Abstract
(1R,3S)-(−)-trans-1-phenyl-3-dimethylamino-1,2,3,4-tetrahydronaphthalene (PAT) is a novel compound that has full-efficacy agonist activity at human 5-HT2C receptors and inverse agonist/antagonist activity at 5HT2A and 5HT2B receptors. In the present paper we describe its effects on food intake in non-deprived C57BL/6 mice adapted to eating a palatable dessert meal each day. PAT showed a dose-related inhibition of food intake with a 50% inhibitory dose of 4.2 mg/kg. The dose–effect curve was similar to that obtained using WAY-161503. Abnormal behaviors were not observed by casual inspection following administration of PAT. The anorectic effect of PAT was additive with that of amphetamine. When PAT, or PAT+amphetamine, were injected 2 h before access to food, most of the anorectic activity had dissipated, indicating that PAT has a biologically effective period of about 1 h. Four daily injections of PAT were associated with some, but not complete loss of the initial anorectic effect; this differs from the rapid tolerance that has been reported to fenfluramine anorexia and suggests that different mechanism(s) are involved in the loss of anorexia.
Keywords: Serotonin type 2C receptor, Food intake, Dose–effect curve, WAY-161503, Amphetamine, Isobologram, Tolerance
1. Introduction
Increased activation of brain serotonin (5-HT) receptors reduces food intake in animals and humans (Halford et al., 2007); these receptors are therapeutic targets to prevent overeating. The 14 mammalian serotonin receptor subtypes are grouped into the 5-HT1–5-HT7 families (Sanders-Bush and Mayer, 2006). The 5-HT2 receptor family consists of the 5-HT2A, 5-HT2B, and 5-HT2C membrane-bound G protein-coupled receptors (GPCRs) that signal primarily through Gαq protein to activate phospholipase C (PLC) and formation of inositol phosphates (IP) and diacylglycerol second messengers (Raymond et al., 2001). The human 5-HT2C receptor (Saltzman et al.,1991) is found exclusively in brain where it is expressed in many regions and seems to be involved in several physiological or psychological processes, including eating behavior (Tecott et al.,1995). For example, 5-HT2C receptor knockout mice demonstrate increased feeding and obesity, and they are resistant to the anorectic effects of S-(+)-fenfluramine (Heisler et al., 2002; Tecott et al., 1995; Vickers et al., 1999, 2001; Weintraub et al., 1992). Moreover, the hypophagic effects of S-(+)-fenfluramine in rats are fully blocked by pretreatment with SB242084 (Bromidge et al., 1997; Vickers et al., 2001), a 5-HT2C receptor antagonist with 100-times and 10-times selectivity over 5-HT2A and 5-HT2B receptors, respectively (Knight et al., 2004).
There is considerable interest to develop a highly selective serotonin 5-HT2C receptor agonist. Recently, we reported preliminary results concerning (1R,3S)-(−)-trans-1-phenyl-3-dimethylamino-1,2,3,4-tetra-hydronaphthalene (PAT), a novel compound synthesized in our laboratories that has full-efficacy agonist activity at human 5-HT2C receptors and inverse agonist/antagonist activity at 5-HT2A and 5-HT2B receptors (Bivens et al., 2007; Booth et al., 2008). The affinity (Ki) and functional activity (activation of PLC/IP formation) for PAT relative to basal constitutive activity was obtained using clonal cells expressing recombinant human 5-HT2A, 5-HT2B, or 5HT2C receptors. The affinity of PAT (Ki=410, 1200, 37.6 nM, at 5-HT2A, 5-HT2B, or 5-HT2C receptors, respectively) is about 10-times and 30-times greater for 5-HT2C versus 5-HT2A and 5-HT2B, respectively. Meanwhile,. PAT is a full-efficacy agonist with regard to activation of PLC/IP formation (concentration required to produce 50% maximal stimulation of IP formation=19.9 nM) at 5-HT2C receptors, but, is an inverse agonist/antagonist in the same assay at 5-HT2A and 5-HT2B receptors (concentration required to produce 50% maximal inhibition of IP formation=490 and 1000 nM, respectively). We believe PAT is the first 5-HT2C agonist reported that does not also activate 5-HT2A and/or 5-HT2B receptors (Nilsson, 2006; Richter et al., 2006; Siuciak et al., 2007; Wacker et al., 2007). The purpose of this paper is to describe the effects of PAT on feeding behavior in mice.
