Abstract
Background
Red meat consumption has been associated with an increased risk of chronic diseases. However, its relationship with mortality remains uncertain.
Methods
We prospectively followed 37698 men from the Health Professionals Follow-up Study (1986-2008) and 83644 women from the Nurses' Health Study (1980-2008), who were free of cardiovascular disease (CVD) and cancer at baseline. Diet was assessed by validated food-frequency questionnaires and updated every four years.
Results
We documented 23926 deaths (including 5910 CVD and 9464 cancer deaths) during 2.96 million person-years of follow-up. After multivariate adjustment for major lifestyle and dietary risk factors, the pooled hazard ratio (HR) and 95% confidence interval of total mortality was 1.13 (1.07-1.20) for 1-serving per day increase of unprocessed red meat, 1.20 (1.15-1.24) for processed red meat. The corresponding HRs were 1.18 (1.13-1.23) and 1.21 (1.13-1.31) for CVD mortality, 1.10 (1.06-1.14) and 1.16 (1.09-1.23) for cancer mortality. We estimated that substitutions of 1-serving per day of other foods (including fish, poultry, nuts, legumes, low-fat dairy, and whole grains) for 1-serving per day of red meat were associated with a 7%-19% lower mortality risk. We also estimated that 9.3% of deaths in men and 7.6% in women in our cohorts could be prevented at the end of follow-up if all individuals consumed <0.5 serving/d (≈42 g/d) of red meat.
Conclusions
Red meat consumption is associated with an increased risk of total, CVD and cancer mortality. Substitution of other healthy protein sources for red meat is associated with a lower mortality risk.
Introduction
Meat is a major source of protein and fat in most diets. Substantial evidence from epidemiological studies shows that meat intake, particularly red meat, is associated with increased risks of diabetes,1 cardiovascular disease (CVD),2 and certain cancers.3 Several studies also suggest an elevated risk of mortality associated with red meat intake. However, most of these studies have been carried out in populations with a particularly high proportion of vegetarians (such as the Seventh-Day Adventists in the U.S.4 and several studies in Europe5). A recent large cohort study with 10 years of follow-up found that a higher intake of total red meat and total processed meat was associated with an increased risk of mortality.6 However, this study did not differentiate unprocessed from processed red meat, and diet and other covariates were only assessed at baseline. Furthermore, no study has so far examined whether substitution of other dietary components for red meat is associated with a reduced mortality risk.
Therefore, we investigated the association between red meat intake and total and cause-specific mortality in two large cohorts with repeated measures of diet and up to 28 years of follow-up: the Health Professionals Follow-up Study (HPFS) and Nurses' Health Study (NHS). We also estimated the associations of substituting other healthy protein sources for red meat with total and cause-specific mortality.
Subjects and Methods
Study Population
We analyzed data from two prospective cohort studies: HPFS (initiated in 1986, n=51529 men, age 40-75 years), and NHS (started in 1976, n=121700 women, age 30-55 years). Detailed descriptions of the cohorts are provided elsewhere.7-8 Questionnaires were administered biennially to collect and update medical, lifestyle and other health-related information, and the follow-up rates exceed 90% in each 2-year cycle for both cohorts.
In the current analysis, we used 1986 for HPFS and 1980 for NHS as baseline, when we assessed diet using validated food-frequency questionnaire (FFQ; 49934 men and 92468 women returned the baseline FFQ). We excluded men and women who had a history of CVD or cancer at baseline (5617 men and 5613 women), who left more than nine blank responses on the baseline FFQ, had missing information on meat intake, or reported implausible energy intake levels (<500 or >3500 kcal/d; 6619 men and 3211 women). After exclusions, data from 37698 men and 83644 women were available for the analysis. The excluded participants and those who remained in the study were similar with respect to red meat intake and obesity status at baseline. The study protocol was approved by the institutional review boards of Brigham and Women's Hospital and Harvard School of Public Health.
Assessment of Meat Consumption
In 1980, a 61-item FFQ was administered among the NHS participants to collect information on their usual intake of foods and beverages in the previous year. In 1984, 1986, 1990, 1994, 1998, 2002 and 2006, similar but expanded FFQs with 131 to 166 items were sent to these participants to update their diet. Using the expanded FFQ employed in the NHS, dietary data were collected in 1986, 1990, 1994, 1998, 2002 and 2006 among the HPFS participants. In each FFQ, we asked the participants how often, on average, they consumed each food of a standard portion size. There were nine possible responses, ranging from “never or less than once per month” to “six or more times per day”. Questionnaire items on unprocessed red meat consumption included “beef, pork or lamb as main dish” (pork was separately asked since 1990), “hamburger”, and “beef, pork, or lamb as a sandwich or mixed dish”. The standard serving size was 85 g (3 ounces) for unprocessed red meat. Processed red meat included “bacon” (two slices, 28 g), “hot dogs” (one, 45 g), and “sausage, salami, bologna, and other processed red meats” (one piece, 45 g). The reproducibility and validity of these FFQs have been described in detail elsewhere.9,10 The corrected correlation coefficients between FFQ and multiple dietary records were 0.59 for unprocessed red meat and 0.52 for processed red meat in HPFS,9 and similar correlations were found in NHS.10
Death Ascertainment
The ascertainment of death has been documented in previous studies.14-15 Briefly, deaths were identified by reports from the next of kin, postal authorities, or by searching National Death Index, and at least 95% of deaths were identified.11 The cause of death was determined after review by physicians and primarily based on medical records and death certificates. We used ICD-8 codes (International Classification of Diseases, 8th revision), which was widely used at the start of the cohorts, to distinguish deaths due to cancer (140-207) and cardiovascular diseases (390-459 and 795).
Assessment of Covariates
In the biennial follow-up questionnaires, we inquired and updated information on medical, lifestyle or other health-related factors, such as body weight, cigarette smoking, physical activity, medication or supplements use, and family history of diabetes, myocardial infarction, and cancer, as well as a history of diabetes, hypertension and hypercholesterolemia. Among NHS participants, we ascertained menopausal status and postmenopausal hormone use.
Statistical Analysis
We used time-dependent Cox proportional hazards models to assess the association of red meat consumption with total and cause-specific mortality risks during the follow-up. We conducted analyses separately for each cohort. In multivariate analysis, we simultaneously controlled for intakes of total energy, whole grain, fruit and vegetables (all in quintiles), as well as other potential non-dietary confounding variables with updated information at each 2 or 4-year questionnaire cycle. These variables included age, body mass index (BMI; <23.0, 23.0-24.9, 25.0-29.9, 30.0-34.9, ≥35.0 kg/m2), ethnicity (whites, non-whites), smoking status (never, past, current 1-14 cigarettes/d, 15-24 cigarettes/d, ≥25 cigarettes/d), alcohol intake (0, 0.1-4.9, 5.0-14.9, ≥15.0 g/d in women; 0, 0.1-4.9, 5.0-29.9, ≥30.0 g/d in men), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 hours of metabolic equivalent tasks per week), multivitamin use (yes, no), aspirin use (yes, no), family history of diabetes, myocardial infarction or cancer, and baseline histories of diabetes, hypertension, or hypercholesterolemia. Among women, we also adjusted for postmenopausal status and menopausal hormone use.
