Abstract
Objective
A small randomized controlled trial suggested that vitamin D might increase the production of testosterone in men, which is supported by experimental studies in animals and a cross-sectional study showing positive associations between plasma 25-hydroxyvitamin D [25(OH)D] and testosterone and concordant seasonal variation of both biomarkers.
Design and Measurements
We investigated the cross-sectional association of plasma 25(OH)D levels and total and free testosterone measured by immunoassay in 1,362 male participants of the Health Professionals Follow-up Study who were selected for a nested case-control study on prostate cancer using multivariate adjusted linear and restricted cubic spline regression models.
Results
25(OH)D was positively associated with total and free testosterone levels. From the lowest to the highest 25(OH)D quintile, multivariate adjusted means (95% confidence interval) were 18.5 (17.7; 19.4), 19.4 (18.6; 20.2), 19.6 (18.8; 20.4), 20.1 (19.3; 20.9) and 20.0 (19.1; 20.8); p-trend=0.003) for total testosterone and 97.7 (93.9; 101.5), 98.2 (94.1; 102.2), 99.2 (95.2; 103.2), 100.7 (96.9; 104.5) and 101.5 (97.6; 105.4; p-trend=0.03) for free testosterone. The shapes of the dose-response curves indicate that the association between 25(OH)D and total and free testosterone is linear at lower levels of 25(OH)D (below approximately 75-85 nmol/l), reaching a plateau at higher levels. Unlike for 25(OH)D, we did not observe any seasonal variation of testosterone concentrations.
Conclusion
This study supports previously reported positive associations between vitamin D and testosterone although we did not observe parallel seasonal variation patterns. Possible causality and direction of the vitamin D-testosterone association deserve further scientific investigation.
Keywords: vitamin D, testosterone
Introduction
Data from a small randomized controlled trial suggest that vitamin D supplementation might increase the production of testosterone in men1. In that trial, 54 healthy men whose mean 25(OH)D levels were in the deficiency range (mean 29.7 nmol/l in the placebo group and 32.5 nmol/l in the vitamin D group; to convert nmol/l to ng/ml, divide by 2.5) received either 83 μg vitamin D daily for 1 year (n=31) or placebo (n=23). Compared to baseline values, a significant increase in total testosterone levels (from 10.7±3.9 nmol/l to 13.4±4.7 nmol/l; p<0.001) was observed in the vitamin D supplementation group. Similar increases were observed for free testosterone and bioactive testosterone. No changes in testosterone concentrations were observed in the placebo group. In a cross-sectional study among 2,299 men who were routinely referred for coronary angiography, positive associations between 25-hydroxy vitamin D (25(OH)D) and total testosterone levels were observed 2. In the same study, 25(OH)D and total testosterone followed a similar seasonal pattern. A recently published cross-sectional study among 3,369 healthy European men observed weak positive associations between 25(OH)D and testosterone levels and an inverse association between 25(OH)D and estradiol 3. While these studies suggest a link between 25(OH)D and sex steroids, more studies are warranted, and especially the shape of the dose-response relationship has not been explored so far. There is biological support for a possible association between vitamin D and male reproductive hormones. The vitamin D receptor and vitamin D metabolizing enzymes are widely expressed, including in the male reproductive tract 4. Furthermore, vitamin D levels have been associated with sperm count and motility 5 and vitamin D receptor (VDR) knockout mice have significant gonadal insufficiency, decreased sperm count and motility 6.
The aim of this study was to cross-sectionally investigate the association between vitamin D status as reflected by 25(OH) D concentrations and plasma testosterone and estradiol in participants of the Health Professionals Follow-up study (HPFS).
Material and Methods
The HPFS is an ongoing prospective cohort study including 51,529 male health professionals who were between 40 and 75 years old at enrollment in 1986. At baseline, the men completed a mailed questionnaire on lifestyle characteristics, medical history and a food frequency questionnaire. Since then, participants have been followed every two years by mailed questionnaires to ascertain the occurrence of new medical diagnoses as well as to update exposure status. Information on dietary intake has been updated every 4 years. Between 1993 and 1995 18,225 participants provided a blood sample, collected in blood tubes containing liquid EDTA and shipped to the laboratory via overnight courier while chilled on ice. At the laboratory, the blood was centrifuged, divided into plasma, erythrocytes, and buffy coat and stored in liquid nitrogen freezers. The study was approved by the Institutional Review Board of Harvard School of Public Health and Brigham and Women's Hospital in Boston, Massachusetts and written informed consent was obtained from all participants.