The use of mice rather than rats in preclinical feeding studies has several advantages among which are the lower amounts of compounds needed and potential extrapolation to genetic interactions. Previously, we developed a feeding protocol in mice that did not involve food restriction by using highly palatable sweetened milk “dessert” as the test meal (Rowland et al., 2001). In that study, we showed that the indirect 5-HT agonist S-(+)-fenfluramine had an anorectic effect that was additive with that of phentermine.
In the present work, we use a palatable solid food dessert having a nutrient composition similar to chow (Robertson and Rowland, 2005). We examine the acute dose–effect function of PAT, its effective time course of action, and its efficacy with repeated administration. Combinations of different classes of anorectic agents can sometimes be more effective than either alone, so we tested for supra-additive effects of PAT administered with amphetamine, a catecholamine releasing agent. The combination effect was assessed using isobologram analysis (Rowland et al., 2001; Roth and Rowland, 1999).
2. Materials and methods
2.1. Animals and housing
Several batches of mice were used in these studies. All were female C57BL/6 mice (Jax Labs), mostly about 3 months of age at the start of the study, but in one case 15 months of age. There were no obvious differences in the baseline intakes between batches, and in some cases the same treatments yielded similar results in different batches, so the results will be presented without regard to batch. All mice were housed singly in standard polycarbonate cages with Sani Chips (Harlan) bedding and wire mesh lids. Chow pellets (#5001, Purina) were available ad libitum from this lid, as well as tap water from a bottle. Each cage contained a red translucent polycarbonate refuge (Mouse Igloo®, Bio-Serv, Frenchtown NJ) in which most animals chose to rest. The vivarium was maintained at 22 °C and a 12:12 light cycle (on 0500 h) was in effect. The animal procedures in this protocol were approved by the Institutional Animal Care and Use Committee and are in accordance with the principles in the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
2.2. Dessert
The palatable dessert was comprised of small (190 mg) fruit flavored pellets of similar composition to chow (Fruit Crunchies®; Bio-Serv). Ten to 15 pellets were placed in glass 10 ml beakers, and these were presented inside the home cage using a stirrup and ring holder. Some mice removed pellets from the beaker, but these pellets could be easily retrieved from the bedding since they are brightly colored. During a familiarization period (1–2 days), the pellets were presented for 24 h, but thereafter for only 30 min per day (at about 1400 h) and 5 days per week. The number of pellets consumed was counted daily; we found this to be a suitably accurate measure because very few crumbs were observed, although on occasion half pellets were retrieved and counted.
2.3. Pharmacological tests
On designated test days, no more frequently than once per week except in the chronic studies, mice were injected intraperitoneally with a drug or its vehicle in a volume of .02 ml/10 g body weight 15 min before presentation of the dessert. The number of pellets consumed was counted as usual and, for each mouse, was expressed as a % of baseline computed as the average intake over the preceding 3 days. For the chronic studies, this baseline refers to the 3 days before the start of treatment.
2.4. Experiment 1: Acute dose–effect of PAT
In a preliminary study to compare this protocol with other published work, a dose–effect curve was established using the commercially available 5HT2C agonist, WAY-161503.
In the main study, a dose–effect curve for PAT was established. Mice (N=16) received Crunchies 5 days per week, and tests were performed on the 5th day. Mice were injected IP with the designated dose of PAT (1,3,6 or 10 mg/kg), or its vehicle (10% DMSO in saline, 0.02 ml/10 g body weight), 15 min before presentation of Crunchies. Intake was measured after 30 min. Mice were used in several tests using different doses in random order, and did not receive the same dose twice. On each test day, all doses were tested. No systematic differences were noted between tests so data were combined by dose, as planned.
