Abstract
Heart failure with a preserved ejection fraction (HFpEF) is the dominant form of heart failure in the older population. The primary chronic symptom in HFpEF is severe exercise intolerance, however, its pathophysiology and therapy are not well understood. We tested the hypothesis that older patients with HFpEF have increased arterial stiffness beyond that which occurs with normal aging and that this contributes to their severe exercise intolerance.
Sixty-nine patients ≥ 60 years with HFpEF and 62 healthy volunteers (24 young healthy subjects ≤ 30 years (YHC) and 38 older healthy subjects ≥ 60 years old (OHC) were examined. Carotid arterial stiffness was assessed using high-resolution ultrasound and peak exercise oxygen consumption (VO2) was measured using expired gas analysis.
Peak VO2 was severely reduced in the HFpEF patients compared to OHC (14.1±2.9 vs. 19.7±3.7 ml/kg/min; p<0.001) and in both was reduced compared to YHC subjects, (32.0±7.2 ml/kg/min; both p<0.001). In HFpEF compared to OHC, carotid arterial distensibility was reduced (0.97±0.45 vs. 1.33±0.55 × 10−3 mmHg−1, p=0.008) and Young’s elastic modulus (YEM) was increased (1320±884 vs. 925±530 kPa, p<0.02). Carotid arterial distensibility was directly (0.28; p=0.02) and YEM was inversely (−0.32; p=0.01) related with peak VO2.
Carotid arterial distensibility is decreased in HFpEF beyond the changes due to normal aging and is related to peak VO2. This supports the hypothesis that increased arterial stiffness contributes to exercise intolerance in HFpEF and is a potential therapeutic target.
Keywords: Aging, heart failure with preserved ejection fraction, arterial stiffness, exercise capacity
Introduction
Approximately 50% or more of heart failure (HF) patients have preserved left ventricular ejection fraction (HFpEF), and the proportion is greater among women and the elderly.1,2 The primary symptom in patients with chronic HFpEF, even well-compensated, is severe exercise intolerance, which can be measured objectively as decreased peak exercise pulmonary oxygen uptake (peak VO2).3-8
It has been shown in population-based studies that increased arterial stiffness is associated with cardiovascular morbidity and mortality, including in older populations.9-12 We and others have shown that increased stiffness of the aorta is associated with exercise intolerance in older patients with either HFpEF or HF and reduced ejection fraction (HFrEF).13,14 It is known that arterial function may have substantial regional variation.15 In HFrEF, it has been shown that the increase in arterial stiffness is generalized and is observed in the peripheral arteries as well as the aorta, and has been shown to be modifiable with angiotensin receptor antagonism.16 Moreover, the pulsatile components of arterial afterload are greater in HFpEF compared with HFrEF and may play a greater role in the pathophysiology of HFpEF than HFrEF.17 It is known that normal aging alone is associated with increased arterial stiffness, and that this can be exacerbated in the setting of systemic hypertension18. In addition, Borlaug et al5,6,19 and others20,21 reported in older HFpEF patients that systemic vascular resistance was increased and appeared to contribute to their reduced peak VO2. However, it is unknown whether arterial stiffness is generalized in HFpEF and whether it contributes to exercise intolerance.
We hypothesized that peripheral arterial stiffness is increased in older HFpEF patients, beyond the extent that occurs with normal aging alone, similar to the changes in the thoracic aorta, and that these changes may contribute to their severe exercise intolerance. To test these hypotheses, we non-invasively measured indexes of stiffness of the carotid artery, a large vessel easily accessible by high resolution ultrasound, and exercise capacity in older patients with HFpEF in comparison to groups of young and old healthy normal volunteers.
Methods
Subjects
Data regarding flow mediated arterial dilation have been recently reported from a subset of these subjects.22 As previously described,4,8,22,23 HFpEF was defined as HF with normal systolic function (LV ejection fraction ≥ 50% and no segmental wall motion abnormalities) and no evidence of significant coronary or valvular heart disease or pulmonary disease. The diagnosis of HF was based on clinical criteria as previously described,4,8,23 that included a HF clinical score from National Health and Nutrition Examination Survey-I of ≥3,24 and those utilized by Rich et al,25 that included a history of acute pulmonary edema, or the occurrence of at least 2 of the following with no other identifiable cause and with improvement following diuresis: dyspnea on exertion, paroxysmal nocturnal dyspnea, orthopnea, bilateral lower extremity edema, or exertional fatigue. Final determination was made by a board-certified cardiologist following review of medical records, interview, examination, and rest and exercise echocardiography (the latter to exclude inducible ischemia as confounder). There were 69 patients with HFpEF.
As previously described, normal, healthy volunteers were screened and excluded if they had any chronic medical illness, were on any chronic medication, had current complaints or an abnormal physical examination (including blood pressure ≥ 140/90 mmHg), had abnormal results on the screening tests (electrocardiogram, rest and exercise echocardiogram, and spirometry), or were regularly exercising.4,22,23 There were 24 young healthy volunteers (age < 30 years, YHC) and 38 older healthy participants (age > 60 years, OHC).