To better represent long-term diet and to minimize the within-person variation, we created cumulative averages of food intake from baseline to death from our repeated FFQs.12 We replaced missing values in each follow-up FFQ with the cumulative averages prior to the missing values. We stopped updating the dietary variables when the participants reported a diagnosis of diabetes, stroke, coronary heart disease, angina, or cancer, because these conditions might lead to changes in diet.
We conducted several sensitivity analyses to test the robustness of our results: (1) we further adjusted for intakes of other major dietary variables (fish, poultry, nuts, legumes, and dairy products, all in quintiles), or several nutrients or dietary components (glycemic load, cereal fiber, magnesium, polyunsaturated and trans fatty acids, all in quintiles) instead of foods; (2) we corrected for measurement error13 in the assessment of red meat intake by using a regression calibration approach using data from validation studies conducted in HPFS9 in 1986 and NHS10 in 1980 and 1986; (3) we repeated the analysis by using simply updated dietary methods (using the most recent dietary variables to predict the mortality risk in the next 4 years)14, or continuing updating a participant's diet even after he/she reported a diagnosis of major chronic disease, or using only baseline dietary variables; (4) we used energy density of red meat intake (serving·1000kcal-1·d-1) as the exposure instead of the crude intake. In addition, we used restricted cubic spline regressions with 4 knots to examine a dose-response relation between red meat intake and risk of total mortality.
We estimated the associations of substituting one serving of an alternative food for red meat with mortality by including both as continuous variables in the same multivariate model, which also contained non-dietary covariates and total energy intake. The difference in their beta coefficients, as well as their variances and covariance were used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for the substitution associations.15 We calculated population attributable risk and 95% CI to estimate the proportion of deaths in the two cohorts that would be prevented at the end of the follow-up if all participants were in the low intake group.16 For these analyses, we compared participants in the low red meat intake category (<0.5 serving/d, or 42 g/d) with the rest of the participants in the cohorts.
The HRs from the final multivariate-adjusted models in each cohort were pooled to obtain a summary risk estimate with the use of an inverse variance-weighted meta-analysis by random-effects model, which allowed for between-study heterogeneity. Data were analyzed with the SAS software, version 9.2 (SAS Institute), at a two-tailed alpha level of 0.05.
Results
In HPFS, with up to 22 years of follow-up (758524 person-years), we documented 8926 deaths, of which 2716 were CVD deaths and 3073 were cancer deaths. In NHS, with up to 28 years of follow-up (2199892 person-years), we documented 15000 deaths, of which 3194 were CVD deaths and 6391 were cancer deaths. Men and women with higher intake of red meat were less likely to be physically active, and more likely to be current smokers, drink alcohol and have higher BMI (Table 1). In addition, a higher red meat intake was associated with a higher intake of total energy, but lower intakes of whole grain, fruit and vegetables. Unprocessed and processed red meat consumption was moderately correlated (0.40 in HPFS and 0.37 in NHS). However, red meat consumption was less correlated with intakes of poultry or fish (Spearman correlation coefficients, −0.04 and −0.18 in HPFS, 0.05 and −0.12 in NHS, respectively). During the follow-up, red meat intake declined in both men and women (eFigure 1). For example, the mean intake of unprocessed red meat dropped from 0.75 to 0.63 serving/d from 1986 to 2006 in men, and from 1.10 to 0.55 serving/d from 1980 to 2006 in women.
Table 1. Baseline age-standardized characteristics of participants in the two cohorts according to quintiles of total red meat consumption.
| Characteristics | Total red meat intake quintile, servings/d | ||||
|---|---|---|---|---|---|
|
| |||||
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| HPFS | |||||
|
| |||||
| n | 7431 | 7813 | 7308 | 7606 | 7540 |
| Age (year) | 53.8 | 52.6 | 52.5 | 52.5 | 52.2 |
| Total red meat intake (serving/d) | 0.22 | 0.62 | 1.01 | 1.47 | 2.36 |
| Physical activity (MET-h/wk) | 27.5 | 22.7 | 20.2 | 18.8 | 17.2 |
| Body mass index (kg/m2) | 24.7 | 25.3 | 25.5 | 25.7 | 26.0 |
| Race, white (%) | 93.1 | 95.1 | 95.2 | 95.8 | 95.8 |
