Abstract
Background
To better target future immunization efforts, we assessed trends and disparities in human papillomavirus (HPV) vaccine initiation among female adolescents in North Carolina over 3 years.
Methods
We analyzed data from a stratified random sample of 1,427 parents who, between 2008 and 2010, completed two linked telephone surveys: the Behavioral Risk Factor Surveillance System and the Child Health Assessment and Monitoring Program surveys. Weighted analyses examined HPV vaccine initiation for girls ages 11 to 17 years.
Results
HPV vaccine initiation increased modestly over time (2008, 34%; 2009, 41%; 2010, 44%). This upward trend was present within 11 subpopulations of girls, including those who lived in rural areas, were of minority (non-black/non-white) race, or had not recently received a preventive check-up. Looking at differences between groups, HPV vaccine initiation was less common among girls who attended private versus public school, were younger, or lacked a recent check-up. However, the latter difference narrowed over time. The low level of initiation among girls without recent check-ups increased substantially (from 11% to 41%), whereas initiation among girls with recent visits improved little (from 39% to 44%, Pinteraction = 0.007).
Conclusions
Although HPV vaccine initiation improved among several groups typically at higher risk for cervical cancer, the lack of progress among girls with recent check-ups suggests that missed opportunities for administration have hampered broader improvements.
Impact
Achieving widespread coverage of HPV vaccine will require redoubled efforts to vaccinate adolescents during routine care.
Introduction
Guidelines recommend administering human papillomavirus (HPV) vaccine to females ages 11 to 26 years to confer protection from several HPV-related cancers, as well as genital warts (1, 2). Despite evidence that HPV vaccine is safe and effective (3-5), uptake remains low in the United States. Just half (53.0%) of girls ages 13 to 17 years have initiated HPV vaccine, and only one third (34.8%) have completed the 3-dose series as of 2011 (6). These rates are far below the Healthy People 2020 goal of 80% completion (7) and also pale in comparison to levels of initiation achieved in several other Western countries. For example, HPV vaccine initiation is above 80% for 12- to 13-year-old girls in England (8) and Australia (9).
Unfortunately, available data do not suggest that current immunization efforts in the United States are leading to rapid improvements in HPV vaccine coverage. The annual gains in initiation among girls ages 13 to 17 years have decreased from 12.1% (2007–2008) to 4.3% (2010–2011, refs. 6, 10, 11). In contrast, the uptake of the 2 other adolescent vaccines recently introduced in the United States (tetanus, diphtheria, and pertussis booster and meningitis vaccine) has increased steadily since 2006 (6, 10). Without a concerted effort to identify and address the unique challenge of HPV vaccination in the U.S. context, coverage levels may remain far short of national goals.
To better understand the trajectory of HPV vaccine uptake, we sought to characterize changes in HPV vaccination among adolescent girls in North Carolina over a 3-year period. Our analyses focused on variables identified in previous studies as correlates of HPV vaccination, including daughter’s age, daughter and parent healthcare utilization, and urbanicity (12-15). We aimed (i) to assess trends in HPV vaccine initiation among subgroups to determine who is and is not improving, (ii) to identify correlates of initiation across all 3 years to understand differences and disparities in initiation, and (iii) to assess how these correlates have changed over time. We use the term difference to refer to correlates related to healthcare access factors (e.g., having a recent preventive check-up) and disparity when describing correlates tied to socio-demographic factors (e.g., race), after the definition offered by the Institute of Medicine (16), whereas we use correlate when generally discussing both differences and disparities. By investigating trends in HPV vaccination in both absolute and relative terms, this study aims to guide efforts to eliminate disparities in HPV vaccination uptake while at the same time raising overall coverage levels.
Materials and Methods
Study design
This study used data collected over 3 years (2008–2010) from North Carolina residents via the Behavioral Risk Factor Surveillance System (BRFSS) and the Child Health Assessment and Monitoring Program (CHAMP) surveys. BRFSS monitors health-related behaviors with an annual telephone survey of a nationally representative sample of noninstitutionalized adults (17). Using a subset of BRFSS respondents, CHAMP is a follow-up survey that measures the health characteristics of children younger than 18 years via parental report (18). In North Carolina, the State Center for Health Statistics Survey Center conducts BRFSS and CHAMP using random digit dialing and a computer-assisted telephone interviewing system. The Survey Center uses disproportionate stratified random sampling (18).
During the BRFSS surveys, interviewers gathered identifying information about a randomly selected child in the household (e.g., the child’s birth month, birth year, and sex) and scheduled an appointment to call back about 2 weeks later to complete the CHAMP survey with the person most knowledgeable about the child’s health (18). A unique identifier linked CHAMP and BRFSS responses to allow use of caregiver data from the BRFSS survey. Our analyses of the 2008–2010 data included only girls because assessment of boys’ HPV vaccination status did not begin until 2010, a year after the vaccine received approval for use in boys (19).
