar]
- 93.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21:1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 94.Speck RM, Gross CR, Hormes JM, et al. Changes in the body image and relationship scale following a one-year strength training trial for breast cancer survivors with or at risk for lymphedema. [Published online ahead of print September 22 2009] Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0550-7. http://www.springerlink.com/content/1428112433361g45/. Accessed April 22, 2010. [DOI] [PubMed]
- 95.Tang M-F, Liou T-H, Lin CC. Improving sleep quality for cancer patients: Benefits of a home-based exercise intervention. [Published online ahead of print October 16 2009] Support Cancer Care. 2009 doi: 10.1007/s00520-009-0757-5. http://www.springerlink.com/content/061wq41587x60560/. Accessed April 22, 2010. [DOI] [PubMed]
- 96.Taylor CLC, Demoor CL, Smith MA, et al. Active for Life after cancer: A randomized trial examining a lifestyle physical activity program for prostate cancer patients. Psycho-Oncol. 2006;15:847–862. doi: 10.1002/pon.1023. [DOI] [PubMed] [Google Scholar]
- 97.Thorsen L, Skovlund E, Stromme SB, Hornslien K, Dahl AA, Fossa SD. Effectiveness of physical activity on cardiorespiratory fitness and health-related quality of life in young and middle-aged cancer patients shortly after chemotherapy. J Clin Oncol. 2005;23:2378–2388. doi: 10.1200/JCO.2005.04.106. [DOI] [PubMed] [Google Scholar]
- 98.Todd J, Scally A, Dodwell D, Horgan K, Topping A. A randomized controlled trial of two programmes of shoulder exercise following axillary node dissection for invasive breast cancer. Physiother. 2008;94:265–273. [Google Scholar]
- 99.Turner J, Hayes S, Reul-Hirche H. Improving the physical status and quality of life of women treated for breast cancer: A pilot study of a structured exercise intervention. J Surg Oncol. 2004;86:141–146. doi: 10.1002/jso.20065. [DOI] [PubMed] [Google Scholar]
- 100.Vallance JK, Courneya KS, Plotnikoff RC, Dinu I, Mackey JR. Maintenance of physical activity in breast cancer survivors after a randomized trial. Medi Sci Sports Exerc. 2007;40:173–180. doi: 10.1249/mss.0b013e3181586b41. [DOI] [PubMed] [Google Scholar]
- 101.Vito NL. The effects of a yoga intervention on physical and psychological functioning for breast cancer survivors. California School of Professional Psychology; Unpublished doctoral dissertation. [Google Scholar]
- 102.von Gruenigen VE, Gibbons HE, Kavanagh MB, Janata JW, Lerner E, Courneya KS. A randomized trial of a lifestyle intervention in obese endometrial cancer survivors: Quality of life outcomes and mediators of behavior change. Health Qual Life Outcomes. 2009;7:17. doi: 10.1186/1477-7525-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wiggins MS, Simonavice EM. Quality of life benefits in cancer survivorship with supervised exercise. Psych Rep. 2009;104:421–424. doi: 10.2466/PR0.104.2.421-424. [DOI] [PubMed] [Google Scholar]
- 104.Wilson RW, Jacobsen PB, Fields KK. Pilot study of a home-based aerobic exercise program for sedentary cancer survivors treated with hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35:721–727. doi: 10.1038/sj.bmt.1704815. [DOI] [PubMed] [Google Scholar]
- 105.Wilson RW, Taliaferro LA, Jacobsen PB. Pilot study of a self-administered stress management and exercise intervention during chemotherapy for cancer. Support Cancer Care. 2006;14:928–935. doi: 10.1007/s00520-006-0021-1. [DOI] [PubMed] [Google Scholar]
- 106.Yang Y. The effects of a Taiji Quan exercise intervention on balance, mobility, and quality of life of people treated for cancer. Lakehead University; Unpublished master’s thesis. [Google Scholar]
- 107.Verhagen AP, de Vet HC, de Brie RA, et al. The Delphi list: A criteria list for quality assessment of randomised clinical trials for conducting systematic reviews developed by Delphi consensus. Journal of Clinical Epidemiology. 1998;51:1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 108.Moseley AM, Herber RD, Sherrington C, Maher CC. Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database (PEDro) Australian Journal of Physiotherapy. 2002;49:43–49. doi: 10.1016/s0004-9514(14)60281-6. [DOI] [PubMed] [Google Scholar]
- 109.Sherrington C, Herbert RD, Maher CC, Moseley AM. PEDro: A database of randomised trials and systematic reviews in physiotherapy. Man Ther. 2000;5:223–226. doi: 10.1054/math.2000.0372. [DOI] [PubMed] [Google Scholar]
- 110.Fairclough DL, Cella DF. Functional Assessment of Cancer Therapy (FACT-G): Non-response to individual questions. Qual Life Res. 1996;5:321–329. doi: 10.1007/BF00433916. [DOI] [PubMed] [Google Scholar]
- 111.Hedges LV, Olkin I. Statistical methods for meta-analysis. Appl Psychol Meas. 1985;11:104–106. [Google Scholar]
- 112.Becker BJ. Synthesizing standardized mean-change measures. Br J Math Stat Psychol. 1988;41:257–278. [Google Scholar]
- 113.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psych Method. 2002;7:105–125. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 114.Johnson BT, Eagly AH. Quantitative synthesis of social psychological research. In: Reis HT, Judd CM, editors. Handbook of research methods in social and personality psychology. New York: Cambridge; 2002. pp. 496–528. [Google Scholar]