| Current smoker (%) | 5.0 | 7.3 | 9.8 | 11.3 | 14.5 |
| Diabetes (%) | 2.0 | 2.0 | 2.2 | 2.4 | 3.5 |
| Hypertension (%) | 19.5 | 19.7 | 19.3 | 19.6 | 20.2 |
| High cholesterol (%) | 14.8 | 11.1 | 9.7 | 9.0 | 7.9 |
| Family history of diabetes (%) | 19.5 | 18.6 | 19.1 | 20.0 | 19.3 |
| Family history of myocardial infarction (%) | 35.1 | 31.8 | 30.9 | 31.4 | 30.0 |
| Family history of cancer (%) | 33.7 | 34.5 | 35.0 | 33.9 | 33.6 |
| Current multivitamin use (%) | 49.1 | 42.5 | 40.3 | 39.5 | 36.6 |
| Current aspirin use (%) | 24.6 | 26.4 | 25.9 | 27.8 | 27.4 |
| Dietary intake | |||||
| Total energy (kcal/d) | 1659 | 1752 | 1886 | 2091 | 2396 |
| Alcohol (g/day) | 8.4 | 10.7 | 11.2 | 12.4 | 13.4 |
| Fruit, serving/d | 2.83 | 2.35 | 2.21 | 2.13 | 2.04 |
| Vegetables, serving/d | 3.29 | 2.89 | 2.91 | 2.97 | 3.07 |
| Whole grain, serving/d | 1.93 | 1.58 | 1.50 | 1.51 | 1.48 |
| Nuts, serving/d | 0.45 | 0.45 | 0.44 | 0.47 | 0.49 |
| Legume, serving/d | 0.45 | 0.38 | 0.39 | 0.43 | 0.47 |
| Dairy products, serving/d | 1.65 | 1.80 | 1.89 | 2.02 | 2.14 |
| Fish, serving/d | 0.55 | 0.43 | 0.38 | 0.36 | 0.32 |
| Poultry, serving/d | 0.64 | 0.58 | 0.55 | 0.55 | 0.53 |
|
| |||||
| NHS | |||||
|
| |||||
| n | 16499 | 17247 | 16461 | 16603 | 16834 |
| Age (year) | 47.3 | 46.0 | 45.8 | 45.3 | 46.0 |
| Total red meat intake (serving/d) | 0.53 | 1.04 | 1.52 | 2.01 | 3.10 |
| Physical activity (MET-h/wk) | 16.9 | 13.9 | 13.8 | 13.3 | 12.4 |
| Body mass index (kg/m2) | 23.9 | 24.3 | 24.4 | 24.5 | 24.7 |
| Race, white (%) | 96.9 | 97.9 | 97.8 | 98.0 | 97.2 |
| Current smoker (%) | 25.5 | 29.1 | 28.2 | 29.7 | 31.6 |
| Diabetes (%) | 1.6 | 1.8 | 2.1 | 2.2 | 2.9 |
| Hypertension (%) | 15.2 | 15.7 | 15.5 | 15.4 | 16.4 |
| High cholesterol (%) | 6.0 | 5.3 | 5.2 | 4.5 | 4.7 |
| Family history of diabetes (%) | 26.7 | 27.9 | 28.1 | 29.0 | 29.9 |
| Family history of myocardial infarction (%) | 19.4 | 19.0 | 19.0 | 18.6 | 19.0 |
| Family history of cancer (%) | 17.1 | 16.7 | 16.1 | 16.6 | 16.3 |
| Postmenopausal (%) | 31.3 | 31.3 | 30.8 | 31.1 | 31.1 |
| Current menopausal hormone use (%)a | 20.6 | 20.4 | 21.0 | 21.3 | 20.7 |
| Current multivitamin use (%) | 37.9 | 33.6 | 33.1 | 32.8 | 32.3 |
| Current aspirin use (%) | 43.2 | 46.9 | 46.3 | 48.3 | 49.1 |
| Dietary intake | |||||
| Total energy (kcal/day) | 1202 | 1371 | 1523 | 1705 | 2030 |
| Alcohol (g/d) | 5.8 | 6.3 | 6.6 | 6.5 | 6.6 |
| Fruit, serving/d | 2.21 | 2.05 | 2.04 | 2.03 | 2.02 |
| Vegetables, serving/d | 1.89 | 1.83 | 1.92 | 1.98 | 2.08 |
| Whole grain, grams/d | 1.53 | 1.37 | 1.35 | 1.36 | 1.28 |
| Nuts, serving/d | 0.16 | 0.13 | 0.13 | 0.14 | 0.15 |
| Legume, serving/d | 0.44 | 0.44 | 0.45 | 0.49 | 0.52 |
| Dairy products, serving/d | 1.81 | 1.80 | 1.82 | 1.87 | 1.83 |
| Fish, serving/d | 0.50 | 0.40 | 0.39 | 0.35 | 0.33 |
| Poultry, serving/d | 0.64 | 0.59 | 0.59 | 0.58 | 0.58 |
Abbreviation: HPFS, Health Professionals Follow-up Study; MET-h, hours of metabolic equivalent tasks; NHS, Nurses' Health Study.
Current menopausal hormone use among postmenopausal women.
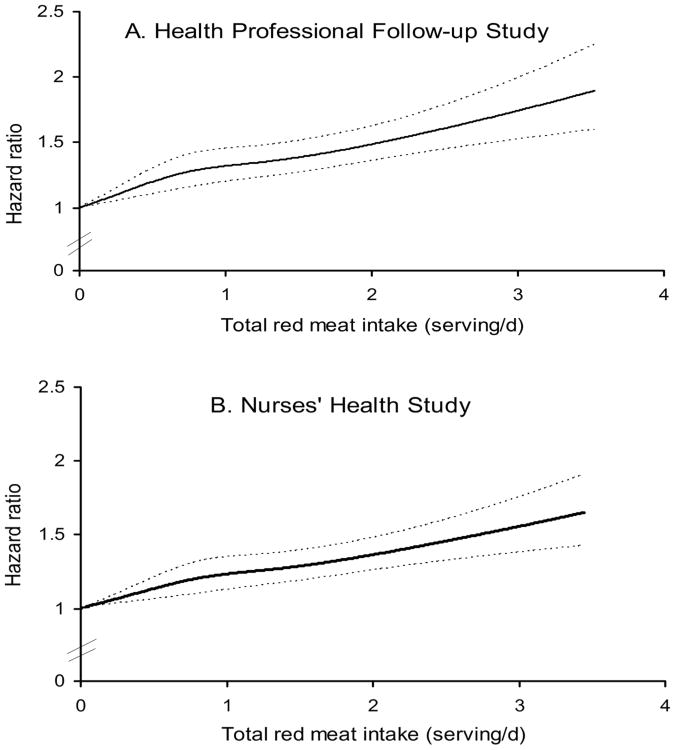
Both unprocessed and processed red meat intakes were associated with an increased risk of total, CVD and cancer mortality in both men and women in the age-adjusted and fully-adjusted models (Tables 2-4). When treating red meat intake as a continuous variable, the elevated risk of total mortality in the pooled analysis was 12% (HR, 1.12; 95% CI, 1.09-1.15) for 1-serving/d increase of total red meat, 13% (HR, 1.13; 95% CI, 1.07-1.20) for unprocessed red meat, and 20% (HR, 1.20; 95% CI, 1.15-1.24) for processed red meat. The HRs (95% CIs) for CVD mortality were 1.16 (1.12-1.20) for total red meat, 1.18 (1.13-1.23) for unprocessed red meat, and 1.21 (1.13-1.31) for processed red meat. The HRs (95% CIs) for cancer mortality were 1.10 (1.07-1.13) for total red meat, 1.10 (1.06-1.14) for unprocessed red meat, and 1.16 (1.09-1.23) for processed red meat. We found no statistically significant differences among specific unprocessed red meat items, or among specific processed red meat items for the associations with total mortality (eTable 1). However, bacon and hot dogs tended to be associated with a higher risk than other items. Spline regression analysis showed that the association between red meat intake and risk of total mortality was linear (P for linearity <0.001; Figure 1). Furthermore, no significant interaction was detected between red meat intake and BMI or physical activity (P >0.10 for both tests).
Table 2. Hazard ratio (HR) and 95% confidence interval (CI) of all-cause mortality according to red meat intake in the HPFS and NHS.