In the present study, we included participants who were selected for a nested case-control study on prostate cancer. The nested case-control study included prostate cancer cases verified by medical records and control subjects selected among participants who were alive and free of diagnosed cancer at the date of the case's diagnosis, and who had a PSA (prostate specific antigen) test after the date of blood draw. For each prostate cancer case one control participant was matched on year of birth (±1 year), PSA test prior to blood draw (yes/no), and timing of blood draw – time of day (midnight to before 9 am, 9 am to before noon, noon to before 4 pm, 4 pm to before midnight), season (winter, spring, summer, fall), and year (exact).
Measurement of vitamin D and testosterone
The cases and corresponding controls were identified in three waves of follow-up resulting in three assay batches: blood-draw to 1996, 1996-1998, 1998-2000. Case-control pairs were analyzed together with the laboratory personnel being blinded to case-control status.
Plasma concentrations of 25(OH)D were determined by radioimmunosorbant assay (RIA, DiaSorin Inc., Stillwater, MN) in the laboratory of Dr. Bruce Hollis as described previously 7. The mean intrapair coefficient of variation calculated from blinded quality control samples was 5.4%. Missing data occurred due to too low plasma volume (2 samples).
Plasma concentrations of sex steroid hormones and SHBG (sex-hormone binding globuline) were measured in the laboratory of Dr. Nader Rifai at the Children's Hospital, Boston, Massachusetts. Total testosterone was measured by means of a chemiluminescent immunoassay (Elecsys autoanalyzer, Roche Diagnostics, Indianapolis, IN), free testosterone by means of enzyme immunoassay (Diagnostic System Laboratories, Webster, TX), estradiol by means of a third-generation RIA (Diagnostic Systems Laboratory, Webster, TX) and SHBG by means of coated tube noncompetitive immunoradiometric assay (Diagnostic Systems Laboratory, Webster, TX). The mean intrapair correlation coefficients of variation were 4.9% for total testosterone, 8.4% for free testosterone, 5.2% for estradiol and 10.7% for SHBG. Missing data occurred because samples were too lipemic for free testosterone assay (13 samples) or due to too low plasma volume (3 samples).
Plasma total cholesterol concentration was measured by means of Infinity Total Cholesterol Enzymatic Assay kit (Sigma Diagnostics, St. Louis, MO) for the first and second analysis batches and an enzymatic assay using reagents from Equal Diagnostics (Exton, PA) for the third analysis batch. The mean intrapair coefficient of variation for cholesterol calculated from blinded quality control samples was 10.9% for the Infinity assay and 9.1% for the Equal Diagnostics assay.
As reported previously 8, 9, to assess the intraperson consistency of 25(OH)D or sex steroid hormones over time, 25(OH)D, total testosterone, free testosterone, estradiol and SHBG were measured for 144 HPFS participants who were free of a cancer diagnosis and who provided a blood sample in 1993/94 and again in 1997 (mean of 3.03±0.46 years apart). Adjusting for age, race, and season of the year, the Pearson correlation coefficient between the two time points was 0.70 for 25(OH)D and Spearman correlation coefficients were 0.68 for total testosterone, 0.39 for free testosterone, 0.55 for estradiol and 0.74 for SHBG (all p<0.0001).
Statistics
To avoid confounding by race/ethnicity we excluded 9 African-American and 5 Asian participants. All remaining participants from the nested case-control study for whom both 25(OH)D and plasma sex steroid hormones were available (n=1,362) were included in the present analysis. Neither 25(OH)D nor sex steroid hormones have been related to total prostate cancer in HPFS 8, 9. Thus, to increase power, we included both cases (who were not diagnosed with cancer at time of blood draw) and controls in our final analysis, but all analyses were repeated including control participants (n=678) only. In sensitivity analyses, we restricted the study population to controls and additionally excluded participants who reported major morbidities such as diabetes, high cholesterol and high blood pressure, which could be an indicator for lower 25(OH)D levels.