2.5. Experiment 2: Acute dose–effect of PAT with amphetamine
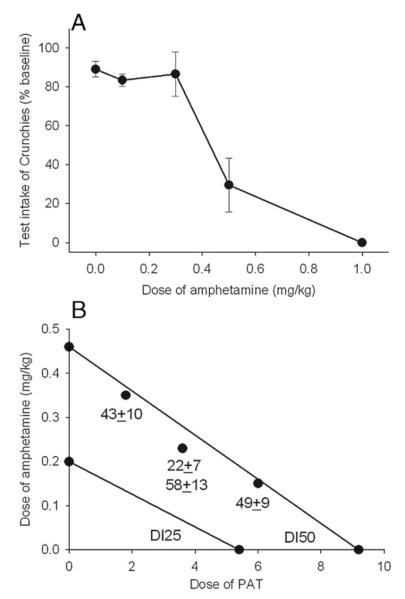
To examine whether the anorectic potency of PAT was either additive or synergistic with amphetamine, dose–effect curves were first established for the catecholaminergic agent as described in Experiment 1. Interpolation of these curves yielded values that suppressed food intake by either 25% or 50% (inhibitory dose, DI25 and DI50, respectively). Different combinations of the amphetamine and PAT, with additive predicted intakes of 50% baseline from the isobologram, were then administered and the effects on intake determined as in Experiment 1.
2.6. Experiment 3: Time-course of action of PAT alone or with amphetamine
To determine the time-course of the anorectic action of PAT, mice were injected with strongly anorectic doses (6 or 10 mg/kg) of PAT, or the 10% DMSO vehicle, either 15, 60 or 120 min before access to Crunchies. Other details were as in the previous studies. As, expected, there were no differences among the groups injected with vehicle at the different times, so these were combined to a single placebo group for statistical analysis.
The study was repeated using the combination of PAT (6 mg/kg) with amphetamine (0.4 mg/kg), injected simultaneously, again given either 15, 60 or 120 min before the dessert.
2.7. Experiment 4: Chronic action of PAT alone or with amphetamine
To determine whether tolerance develops to the anorectic action of PAT with repeated administration, we examined the effect of daily injections of PAT, or PAT plus amphetamine, on acute dessert intake. For this study, a baseline was established the previous week and 2 groups of 8 mice matched for intake were formed. Baseline was checked on Monday of the test week, then for the next 4 days mice were injected with either the vehicle(s), or PAT (6 mg/kg) alone or with amphetamine (0.4 mg/kg), 15 min before presentation of Crunchies. For the statistical analysis, data were expressed as % of the previous week’s baseline.
2.8. Test compounds
The full description of the synthesis of PAT hydrochloride (FW. 287.5) is reported elsewhere (Booth et al., 2008; Wyrick et al., 1993). Briefly, the benzylstyrylketone was cyclized to the tetralone intermediate and reduced to the (±)-cis- and (±)-trans-tetralols. This diastereomeric tetralol mixture was converted to the free amine, followed by dimethylation and fractional recrystallization, to isolate (±)-trans-PAT. The (±)-trans-PAT enantiomeric mixture was converted to the (−)-camphorsulfonic acid diastereomeric salt and resolved by fractional recrystallization. The pure (1R,3S)-(−)-trans-PAT enantiomer was converted to the hydrochloride salt, dissolved in 10% DMSO in saline, and injected IP 15 min before test food presentation. WAY-161503 (8,9-dichloro-2,3,4,4a-tetrahydro-1H-pyrazino[1,2-a]-quinozalin-5(6H)-one) hydrochloride (FW 308.6) was purchased from Tocris (Ellisville, MO), dissolved in warmed saline, and was injected IP 30 min before test food presentation. Amphetamine hydrochloride (FW 368.5) was purchased from Sigma Chemical Co (St Louis, MO). Drugs, or equivalent volumes of the vehicle solution, were administered IP to mice 15 min before test food presentation. All injection volumes were 0.02 ml/10 g. When mice received drug combinations, or vehicles, two injections were given at the times indicated. Drug doses refer to weights of the salts.