Because of the significant influence of atherosclerosis on vascular function,26 in both patients and healthy subjects, the protocol excluded those at high risk (known hyperlipidemia, cigarette smoking) and those with known or suspected coronary, cerebrovascular, and peripheral arterial disease by record review, history, physical examination, exercise echocardiography, and carotid ultrasound.
Study Protocol
The study protocol was approved by the Institutional Review Board at Wake Forest University Health Sciences. All subjects were familiarized with the testing environment and procedures and informed consent was obtained during the screening visit. During a subsequent visit, patients reported in the morning following an overnight fast and prior to taking their morning medications, and in a single visit the outcome measures of carotid artery stiffness, LV function, and exercise capacity were obtained. All testing was performed by and all results were analyzed by individuals blinded to patient groups.
Echocardiography
As previously described,4,8,23,27 resting echocardiography was performed by an experienced, registered echosonographer using a Philips Sonos 5500 (Andover, Massachusetts) ultrasound imaging system fitted with multifrequency transducer. Standard 2-dimensional images were obtained in the parasternal long-axis and short-axis views and apical 4- and 2-chamber views. Off-line analyses were performed using a digital analysis workstation according to ASE standards4. Doppler mitral filling patterns were categorized as normal, impaired relaxation, pseudonoramal, and restrictive.28,29
Exercise Testing Protocol
As previously described,4,8,13,23,27,30 exercise testing was performed with participants in the upright position on an electronically braked cycle ergometer. A detailed description of the exercise testing protocol methods is available on the online supplement.
Carotid artery stiffness measurements
As previously described,31 standardized longitudinal B-mode images of the left common carotid artery were recorded with the subject in the supine position using a commercially available ultrasound instrument (Sequoia, Accuson, Inc.) fitted with a high frequency (10 MHz) linear probe and using vascular software and optimized settings. A detailed description of the carotid artery stiffness measurements methods is available on the online supplement.
Statistical Analysis
Values were expressed as means ± SD unless otherwise noted. Comparisons were performed using analysis of covariance. Intergroup comparisons of baseline characteristics and measures of exercise performance, and carotid artery stiffness were made using the independent t-test for continuous measures and Fisher’s exact test for categorical measures. Carotid arterial distensibility was selected as the primary outcome measure of arterial stiffness prior to study initiation. Because of non-normal distribution, carotid stiffness measures were log-transformed. Multiple linear regression analysis was performed to determine significant predictors of peak VO2 and to determine the independent effects of these variables on peak VO2 after adjustment for differences in sex, race and body mass index. For all analyses, a two-tailed p-value of <0.05 was required for significance.
Results
Carotid artery imaging and exercise testing were well tolerated in all individuals and there were no adverse events associated with either.
Participant Characteristics
Characteristics of the HFpEF, YHC and OHC are shown in Table 1. The OHC and HFpEF groups were well matched for age, however, body mass was higher for HFpEF than YHC or OHC. There was a higher percentage of women in the OHC and HFpEF groups than in YHC. The HFpEF patients had characteristics similar to those reported from population studies, and from previous reports from our laboratory.4,8,23,29 Specifically, they had increased left atrial diameter, LV posterior and septal wall thickness, mass and mass to volume ratio compared to OHC (Table 1). HFpEF patients were stable, New York Heart Association (NYHA) class II (46%) and III (54%). They had a mean LV ejection fraction by echocardiography of 59 ± 8%, and most had abnormal Doppler mitral flow patterns.29 Chronic systemic hypertension was present in 90% of HFpEF patients and diabetes was present in 28%. HFpEF patients were being clinically treated with the following medications: ACE-Inhibitors/angiotensin receptor antagonists (45%), digoxin (6%), diuretics (61%), nitrates (12%), beta blockers (21%) and calcium channel blockers (30%). All patients were in sinus rhythm.
Table 1.