We used multivariate linear regression with robust variance (PROC MIXED with empirical statement, version 9.2; SAS Institute, Cary, NC) 10 to investigate the association between 25(OH)D and total testosterone, free testosterone or estradiol. This method allows for valid inference without assumption of normal distribution in the dependent variable. To account for differences in absolute concentrations between analysis batches, free testosterone levels were batch-standardized11. Using regression coefficients for batch derived from a linear regression model with free testosterone as dependent variable and batch-indicators as independent variable, free testosterone levels were standardized to the levels that would be obtained if all measurements were from batch 1. Batch-standardization of total testosterone and estradiol was not necessary since mean values were similar across batches. As an alternative approach we calculated free testosterone from total testosterone and SHBG under the assumption of a constant albumin concentration of 43 g/l according to Vermeulen et al. 12. Multivariate adjusted mean sex steroid hormone concentrations and corresponding 95% confidence intervals (95% CI) were calculated by batch-specific quintiles of 25(OH)D. To test for linear trend, participants were assigned the median values of the batch-specific 25(OH)D quintiles and the resulting variable was entered continuously to the model, deriving the P values for linear trend from this variable's Wald's test. In addition, continuous parameter estimates representing the change in testosterone per 25 nmol/l increment in 25(OH)D (based on trend-variable) are presented. The fully multivariate adjusted model included age, BMI, analysis batch, time of blood collection (4 categories: before 9am, 9am-12pm, 12pm-4pm, after 4pm), season, geographical region (West, Midwest, South, Northeast), smoking status, and physical activity (MET-hours/week). Total testosterone was additionally adjusted for SHBG in a separate model, which can be interpreted similarly to the multivariate model for free testosterone. The presented age- and batch-adjusted mean values were centered to age 60 years and batch 1; age-, batch- and BMI-adjusted models were centered to age 60 years, batch 1 and BMI 25 kg/m2; multivariate adjusted models means were centered to age 60 years, batch 1, BMI 25 kg/m2 and mean physical activity (40 MET hours/week). In a sensitivity analysis, we tested whether additional adjustment for batch-specific deciles of plasma cholesterol, which is a precursor for both testosterone and vitamin D, changes associations. We examined possible non-linear associations using restricted cubic splines 13 with 4 knots to divide continuous 25(OH)D concentration into 5 intervals. To make the graph more stable highest and lowest percentile of 25(OH)D levels were excluded.
To investigate if the association between 25(OH) vitamin D and testosterone varies by age, BMI, vasectomy or season analyses stratified by these factors were performed. Tests for statistical interaction were performed by creating a cross-product variable of the medians of 25(OH)D quintiles and the respective stratification variable and testing the difference of the model with and without this variable by means of the likelihood ratio test. Because testosterone levels measured in morning samples are considered more accurate 14, we repeated all analyses excluding blood samples drawn in the afternoon (n=236).
In an additional analysis we defined hypogonadism based on low total testosterone levels (<11 nmol/l) 15 and estimated the relative risk (approximated by the odds ratio) of hypogonadism by means of logistic regression. The multivariate adjusted model included all covariates described above.
We determined the seasonal variation of 25(OH)D, total testosterone, free testosterone and estradiol by calculating age- and batch-adjusted mean concentrations by month of blood collection for both bi omarkers using linear regression with robust variance as described above.
Results
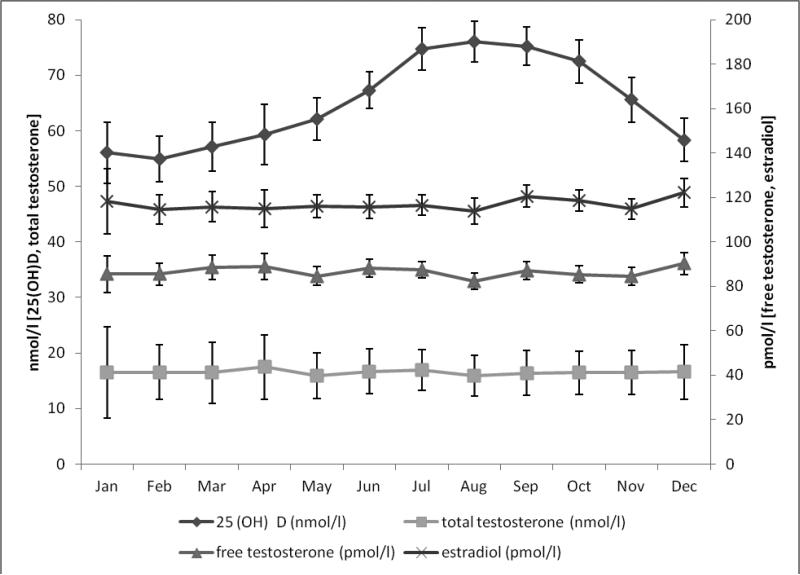
Using common cut-offs for vitamin D status, 24% (n=333) of participants had 25(OH)D levels <50 nmol/l (vitamin D deficiency), 44% (n=601) had 25(OH)D levels between 50 and 74.9 nmol/l (insufficiency) and 31% (n=428) had 25(OH)D levels ≥75 nmol/l (sufficiency). Men in the highest quintile of 25(OH)D were slightly younger, had a lower BMI and higher physical activity) than men in the lowest quintile (table 1). Circulating 25(OH) vitamin D showed seasonal variation with highest concentrations in summer and fall (peak: August) and lowest in winter and spring (nadir: February), whereas no seasonal variation was seen for total testosterone, free testosterone or estradiol (figure 1).
Table 1.