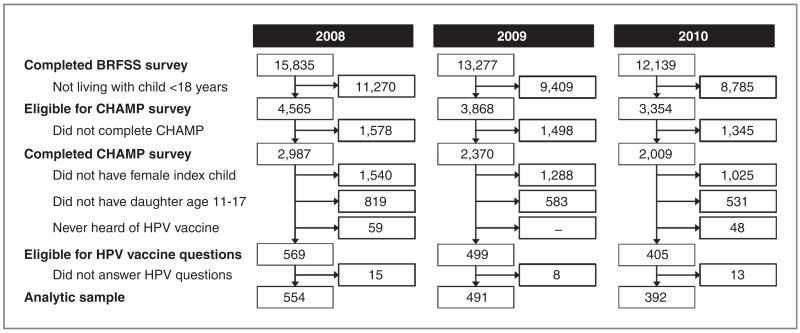
Of 7,366 caregivers who participated in the CHAMP survey in 2008–2010 (Fig. 1), 1,473 caregivers completed the HPV vaccine section of the survey about a female child age 11 through 17 years. A total of 36 of these caregivers were excluded from the study sample because they either declined to answer HPV vaccine questions or did not know if the index child had received the vaccine. The final analytic sample consisted of 1,437 caregivers. Most of these caregivers were the same respondents who completed the initial BRFSS surveys (79%). Because most caregivers (94% each year) reported being parents of the index children, we refer to them as parents and the female children as daughters. Among the original BRFSS respondents who were different from the CHAMP respondents, 95% were also parents to the index child. An Institutional Review Board at the University of North Carolina (Chapel Hill, NC) determined that this study did not require review.
Figure 1.
Flow diagram of parents’ participation in the 2008–2010 North Carolina CHAMP surveys.
Measures
The CHAMP survey assessed HPV vaccine initiation, the main study outcome, with the question, “Has (child’s name) had any shots of the HPV vaccine?” The survey collected data on HPV vaccine initiation rather than series completion because population-level adoption of the vaccine remains in an early stage in the Unites States, making initiation a more appropriate outcome to study (20). Furthermore, while clinical trials have shown that completing the vaccine series confers protection from HPV infections and associated cervical dysplasia (1, 4), accumulating data suggest that fewer than 3 doses may provide substantial health benefits (1, 3, 4). Unless otherwise noted, we use initiation and coverage to refer to having received at least one dose of HPV vaccine.
The CHAMP survey collected data on daughter’s age, race and ethnicity (non-Hispanic white, non-Hispanic black, or other), and school type (attended public school vs. private or home school). Healthcare-related measures included whether the daughter had health insurance (private, public, or no insurance), had a regular healthcare provider, or had a preventive check-up in the last 12 months. The CHAMP survey also assessed the highest education level completed by anyone in the household, as well as location of household residence, which we later classified as either “urban” (in a metropolitan statistical area) or “rural” (outside of a metropolitan statistical area; ref. 21). The BRFSS survey gathered information about parents’ demographic characteristics and whether they had received the influenza vaccine (either shot or spray) in the last year.
Data analysis
To explore trends, we calculated the proportion of adolescents in each subgroup (e.g., non-Hispanic whites) who initiated HPV vaccination for each study year. For each subgroup, we then used logistic regression to assess changes in HPV vaccine initiation over the 3-year period.
Next, we identified correlates of HPV vaccine initiation and explored how those correlates changed over time. To maximize the power of our analysis, we pooled data from all 3 years. We then used logistic regression to identify bivariate correlates of HPV vaccine initiation and entered statistically significant (P < 0.05) correlates into a multi-variate model. To assess how correlates changed over time, we tested the interaction between each statistically significant multivariate correlate with year. We then used adjusted Wald tests to derive an overall P value for the interaction term for all levels of the variables of interest. Finally, we conducted a sensitivity analysis that excluded cases in which different respondents provided BRFSS and CHAMP data. Because this analysis yielded the same pattern of findings as the primary analysis, we report on the analysis of the full sample only.
We applied sampling weights to percentages and tests of association to account for the complex survey design (18); frequencies were not weighted. Analyses used Stata version 12 with 2-tailed tests and a critical α of 0.05.
Results
Demographic and healthcare characteristics
Most parents reported that their daughters were non-Hispanic white (64%) or non-Hispanic black (21%; Table 1). A majority of daughters had private (66%) or public (24%) health insurance, had a regular healthcare provider (82%), and had received a preventive check-up in the previous 12 months (80%). Most parents were 40 years old or older (71%) and female (86%). Two-thirds of parents (66%) had not received seasonal flu vaccine in the year before the interview. Most households included a person with at least some college education (79%) and were located in an urban area (74%). Demographic, healthcare, and household characteristics were similar across years on all variables except that the sample included more female parents over time.
Table 1.