2.9. Statistical analysis
Mice were used repeatedly, with about 1 week between treatments, and for statistical purposes were assumed to be separate subjects. The intakes, expressed as % baseline, were subjected to 1-way ANOVA to establish dose–effect curves. The 25% and 50% inhibitory doses (DIs) were derived from the best fit line (Sigma Plot). For isobolographic analysis, the ratio of doses of the component drugs were derived from the ratio of their DI25 or DI50 inhibitory doses obtained from the dose–effect curves. In Experiment 4, 2-way ANOVA (days and group) was used to assess main and interactive effects, with post hoc t-tests (P<0.05) for significant differences.
3. Results
3.1. Experiment 1: Acute dose–effect of PAT
Mice adapted in less than one week to avidly eating the dessert, averaging 8 Crunchies or ~1.5 g in 30 min access. This did not differ between batches of mice or across the 5 months of experimentation for each batch. Mice gained weight during the experiments by ~25%.
In the preliminary study, WAY-161503 produced a dose-related inhibition of intake (Fig. 1). Linear regression indicated that the doses of WAY-161503 interpolated to reduce intake to 75% (DI25) or 50% (DI50) of baseline were 4.2 and 8.4 mg/kg, respectively.
Fig. 1.
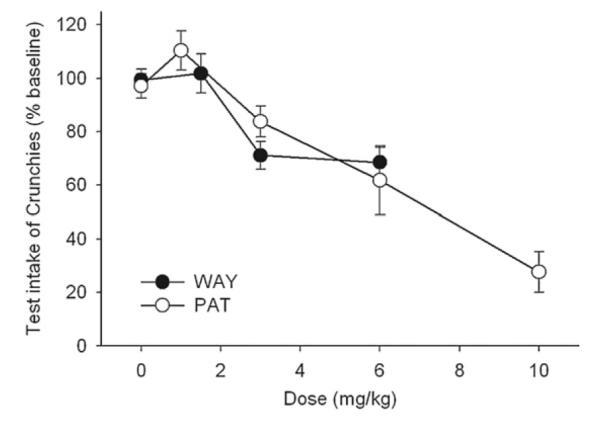
Dose–effect curves for WAY-161503 and (−)-trans-PAT on 30 min intake of Crunchies by non-deprived mice. Each point is the mean±SE of 8 or more observations.
The dose–effect curve for PAT (Fig. 1) was quite similar to that of WAY-161503, but included a higher dose which suppressed intake by ~75%. Linear regression of these data indicated that the DI25 and DI50 doses of PAT were 5.4 and 9.2 mg/kg, respectively.
3.2. Experiment 2: Acute dose–effect of PAT with catecholamine agents
Amphetamine reduced food intake in a dose-related manner (Fig. 2A) and from the linear regression the DI25 and DI50 were 0.20 and 0.46 mg/kg, respectively. The DI25 and DI50 isobolograms for the combinations of PAT and amphetamine are shown in Fig. 2B. Four combinations, all near DI50s, of PAT and amphetamine were tested. The observed results are indicated. In three of the four cases, the observed effect was close to additive as predicted from the isobologram, whereas in one case the effect was greater than expected (intake was 22±7% of baseline). This combination was subsequently replicated with the result, as shown, close to additive (58±13%). The reason for this discrepancy is unclear.
Fig. 2.
Panel A: Dose–effect curve amphetamine on 30 min intake of Crunchies by non-deprived mice. Each point is the mean±SE of 6 or more observations. Panel B: DI25 and DI50 isobolograms for combinations of amphetamine and (−)-trans-PAT, determined from regression of the individual drug data. The points shown are specific combinations that were tested and the numbers beneath are the observed intakes (mean±SE; Ns 8 or more).