Characteristics of study participants according to 25(OH)D quintiles
| Q1 (n=272) | Q2 (n=272) | Q3 (n=272) | Q4 (n=275) | Q5 (n=271) | |
|---|---|---|---|---|---|
| Age at blood collection* | 66.4(7.2) | 66.1(7.3) | 65.5(7.3) | 65.6(7.9) | 65.3(7.3) |
| Prostate cancer cases, % | 46 | 52 | 49 | 50 | 57 |
| Analysis batch | |||||
| Blooddraw-1996, % | 29 | 29 | 29 | 30 | 30 |
| 1996-1998, % | 36 | 37 | 36 | 36 | 36 |
| 1998-2000, % | 35 | 34 | 35 | 34 | 34 |
| Season of blood collection | |||||
| Spring , % | 30 | 19 | 17 | 13 | 9 |
| Summer , % | 16 | 30 | 30 | 36 | 39 |
| Fall , % | 22 | 24 | 31 | 36 | 37 |
| Winter , % | 25 | 19 | 15 | 8 | 6 |
| Time of blood collection | |||||
| midnight to before 9am , % | 27 | 26 | 24 | 27 | 33 |
| 9am to before noon , % | 45 | 47 | 45 | 44 | 43 |
| noon to before 4 pm , % | 13 | 13 | 16 | 15 | 10 |
| 4pm to before midnight , % | 3 | 3 | 4 | 4 | 4 |
| Geographical region | |||||
| West , % | 24 | 22 | 28 | 23 | 23 |
| Midwest , % | 21 | 32 | 27 | 29 | 29 |
| South , % | 28 | 26 | 23 | 29 | 34 |
| Northeast , % | 26 | 20 | 21 | 18 | 14 |
| Height (cm) | 178(7) | 178(7) | 179(6) | 178(6) | 179(6) |
| BMI (kg/m2) | 26.9(4.2) | 26.5(3.9) | 25.4(3.1) | 25.5(3.0) | 25.3(3.1) |
| Smoking status | |||||
| never , % | 43 | 45 | 45 | 46 | 39 |
| past , % | 46 | 48 | 49 | 47 | 54 |
| current , % | 5 | 4 | 5 | 5 | 3 |
| Physical activity (METs/week) | 29.0(38.0) | 35.3(35.3) | 42.4(55.1) | 40.9(42.6) | 51.5(41.0) |
Q=Quintile; Quintile cutpoints were 43.7, 52.9, 61.2 and 71.1 nmol/l for batch 1; 45.4, 53.9, 63.6 and 77.4 nmol/l for batch 2 and 58.4, 71.6, 84.6 and 101.8 nmol/l for batch 3
Values are means (SD) or percentages and are standardized to the age distribution of the study population.
Value is not age adjusted
Figure 1.
Monthly variation of mean (age- and batch-adjusted) 25-hydroxyvitamin D [25(OH)D], total testosterone, free testosterone and estradiol. Bars indicate 95% confidence intervals.
Total testosterone concentrations significantly increased across quintiles of circulating 25(OH) vitamin D levels (table 2) in all adjustment models. The age- and batch-adjusted associations were slightly attenuated by additional adjustment for BMI, and further potentially confounding factors including time and season of blood collection, smoking, geographical region and physical activity. When including only control participants (n=678), multivariate adjusted mean testosterone concentrations (95% CI) from the lowest to the highest quintile of 25 (OH) vitamin D were 18.0 (16.9; 19.2), 19.4 (18.1; 20.6), 19.4 (18.2; 20.5), 19.7 (18.6; 20.8), 20.1 (18.9; 21.3) nmol/l, (p-trend=0.01). A positive association persisted when including only control participants without major morbidities (diabetes, high blood pressure or high blood cholesterol; n=316; data not shown). Additional adjustment for plasma total cholesterol did not change associations remarkably (data not shown).
Table 2.