| Frequency of consumption (quintiles) | P for trend | HR (95% CI) for 1 serving/d increase | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| HPFS | |||||||
|
| |||||||
| Total red meat (servings/d)a | 0.25 (0.13-0.37) | 0.61 (0.53-0.70) | 0.95 (0.87-1.04) | 1.36 (1.24-1.49) | 2.07 (1.83-2.47) | ||
| Cases/person-years | 1713/151212 | 1610/152120 | 1679/151558 | 1794/152318 | 2130/151315 | ||
| Age-adjusted model | 1.00 | 1.06 (0.99-1.14) | 1.14 (1.06-1.21) | 1.21 (1.14-1.30) | 1.45 (1.36-1.54) | <0.001 | 1.19 (1.16-1.23) |
| Multivariate modelb | 1.00 | 1.12 (1.05-1.20) | 1.21 (1.13-1.30) | 1.25 (1.16-1.34) | 1.37 (1.27-1.47) | <0.001 | 1.14 (1.10-1.17) |
| Unprocessed red meat (servings/d)a | 0.17 (0.07-0.24) | 0.43 (0.37-0.47) | 0.66 (0.58-0.73) | 0.95 (0.87-1.04) | 1.46 (1.29-1.67) | ||
| Cases/person-years | 1855/150676 | 1722/149097 | 1535/154352 | 1819/150925 | 1995/153474 | ||
| Age-adjusted model | 1.00 | 1.06 (0.99-1.13) | 1.00 (0.94-1.07) | 1.15 (1.08-1.23) | 1.34 (1.25-1.42) | <0.001 | 1.22 (1.18-1.27) |
| Multivariate modelb | 1.00 | 1.11 (1.04-1.18) | 1.14 (1.06-1.22) | 1.20 (1.12-1.28) | 1.29 (1.20-1.38) | <0.001 | 1.17 (1.12-1.21) |
| Processed red meat (servings/d)a | 0.02 (0-0.07) | 0.13 (0.10-0.14) | 0.21 (0.20-0.26) | 0.39 (0.34-0.46) | 0.74 (0.64-1.00) | ||
| Cases/person-years | 1917/171619 | 1395/131069 | 1661/152481 | 1717/152128 | 2236/151227 | ||
| Age-adjusted model | 1.00 | 0.99 (0.93-1.06) | 1.13 (1.05-1.20) | 1.14 (1.07-1.22) | 1.38 (1.30-1.47) | <0.001 | 1.34 (1.28-1.40) |
| Multivariate modelb | 1.00 | 1.06 (0.99-1.14) | 1.15 (1.07-1.23) | 1.18 (1.10-1.27) | 1.27 (1.19-1.36) | <0.001 | 1.18 (1.12-1.24) |
|
| |||||||
| NHS | |||||||
|
| |||||||
| Total red meat (servings/d)a | 0.51 (0.37-0.61) | 0.85 (0.76-0.96) | 1.14 (1.03-1.32) | 1.49 (1.33-1.71) | 2.17 (1.85-2.66) | ||
| Cases/person-years | 2946/438326 | 2759/442134 | 2658/439712 | 2872/440329 | 3765/439391 | ||
| Age-adjusted model | 1.00 | 1.07 (1.01-1.12) | 1.09 (1.04-1.15) | 1.24 (1.18-1.30) | 1.61 (1.53-1.69) | <0.001 | 1.30 (1.28-1.33) |
| Multivariate modelb | 1.00 | 1.08 (1.02-1.14) | 1.11 (1.05-1.17) | 1.18 (1.12-1.24) | 1.24 (1.17-1.30) | <0.001 | 1.11 (1.08-1.13) |
| Unprocessed red meat (servings/d)a | 0.37 (0.28-0.46) | 0.61 (0.56-0.68) | 0.86 (0.77-1.00) | 1.13 (1.01-1.28) | 1.64 (1.43-2.05) | ||
| Cases/person-years | 3079/441041 | 2885/441207 | 2545/439306 | 2709/431097 | 3782/447240 | ||
| Age-adjusted model | 1.00 | 1.05 (1.00-1.11) | 0.98 (0.93-1.03) | 1.09 (1.03-1.14) | 1.48 (1.41-1.55) | <0.001 | 1.31 (1.28-1.35) |
| Multivariate modelb | 1.00 | 1.07 (1.01-1.12) | 1.07 (1.01-1.12) | 1.10 (1.05-1.16) | 1.19 (1.13-1.25) | <0.001 | 1.10 (1.06-1.13) |
| Processed red meat (servings/d)a | 0.05 (0-0.11) | 0.14 (0.13-0.16) | 0.23 (0.21-0.28) | 0.36 (0.33-0.42) | 0.64 (0.56-0.87) | ||
| Cases/person-years | 3076/442594 | 2799/420403 | 2778/455365 | 2814/441369 | 3533/440161 | ||
| Age-adjusted model | 1.00 | 1.06 (1.01-1.12) | 1.10 (1.04-1.16) | 1.18 (1.12-1.24) | 1.49 (1.42-1.56) | <0.001 | 1.61 (1.54-1.69) |
| Multivariate modelb | 1.00 | 1.04 (0.99-1.10) | 1.08 (1.03-1.14) | 1.14 (1.08-1.20) | 1.20 (1.14-1.27) | <0.001 | 1.21 (1.15-1.27) |
|
| |||||||
| Pooled Resultsc | |||||||
|
| |||||||
| Total red meat | 1.00 | 1.10 (1.05-1.14) | 1.15 (1.06-1.26) | 1.21 (1.14-1.28) | 1.30 (1.18-1.43) | <0.001 | 1.12 (1.09-1.15) |
| Unprocessed red meat | 1.00 | 1.08 (1.05-1.12) | 1.10 (1.03-1.17) | 1.15 (1.05-1.25) | 1.23 (1.14-1.34) | <0.001 | 1.13 (1.07-1.20) |
| Processed red meat | 1.00 | 1.05 (1.00-1.09) | 1.11 (1.04-1.18) | 1.15 (1.11-1.20) | 1.23 (1.16-1.30) | <0.001 | 1.20 (1.15-1.24) |
Abbreviation: HPFS, Health Professionals Follow-up Study; NHS, Nurses' Health Study.
Data were presented as median (interquartile range).
Multivariate model: adjusted for age (continuous), body mass index category (<23, 23-24.9, 25-29.9, 30-34.9, ≥35 kg/m2), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15 g/d), physical activity level (<3, 3-8.9, 9-17.9, 18-26.9, ≥27 hours of metabolic equivalent tasks per week), smoking status (never, past, current 1-14 cigarettes/d, current 15-24 cigarettes/d, current ≥25 cigarettes/d,), race (white/non-white), menopausal status and hormone use in women (premenopausal, postmenopausal never users, postmenopausal past users, postmenopausal current users), family history of diabetes, myocardial infarction, or cancer, history of diabetes, hypertension, or hypercholesterolemia, quintiles of total energy intake, whole grains, fruits, and vegetables.
Results from multivariate model were combined using random-effects model.