Characteristics of the Study Population
| Characteristic | YHC (n=24) |
OHC (n=38) |
HFpEF (n=69) |
p-Value |
|---|---|---|---|---|
| Age (ys) | 26 ± 3 | 69 ± 6 | 70 ± 7 | 0.65 |
| Women | 12 (50) | 25 (66) | 52 (77) | 0.37 |
| Caucasian | 23 (96) | 36 (95) | 47 (68) | 0.001 |
| Body weight (kg) | 73 ± 15 | 72 ± 14 | 87 ± 19 | <0.001 |
| Body surface area (m2) | 1.86 ± 0.24 | 1.80 ± 0.20 | 1.94 ± 0.23 | 0.01 |
| Body mass index (kg/m2) | 24 ± 3 | 26 ± 3 | 32 ± 7 | <0.001 |
| Ejection fraction (%) | -- | 65 ± 8 | 59 ± 8 | 0.01 |
| Septal wall thickness (cm) | -- | 1.0 ± 0.1 | 1.4 ± 0.3 | <0.001 |
| Posterior wall thickness (cm) | -- | 0.9 ± 0.1 | 1.2 ± 0.2 | <0.001 |
| Mass (g) | -- | 167 ± 46 | 261 ± 88 | <0.001 |
| Mass/volume ratio | -- | 2.52 ± 1.06 | 3.22 ± 1.60 | 0.07 |
| Left atrial diameter (cm) | -- | 3.1 ± 0.5 | 3.5 ± 0.7 | 0.02 |
| Mitral early velocity (cm/s) | -- | 68 ± 18 | 74 ± 20 | 0.21 |
| Mitral atrial velocity (cm/s) | -- | 73 ± 13 | 84 ± 24 | 0.006 |
| Early/atrial ratio | -- | 0.94 ± 0.19 | 1.01 ± 0.81 | 0.51 |
| Deceleration time (ms) | -- | 217 ± 49 | 228 ± 71 | 0.41 |
| Isovolumic relaxation time (ms) | -- | 74 ± 17 | 82 ± 23 | 0.08 |
| Diastolic Filling | ||||
| Normal | -- | 30 (79) | 0 (0) | <0.001 |
| Impaired relaxation | -- | 8 (21) | 32 (50) | 0.006 |
| Pseudonormal | -- | 0 (0) | 29 (45) | <0.001 |
| Restrictive | -- | 0 (0) | 3 (5) | 0.29 |
| History of hypertension | -- | -- | 62 (90) | -- |
| Diabetes mellitus | -- | -- | 19 (28) | -- |
| ACE | -- | -- | 27 (39) | -- |
| Angiotensin receptor blockers | -- | -- | 4 (6) | -- |
| Digoxin | -- | -- | 4 (6) | -- |
| Diuretics | -- | -- | 42 (61) | -- |
| Beta-blockers | -- | -- | 15 (22) | -- |
| Calcium channel blockers | -- | -- | 21 (30) | -- |
| Nitrates | -- | -- | 8 (12) | -- |
Values are mean ± SD or count (%). P-value indicates comparison of HFpEF versus OHC. ACE, angiotensin converting enzyme inhibitor.
Exercise Performance
Participants gave an exhaustive exercise effort and mean peak respiratory exchange ratios were >1.13 for all 3 groups (Table 2). Peak VO2 (L/min or ml/kg/min) was severely reduced in HFpEF patients compared to OHC subjects and was greater in YHC subjects than in both older groups (Table 2). Peak exercise workload, time, heart rate were also markedly reduced in HFpEF patients compared to OHC subjects and were greater in YHC subjects than in both older groups (all p<0.001). Peak exercise systolic blood pressure was similar in HFpEF patients and OHC subjects (p=0.42) and in both was higher than in YHC subjects (p<0.01 for both). No significant difference was found between YHC and OHC or OHC and HFpEF patients for the Ve/VCO2 slope (Table 2). Six minute walk distance was also reduced in HFpEF patients compared to OHC subjects (p<0.001) and in both was reduced compared to YHC subjects (p<0.001 for both).
Table 2.
Peak Exercise Performance
| Parameter | YHC | OHC | HFpEF | YHC vs OHC p-Value |
OHC vs HFpEF p-Value |
|---|---|---|---|---|---|
| VO2 (ml/kg/min) | 32.0 ± 7.2 | 19.7 ± 3.7 | 14.1 ± 2.9 | <0.001 | <0.001 |
| VO2 (ml/min) | 2310 ± 665 | 1418 ± 397 | 1218 ± 360 | <0.001 | 0.010 |
| Time (seconds) | 1243 ± 394 | 737 ± 190 | 540 ± 197 | <0.001 | <0.001 |
| Workload (Watts) | 163 ± 55 | 89 ± 28 | 67 ± 29 | <0.001 | <0.001 |
| Heart Rate (bpm) | 176 ± 16 | 148 ± 14 | 128 ± 19 | <0.001 | <0.001 |
| Systolic BP (mmHg) | 176 ± 19 | 195 ± 23 | 191 ± 24 | 0.001 | 0.419 |
| Diastolic BP (mmHg) | 83 ± 12 | 88 ± 9 | 90 ± 13 | 0.056 | 0.458 |
| Oxygen pulse (ml/beat) | 13.3 ± 4.2 | 9.6 ± 2.7 | 9.7 ± 3.0 | 0.001 | 0.906 |
| VE (l/min) | 85 ± 20 | 59 ± 17 | 48 ± 16 | <0.001 | 0.001 |
| RER | 1.16 ± 0.09 | 1.21 ± 0.09 | 1.13 ± 0.08 | 0.052 | <0.001 |
| 6 minute walk (m) | 665 ± 70 | 551 ± 59 | 425 ± 103 | <0.001 | <0.001 |
| Ve/VCO2 Slope | 28.6 ± 4.5 | 31.1 ± 3.6 | 32.4 ± 5.8 | 0.084 | 0.293 |
Values are mean ± SD. BP, blood pressure; RER, respiratory exchange ratio; VE, minute ventilation; VO2, oxygen consumption; VCO2, carbon dioxide production.