Mean testosterone concentrations according to quintiles of 25-OH vitamin D
| 25-OH Vitamin D (quintiles) | |||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ptrend* | β † | |
| Total testosterone (nmo/l) age and batch-adjusted | 17.4 | 18.3 | 19.0 | 19.5 | 19.6 | 0.96 | |
| (95% CI) | (16.5; 18.3) | (17.4; 19.2) | (18.1; 19.9) | (18.6; 20.4) | (18.7; 20.5) | <0.0001 | (0.56; 1.37) |
| age-, batch- and bmi-adjusted | 18.7 | 19.4 | 19.5 | 20.0 | 20.0 | 0.56 | |
| (95% CI) | (17.8; 19.6) | (18.6; 20.3) | (18.7; 20.4) | (19.2; 20.9) | (19.1; 20.9) | 0.01 | (0.17; 0.95) |
| multivariate‡ adjusted | 18.5 | 19.4 | 19.6 | 20.1 | 20.0 | 0.61 | |
| (95% CI) | (17.7; 19.4) | (18.6; 20.2) | (18.8; 20.4) | (19.3; 20.9) | (19.1; 20.8) | 0.003 | (0.21; 1.02) |
| multivariate‡ adjusted plus SHBG | 13.8 | 14.5 | 14.7 | 15.1 | 14.9 | 0.48 | |
| (95% CI) | (13.0; 14.7) | (13.7; 15.3) | (13.9; 15.5) | (14.3; 15.9) | (14.1; 15.7) | 0.01 | (0.14; 0.83) |
| Free testosterone§ (pmol/l) age and batch-adjusted | 92.7 | 93.7 | 95.0 | 98.9 | 99.6 | 2.25 | |
| (95% CI) | (87.8; 97.6) | (88.1; 99.3) | (89.5; 100.5) | (93.2; 104.7) | (94.1; 105.1) | 0.003 | (0.80; 3.70) |
| age-, batch- and bmi-adjusted | 98.5 | 98.4 | 98.8 | 100.3 | 100.9 | 1.01 | |
| (95% CI) | (94.7; 102.4) | (94.3; 102.4) | (94.8; 102.8) | (96.5; 104.1) | (97.0; 104.8) | 0.17 | (−0.43; 2.45) |
| multivariate‡ adjusted | 97.7 | 98.2 | 99.2 | 100.7 | 101.5 | 1.60 | |
| (95% CI) | (93.9; 101.5) | (94.1; 102.2) | (95.2; 103.2) | (96.9; 104.5) | (97.6; 105.4) | 0.03 | (0.15; 3.06) |
| Estradiol (pmol/l) age and batch-adjusted | 87.1 | 88.0 | 86.5 | 88.1 | 90.0 | 1.26 | |
| (95% CI) | (82.4; 91.7) | (83.6; 92.4) | (82.1; 91.0) | (83.6; 92.6) | (85.4; 94.7) | 0.25 | (−0.89; 3.41) |
| age-, batch- and bmi-adjusted | 83.2 | 84.7 | 84.9 | 86.5 | 88.7 | 2.47 | |
| (95% CI) | (78.6; 87.8) | (80.3; 89.1) | (80.5; 89.3) | (81.9; 91.0) | (84.1; 93.3) | 0.02 | (0.38; 4.57) |
| multivariate‡ adjusted | 81.9 | 84.3 | 84.6 | 86.5 | 89.1 | 3.15 | |
| (95% CI) | (77.3; 86.5) | (80.0; 88.6) | (80.2; 88.9) | (81.9; 91.0) | (84.4; 93.8) | 0.01 | (0.91; 5.39) |
Q=Quintile, BMI=body mass index;
trend-variable based on quintile medians
continuous parameter estimate (change in testosterone per 25 nmol/l increment in 25-OH vitamin D based on trend-variable)
adjusted for age (at blood collection), batch, time of blood collection, season, BMI at blood collection (continuous), smoking status, geographical region, physical activity (METs/week)
standardized by batch
Note: Age- and batch-adjusted mean values were centered to age 60 years and batch 1; age-, batch- and BMI-adjusted models were centered to age 60 years, batch 1 and BMI 25 kg/m2; multivariate adjusted models means were centered to age 60 years, batch 1, BMI 25 kg/m2 and mean physical activity (40 MET hours/week).
Circulating 25(OH) vitamin D levels were significantly positively associated with free testosterone in the age- and batch-adjusted as well as in the multivariate-adjusted model. These findings were not changed when only control participants were included (multivariate adjusted mean free testosterone concentrations (95% CI) from the lowest to the highest quintile of 94.6 (89.7; 99.5), 96.6 (91.0; 102.2), 96.7 (91.3; 102.1), 100.3 (94.7; 106.0). 100.8 (95.4; 106.2), (p-trend=0.02).
When estimating the association between 25(OH)D and free testosterone calculated according to Vermeulen et al. 12 positive associations of 25(OH) D and free testosterone were observed in all models (data not shown). We observed a significant positive association between 25(OH)D and estradiol in the multivariate adjusted model.