3.3. Experiment 3: Time-course of action of PAT alone or with amphetamine
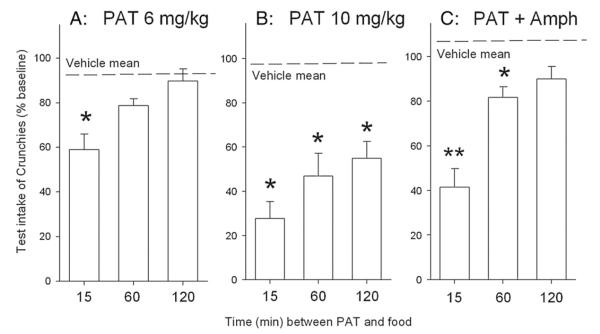
The time–effect curve for 6 mg/kg PAT is shown in Fig. 3A. One way ANOVA showed a significant group difference (F3,43=9.21, P<0.001), with the 15 min group significantly lower than all other groups (P<0.05, Tukey); these latter did not differ among themselves.
Fig. 3.
Time course of acute anorectic effect of (−)-trans-PAT at 6 mg/kg (A), 10 mg/kg (B), or a combination of PAT (6 mg/kg) plus amphetamine (0.4 mg/kg). Drugs were injected at the times shown before access to food. *P<0.05 lower than mean of vehicle-injected controls.
The time–effect curve for 10 mg/kg PAT is shown in Fig. 3B. One way ANOVA showed a significant group difference (F3,27=15.01, P<0.001), with all groups reliably lower than vehicle-injected controls and the 120 min group higher than the 15 min group (Ps<0.05).
The time–effect curve for 6 mg/kg PAT plus 0.4 mg/kg amphetamine is shown in Fig. 3C. One way ANOVA showed a significant group difference (F3,28=21.86, P<0.001), with the 15 min group differing from 60 min, which was still significantly reduced relative to controls.
3.4. Experiment 4: Chronic action of PAT alone or with amphetamine
The effects of chronic (4 day) administration of PAT (6 mg/kg) are shown in Fig. 4A. Two-way ANOVA revealed significant main effects of both day (P<0.001) and treatment (P<0.001), but no significant interaction. Intakes of both the PAT-treated and control groups rose across days, although the groups differed (t-tests, P<0.05) on each day.
Fig. 4.
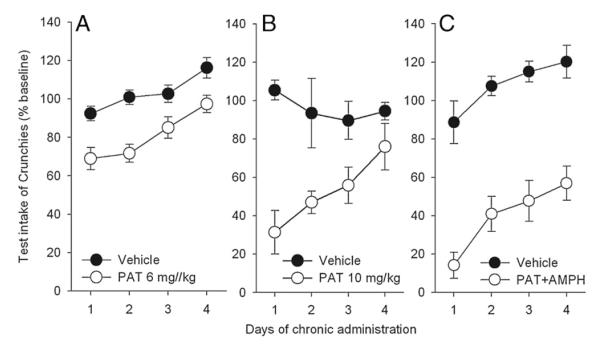
Effect of chronic administration of (−)-trans-PAT 6 mg/kg (A), 10 mg/kg (B), or 6 mg/kg with amphetamine (0.4 mg/kg) on intake of Crunchies. Shown are mean±SE for 8 mice per group. Drug treated groups lower (Ps<0.05) than vehicle except panel B, day 4.
The effects of chronic administration of PAT (10 mg/kg) are shown in Fig. 4B. Two-way ANOVA revealed a significant main effect of drug (P<0.001) but not days, and a drug×days interaction (P<0.02). In this case, the intake of the vehicle group did not increase across the study, but that of the PAT-injected group did, so their intake on day 4 was higher than on day 1 (P<0.05) but no longer differed from the vehicle control group.
The effects of chronic administration of PAT (6 mg/kg) with amphetamine (0.4 mg/kg) are shown in Fig. 4C. Two-way ANOVA revealed significant main effects of drug (P<0.001) and day (P<0.01), but no interaction. The intake of both controls and drug group rose across the test period, but without convergence of the intakes. Further, all of the mice in the combination group consumed less than their own baseline on each of the 4 test days.