- 115.Huedo-Medina T, Sanchez-Meca J, Martin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2. Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 116.Duval S, Tweedlie Trim and Fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 117.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;4:1088–1101. [PubMed] [Google Scholar]
- 118.Egger M, Davey G, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. Bristish Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kirsch I, Deacon BJ, Huedo-Medina TB, Scobria A, Moore TJ, Johnon BT. Initial severity and antidepressant benefits: A meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 121.Johnson BT, Scott-Sheldon LAJ, Carey MP. Meta-synthesis of health behavior change meta-analyses. [Published online ahead of print February 12 2010] American Journal of Public Health. 2010 doi: 10.2105/AJPH.2008.155200. http://ajph.aphapublications.org/cgi/content/abstract/AJPH.2008.155200v2. Accessed May 4, 2010. [DOI] [PMC free article] [PubMed]
- 122.Stram DO. Meta-analysis of published data using linear mixed-effects models. Biometrics. 1996;52:536–544. [PubMed] [Google Scholar]
- 123.Lipsey MW. Those confounded moderators in meta-analysis: Good, bad, and ugly. Ann Am Acad Political Soc Sci. 2003;587:69–81. [Google Scholar]
- 124.Nausheen B, Gidron Y, Peveler R, Moss-Morris R. Social support and cancer progression: A systematic review. J Psychosom Res. 2009;67:403–415. doi: 10.1016/j.jpsychores.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 125.Noar SM. Behavioral interventions to reduce HIV-related sexual risk behavior: Review and synthesis of meta-analytic evidence. AIDS Beh. 2008;12:335–353. doi: 10.1007/s10461-007-9313-9. [DOI] [PubMed] [Google Scholar]
- 126.Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: A systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12:e4. doi: 10.2196/jmir.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions. Annu Rev Public Health. 2010;31:399–418. doi: 10.1146/annurev.publhealth.012809.103604. [DOI] [PubMed] [Google Scholar]
- 128.Holmes MD, Chen WY, Feskanich D, Croenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. J Am Med Assoc. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 129.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 130.Mancini D, LeJemetel T, Aaronson K. Peak VO2: A simple yet enduring standard. Circulation. 2008;101:1080. doi: 10.1161/01.cir.101.10.1080. [DOI] [PubMed] [Google Scholar]
- 131.Schag CAC, Heinrich RL. Development of a comprehensive quality of life measurement tool: CARES. Oncology. 1990;4:135–147. [PubMed] [Google Scholar]
- 132.Schag CA, Ganz PA, Heinrich RL. Cancer rehabilitation evaluation system—short form (CARES-SF). A cancer specific rehabilitation and quality of life instrument. Cancer. 1991;68:1406–1413. doi: 10.1002/1097-0142(19910915)68:6<1406::aid-cncr2820680638>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 133.Bjordal K, Kaasa S. Psychometric validation of the EORTC Core Quality of Life Questionnaire, 30-item version and a diagnosis-specific module for head and neck cancer patients. Acta Oncol. 1992;31:311–321. doi: 10.3109/02841869209108178. [DOI] [PubMed] [Google Scholar]
- 134.Cella D, Elton DT, Lai JS, et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 135.Brady MJ, Cella DF, Mo F. Reliability and validity of the Functional Assessment of Cancer Therapy—Breast quality of life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 136.List MA, D’Antonio LL, Cella DF, et al. The performance status scale for head and neck cancer patients and the Functional Essessment of Cancer Therapy—Head and Neck Scale. A study of utility and validity. Cancer. 1996;77:2294–2301. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2294::AID-CNCR17>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 137.Esper P, Mo F, Chodak G, et al. Measuring quality of life in men with prostate cancer using the Functional Assessment of Cancer Therapy—Prostate instrument. Urology. 1997;50:920–928. doi: 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
- 138.Webster ZK, Odom L, Peterman A, Lent L, Cella D. The functional assessment of chronic illness therapy (FACTIT) measurement system: Validation of version 4 of the core questionnaire. Qual Life Res. 1999;8:604. [Google Scholar]
- 139.Frank-Stromborg M, Olsen S. Instruments for clinical healthcare research. 2. Boston: Jones and Bartlett; [Google Scholar]
- 140.Ware JE, Kosinski M, Gandek B. SF-36 Health Survey: Manual and interpretation guide. Lincoln RI: Quality Metric Incorporated; 2000. [Google Scholar]
- 141.de Jeu JH, Pedersen SS, Balk RT, van Domburg RT, Vantrimpont PJMJ, Erdman RAM. Development of the Rotterdam Quality of Life Questionnaire for heart transplant recipients. Neth Heart J. 2003;11:289–293. [PMC free article] [PubMed] [Google Scholar]
- 142.The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28:551–558. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