Carotid artery measurements
Arterial and lumen diameters were increased in HFpEF compared to OHC and YHC (p<0.001; Table 3). Compared to YHC, OHC had significantly increased carotid arterial stiffness indexes (decreased arterial compliance, increased PEM, YEM, and beta index, Table 3). Compared to OHC, patients with HFpEF had further reductions in arterial distensibility (p=0.008), and increased YEM (p=0.018). The decreased carotid arterial distensibility in HFpEF patients appeared primarily due to increased pulse pressure, since phasic arterial diameter change was similar to OHC. Carotid intimal media thickness was increased in OHC compared to YHC and also similar in HFpEF compared to OHC. These results were not significantly altered following adjustments for gender or medications.
Table 3.
Carotid Artery Stiffness Measurements
| Parameter | YHC | OHC | HFpEF | YHC vs OHC p-Value |
OHC vs HFpEF p-Value |
|---|---|---|---|---|---|
| Primary Carotid Stiffness Measure | |||||
| Carotid distensibility (×10−3 mmHg−1) | 1.96 ± 1.18 | 1.33 ± 0.55 | 0.97 ± 0.45 | 0.211 | 0.008 |
| Secondary Carotid Stiffness Measures | |||||
| Carotid compliance (×10−3 mm*mmHg−1) | 97.7 ± 57.3 | 66.3 ± 34.8 | 58.6 ± 24.0 | 0.010 | 0.295 |
| Peterson’s elastic modulus (kPa) | 76 ± 37 | 194 ± 120 | 237 ± 129 | <0.001 | 0.131 |
| Young’s elastic modulus (kPa) | 450 ± 229 | 925 ± 530 | 1320 ± 884 | <0.001 | 0.018 |
| Beta Index | 6.46 ± 3.21 | 14.61 ± 8.86 | 16.54 ± 8.35 | <0.001 | 0.315 |
| Carotid Artery Dimensions | |||||
| Intima-medial thickness (mm) | 0.57 ± 0.06 | 0.75 ± 0.12 | 0.79 ± 0.14 | <0.001 | 0.207 |
| Systolic arterial diameter (mm) | 7.12 ± 0.78 | 7.48 ± 0.94 | 8.34 ± 0.98 | 0.169 | <0.001 |
| Diastolic arterial diameter (mm) | 6.53 ± 0.70 | 7.14 ± 0.85 | 7.95 ± 0.97 | 0.012 | <0.001 |
| Systolic luminal diameter (mm) | 6.03 ± 0.83 | 6.02 ± 0.79 | 6.80 ± 1.06 | 0.986 | 0.001 |
| Diastolic luminal diameter (mm) | 5.59 ± 0.74 | 5.63 ± 0.71 | 6.34 ± 1.02 | 0.885 | 0.002 |
| Arterial diameter change (mm) | 0.59 ± 0.23 | 0.34 ± 0.17 | 0.39 ± 0.17 | <0.001 | 0.183 |
| Luminal diameter change (mm) | 0.44 ± 0.20 | 0.39 ± 0.18 | 0.42 ± 0.18 | 0.493 | 0.509 |
| Blood Pressure | |||||
| Systolic blood pressure (mmHg) | 112 ± 12 | 131 ± 15 | 146 ± 18 | <0.001 | <0.001 |
| Diastolic blood pressure (mmHg) | 69 ± 10 | 75 ± 9 | 76 ± 11 | 0.023 | 0.729 |
| Pulse pressure (mmHg) | 43 ± 7 | 56 ± 13 | 70 ± 16 | <0.001 | <0.001 |
Values are means ± SD.