.Demographic characteristics of 11- to 17-year-old daughters and their parents who participated in the 2008–2010 North Carolina CHAMP surveys, 2008–2010
| 2008 n (%) |
2009 n (%) |
2010 n (%) |
Overall n (%) |
|
|---|---|---|---|---|
| Total | 554 (100) | 491 (100) | 392 (100) | 1,437 (100) |
| Daughter characteristics | ||||
| Age, y | ||||
| 11–12 | 135 (29) | 115 (27) | 100 (29) | 350 (29) |
| 13–15 | 235 (39) | 202 (42) | 172 (44) | 609 (41) |
| 16–17 | 184 (32) | 174 (31) | 120 (27) | 478 (30) |
| Race/ethnicity | ||||
| Non-Hispanic white | 400 (67) | 359 (60) | 289 (67) | 1,048 (64) |
| Non-Hispanic black | 82 (21) | 65 (22) | 58 (20) | 205 (21) |
| Other | 72 (12) | 67 (18) | 45 (13) | 184 (15) |
| School typea | ||||
| Public | 487 (88) | 429 (86) | 351 (90) | 1,267 (88) |
| Private/home schooled | 63 (12) | 61 (14) | 41 (10) | 165 (12) |
| Healthcare coverage | ||||
| No insurance | 32 (5) | 31 (6) | 27 (7) | 90 (6) |
| Public insurance | 109 (21) | 105 (27) | 86 (24) | 300 (24) |
| Private insurance | 384 (69) | 336 (63) | 268 (65) | 988 (66) |
| Not reported | 29 (6) | 19(4) | 11 (4) | 59 (5) |
| Regular healthcare provider | ||||
| No or do not know (n = 4) | 80 (14) | 106 (22) | 65 (17) | 251 (18) |
| Yes | 474 (86) | 385 (78) | 327 (89) | 1,186 (82) |
| Preventive check-up in last 12 mo | ||||
| No or do not know (n = 7) | 104 (18) | 103 (21) | 75 (20) | 282 (20) |
| Yes | 450 (82) | 388 (79) | 317 (80) | 1,155 (80) |
| Parent characteristics b | ||||
| Age, y | ||||
| ≤39 | 138 (30) | 123 (31) | 88 (86) | 349 (29) |
| 40–49 | 272 (49) | 234 (46) | 203 (52) | 709 (79) |
| ≥50 | 144 (21) | 134 (23) | 101 (22) | 379 (22) |
| Sex | ||||
| Female | 462 (83) | 403 (84) | 346 (90) | 1,211 (86) |
| Male | 92 (17) | 85 (16) | 46 (10) | 223 (14) |
| Marital status | ||||
| Married/member of unmarried couple | 421 (76) | 363 (72) | 291 (75) | 1,075 (74) |
| Other (divorced, widowed, separated, never married) |
133 (24) | 128 (28) | 101 (26) | 362 (26) |
| Employment status | ||||
| Employed for wages/self-employed | 404 (69) | 344 (67) | 274 (68) | 1,022 (68) |
| Other (unemployed, homemaker, student, retired, unable to work) |
150 (31) | 147 (33) | 118 (32) | 415 (32) |
| Flu vaccine in the past y | ||||
| No or do not know (n = 9) | 354 (63) | 328 (70) | 243 (66) | 925 (66) |
| Yes | 200 (37) | 163 (30) | 149 (34) | 512 (34) |
| Household characteristics | ||||
| Annual household income | ||||
| <$50,000 | 213 (37) | 199 (45) | 136 (37) | 548 (40) |
| ≥$50,000 | 302 (55) | 252 (49) | 216 (54) | 770 (52) |
| Not reported | 39 (8) | 40 (7) | 40 (10) | 119 (8) |
| Highest education level in household | ||||
| High school or less | 111 (20) | 108 (23) | 70 (18) | 289 (21) |
| Some college or more | 443 (80) | 383 (77) | 322 (82) | 1,148 (79) |
| Urbanicity | ||||
| Rural | 170 (29) | 124 (25) | 93 (24) | 387 (26) |
| Urban | 384 (71) | 367 (75) | 299 (76) | 1,050 (74) |
NOTE: The percentages reflect weighted data to account for complex survey design.
Excludes parents who did not know their daughters' school type (n = 2 in 2008) or whose daughters were not in school (n = 2 in 2008; n = 1 in 2009). Because of the small number of parents reporting their daughters were home schooled (n = 18 in 2008; n = 19 in 2009; n = 13 in 2010), analyses combined data from private- and home-schooled daughters.
Most caregivers (94%) reported being parents of the index children. Among the original BRFSS respondents who did not complete the CHAMP survey, 95% were also parents to the index children.
Trends in HPV vaccine initiation by subgroup
In the study population as a whole, HPV vaccine initiation increased over time (2008, 34%; 2009, 41%; 2010, 44%). Stratified analyses identified 11 subgroups of daughters who experienced statistically significant increases in initiation, including daughters who were ages 13–15 (P = 0.039), whose race was non-white or non-black (P = 0.048), or who attended public school (P = 0.013; Table 2). With respect to healthcare access and utilization, initiation also improved among girls with a regular healthcare provider (P = 0.048) or without a recent preventive check-up (P = 0.001). In terms of parental characteristics, HPV vaccine initiation increased among girls of respondents who were female (P = 0.035), married (P = 0.008), or who had not received flu vaccine in the previous year (P = 0.009). Initiation also increased among daughters who lived in households with higher annual incomes (P = 0.007), with higher levels of education (P = 0.012), or located in rural areas (P = 0.011). In most other subgroups, HPV vaccine initiation increased over time without achieving statistical significance. By 2010, no subgroup’s rate of initiation was greater than 57%, and most groups had initiation rates between 40% and 50%.
Table 2.