Table 4. Hazard ratio (HR) and 95% confidence interval (CI) of cancer mortality according to red meat intake in the HPFS and NHS.
| Frequency of consumption (quintiles) | P for trend | HR (95% CI) for 1 serving/d increase | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| HPFS | |||||||
|
| |||||||
| Total red meat | |||||||
| Cases/person-years | 598/152206 | 558/153082 | 561/152574 | 646/153343 | 710/152584 | ||
| Age-adjusted model | 1.00 | 1.03 (0.91-1.15) | 1.05 (0.93-1.18) | 1.20 (1.07-1.34) | 1.33 (1.20-1.49) | <0.001 | 1.17 (1.12-1.22) |
| Multivariate modela | 1.00 | 1.05 (0.94-1.18) | 1.07 (0.95-1.20) | 1.18 (1.05-1.33) | 1.24 (1.09-1.40) | <0.001 | 1.12 (1.06-1.17) |
| Unprocessed red meat | |||||||
| Cases/person-years | 650/151745 | 588/150121 | 540/155255 | 613/152008 | 682/154661 | ||
| Age-adjusted model | 1.00 | 1.00 (0.89-1.12) | 0.97 (0.86-1.08) | 1.06 (0.95-1.18) | 1.25 (1.12-1.39) | <0.001 | 1.18 (1.11-1.26) |
| Multivariate modela | 1.00 | 1.01 (0.90-1.13) | 1.03 (0.91-1.15) | 1.05 (0.94-1.18) | 1.18 (1.05-1.33) | <0.001 | 1.13 (1.05-1.21) |
| Processed red meat | |||||||
| Cases/person-years | 669/172756 | 487/131895 | 580/153463 | 589/153122 | 748/152551 | ||
| Age-adjusted model | 1.00 | 0.97 (0.86-1.09) | 1.09 (0.98-1.22) | 1.09 (0.97-1.21) | 1.28 (1.15-1.42) | <0.001 | 1.31 (1.21-1.41) |
| Multivariate modela | 1.00 | 1.00 (0.89-1.12) | 1.07 (0.96-1.20) | 1.07 (0.95-1.20) | 1.15 (1.02-1.29) | <0.001 | 1.17 (1.07-1.27) |
|
| |||||||
| NHS | |||||||
|
| |||||||
| Total red meat | |||||||
| Cases/person-years | 1264/439774 | 1191/443495 | 1185/440970 | 1263/441727 | 1488/441393 | ||
| Age-adjusted model | 1.00 | 1.04 (0.96-1.13) | 1.08 (1.00-1.17) | 1.19 (1.10-1.29) | 1.39 (1.29-1.50) | <0.001 | 1.21 (1.17-1.25) |
| Multivariate modela | 1.00 | 1.05 (0.97-1.14) | 1.10 (1.01-1.19) | 1.15 (1.06-1.25) | 1.17 (1.08-1.28) | <0.001 | 1.09 (1.05-1.13) |
| Unprocessed red meat | |||||||
| Cases/person-years | 1308/442572 | 1222/442671 | 1120/440530 | 1215/432361 | 1526/449225 | ||
| Age-adjusted model | 1.00 | 1.02 (0.94-1.10) | 0.97 (0.90-1.06) | 1.09 (1.01-1.18) | 1.33 (1.24-1.44) | <0.001 | 1.22 (1.17-1.27) |
| Multivariate modela | 1.00 | 1.04 (0.96-1.12) | 1.03 (0.95-1.12) | 1.11 (1.02-1.20) | 1.17 (1.08-1.27) | <0.001 | 1.09 (1.04-1.14) |
| Processed red meat | |||||||
| Cases/person-years | 1294/444119 | 1230/421760 | 1236/456687 | 1204/442791 | 1427/442002 | ||
| Age-adjusted model | 1.00 | 1.08 (1.00-1.17) | 1.11 (1.03-1.20) | 1.14 (1.05-1.23) | 1.35 (1.25-1.46) | <0.001 | 1.41 (1.31-1.52) |
| Multivariate modela | 1.00 | 1.05 (0.97-1.14) | 1.08 (1.00-1.17) | 1.08 (1.00-1.17) | 1.14 (1.05-1.23) | 0.001 | 1.14 (1.05-1.24) |
|
| |||||||
| Pooled Resultsb | |||||||
|
| |||||||
| Total red meat | 1.00 | 1.05 (0.98-1.12) | 1.09 (1.02-1.16) | 1.16 (1.08-1.24) | 1.19 (1.11-1.28) | <0.001 | 1.10 (1.07-1.13) |
| Unprocessed red meat | 1.00 | 1.03 (0.97-1.10) | 1.03 (0.96-1.10) | 1.09 (1.02-1.16) | 1.17 (1.10-1.26) | <0.001 | 1.10 (1.06-1.14) |
| Processed red meat | 1.00 | 1.03 (0.97-1.10) | 1.08 (1.01-1.15) | 1.08 (1.01-1.15) | 1.14 (1.07-1.22) | <0.001 | 1.16 (1.09-1.23) |
Abbreviation: HPFS, Health Professionals Follow-up Study; NHS, Nurses' Health Study.
Multivariate model: adjusted for age (continuous), body mass index category (<23, 23-24.9, 25-29.9, 30-34.9, ≥35 kg/m2), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15 g/d), physical activity level (<3, 3-8.9, 9-17.9, 18-26.9, ≥27 hours of metabolic equivalent tasks per week), smoking status (never, past, current 1-14 cigarettes/d, current 15-24 cigarettes/d, current ≥25 cigarettes/d,), race (white/non-white), menopausal status and hormone use in women (premenopausal, postmenopausal never users, postmenopausal past users, postmenopausal current users), family history of diabetes, myocardial infarction, or cancer, history of diabetes, hypertension, or hypercholesterolemia, quintiles of total energy intake, whole grains, fruits, and vegetables.
Results from multivariate model were combined using random-effects model.
Figure 1. Dose-response relationship between red meat intake and risk of all-cause mortality in (A) Health Professionals Follow-up Study and (B) Nurses' Health Study.
The results were adjusted for age (continuous), body mass index category (<23, 23-24.9, 25-29.9, 30-34.9, ≥35 kg/m2), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15 g/d), physical activity level (<3, 3-8.9, 9-17.9, 18-26.9, ≥27 hours of metabolic equivalent tasks per week), smoking status (never, past, current 1-14 cigarettes/d, current 15-24 cigarettes/d, current ≥25 cigarettes/d,), race (white/non-white), menopausal status and hormone use in women (premenopausal, postmenopausal never users, postmenopausal past users, postmenopausal current users), family history of diabetes, myocardial infarction, or cancer, history of diabetes, hypertension, or hypercholesterolemia, quintiles of total energy intake, whole grains, fruits, and vegetables.
Additional adjustment for other foods (fish, poultry, nuts, beans, and dairy products) or nutrients (glycemic load, cereal fiber, magnesium, polyunsaturated and trans fatty acids) did not appreciably alter the results. Additional adjustment for saturated fat and cholesterol moderately attenuated the association between red meat intake and risk of CVD death, and the pooled HR dropped from 1.16 (95% CI, 1.12-1.20) to 1.12 (95% CI, 1.07-1.18). Similarly, additional adjustment for heme iron moderately attenuated the association, and the pooled HR dropped from 1.16 (95% CI, 1.12-1.20) to 1.11 (95% CI, 1.05-1.17). Additional adjustment for husband's education level as a surrogate of social-economic status in women did not change the results.
The results were not materially changed when we continuously updated dietary information even after diagnosis of chronic diseases (eTable 2) or simply updated the dietary variables (eTable 3). Also, using energy density of red meat intake as the exposure showed similar findings (eTable 4). In the sensitivity analysis that accounted for measurement error in diet, the associations became even stronger. For example, the HR was 1.25 (95% CI, 1.16-1.35) for 1-serving/d increase of total red meat intake with mortality in HPFS, and it was 1.83 (95% CI, 1.54-2.20) in NHS. However, the associations were attenuated in analyses using only baseline dietary data (eTable 5).