4. Discussion
Crunchies® proved to be an extremely readily accepted and convenient food to promote eating of a very large meal in non-deprived mice. They ate some 40% of their normal daily intake in 30 min. More often than not, each pellet was eaten in an all-or-none manner, since there was little spillage and partly eaten fragments were rarely found. This is an interesting analog of the human eating situation where portion size is often fixed or predetermined. Thus, reductions in food intake were because the mice started fewer new food items, suggesting that they were satiated by a smaller number. This is substantiated further by informal observation that the latency to eat was usually only a few seconds in all drug conditions. Since the mice did not gain excessive weight over 5 months of this study, it is likely that they reduced their intake of maintenance chow in proportion to the dessert intake (Robertson and Rowland, 2005).
PAT reduced food intake in this model in a dose-related manner, with a suppression of about 75% at the highest dose tested. We noted no unusual behaviors in PAT-treated mice when food was put in or taken out of their cages. The dose–effect curve for WAY-161503 was extremely similar to PAT, but we did not test doses above 6 mg/kg. A monotonic dose–effect for WAY-161503 has been reported in fasted, obese mice in which 5 mg/kg was the DI50, and 30 mg/kg completely suppressed intake (Rosenzweig-Lipson et al., 2006). The absolute food intake in our dessert protocol (~1.4 g) is considerably higher than the ~0.9 g chow consumed by the fasted mice used by Rosenzweig-Lipson et al. (2006).
In three out of four tests, amphetamine had a strictly additive action with low (DI25) doses of PAT. Thus, there is no consistent evidence for synergy of action. In studies not reported here, we tested both PAT and WAY combinations with phentermine, and the results again were indicative of additivity and not synergy of effect. This additivity suggests that the anorectic mechanisms of PAT and amphetamine are different, and are consistent with the conclusion that the anorectic effect of PAT is mediated via 5HT2C receptors. However, confirmatory work with selective antagonists will be needed.
While WAY-161503 and PAT have similar Ki values, 32 and 38 nM respectively in their displacement of [3H]-mesulergine from human 5-HT2C receptors expressed in CHO cells (Booth et al., 2008; Rosenzweig-Lipson et al., 2007), these agents differ significantly in their action at 5-HT2A and 5-HT2B receptors that share a transmembrane domain sequence identity of about 75% with each other and the 5-HT2C receptor (Julius et al., 1990). PAT is devoid of agonist activity at 5-HT2A and 5-HT2B receptors, as measured by activation of PLC/IP formation, but has the full agonist activity of 5-HT at 5-HT2C receptors. In contrast, WAY-161503 has comparable agonist activity at 5-HT2B and 5-HT2C receptors, and some activity at 5-HT2A receptors. Such nonselective activation of 5-HT2 receptors subtypes likely is not desirable because 5-HT2A receptor activation is associated with psychomimetic (hallucinogenic) effects (Nichols, 2004) and 5-HT2B activation can lead to valvular heart disease (Connolly et al.,1997; Fitzgerald et al., 2000; Rothman et al., 2000; Setola et al., 2005; Roth, 2007) and pulmonary hypertension (Pouwels et al., 1990; Launay et al., 2002). In the present animal model, the efficacies and DI25s of WAY-161503 and PAT on food intake were comparable, in agreement with their similar pharmacology at 5-HT2C receptors, and suggesting that this receptor accounts for all of the anorectic action of this drug.
The anorectic half-life of PAT appears to be about 1 h. Thus, injected either alone or in combination with amphetamine, delaying access to food for 2 h after injection of the drug(s) was associated with sub-stantial if not complete loss of anorexia, except at the highest dose. This relatively short half-life, coupled with the relatively poor solubility of PAT, made us opt for daily injections in the chronic study, rather than truly continuous exposure by minipump administration. After at least for 4 daily injections, PAT retained a substantial amount of its anorectic potency; in similar protocols using fenfluramine, we have shown that statistically complete reversal of anorexia occurs within this time frame in rats (Rowland and Carlton,1984). This suggests that while desensitization of 5-HT2C receptors may occur with chronic administration of an agonist, and be responsible for partial tolerance to PAT anorexia in the present study, the rapid tolerance to fenfluramine involves other or additional mechanisms.