.Trends in HPV vaccine initiation among girls ages 11–17 by subgroup, 2008–2010 North Carolina CHAMP surveys
| 2008 % (95% CI) |
2009 % (95% CI) |
2010 % (95% CI) |
P | |
|---|---|---|---|---|
| Daughter characteristics | ||||
| Age, y | ||||
| 11–12 | 24 (16–34) | 22 (13–36) | 28 (18–42) | 0.518 |
| 13–15 | 33 (26–41) | 40 (31–49) | 46 (37–55) | 0.039 |
| 16–17 | 45 (36–54) | 59 (49–69) | 57 (45–68) | 0.104 |
| Race/ethnicity | ||||
| Non-Hispanic white | 35 (29–41) | 42 (35–49) | 43 (36–50) | 0.084 |
| Non-Hispanic black | 35 (24–48) | 48 (33–63) | 40 (27–55) | 0.601 |
| Other | 30 (17–47) | 30 (18–45) | 54 (36–72) | 0.048 |
| School type | ||||
| Public | 35 (30–41) | 43 (37–50) | 46 (40–53) | 0.013 |
| Private/home schooled | 26 (16–38) | 28 (16–43) | 22 (10–39) | 0.693 |
| Healthcare coverage | ||||
| No insurance | 18 (6–43) | 51 (28–73) | 41 (19–66) | 0.182 |
| Public insurance | 37 (27–50) | 48 (35–61) | 50 (37–62) | 0.191 |
| Private insurance | 34 (29–40) | 37 (31–44) | 42 (35–50) | 0.094 |
| Not reported | 37 (17–64) | 40 (13–74) | 38 (9–78) | 0.979 |
| Regular healthcare provider | ||||
| No/do not know | 19 (11–30) | 59 (46–70) | 34(21–49) | 0.174 |
| Yes | 37 (31–43) | 36 (30–43) | 46 (39–53) | 0.048 |
| Preventive check-up in last 12 mo | ||||
| No/do not know | 11 (6–19) | 24 (14–39) | 41 (27–56) | 0.001 |
| Yes | 39 (34–45) | 45 (39–52) | 44 (38–51) | 0.269 |
| Parent characteristics | ||||
| Age, y | ||||
| ≤39 | 31 (22–42) | 41 (30–53) | 47 (35–61) | 0.054 |
| 40–49 | 34 (27–42) | 40 (32–49) | 42 (34–51) | 0.165 |
| ≥50 | 38 (29–48) | 43 (33–55) | 44 (32–56) | 0.506 |
| Sex | ||||
| Female | 34 (29–39) | 40 (33–46) | 43 (37–50) | 0.035 |
| Male | 37 (25–49) | 47 (33–62) | 51 (34–67) | 0.159 |
| Marital status | ||||
| Married/member of unmarried couple | 33 (27–39) | 39 (32–46) | 45 (38–52) | 0.008 |
| Other (divorced, widowed, separated, never married) |
40 (30–50) | 48 (36–60) | 40 (29–53) | 0.973 |
| Employment status | ||||
| Employed for wages/self-employed | 38 (32–44) | 40 (33–48) | 47 (40–55) | 0.053 |
| Other (unemployed, homemaker, student, retired, unable to work) |
27 (18–37) | 43 (32–54) | 37 (27–48) | 0.167 |
| Flu vaccine in past y | ||||
| No/do not know | 27 (22–33) | 37 (30–45) | 40 (33–48) | 0.009 |
| Yes | 47 (38–56) | 50 (40–61) | 51 (41–61) | 0.532 |
| Household characteristics | ||||
| Annual household income | ||||
| <$50,000 | 34 (26–43) | 42 (33–52) | 38 (29–49) | 0.541 |
| ≥$50,000 | 34 (27–41) | 41 (33–49) | 49 (40–57) | 0.007 |
| Not reported | 37 (21–58) | 36 (17–61) | 37 (20–58) | 0.982 |
| Highest education level in household | ||||
| High school or less | 38 (26–51) | 42 (29–56) | 40 (27–55) | 0.809 |
| Some college or more | 33 (28–39) | 41 (34–48) | 45 (38–52) | 0.012 |
| Urbanicity | ||||
| Rural | 22 (16–29) | 39 (27–53) | 41 (28–55) | 0.011 |
| Urban | 39 (33–46) | 42 (35–49) | 45 (28–52) | 0.256 |
NOTE: P values are from logistic regression. All analyses used weighted data to account for complex survey design.
Overall correlates of HPV vaccine initiation
In analyses of correlates in the pooled sample, more older daughters (ages 13–15 and 16–17) than younger daughters (ages 11–12) had initiated HPV vaccination, resulting in ORs of 2.02 [95% confidence interval (CI), 1.34–3.04] and 3.71 (95% CI, 2.42–5.69), respectively (Table 3). Differences in initiation included higher initiation among girls with a recent preventive check-up (OR, 2.09; 95% CI, 1.37–3.20) and for those whose parents had recently received flu vaccine (OR, 1.74; 95% CI, 1.29–2.35). Initiation was lower for girls attending private or home school rather than public school (OR, 0.48; 95% CI, 0.30–0.78). Although urbanicity was correlated with HPV vaccine initiation in bivariate analysis, this variable did not retain statistical significance in the multivariate model. We did not observe statistically significant disparities by other participant characteristics (e.g., child’s race/ethnicity) or other differences related to healthcare utilization (e.g., type of health insurance).