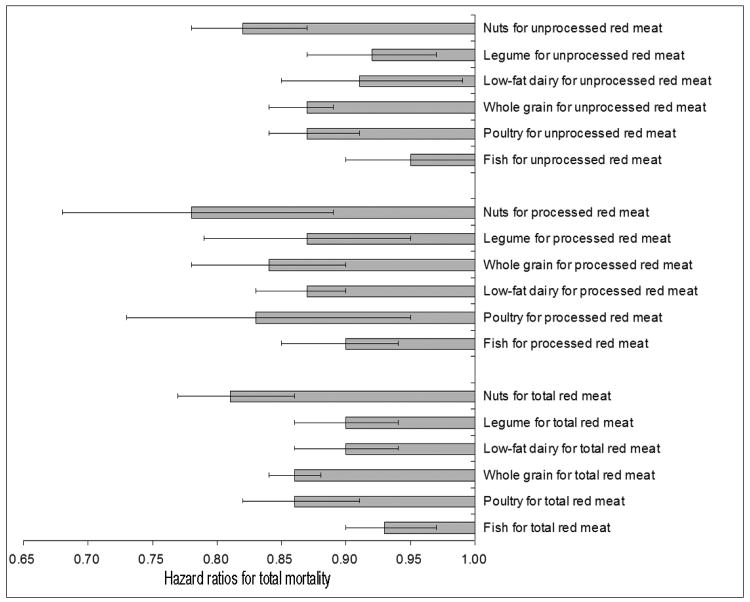
In the substitution analyses, replacing 1-serving/d of total red meat with 1-serving/d of fish, poultry, nuts, legumes, low-fat dairy products, or whole grains was associated with a lower risk of total mortality (Figure 2): 7% (HR, 0.93; 95% CI, 0.90-0.97) for fish, 14% (HR, 0.86; 95% CI, 0.82-0.91) for poultry, 19% (HR, 0.81; 95% CI, 0.77-0.86) for nuts, 10% (HR, 0.90; 95% CI, 0.86-0.94) for legumes, 10% (HR, 0.90; 95% CI, 0.86-0.94) for low-fat dairy products, and 14% (HR, 0.86; 95% CI, 0.82-0.88) for whole grain. The corresponding substitution estimates were 5%, 13%, 18%, 8%, 9%, and 13% for replacement of unprocessed red meat, and 10%, 17%, 22%, 13%, 13%, and 16% for replacement of processed red meat.
Figure 2. Hazard ratios and 95% confidence intervals for total mortality associated with replacement of other food groups for red meat intake.
Adjusted for age (continuous), body mass index category (<23, 23-24.9, 25-29.9, 30-34.9, ≥35 kg/m2), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15 g/d), physical activity level (<3, 3-8.9, 9-17.9, 18-26.9, ≥27 hours of metabolic equivalent tasks per week), smoking status (never, past, current 1-14 cigarettes/d, current 15-24 cigarettes/d, current ≥25 cigarettes/d,), race (white/non-white), menopausal status and hormone use in women (premenopausal, postmenopausal never users, postmenopausal past users, postmenopausal current users), family history of diabetes, myocardial infarction, or cancer, history of diabetes, hypertension, or hypercholesterolemia, total energy intake, and the corresponding two dietary variables in the models.
We estimated that 9.3% (95% CI, 5.9%-12.7%) in men and 7.6% (95% CI, 3.5%-11.7%) in women of total deaths during the follow-up could be prevented if all participants consumed <0.5 serving/d of total red meat in our cohorts; the estimates were 8.6% (95% CI, 2.3%-14.7%) in men and 12.2% (95% CI, 3.3%-21.0%) in women for CVD deaths. However, only 22.8% of men and 9.6% of women were in the low risk category of total red meat.
Comment
In these two large prospective cohorts of U.S. men and women, we found that a higher intake of red meat was associated with a significantly elevated risk of total, CVD and cancer mortality, and this association was observed for both unprocessed and processed red meat, with a relatively greater risk for processed red meat. Substitution of fish, poultry, nuts, legumes, low-fat dairy products, and whole grains for red meat was associated with a significantly lower risk of mortality.
Red meat is a major food source of protein and fat, and its potential associations with risks of diabetes,1 CVD,2 cancer,3 and mortality4-6 have attracted much attention. Several studies suggested that vegetarians had a greater longevity compared with non-vegetarians,4-5 but this might not be ascribed only to the absence of red meat. Sinha et al.6 showed in the NIH-AARP study that higher intakes of red and processed meats were associated with an elevated risk of mortality. However, that study did not distinguish unprocessed and processed red meats and did not update dietary information during the follow-up.
The strengths of the current study include a large sample size, the high rates of long-term follow-up, detailed and repeated assessments of diet and lifestyle. All participants were health professionals, minimizing potential confounding by educational attainment or differential access to health care. In addition, the FFQs used in these studies were validated against multiple diet records.9,10 However, the measurement errors inherent in dietary assessments were inevitable; these include misclassification of ham or cold-cuts as unprocessed red meat, and inaccurate assessment of red meat content in the mixed dishes. Because of the prospective study design, any measurement errors of meat intake are independent of study outcome ascertainment, and therefore, are likely to attenuate the associations towards the null.17 In the sensitivity analysis accounting for measurement errors, the risk estimates became stronger. Moreover, we calculated cumulative averages for dietary variables to better represent a person's long-term diet pattern and minimize the random measurement error caused by within-person variation. As expected, the analyses using baseline diet only yielded weaker associations. We also stopped updating the dietary information after a diagnosis of major chronic disease, under the assumption that participants could have changed their diet after receiving the diagnosis. Finally, because our participants were predominantly non-Hispanic white health professionals, the generalizability of the observed associations may be limited to similar populations.
Several mechanisms may explain the adverse effect of red meat intake on mortality risk. In regard to CVD mortality, we previously reported that red meat intake was associated with an increased risk of coronary heart disease (CHD),2,15 and saturated fat and cholesterol from red meat may partially explain this association.14 The association between red meat and CVD mortality was moderately attenuated after further adjustment for saturated fat and cholesterol, suggesting a mediating role of these nutrients. However, we could not assess whether lean meat has the same health risks compared to meat with higher fat content. Furthermore, dietary iron, particularly heme iron primarily from red meat, has been positively associated with myocardial infarction and fatal CHD.18-21 The association between red meat and CVD mortality were moderately attenuated after additional adjustment for heme iron. This suggests that heme-iron intake may partially explain this association, although some studies using biomarkers of iron status found no association between ferritin and transferrin saturation levels with risk of total mortality.22 Unprocessed and processed meats contain similar saturated fat and heme iron; however, other constituents in processed meat, particularly sodium and nitrites, might explain the additional harm of processed meats. The high sodium content may increase CVD risk through its effect on blood pressure.23-24 Nitrites and nitrates are frequently used in preservation of processed meats, and blood nitrite concentrations have been related to endothelial dysfunction25 and impaired insulin response in adults.26
With regard to cancer mortality, red meat intake has been associated with increased risks of colorectal cancer and several other cancers.27 Several compounds in red meat or created by high-temperature cooking, including N-nitroso compounds (nitrosamines or nitrosamides) converted from nitrites,28 polycyclic aromatic hydrocarbons, and heterocyclic amines,29-31 are potential carcinogens. Heme iron and iron overload might also be associated with increased cancer risk through promotion of N-nitroso compounds formation,32 increased colonic cytotoxicity and epithelial proliferation,33 increased oxidative stress and iron-induced hypoxia signaling.34
In conclusion, we found that a greater consumption of unprocessed and processed red meat is associated with a higher mortality risk. Compared with red meat, other dietary components, such as fish, poultry, nuts, legumes, low-fat dairy products and whole grains, were associated with a lower risk. These results indicate that replacement of red meat with alternative healthy dietary components may lower the mortality risk.