Relationship of carotid arterial stiffness indexes with exercise performance
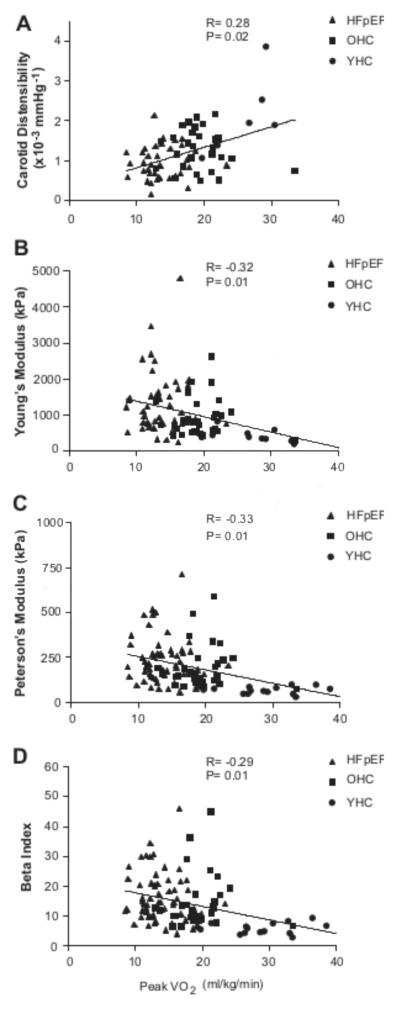
Indices of carotid arterial stiffness including PEM, YEM, β-index and distensibility, adjusted for sex, race and BMI, were significantly correlated with peak exercise VO2 (Figure 1). Given that HFpEF had significantly lower maximal HR, the relationship between oxygen pulse (O2P, equal to VO2/HR) and carotid arterial stiffness indices was performed. The O2P was significantly correlated with carotid arterial distensibility (r=0.26, p=0.04) but was not was related to PEM (r=−0.20, p=0.11), YEM (r=−0.20, p=0.10) or β-index(r=−0.16, p=0.18). No significant association was found between Ve/VCO2 slope and carotid arterial distensibility (r=−0.02, p=0.88), YEM (r=−0.06, p=0.65), PEM (r=0.01, p=0.97) and Beta index (r=0.01, p=0.91). Carotid arterial distensibility was also correlated with 6-minute walk distance (r=0.25; p<0.03).
Figure 1.
Relationship of carotid arterial distensibility (A), Young’s elastic modulus (B), Peterson’s modulus (C), and Beta index (D) to peak exercise VO2 for the study group. Young normals (circles); old normals (squares); HFpEF (triangles). Correlation coefficients and p-values shown are following log transformation and adjustment for sex, race and body mass index.
Discussion
This study demonstrates that although healthy, normal aging alone is accompanied by increased carotid arterial stiffness and reduced peak VO2, older patients with HFpEF have severely reduced exercise capacity and increased carotid arterial stiffness beyond that which occurs with normal ‘non-hypertensive’ aging. Measures of carotid arterial stiffness performed at rest correlated significantly with peak VO2 and 6-minute walk distance. These data support the hypothesis that increased arterial stiffness may play a role in the pathophysiology of HFpEF, the dominant form of HF in the older population. In addition, because exercise intolerance is the primary chronic symptom in HFpEF, these data suggest that increased arterial stiffness may be a potential therapeutic target in older patients with this disorder. To this end, endurance training may be an important therapy to improve arterial stiffness in older HFpEF patients. Finally, because the carotid artery is a large vessel easily assessable by high resolution ultrasound imaging, this technique may provide a practical means for serial evaluation of arterial stiffness during future clinical intervention trials in HFpEF.
These data significantly extend previous reports from our group and others suggesting that arterial stiffness is increased in older patients with HFpEF.5,13,32,33 We have previously reported that stiffness of the proximal thoracic aorta is increased in older patients with both HFpEF and HFrEF and that in both types of HF it is a strong, independent predictor of the severity of exercise intolerance.13,14 Melenovsky et al reported that carotid mean blood pressure and carotid pulse pressure were increased while total arterial compliance (measured as stroke volume divided by carotid pulse pressure) was significantly decreased in HFpEF compared to age-and sex-matched healthy control subjects.34 Arterial sites other than the aorta, including the carotid and radial arteries, have been shown to have increased stiffness in patients with HFrEF.16 The present findings indicate that arterial stiffness extends beyond the thoracic aorta in HFpEF as well. We have previously reported other similarities in the pathophysiology of HFpEF and HFrEF, including severely reduced exercise capacity, exercise cardiac output, and quality of life and increased neurohormonal activation.4,13,14,35
The present study utilized high resolution ultrasound to assess carotid arterial stiffness. While this technique can provide high quality data at rest, it is not feasible during upright maximal exercise. However, Tartiere-Kesri et al. recently measured carotid artery stiffness at rest and during low-intensity (30 watts, equal to 39% peak power output) sub-maximal cycling in older HFpEF patients (n=23) and age-matched healthy controls (n-15).32 They reported that rest and exercise carotid systolic blood pressure, carotid pulse pressure, and proximal arterial load (carotid Peterson modulus, aortic pulse wave velocity) were significantly increased while arterial compliance was significantly lower in HFpEF patients versus healthy controls.32 Also, the change in carotid Peterson’s modulus from rest to 30 watt cycling was significantly increased in older HFpEF compared controls (+155% vs. −5%, respectively), and was inversely related to peak power output or 6-minute walk distance. We extend these findings by studying a larger group of older HFpEF patients and by including both young and old healthy control subjects to demonstrate that carotid arterial distensibility is decreased in HFpEF beyond the changes due to normal aging alone, and by correlating these changes with peak exercise VO2 as well as 6-minute walk distance.