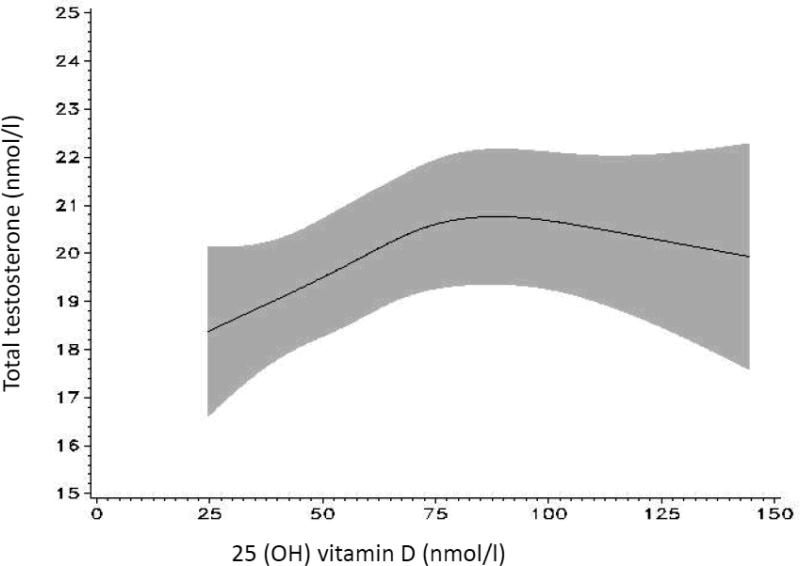
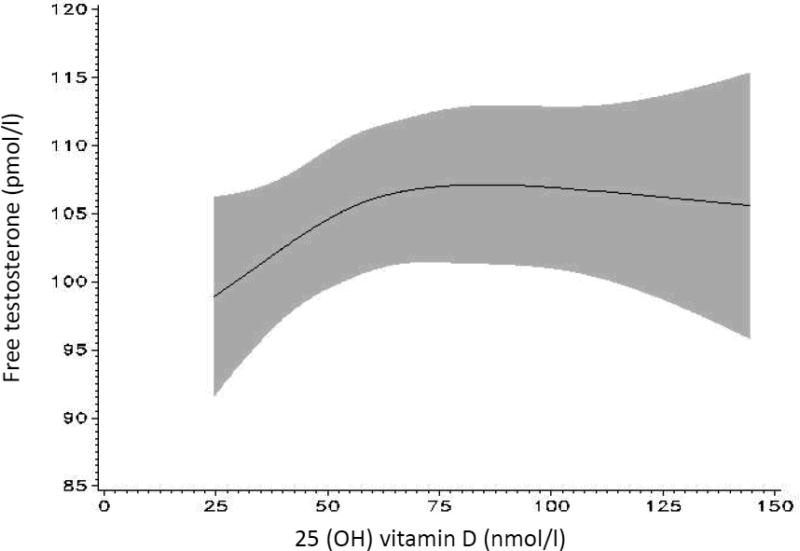
The spline models show graphically that the positive association between 25(OH)D and total and free testosterone is linear at lower 25(OH)D levels, below approximately 75-85 nmol/l in the case of total testosterone and 75 nmol/l in the case of free testosterone (figure 2a and 2b), reaching a plateau at higher levels (p for non-linearity 0.05 for total testosterone and 0.14 for free testosterone).
Figure 2a.
Association between 25 (OH) vitamin D and total testosterone modeled continuously using splines; 95% confidence interval in gray [model adjusted for age (at blood collection), batch, time of blood collection, season, BMI at blood collection (continuous), smoking status, geographical region, physical activity (METs/week)].
Figure 2b.
Association between 25 (OH) vitamin D and free testosterone modeled continuously using splines; 95% confidence interval in gray [model adjusted for age (at blood collection), batch, time of blood collection, season, BMI at blood collection (continuous), smoking status, geographical region, physical activity (METs/week)].
No significant differences in the multivariate adjusted association between 25(OH)D and total testosterone were observed after stratification by age at blood collection or BMI categories (data not shown). There was some suggestion of differences in the association between 25(OH)D and free testosterone by age at blood collection and BMI categories: a positive association between 25 (OH) D and free testosterone was only seen in men aged 70 years or older (p-trend= 0.002), but not in men below age 70 years (p-trend=0.53; p for interaction 0.02). The association between 25(OH)D and free testosterone was stronger in men with a BMI ≥30 kg/m2 (p-trend=0.07) than in lower BMI categories (<23, 23-24.9, 25-29.9 kg/m2) (p for interaction=0.03). When we stratified models by vasectomy, significant positive associations between 25(OH) vitamin D and total and free testosterone were observed in men who had not had a vasectomy (n=1,002) (p-trend=0.001 for total testosterone and 0.004 for free testosterone), while no significant associations were observed in men who had had a vasectomy (n=360) (p for interaction 0.24 for total testosterone, and 0.02 for free testosterone).
We did not observe a clear geographical trend when stratifying by geographical region: significant positive associations (in multivariate adjusted models) between 25(OH)D and total testosterone were observed among men residing in the South (n=381, p-trend=0.01) and in the Northeast (n=268, p-trend=0.03), but not in the West (n=331, p-trend=0.34) or Midwest (n=378, p-trend=0.13). A significant positive association between 25(OH)D and free testosterone was observed only in men residing in the Northeast (p-trend=0.04).