Supplementary Material
Table 3. Hazard ratio (HR) and 95% confidence interval (CI) of cardiovascular mortality according to red meat intake in the HPFS and NHS.
| Frequency of consumption (quintiles) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | P for trend | HR (95% CI) for 1 serving/d increase | |
| HPFS | |||||||
|
| |||||||
| Total red meat | |||||||
| Cases/person-years | 537/152293 | 490/153126 | 506/152623 | 518/153454 | 665/152647 | ||
| Age-adjusted model | 1.00 | 1.05 (0.93-1.19) | 1.11 (0.98-1.26) | 1.15 (1.02-1.30) | 1.48 (1.32-1.66) | <0.001 | 1.21 (1.16-1.27) |
| Multivariate modela | 1.00 | 1.09 (0.96-1.24) | 1.16 (1.03-1.32) | 1.17 (1.03-1.33) | 1.35 (1.19-1.53) | <0.001 | 1.14 (1.08-1.20) |
| Unprocessed red meat | |||||||
| Cases/person-years | 578/151850 | 528/150172 | 446/155316 | 532/152087 | 632/154719 | ||
| Age-adjusted model | 1.00 | 1.08 (0.95-1.20) | 0.97 (0.86-1.10) | 1.11 (0.98-1.25) | 1.41 (1.26-1.58) | <0.001 | 1.26 (1.18-1.34) |
| Multivariate modela | 1.00 | 1.10 (0.97-1.24) | 1.08 (0.95-1.22) | 1.14 (1.01-1.29) | 1.32 (1.16-1.49) | <0.001 | 1.19 (1.10-1.27) |
| Processed red meat | |||||||
| Cases/person-years | 594/172817 | 423/131953 | 510/153537 | 512/153206 | 677/152631 | ||
| Age-adjusted model | 1.00 | 0.99 (0.88-1.12) | 1.14 (1.01-1.29) | 1.13 (1.00-1.27) | 1.37 (1.23-1.53) | <0.001 | 1.34 (1.24-1.46) |
| Multivariate modela | 1.00 | 1.05 (0.93-1.19) | 1.15 (1.01-1.30) | 1.15 (1.02-1.31) | 1.25 (1.11-1.41) | 0.003 | 1.17 (1.07-1.29) |
|
| |||||||
| NHS | |||||||
|
| |||||||
| Total red meat | |||||||
| Cases/person-years | 601/440429 | 570/444046 | 517/441619 | 598/442319 | 908/441994 | ||
| Age-adjusted model | 1.00 | 1.11 (0.99-1.25) | 1.09 (0.97-1.22) | 1.33 (1.19-1.49) | 1.98 (1.79-2.20) | <0.001 | 1.44 (1.38-1.50) |
| Multivariate modela | 1.00 | 1.14 (1.01-1.27) | 1.11 (0.99-1.26) | 1.28 (1.13-1.43) | 1.45 (1.30-1.63) | <0.001 | 1.17 (1.11-1.22) |
| Unprocessed red meat | |||||||
| Cases/person-years | 617/443224 | 646/443182 | 481/441163 | 549/432988 | 901/449850 | ||
| Age-adjusted model | 1.00 | 1.21 (1.08-1.35) | 0.96 (0.85-1.09) | 1.15 (1.03-1.29) | 1.82 (1.65-2.02) | <0.001 | 1.46 (1.39-1.54) |
| Multivariate modela | 1.00 | 1.22 (1.09-1.37) | 1.09 (0.96-1.23) | 1.19 (1.06-1.34) | 1.39 (1.24-1.55) | <0.001 | 1.17 (1.10-1.24) |
| Processed red meat | |||||||
| Cases/person-years | 671/444737 | 551/422411 | 586/457265 | 572/443383 | 814/442609 | ||
| Age-adjusted model | 1.00 | 0.98 (0.88-1.10) | 1.10 (0.99-1.23) | 1.16 (1.03-1.29) | 1.65 (1.49-1.83) | <0.001 | 1.79 (1.64-1.95) |
| Multivariate modela | 1.00 | 0.97 (0.87-1.09) | 1.10 (0.99-1.23) | 1.12 (0.99-1.25) | 1.29 (1.15-1.43) | <0.001 | 1.26 (1.15-1.39) |
|
| |||||||
| Pooled Resultsb | |||||||
|
| |||||||
| Total red meat | 1.00 | 1.12 (1.03-1.22) | 1.13 (1.04-1.24) | 1.23 (1.13-1.34) | 1.40 (1.29-1.53) | <0.001 | 1.16 (1.12-1.20) |
| Unprocessed red meat | 1.00 | 1.16 (1.05-1.28) | 1.09 (1.00-1.18) | 1.17 (1.07-1.27) | 1.36 (1.25-1.47) | <0.001 | 1.18 (1.13-1.23) |
| Processed red meat | 1.00 | 1.01 (0.92-1.10) | 1.12 (1.03-1.22) | 1.13 (1.04-1.23) | 1.27 (1.18-1.38) | <0.001 | 1.21 (1.13-1.31) |
Abbreviation: HPFS, Health Professionals Follow-up Study; NHS, Nurses' Health Study.
Multivariate model: adjusted for age (continuous), body mass index category (<23, 23-24.9, 25-29.9, 30-34.9, ≥35 kg/m2), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15 g/d), physical activity level (<3, 3-8.9, 9-17.9, 18-26.9, ≥27 hours of metabolic equivalent tasks per week), smoking status (never, past, current 1-14 cigarettes/d, current 15-24 cigarettes/d, current ≥25 cigarettes/d,), race (white/non-white), menopausal status and hormone use in women (premenopausal, postmenopausal never users, postmenopausal past users, postmenopausal current users), family history of diabetes, myocardial infarction, or cancer, history of diabetes, hypertension, or hypercholesterolemia, quintiles of total energy intake, whole grains, fruits, and vegetables.
Results from multivariate model 2 were combined using random-effects model.