Table 3.
Correlates of HPV vaccine initiation among girls ages 11–17, 2008–2010 North Carolina CHAMP surveys
| No. vaccinated/ No. in category |
(%) | Bivariate OR (95% CI) |
Multivariate OR (95% CI) |
|
|---|---|---|---|---|
| Overall | 573/1,437 | 40 | ||
| Year | ||||
| 2008 | 194/554 | 34 | Ref | Ref |
| 2009 | 205/491 | 41 | 1.34 (0.96–1.87) | 1.46 (1.04–2.06)a |
| 2010 | 174/392 | 44 | 1.50 (1.07–2.09)a | 1.62 (1.13–2.32)a |
| Daughter characteristics | ||||
| Age, y | ||||
| 11–12 | 85/350 | 25 | Ref | Ref |
| 13–15 | 247/609 | 40 | 2.00 (1.36–2.95)b | 2.02 (1.34–3.04)b |
| 16–17 | 241/478 | 54 | 3.54 (2.37–5.28)b | 3.71 (2.42–5.69)b |
| Race/ethnicity | ||||
| Non-Hispanic white | 425/1,048 | 40 | Ref | |
| Non-Hispanic black | 83/205 | 41 | 1.06 (0.73–1.55) | |
| Other | 65/184 | 37 | 0.89 (0.57–1.36) | |
| School type | ||||
| Public | 523/1,267 | 42 | Ref | Ref |
| Private/home schooled | 47/165 | 25 | 0.47 (0.30–0.75)a | 0.48 (0.30–0.78)a |
| Healthcare coverage | ||||
| No insurance | 24/90 | 38 | Ref | |
| Public insurance | 136/300 | 46 | 1.38 (0.71–2.69) | |
| Private insurance | 392/988 | 38 | 1.01 (0.54–1.87) | |
| Not reported | 21/59 | 38 | 1.01 (0.38–2.67) | |
| Regular healthcare provider | ||||
| No/do not know | 90/251 | 41 | Ref | |
| Yes | 483/1,186 | 40 | 0.96 (0.66–1.38) | |
| Preventive check-up in last 12 mo | ||||
| No/do not know | 59/282 | 26 | Ref | Ref |
| Yes | 514/1,155 | 43 | 2.20 (1.46–3.31)b | 2.09 (1.37–3.20)b |
| Parent characteristics | ||||
| Age, y | ||||
| ≤39 | 131/349 | 40 | Ref | |
| 40–49 | 276–709 | 39 | 0.96 (0.68–1.36) | |
| ≥50 | 166/379 | 42 | 1.09 (0.74–1.61) | |
| Sex | ||||
| Female | 484/1,211 | 39 | Ref | |
| Male | 88/223 | 44 | 1.23 (0.84–1.80) | |
| Marital status | ||||
| Married/member of unmarried couple | 414/1,075 | 39 | Ref | |
| Other (divorced, widowed, separated, never married) | 159/362 | 43 | 1.20 (0.87–1.65) | |
| Employment status | ||||
| Employed for wages/self-employed | 418/1,022 | 42 | Ref | |
| Other (unemployed, homemaker, student, retired, unable to work) |
155/415 | 36 | 0.78 (0.57–1.06) | |
| Flu vaccine in past y | ||||
| No/do not know | 323/925 | 35 | Ref | Ref |
| Yes | 250/512 | 49 | 1.80 (1.35–2.40)b | 1.74 (1.29–2.35)b |
| Household characteristics | ||||
| Annual household income | ||||
| <$50,000 | 216/548 | 39 | Ref | |
| ≥$50,000 | 315/770 | 41 | 1.10 (0.82–1.48) | |
| Not reported | 42/119 | 37 | 0.93 (0.54–1.61) | |
| Highest education level in household | ||||
| High school or less | 117/289 | 40 | Ref | |
| Some college or more | 456/1,148 | 40 | 0.98 (0.69–1.40) | |
| Urbanicity | ||||
| Rural | 126/387 | 33 | Ref | Ref |
| Urban | 447/1,050 | 42 | 1.44 (1.03–2.02)a | 1.34 (0.92–1.94) |
NOTE: Models include data from all 3 years. Multivariate models include correlates that were statistically significant predictors of HPV vaccine uptake in bivariate models. All analyses used weighted data to account for complex survey design.
P < 0.05.
P < 0.001.