Stratification by season of blood collection revealed slightly stronger positive associations between 25(OH)D and testosterone during winter or spring (n=434, p-trend 0.01) than during summer or fall (n=910, p-trend 0.06), whereas a significant positive association between 25(OH)D and free testosterone was only observed during summer or fall (p-trend 0.02) but not in winter or spring (p-trend 0.92). However, no significant interactions by season were observed. Associations between 25(OH)D and both total and free testosterone were stronger when blood samples drawn in the afternoon were excluded (data not shown).
When defining hypogonadism based on total testosterone levels <11 nmol/l (n=248), after multivariate adjustment for age, analysis batch, time and season of blood collection, BMI, smoking, geographical region, and physical activity, comparing participants in the highest versus lowest quintile of 25(OH) vitamin D had a significantly decreased relative risk of hypogonadism of 0.50 (95% CI 0.31-0.93; p-trend=0.01). This association was stronger when participants whose blood samples were drawn in the afternoon were excluded (OR highest versus lowest quintile of 25(OH)D 0.34 , 95% CI 0.18-0.65, p-trend= 0.001).
Discussion
This study confirms previously observed positive associations between circulating 25(OH) D and total and free testosterone levels before and after adjustment for a variety of potential confounders. The shape of the dose-response curves indicate that associations between 25(OH)D and testosterone are strongest in lower ranges of 25(OH)D. Unlike for vitamin D, we did not observe any seasonal variation of testosterone concentrations.
This is the fourth study to show positive associations between vitamin D and testosterone1-3. Both vitamin D receptor (VDR) and vitamin D metabolizing enzymes were shown to be expressed in human Leydig cells, where testosterone in the male testes is synthesized 4, suggesting that vitamin D might affect the production of male reproductive hormones. This hypothesis is supported by the observation that VDR knockout mice are characterized by hypergonadotropic hypogonadism 6. It cannot be excluded that the here observed associations between 25(OH)D and testosterone reflect non-biological correlations due to similar distributions of confounding factors or due to common biological precursors such as cholesterol. However, positive associations between 25(OH)D and testosterone persisted after adjusting for a variety of factors that are associated with both 25(OH)D levels and testosterone levels such as age, BMI and physical activity. Furthermore, adjustment for total cholesterol did not change the observed associations between 25(OH)D and total or free testosterone.
Vitamin D status as reflected by 25(OH)D levels is mainly determined by sun exposure (ultraviolet light conversion of 7-dehydrocholesteron in the skin), while dietary intake generally plays a more minor role in vitamin D supply 16. Although our findings of a positive association between 25(OH) vitamin D and testosterone confirm the previous cross-sectional findings among 2,299 men routinely referred for angiography in the German LURIC study2, we did not observe any seasonal variation of total testosterone, which contrasts this study's findings of parallel seasonal variation of 25(OH)D and testosterone. It is noteworthy that the two studies differ substantially with respect to vitamin D status. Based on 25(OH)D levels, in the German study 63% of participants had a deficient vitamin D status (25 (OH) D <50 nmol/l), whereas in our sample, only 25% of participants were vitamin D deficient. The poor vitamin D supply in the German sample may be partly explainable by the geographical latitude which is further away from equatorial latitudes than US latitudes. In addition, while in the US milk and other foods are commonly fortified with vitamin D, no fortification is undertaken in Germany, so that the dietary vitamin D intake is substantially lower than in the US. Finally, the German sample of men who were referred for coronary angiography may have had exceptionally low 25(OH)D levels since coronary artery disease has been linked to a poor vitamin D status. In a study relating vitamin D levels to sperm quality in humans, the effect of vitamin D on sperm motility was most pronounced in men with vitamin D deficiency 5. In a recent multicenter cross-sectional study among 3,369 community-dwelling European men, of whom 37% were in the deficiency range, 25(OH)D was positively associated with total and free testosterone in age- and study center-adjusted models, but not after multivariate adjustment for health and lifestyle factors3. In the same study no seasonal variation of testosterone was observed. Seasonal variation of testosterone has been investigated in numerous other cross-sectional and longitudinal studies, yielding inconsistent results17. In line with our finding, no seasonal variation of testosterone has been observed in four studies17-20.
Our investigation of the dose-response relationship suggests that associations between 25(OH)D and testosterone are stronger at the lower end of vitamin D concentrations. However, due to the low proportion of vitamin D deficient men in our study we were not able to investigate associations at the very low end of 25(OH)D levels (only 10 participants had 25(OH)D levels <25 nmol/l).