In summary, targeting brain 5-HT2C receptor activation as novel pharmacotherapy for obesity appears to hold promise, although pharmacokinetic parameters will need to be carefully considered in such a drug discovery process. Given the potential for untoward side effects associated with activation of 5-HT2A (psychiatric) and 5-HT2B (cardio-pulmonary) receptors, and the possibility that any type of anorexic drug might be abused, design and development of drugs selective for 5HT2C activation obviously is preferred.
References
- Bivens NM, Weisstuab NV, Bradley-Moore M, Kumar K, Booth RG, Gingrich JA. (−)-trans-PAT: a novel 5HT2C receptor agonist/5HT2A receptor antagonist exhibits anti-psychotic and appetite suppressant properties. American College of Neuropsychopharmacology 46th Annual Meeting; Boca Raton, Fl. December 9–confproc.2007. [Google Scholar]
- Booth RG, Fang L, Huang Y, Fang L, Sivendran S. (1R, 3S)-(−)-trans-PAT: a full-efficacy serotonin 5-HT2C receptor agonist with 5-HT2A/2B receptor antagonist and inverse agonist activity. Submitted to Eur J Pharmacol. 2008 doi: 10.1016/j.ejphar.2009.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromidge SM, Duckworth M, Forbes IT, Ham P, King FD, Thewlis KM, et al. 6-Chloro-5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]-indoline (SB-242084): the cn selective and brain penetrant 5-HT2C receptoJ Mcnmr antagonist. J Med Chem. 1997;40:3494–6. doi: 10.1021/jm970424c. [DOI] [PubMed] [Google Scholar]
- Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, et al. Valvular heart disease associated with fenfluramine–phentermine. N Engl J Med. 1997;337:581–8. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]; N. Engl. J. Med. 1997;337:1783. Erratum in. [Google Scholar]
- Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, et al. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol. 2000;57:75–81. [PubMed] [Google Scholar]
- Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs: effects on appetite suppression and use for the treatment of obesity. Drugs. 2007;67:27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–11. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- Julius D, Huang KN, Livelli TJ, Axel R, Jessel TM. The 5-HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proc Natl Acad Sci. 1990;87:928–32. doi: 10.1073/pnas.87.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, et al. Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn-Schmiedeberg’s Arch Pharmacol. 2004;370:114–23. doi: 10.1007/s00210-004-0951-4. [DOI] [PubMed] [Google Scholar]
- Launay JM, Herve P, Peoc’h K, Tournois C, Callebert J, Nebigil CG, et al. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med. 2002;8:1129–35. doi: 10.1038/nm764. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–81. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nilsson BM. 5-Hydroxytryptamine 2C (5-HT2C) receptor agonists as potential antiobesity agents. J Med Chem. 2006;49:4023–34. doi: 10.1021/jm058240i. [DOI] [PubMed] [Google Scholar]
- Pouwels HM, Smeets JL, Cheriex EC, Wouters EF. Pulmonary hypertension and fenfluramine. Eur Respir J. 1990;3:606–7. [PubMed] [Google Scholar]
- Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, et al. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- Richter HG, Adams DR, Benardeau A, Bickerdike MJ, Bentley JM, Blench TJ, et al. Synthesis and biological evaluation of novel hexahydro-pyrido[3¢,2¢:4,5]pyrrolo [1,2-a]pyrazines as potent and selective 5-HT(2C) receptor agonists. Bioorg Med Chem Lett. 2006;16:1207–11. doi: 10.1016/j.bmcl.2005.11.083. [DOI] [PubMed] [Google Scholar]