Changes in correlates over time
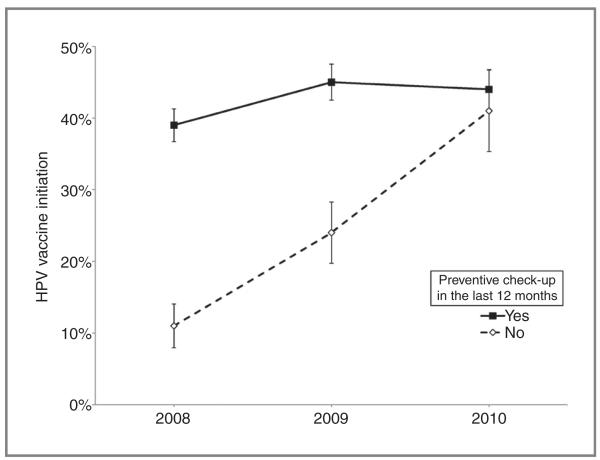
Our analysis of each correlate’s interaction with time indicated that the effect of having a recent preventive check-up changed over the study period (Pinteraction = 0.007). Daughters without recent check-ups had a low level of initial coverage that increased substantially over time (from 11% to 41%), whereas initiation among girls with check-ups improved little (from 39% to 44%; Fig. 2). Probing this interaction, we found that the OR for initiation among those with a recent check-up compared with those without decreased from 5.41 (95% CI, 2.47–11.88) in 2008 to 2.57 (95% CI, 1.26–5.24) in 2009 to 1.16 (95% CI, 0.60–2.25) in 2010. Analyses did not find interactions with time for other correlates identified as statistically significant: child’s age, parent’s use of flu vaccine, or school type.
Figure 2.
Effect of having had a preventive check-up in the last 12 months among girls ages 11–17, 2008–2010 North Carolina CHAMP surveys.
Discussion
Our findings indicate that between 2008 and 2010, HPV vaccine initiation increased modestly among adolescent girls in North Carolina. Vaccine initiation improved among some subpopulations of girls, including several groups with low levels of initiation in 2008 (12-15). Although we found that HPV vaccine initiation correlated with factors such as child’s age and receipt of a recent preventive checkup, the latter difference narrowed over time. Furthermore, unlike an analysis of 2008 CHAMP data (14), when we pooled CHAMP data from 2008 to 2010, we did not find any previously reported disparities, such as higher initiation for girls living in urban versus rural areas.
Although important, the improvements in HPV vaccine initiation were moderate to small and were isolated to pockets of the population. For many subgroups of girls, coverage did not improve substantially. By 2010, most groups had initiation levels between 40% and 50%, but not one achieved 80% initiation, and most fell far short. Although we did not assess series completion, it is likely lower still, suggesting that the Healthy People 2020 (7) goal of 80% series completion is still quite far from reach.
The relationship between HPV vaccine initiation and the use of preventive services best illustrates the limitations of the improvements we observed. In the pooled sample, girls were more likely to have received one or more doses of HPV vaccine if they or their parents received other preventive healthcare services (i.e., preventive check-ups for daughters and flu vaccine for parents). Yet the overall improvements in uptake over time were largely driven by daughters whose families did not receive such services. For instance, daughters with recent preventive check-ups had relatively stable levels of initiation (from 39% in 2008 to 44% in 2010), whereas initiation among daughters without check-ups improved considerably (from 11% in 2008 to 41% in 2010). This finding suggests that although their level of initiation was low initially, many girls from families who had not received recent preventive care did eventually come into contact with the healthcare system and receive the attendant benefits. Ultimately, however, this group constituted a minority of girls in the sample (20%), limiting the population-level benefit of this trend.
The flat vaccination rate among girls who had received preventive check-ups could reflect missed opportunities to administer HPV vaccine. Many studies have found such missed opportunities to be common among adolescents seeking preventive care (14, 22-24), but providers can reduce missed opportunities (25-28). Provider recommendation often strongly correlates with whether an adolescent receives HPV vaccine (13, 29-31), although the causal direction of this relationship is often unclear. Patients need a provider to receive HPV vaccine, but they may initiate the process by requesting vaccination and then receiving a doctor’s advice. This study did not collect data on whether girls received recommendations for HPV vaccine during their preventive visits, but future research should investigate patient, provider, and parent behaviors during these check-ups.
Previous research suggests that HPV vaccine will offer the most protection if administered before sexual debut (1, 32, 33), and as a result, is more cost-effective if given to younger adolescents (33, 34). In our study, as in others (13, 14), younger girls (ages 11–12) were less likely than older girls (ages 13–17) to have initiated the vaccine. Targeting younger adolescents, in keeping with national guidelines, will be important for maximizing the efficacy and cost-effectiveness of HPV vaccination. Across several studies (14, 15, 35), including this one, the initiation of HPV vaccine did not differ by race. Given the higher incidence of cervical cancer among non-Hispanic black versus non-Hispanic white women (36, 37), equivalent vaccination rates may help to reduce racial disparities related to cervical cancer. However, higher rates of vaccination for all races, but particularly for Hispanics and non-Hispanic blacks, could be even more effective in preventing cervical cancer and reducing the observed disparities in mortality associated with the disease (1, 11, 36, 37).
The limitations of our study include a trend design that did not permit us to interview the same respondents over time, limiting causal inference. Although we ascertained HPV vaccine initiation by parent’s self-report, a high percentage of parents can accurately recall HPV vaccination status (31). In some cases, a different person answered the BRFSS and CHAMP surveys. Although we used survey weights to account for differential response rates, survey participation was limited to parents with landline telephones, and therefore, our results may be less generalizable to populations with less access to land line phones, who are more likely to be low income, rural, and of minority race/ethnicity (18).