Acknowledgments
We are indebted to the participants in the Health Professional Follow-up Study and Nurses' Health Study for their continuing outstanding support and colleagues working in these studies for their valuable help. In addition, we would like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY.
Funding/Support: The study was supported by the National Institutes of Health grant (DK58845, CA55075, CA87969, HL34594 and 1U54CA155626-01). Dr. Sun was supported by a career development award K99HL098459 from the National Heart, Lung, and Blood Institute. The funding sources did not involve in the data collection, data analysis, manuscript writing and publication.
Footnotes
Disclosures: None of the authors had any financial or personal conflict of interest to disclose
Author Contributions: Drs. Pan and Hu have full access to the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Pan, Willett, Hu.
Acquisition of data: Manson, Stampfer, Willett, Hu.
Analysis and interpretation of data: Pan, Sun, Bernstein, Schulze, Manson, Stampfer, Willett, Hu.
Drafting of the manuscript: Pan.
Critical revision of the manuscript for important intellectual content: Sun, Bernstein, Schulze, Manson, Stampfer, Willett, Hu.
Statistical analysis: Pan, Sun, Hu.
Administrative, technical, and material support: Manson, Stampfer, Willett, Hu.
Obtaining funding and study supervision: Manson, Stampfer, Willett, Hu.
Final approval: Pan, Sun, Bernstein, Schulze, Manson, Stampfer, Willett, Hu.
References
- 1.Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94(4):1088–1096. doi: 10.3945/ajcn.111.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121(21):2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng W, Lee SA. Well-done meat intake, heterocyclic amine exposure, and cancer risk. Nutr Cancer. 2009;61(4):437–446. doi: 10.1080/01635580802710741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser GE. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am J Clin Nutr. 1999;70(Suppl 3):532S–538S. doi: 10.1093/ajcn/70.3.532s. [DOI] [PubMed] [Google Scholar]
- 5.Key TJ, Fraser GE, Thorogood M, et al. Mortality in vegetarians and nonvegetarians: detailed findings from a collaborative analysis of 5 prospective studies. Am J Clin Nutr. 1999;70(Suppl 3):516S–524S. doi: 10.1093/ajcn/70.3.516s. [DOI] [PubMed] [Google Scholar]
- 6.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med. 2009;169(6):562–571. doi: 10.1001/archinternmed.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care. 2002;25(3):417–424. doi: 10.2337/diacare.25.3.417. [DOI] [PubMed] [Google Scholar]
- 8.Fung TT, Schulze M, Manson JE, Willett WC, Hu FB. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med. 2004;164(20):2235–2240. doi: 10.1001/archinte.164.20.2235. [DOI] [PubMed] [Google Scholar]
- 9.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69(2):243–249. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 10.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–67. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 11.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the national death index and equifax nationwide death search. Am J Epidemiol. 1994;140(11):1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 12.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 13.Qiu W, Rosner B. Measurement error correction for the cumulative average model in the survival analysis of nutritional data: application to Nurses' Health Study. Lifetime Data Anal. 2010;16(1):136–153. doi: 10.1007/s10985-009-9124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122(9):876–883. doi: 10.1161/CIRCULATIONAHA.109.915165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18(5):571–579. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 17.Willett WC. Nutritional Epidemiology. 2nd. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 18.Ascherio A, Willett W, Rimm E, Giovannucci E, Stampfer M. Dietary iron intake and risk of coronary disease among men. Circulation. 1994;89(3):969–974. doi: 10.1161/01.cir.89.3.969. [DOI] [PubMed] [Google Scholar]
- 19.Klipstein-Grobusch K, Grobbee DE, den Breeijen JH, Boeing H, Hofman A, Witteman JC. Dietary iron and risk of myocardial infarction in the Rotterdam Study. Am J Epidemiol. 1999;149(5):421–428. doi: 10.1093/oxfordjournals.aje.a009829. [DOI] [PubMed] [Google Scholar]
- 20.van der A DL, Peeters PH, Grobbee DE, Marx JJM, van der Schouw YT. Dietary haem iron and coronary heart disease in women. Eur Heart J. 2005;26(3):257–262. doi: 10.1093/eurheartj/ehi027. [DOI] [PubMed] [Google Scholar]
- 21.Qi L, van Dam RM, Rexrode K, Hu FB. Heme Iron From Diet as a risk factor for coronary heart disease in women with type 2 diabetes. Diabetes Care. 2007;30(1):101–106. doi: 10.2337/dc06-1686. [DOI] [PubMed] [Google Scholar]
- 22.Menke A, Muntner P, Fernández-Real JM, Guallar E. The association of biomarkers of iron status with mortality in US adults. Nutr Metab Cardiovasc Dis. 2011 doi: 10.1016/j.numecd.2010.11.011. dio: 10.1016/j.numecd.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362(7):590–599. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith-Spangler CM, Juusola JL, Enns EA, Owens DK, Garber AM. Population strategies to decrease sodium intake and the burden of cardiovascular disease: a cost-effectiveness analysis. Ann Intern Med. 2010;152(8):481–487. doi: 10.7326/0003-4819-152-8-201004200-00212. [DOI] [PubMed] [Google Scholar]
- 25.Kleinbongard P, Dejam A, Lauer T, et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006;40(2):295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Pereira EC, Ferderbar S, Bertolami MC, et al. Biomarkers of oxidative stress and endothelial dysfunction in glucose intolerance and diabetes mellitus. Clin Biochem. 2008;41(18):1454–1460. doi: 10.1016/j.clinbiochem.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 27.The World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 28.Hughes R, Cross AJ, Pollock JRA, Bingham S. Dose-dependent effect of dietary meat on endogenous colonic N-nitrosation. Carcinogenesis. 2001;22(1):199–202. doi: 10.1093/carcin/22.1.199. [DOI] [PubMed] [Google Scholar]
- 29.Skog K, Steineck G, Augustsson K, Jägerstad M. Effect of cooking temperature on the formation of heterocyclic amines in fried meat products and pan residues. Carcinogenesis. 1995;16(4):861–867. doi: 10.1093/carcin/16.4.861. [DOI] [PubMed] [Google Scholar]
- 30.Sinha R, Rothman N, Salmon CP, et al. Heterocyclic amine content in beef cooked by different methods to varying degrees of doneness and gravy made from meat drippings. Food Chem Toxicol. 1998;36(4):279–287. doi: 10.1016/s0278-6915(97)00162-2. [DOI] [PubMed] [Google Scholar]
- 31.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44(1):44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 32.Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63(10):2358–2360. [PubMed] [Google Scholar]
- 33.Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R. Red meat and colon cancer: the cytotoxic and hyperproliferative effects of dietary heme. Cancer Res. 1999;59(22):5704–5709. [PubMed] [Google Scholar]
- 34.Huang X. Iron overload and its association with cancer risk in humans: evidence for iron as a carcinogenic metal. Mutat Res. 2003;533(1-2):153–171. doi: 10.1016/j.mrfmmm.2003.08.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.