The present study also extends prior findings by excluding patients with evidence of significant atherosclerotic plaque on carotid imaging, and patients with any history or evidence of cerebrovascular, peripheral vascular, or coronary artery disease. There was also no significant increase in intimal medial thickness in HFpEF compared to healthy age-matched volunteers, suggesting that the observed changes in arterial stiffness and their relationship with peak VO2 were not necessarily due merely to atherosclerosis. The absence of differences in phasic arterial diameter change suggests that the differences in stiffness were due primarily to increased pulse pressure, a finding consistent with that found at rest or low-level cycle exercise by Tartiere-Kesri and associates.32
There are a number of potential mechanisms that could be responsible for the altered arterial stiffness in HF patients.36 First, neurohormonal changes in HF, including activation of the renin-angiotensin system and increased plasma norepinephrine, may cause vasoconstriction and sodium retention in the vascular wall.37,38 We and others have shown that HFpEF patients have activation of both renin-angiotensin system and the adrenergic system.4,39 Second, the hypertrophic effect of angiotensin II and aldosterone on vascular smooth muscle cells results in changes in vascular wall structure.40-42 Endothelial dysfunction also affects vasodilatation properties of the artery and proliferation of smooth muscle cells.40 Finally, atherosclerosis has been associated with impaired arterial elastic properties, and may result in decreased distensibility.42,43 The above changes can be accelerated by chronic hypertension and diabetes. Notably, in population-based studies, similar to our patient group, hypertension is found in approximately 90% of older HFpEF patients and diabetes in approximately 30%.44
Potential Limitations
The present study was designed prior to publication of the criteria proposed by Paulus et al.45 However, the inclusion criteria used have been shown to identify patients who have severe abnormalities in key pathophysiological attributes similar to patients with established, severe systolic heart failure and to have significantly increased rates of death and heart failure rehospitalization.4,24,25,46 The Paulus criteria require 3 obligatory features: signs or symptoms of congestive heart failure; preserved systolic function; and evidence of LV diastolic dysfunction. The first 2 criteria were automatically met by study design. The third criterion as stated by Paulus et al. is best fulfilled by either invasive measurements or tissue Doppler and pulmonary venous flow, which are not available in this study. However, Paulus et al. acknowledge the finding by Zile et al.29 that in patients with the combination of a clear history of HF, normal EF, and concentric LV remodeling (all typical of the patients in the present study), 92% were found to have abnormal diastolic function by subsequent invasive measurements. The present study had 2 additional features supported by Paulus et al., including formal cardiopulmonary exercise testing with measurement of peak VO2 to confirm the presence of severely reduced exercise capacity and pulmonary function testing to prevent misdiagnosis due to pulmonary disease.
Cardiac medications were withheld the evening before exercise testing and carotid artery imaging in HFpEF patients (approximately 12-24 hours). Participants also abstained from caffeine ingestion for 24 hours prior to testing. However, we cannot exclude the potential for residual effects of vasoactive drugs that may confound measures of carotid arterial stiffness. Despite this, most of the medications in the HFpEF patients would be expected to reduce arterial stiffness. By definition, subjects in the healthy older group were taking no chronic medications. Thus, the severity of arterial stiffness observed in the HFpEF patients would be more likely to be underestimated due to medication effects, rather than overestimated. Although current (< 2 years) smokers were excluded, some participants had been smokers in the past.
In the present study, we did not have an older control group with diabetes or hypertensive left ventricular hypertrophy without HF, therefore, it is possible that the increased carotid arterial stiffness observed in our elderly HFpEF patients may have been due to antecedent hypertension. Indeed, Melanovsky previously reported that carotid mean blood pressure, carotid augmentation index and arterial compliance were similar between HFpEF patients and individuals with hypertensive left ventricular hypertrophy without overt HF.34
Measures of carotid distensibility were obtained during supine rest, and therefore the present data do not exclude additional, more severe abnormalities in vascular function, such as impaired vasodilation, that may occur during exercise and potentially contribute to exercise tolerance5. Indeed, our finding of a significant positive (but weak) correlation between peak VO2 or O2P (an indirect measure of stroke volume) and carotid distensibility suggests that increased arterial loading may result in the blunted peak exercise SV found in some studies of clinically stable older HFpEF patients3. Although these data, taken together with previous reports from our group and other investigators, suggest that there is a generalized increase in systemic arterial stiffness in patients with HFpEF, there can be substantial regional and segmental variations in arterial function.13,22 While the carotid artery is relatively proximal to the left ventricle, there are relatively few data examining its role in determining impedance to ventricular ejection and overall systemic vascular resistance, unlike the case with the aorta.13,14
The finding of abnormal arterial stiffness and significant correlations with exercise tolerance in a cross-sectional study cannot prove a causal relationship. Furthermore, peak VO2 is determined by a complex interaction of multiple organ systems and physiological responses. Thus, it is not certain that modification of arterial stiffness, if feasible, would lead to improved exercise capacity.
The present study utilized a variety of indexes of arterial stiffness, each of which has somewhat different determinants and implications, and there is lack of consensus in the literature regarding which of the indexes is optimal for clinical studies. Carotid arterial distensibility was the pre-selected primary outcome, however, most of the indexes of arterial stiffness were in agreement regarding the key findings of the study.