- Robertson KL, Rowland NE. Effect of two types of environmental enrichment for singly housed mice on food intake and weight gain. Lab Anim. 2005;34:29–32. doi: 10.1038/laban1005-29. [DOI] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Zhang J, Mazandarani H, Harrison B, Sabb A, Sabalski J, et al. Antiobesity-like effects of the 5-HT2C receptor agonist WAY-161503. Brain Res. 2006;1073:240–51. doi: 10.1016/j.brainres.2005.12.052. [DOI] [PubMed] [Google Scholar]
- Roth BL. Drugs and valvular heart disease. N Engl J Med. 2007;356 doi: 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]
- Roth JD, Rowland NE. Anorectic efficacy of the fenfluramine/phentermine combination in rats: additivity or synergy? Eur J Pharmacol. 1999;373:127–34. doi: 10.1016/s0014-2999(99)00235-6. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, et al. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–41. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Carlton J. Inhibition of gastric emptying by peripheral and central fenfluramine in rats: correlation with anorexia. Life Sci. 1984;34:2495–9. doi: 10.1016/0024-3205(84)90286-8. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Lo J, Robertson K. Acute anorectic effect of single and combined drugs in mice using a non-deprivation protocol. Psychopharmacology. 2001;157:193–6. doi: 10.1007/s002130100789. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Mayer SE. Serotonin receptor agonists and antagonists. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 11th Edition McGraw-Hill; New York, NY: 2006. pp. 297–315. [Google Scholar]
- Saltzman AG, Morse B, Whitman MM, Ivanshchenko Y, Jaye M, Felder S. Cloning of the human serotonin 5-HT2 and 5-HT1C receptor subtypes. Biochem Biophys Res Commun. 1991;181:1469–7148. doi: 10.1016/0006-291x(91)92105-s. [DOI] [PubMed] [Google Scholar]
- Setola V, Dukat M, Glennon RA, Roth BL. Molecular determinants for the interaction of the valvulopathic anorexigen norfenfluramine with the 5-HT2B receptor. Mol Pharmacol. 2005;68:20–33. doi: 10.1124/mol.104.009266. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P, et al. CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology. 2007;52:279–90. doi: 10.1016/j.neuropharm.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, et al. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–6. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Dourish CT, Kennett GA. Evidence that hypophagia induced by d-fenfluramine and d-norfenfluramine in the rat is mediated by 5-HT2C receptors. Neuropharmacology. 2001;41:200–9. doi: 10.1016/s0028-3908(01)00063-6. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Clifton PG, Dourish CT, Tecott LH. Reduced satiating effect of d-fenfluramine in serotonin 5-HT2C receptor mutant mice. Psychopharmacology. 1999;143:309–14. doi: 10.1007/s002130050952. [DOI] [PubMed] [Google Scholar]
- Wacker DA, Varnes JG, Malmstrom SE, Cao X, Hung CP, Ung T, et al. Discovery of (R)-9-ethyl-1,3,4,10b-tetrahydro-7-trifluoromethylpyrazino[2,1-a]isoindol-6(2H)-one, a selective, orally active agonist of the 5-HT(2C) receptor. J Med Chem. 2007;50:1365–79. doi: 10.1021/jm0612968. [DOI] [PubMed] [Google Scholar]
- Weintraub M, Sundaresan PR, Schuster B, Moscucci M, Stein EC. Long-term weight control study. III (weeks 104 to 156). An open-label study of dose adjustment of fenfluramine and phentermine. Clin Pharmacol Ther. 1992;51:602–7. doi: 10.1038/clpt.1992.71. [DOI] [PubMed] [Google Scholar]
- Wyrick SD, Booth RG, Myers AM, Owens CE, Kula NS, Baldessarini RJ, et al. Synthesis and pharmacological evaluation of 1-phenyl-3-amino-1,2,3,4-tetrahydronaphthalenes as ligands for a novel receptor with sigma-like neuromodulatory activity. J Med Chem. 1993;36:2542–51. doi: 10.1021/jm00069a013. [DOI] [PubMed] [Google Scholar]