HPV vaccination could lead to lower incidence of genital warts and several types of cancer. However, HPV vaccine uptake is not as high as other adolescent vaccines (10, 11). From 2008 to 2010, most of the subgroups among a statewide sample of North Carolina female adolescents and their parents moved toward a middling level of vaccine uptake. Because the majority of girls attended preventive check-ups without receiving the vaccine, reducing missed opportunities to vaccinate at these visits could help to increase HPV vaccine coverage in the United States. Future research should investigate interventions aimed at increasing HPV vaccination during these preventive check-ups, especially among younger adolescents. It is also important to continue to monitor how correlates of HPV vaccine uptake may be changing over time.
Acknowledgments
The authors thank Dr. J. Michael Bowling for his assistance with the statistical analyses in this manuscript.
Grant Support
This research was supported in part by an educational grant from GlaxoSmithKline and the Cancer Control Education Program at UNC Lineberger Comprehensive Cancer Center (R25 CA57726), with additional support from the NIH (P50CA105632 and P30CA016058).
N.T. Brewer has received grants or served on paid advisory boards for GlaxoSmithKline and Merck Sharp & Dohme Corp and is a Consultant/Advisory Board member of Merck. P.L. Reiter has received a past research grant from Merck Sharp & Dohme Corp. but has not received honoraria or consulting fees from this company.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed by the other authors.
Authors’ Contributions
Conception and design: P.L. Reiter, N.T. Brewer
Development of methodology: J.L. Moss, N.T. Brewer
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): N.T. Brewer
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): J.L. Moss, M.B. Gilkey, P.L. Reiter, N.T. Brewer
Writing, review, and/or revision of the manuscript: J.L. Moss, M.B. Gilkey, N.T. Brewer
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): J.L. Moss, N.T. Brewer
Study supervision: N.T. Brewer
References
- 1.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER, et al. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 2.Sheinfeld Gorin SN, Glenn BA, Perkins RB. The human papillomavirus (HPV) vaccine and cervical cancer: uptake and next steps. Adv Ther. 2011;28:615–39. doi: 10.1007/s12325-011-0045-x. [DOI] [PubMed] [Google Scholar]
- 3.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103:1444–51. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459–66. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block SL, Brown DR, Chatterjee A, Gold MA, Sings HL, Meibohm A, et al. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) l1 virus-like particle vaccine. Pediatr Infect Dis J. 2010;29:95–101. doi: 10.1097/INF.0b013e3181b77906. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) National and state vaccination coverage among adolescents aged 13–17 years - United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:671–7. [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services immunization and infectious diseases. HealthyPeople.gov [Internet] [updated 2012 Sep 13; cited 2012 Sep 14]. Available from: http://healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=23.
- 8.Department of Health [Internet] Health Protection Agency; London, England: c2010–2012 [updated 2010 Jan; cited 2012 Jun 22]. Available from: http://data.parliament.uk/DepositedPapers/Files/DEP2012-1386/PQ119371-1.pdf. [Google Scholar]
- 9.Immunise Australia Program [Internet] Australia Government Department of Health and Ageing. c2011-2012 [updated 2012 Jul 12; cited 2012 Jun 22]. Available from: http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/immunise-hpv.
- 10.Centers for Disease Control and Prevention (CDC) National and state vaccination coverage among adolescents aged 13 through 17 years–United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:1117–23. [PubMed] [Google Scholar]
- 11.Stokley S, Cohn A, Dorell C, Hariri S, Yankey D, Messonnier N, et al. Adolescent vaccination-coverage levels in the United States: 2006–2009. Pediatrics. 2011;128:1078–86. doi: 10.1542/peds.2011-1048. [DOI] [PubMed] [Google Scholar]
- 12.Crosby RA, Casey BR, Vanderpool R, Collins T, Moore GR. Uptake of free HPV vaccination among young women: a comparison of rural versus urban rates. J Rural Health. 2011;27:380–4. doi: 10.1111/j.1748-0361.2010.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottlieb SL, Brewer NT, Sternberg MR, Smith JS, Ziarnowski K, Liddon N, et al. Human papillomavirus vaccine initiation in an area with elevated rates of cervical cancer. J Adolesc Health. 2009;45:430–7. doi: 10.1016/j.jadohealth.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Reiter PL, Cates JR, McRee AL, Gottlieb SL, Shafer A, Smith JS, et al. Statewide HPV vaccine initiation among adolescent females in North Carolina. Sex Transm Dis. 2010;37:549–56. doi: 10.1097/OLQ.0b013e3181d73bf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor LD, Hariri S, Sternberg M, Dunne EF, Markowitz LE. Human papillomavirus vaccine coverage in the United States, National Health and Nutrition Examination Survey, 2007–2008. Prev Med. 2011;52:398–400. doi: 10.1016/j.ypmed.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Smedley BA, Stith AY. In: Unequal treatment: confronting racial and ethnic disparities in healthcare. Nelson ARCommittee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Board on Health Sciences Policy, Institute of Medicine of the National Academies, editor. National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- 17.Behavioral risk factor surveillance system: at a glance. 2010 CDC.gov [Internet] [cited 2011 Dec 5]. Available from: http://www.cdc.gov/chronicdisease/resources/publications/aag/pdf/2010/brfss1.pdf.