Perspectives
Carotid arterial distensibility is decreased in older patients with HFpEF beyond the changes which occur with normal aging alone, and is correlated with peak exercise VO2 and 6 minute walk distance. This suggests that a generalized increase in systemic arterial stiffness may contribute to the pathophysiology of exercise intolerance in older patients with HFpEF. Assessment of carotid stiffness by high resolution ultrasound may provide a practical means for serial assessment of arterial stiffness during clinical studies of therapeutic agents aimed at improving exercise intolerance, the primary chronic symptom in HFPEF.
Supplementary Material
Novelty and significance.
What is new?
• Carotid arterial distensibility is decreased in older patients with heart failure with preserved ejection fraction (HFpEF) beyond the changes which occur with normal aging alone, and is correlated with exercise capacity.
What is relevant?
• Increased arterial stiffness may contribute to the pathophysiology of exercise intolerance, the primary symptom in chronic HFpEF, which is the dominant form of heart failure in the community.
Acknowledgments
Sources of Funding Research supported by NIH grants R37-AG18915 and P30-AG021332
Footnotes
Conflicts of Interest Dr. Kitzman is a consultant for Relypsa Inc., Boston Scientific Corp., Abbot, and Servier, has received grant support from Novartis, and owns stock in Gilead Sciences.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Kitzman DW, Gardin JM, Gottdiener JS, Arnold AM, Boineau R, Aurigemma GP, Marino E, Lyles M, Cushman M, Enright P, For the Cardiovascular Health Study Group Importance of heart failure with preserved systolic function in patients greater than or equal to 65 Years of Age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 3.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan M. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 4.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 5.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired Chronotropic and Vasodilator Reserves Limit Exercise Capacity in Patients With Heart Failure and a Preserved Ejection Fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, Johnson BD, Redfield MM. Global Cardiovascular Reserve Dysfunction in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of Exercise Intolerance in Elderly Heart Failure Patients With Preserved Ejection Fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boutouyrie P, Tropeano AI, Asmar R. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 10.Blacher J, Asmar R, Djane S, London GM, Safar M. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 11.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortis pulse-wave velocity and its relationship to mortality in dibetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 12.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness: A Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 13.Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Little WC. Cardiac cycle dependent changes in aortic area and aortic distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 14.Rerkpattanapipat P, Hundley WG, Link KM, Brubaker PH, Hamilton CA, Darty SN, Morgan TM, Kitzman DW. Relation of Aortic Distensibility Determine by Magnetic Resonance Imaging in patients equal to 60 Years of Age to Systolic Heart Failure and Exercise Capacity. Am J Cardiol. 2002;90:1221–1225. doi: 10.1016/s0002-9149(02)02838-2. [DOI] [PubMed] [Google Scholar]
- 15.McKay CR, Rich MW, Vlietstra RE, Kitzman DW, Fleg J, Krumholz HM, Lakatta E, Cooke JP, Cannon CP, Ezekowitz MD, Frohlich ED, Jalife J, Kass DA, Kottke BA, Lorell BH, Muller JE, Saltin B, Shen WK, Somers VK. Executive Summary: Pivotal research in cardiovascular syndromes in the elderly: PRICE-I Executive Summary. Am J Geriatr Cardiol. 2000;9:243–250. doi: 10.1111/j.1076-7460.2000.89991.x. [DOI] [PubMed] [Google Scholar]
- 16.Giannattasio C, Achilli F, Failla M, Capra A, Vincenzi A, Valagussa F, Mancia G. Radial, carotid and aortic distensibility in congestive heart failure: effects of high-dose angiotensin-converting enzyme inhibitor or low-dose association with angiotensin type 1 receptor blockade. J Am Coll Cardiol. 2002;39:1275–1282. doi: 10.1016/s0735-1097(02)01755-2. [DOI] [PubMed] [Google Scholar]
- 17.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of Vasodilation in Heart Failure With Preserved or Reduced Ejection Fraction: Implications of Distinct Pathophysiologies on Response to Therapy. J Am Coll Cardiol. 2012;59:442–451. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 18.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 19.Borlaug BA, Nishimura RA, Sorajja P, Lam CSP, Redfield MM. Exercise Hemodynamics Enhance Diagnosis of Early Heart Failure with Preserved Ejection Fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeder MT, Thompson BR, Brunner-La Rocca H-P, Kaye DM. Hemodynamic Basis of Exercise Limitation in Patients With Heart Failure and Normal Ejection Fraction. J Am Coll Cardiol. 2010;56:855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 21.Maeder MT, Thompson BR, Htun N, Kaye DM. Hemodynamic determinants of the abnormal cardiopulmonary exercise response in heart failure with preserved left ventricular ejection fraction. J Card Fail. 2012;18:702–710. doi: 10.1016/j.cardfail.2012.06.530. [DOI] [PubMed] [Google Scholar]
- 22.Haykowsky MJ, Herrington DM, Brubaker PH, Morgan TM, Hundley WG, Kitzman DW. Relationship of flow mediated arterial; dilation and exercise capacity in older patients with heart failure and preserved ejection fraction. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls099. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise Training in Older Patients With Heart Failure and Preserved Ejection Fraction. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 25.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney R. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 26.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield J. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 27.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Zile MR, Gaasch WH, Carroll JD, Feldman MD, Aurigemma GP, Schaer GL, Ghali JK, Liebson PR. Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation. 2001;104:779–782. doi: 10.1161/hc3201.094226. [DOI] [PubMed] [Google Scholar]
- 30.Marburger CT, Brubaker PH, Pollock WE, Morgan TM, Kitzman DW. Reproducibility of cardiopulmonary exercise testing in elderly heart failure patients. Am J Cardiol. 1998;82:905–909. doi: 10.1016/s0002-9149(98)00502-5. [DOI] [PubMed] [Google Scholar]
- 31.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, Heiss G. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension. 1999;34:201–206. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- 32.Tartiere-Kesri L, Tartiere JM, Logeart D, Beauvais F, Cohen Solal A. Increased Proximal Arterial Stiffness and Cardiac Response With Moderate Exercise in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2012;59:455–461. doi: 10.1016/j.jacc.2011.10.873. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined Ventricular Systolic and Arterial Stiffening in Patients With Heart Failure and Preserved Ejection Fraction. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 34.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular Features of Heart Failure With Preserved Ejection Fraction Versus Nonfailing Hypertensive Left Ventricular Hypertrophy in the Urban Baltimore Community: The Role of Atrial Remodeling/Dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 35.Hundley WG, Bayram E, Hamilton CA, Hamilton EA, Morgan TM, Darty SN, Stewart KP, Link KM, Herrington DM, Kitzman DW. Leg flow-mediated arterial dilation in elderly patients with heart failure and normal left ventricular ejection fraction. Am J Physiol Heart Circ Physiol. 2007;292:H1427–H1434. doi: 10.1152/ajpheart.00567.2006. [DOI] [PubMed] [Google Scholar]
- 36.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 37.Zelis R, Mason DT. Diminished forearm arteriolar dilator capacity produced by mineralocorticoid-induce salt retention in man. Implication concerning congestive heart failure and vascular stiffness. Circulation. 1970;41:589–592. doi: 10.1161/01.cir.41.4.589. [DOI] [PubMed] [Google Scholar]
- 38.Eaton GM, Cody R, Binkley PF. Increased aortic impedance precedes peripheral vasoconstriction at the early stage of ventricular failure in the paced canine model. Circulation. 1993;88:2714–2721. doi: 10.1161/01.cir.88.6.2714. [DOI] [PubMed] [Google Scholar]
- 39.Clarkson PBM, Wheeldon NM, MacFadyen RJ, Pringle SD, MacDonald TM. Effects of brain natriuretic peptide on exercise hemodynamics and neurohormones in isolated diastolic heart failure. Circulation. 1996;93:2037–2042. doi: 10.1161/01.cir.93.11.2037. [DOI] [PubMed] [Google Scholar]
- 40.Khan Z, Millard RW, Gabel M, Walsh RA, Hoit BD. Effect of congestive heart failure on in vivo canine aortic elastic properties. J Am Coll Cardiol. 1999;33:267–272. doi: 10.1016/s0735-1097(98)00530-0. [DOI] [PubMed] [Google Scholar]
- 41.Cohn JN. Pathophysiologic and prognostic implication of measuring arterial compliance in hypertensive disease. Prog Cardiovasc Dis. 1999;41:441–450. doi: 10.1016/s0033-0620(99)70020-4. [DOI] [PubMed] [Google Scholar]
- 42.Ubels FL, Muntinga JH, van Doormaal JJ, Reitsma WD, Smit AJ. Effects of initial and long-term lipid lowering therapy on vascular wall characteristics. Atherosclerosis. 2001;154:155–161. doi: 10.1016/s0021-9150(00)00450-0. [DOI] [PubMed] [Google Scholar]
- 43.Wada T, Kodaira K, Fujishiro, Maie K, Tsukiyama E, Fukumoto T, Uchida T, Yamazaki S. Correlation of ultrasound-measured common carotid artery stiffness with pathological findings. Arterioscler Thromb. 1994;14:479–482. doi: 10.1161/01.atv.14.3.479. [DOI] [PubMed] [Google Scholar]
- 44.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 45.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. European Heart Journal. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 46.Pernenkil R, Vinson JM, Shah AS, Beckham V, Wittenberg C, Rich MW. Course and prognosis in patients ≥ 70 years of age with congestive heart failure and normal versus abnormal left ventricular ejection fraction. Am J Cardiol. 1997;79:216–219. doi: 10.1016/s0002-9149(96)00719-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.