- 18.The North Carolina Child Health Assessment and Monitoring program: survey methodology and data collection. 2010 Epi.PublicHealth.NC.gov [Internet] [cited 2011 Dec 5]. Available from: http://www.epi.state.nc.us/SCHS/pdf/Primer18_WEB_051210.pdf.
- 19.FDA approves new indication for Gardasil to prevent genital warts in men and boys. 2009 doi: 10.1089/apc.2009.9916. FDA.gov [Internet] [cited 2011 Dec 5]. Available from: http://www.fda.gov/NewsEvents/Newsroom/Press-Announcements/ucm187003.htm. [DOI] [PubMed]
- 20.Gierisch JM, Reiter PL, Rimer BK, Brewer NT. Standard definitions of adherence for infrequent yet repeated health behaviors. Am J Health Behav. 2010;34:669–79. doi: 10.5993/ajhb.34.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metropolitan and micropolitan statistical areas. Census.gov [Internet] [cited 2011 Dec 5]. Available from: http://www.census.gov/population/metro/
- 22.Brewer NT, Gottlieb SL, Reiter PL, McRee AL, Liddon N, Markowitz L, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis. 2011;38:197–204. doi: 10.1097/OLQ.0b013e3181f12dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dempsey A, Cohn L, Dalton V, Ruffin M. Patient and clinic factors associated with adolescent human papillomavirus vaccine utilization within a university-based health system. Vaccine. 2010;28:989–95. doi: 10.1016/j.vaccine.2009.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiter PL, McRee AL, Gottlieb SL, Brewer NT. Correlates of receiving recommended adolescent vaccines among adolescent females in North Carolina. Hum Vaccin. 2011;7:67–73. doi: 10.4161/hv.7.1.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briss PA, Rodewald LE, Hinman AR, Shefer AM, Strikas RA, Bernier RR, et al. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. The Task Force on Community Preventive Services. AmJ PrevMed. 2000;18(Suppl):97–140. doi: 10.1016/s0749-3797(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson VJ, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005:CD003941. doi: 10.1002/14651858.CD003941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee GM, Lorick SA, Pfoh E, Kleinman K, Fishbein D. Adolescent immunizations:missed opportunities for prevention. Pediatrics. 2008;122:711–7. doi: 10.1542/peds.2007-2857. [DOI] [PubMed] [Google Scholar]
- 28.Tierney CD, Yusuf H, McMahon SR, Rusinak D, O’Brien MA, Massoudi MS, et al. Adoption of reminder and recall messages for immunizations by pediatricians and public health clinics. Pediatrics. 2003;112:1076–82. doi: 10.1542/peds.112.5.1076. [DOI] [PubMed] [Google Scholar]
- 29.Bartlett JA, Peterson JA. The uptake of human papillomavirus (HPV) vaccine among adolescent females in the United States: a review of the literature. J Sch Nurs. 2011;27:434–46. doi: 10.1177/1059840511415861. [DOI] [PubMed] [Google Scholar]
- 30.Reiter PL, Brewer NT, Gottlieb SL, McRee AL, Smith JS. Parents’ health beliefs and HPV vaccination of their adolescent daughters. Soc Sci Med. 2009;69:475–80. doi: 10.1016/j.socscimed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 31.Dorell CG, Jain N, Yankey D. Validity of parent-reported vaccination status for adolescents aged 13–17 years: National Immunization Survey-Teen, 2008. Public Health Rep. 2011;126(Suppl 2):60–9. doi: 10.1177/00333549111260S208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hildesheim A, Herrero R. Human papillomavirus vaccine should be given before sexual debut for maximum benefit. J Infect Dis. 2007;196:1431–2. doi: 10.1086/522869. [DOI] [PubMed] [Google Scholar]
- 33.Sigurdsson K, Sigvaldason H, Gudmundsdottir T, Sigurdsson R, Briem H. The efficacy of HPV 16/18 vaccines on sexually active 18–23 year old women and the impact of HPV vaccination on organized cervical cancer screening. Acta Obstet Gynecol Scand. 2009;88:27–35. doi: 10.1080/00016340802566770. [DOI] [PubMed] [Google Scholar]
- 34.Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359:821–32. doi: 10.1056/NEJMsa0707052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiro JA, Pruitt SL, Bruce CM, Persaud D, Lau M, Vernon SW, et al. Multilevel correlates for human papillomavirus vaccination of adolescent girls attending safety net clinics. Vaccine. 2012;30:2368–75. doi: 10.1016/j.vaccine.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 36.Singh GK. Rural-urban trends and patterns in cervical cancer mortality, incidence, stage, and survival in the United States, 1950-2008. J Community Health. 2012;37:217–23. doi: 10.1007/s10900-011-9439-6. [DOI] [PubMed] [Google Scholar]
- 37.Watson M, Saraiya M, Benard V, Coughlin SS, Flowers L, Cokkinides V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer. 2008;113(Suppl):2855–64. doi: 10.1002/cncr.23756. [DOI] [PubMed] [Google Scholar]