The observed statistical interaction by vasectomy requires confirmation by further studies. A biological explanation why vitamin D may be associated with higher testosterone levels only in men who had not had a vasectomy is lacking so far and it cannot be excluded that this finding was due to chance. Vasectomy is not strongly associated with changes in hormonal profiles 21, 22. In our study testosterone, SHBG and vitamin D levels did not differ substantially by vasectomy status, but men who had had a vasectomy were younger and more likely to reside in sunny areas than men who had not had a vasectomy. Thus, it is possible that the observed effect modification was due to differences in the distribution of confounding factors by vasectomy status. Strengths of our study include the ability to adjust for a variety of potential confounders. Although residual confounding cannot be excluded, our findings suggest an association between 25(OH)D and testosterone independent of major determinants of 25(OH)D/testosterone such as BMI, smoking and physical activity. Our study also has several limitations. Due to the cross-sectional design no conclusions with respect to causality or directionality of the vitamin D-testosterone association can be drawn. Another drawback of our study is that total testosterone was measured by an immunoassay, which has lower accuracy and sensitivity than methods based on mass spectrometry, which is considered the gold standard23. Due to the strong variation by analysis batch and the low intra-person consistency of free testosterone measurements, the associations observed with free testosterone should be interpreted with caution. Furthermore, our findings are only generalizable to a limited extent, since this is a sample of middle aged mainly Caucasian men with a rather homogenous socio-economic status.
In conclusion, the present study confirms previous findings of a positive association between vitamin D status as reflected by 25(OH) vitamin D levels and testosterone levels, although in our study this finding was not supported by parallel seasonal variation patterns of 25(OH) vitamin D and testosterone. Whether these cross-sectional findings reflect a causal relationship deserves further scientific examination.
Acknowledgements
This study was supported by grants CA55075 and CA133891 from the National Cancer Institute, National Institutes of Health. Katharina Nimptsch is recipient of a scholarship within the Postdoc-Program of the German Academic Exchange Service (DAAD).
Footnotes
The authors declare no conflict of interest.
References
- 1.Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B, Wehr E, Zittermann A. Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res. 2011;43:223–225. doi: 10.1055/s-0030-1269854. [DOI] [PubMed] [Google Scholar]
- 2.Wehr E, Pilz S, Boehm BO, Marz W, Obermayer-Pietsch B. Association of vitamin D status with serum androgen levels in men. Clin Endocrinol (Oxf) 2010;73:243–248. doi: 10.1111/j.1365-2265.2009.03777.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee DM, Tajar A, Pye SR, Boonen S, Vanderschueren D, Bouillon R, O'Neill TW, Bartfai G, Casanueva FF, Finn JD, Forti G, Giwercman A, Han TS, Huhtaniemi I, Kula K, Lean ME, Pendleton N, Punab M, Wu F. Association of hypogonadism with vitamin D status: the European Male Ageing Study. Eur J Endocrinol. 2011 doi: 10.1530/EJE-11-0743. [DOI] [PubMed] [Google Scholar]
- 4.Blomberg Jensen M, Nielsen JE, Jorgensen A, Rajpert-De Meyts E, Kristensen DM, Jorgensen N, Skakkebaek NE, Juul A, Leffers H. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod. 2010;25:1303–1311. doi: 10.1093/humrep/deq024. [DOI] [PubMed] [Google Scholar]
- 5.Blomberg Jensen M, Bjerrum PJ, Jessen TE, Nielsen JE, Joensen UN, Olesen IA, Petersen JH, Juul A, Dissing S, Jorgensen N. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum Reprod. 2010 doi: 10.1093/humrep/der059. [DOI] [PubMed] [Google Scholar]
- 6.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39:529–533. [PubMed] [Google Scholar]
- 8.Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15:255–265. doi: 10.1023/B:CACO.0000024245.24880.8a. [DOI] [PubMed] [Google Scholar]
- 9.Platz EA, Leitzmann MF, Rifai N, Kantoff PW, Chen YC, Stampfer MJ, Willett WC, Giovannucci E. Sex steroid hormones and the androgen receptor gene CAG repeat and subsequent risk of prostate cancer in the prostate-specific antigen era. Cancer Epidemiol Biomarkers Prev. 2005;14:1262–1269. doi: 10.1158/1055-9965.EPI-04-0371. [DOI] [PubMed] [Google Scholar]
- 10.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- 11.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008;167:653–666. doi: 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- 12.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 13.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6:356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 15.Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O'Neill TW, Bartfai G, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Boonen S, Vanderschueren D, Labrie F, Huhtaniemi IT. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 363:123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 17.Brambilla DJ, O'Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 2007;67:853–862. doi: 10.1111/j.1365-2265.2007.02976.x. [DOI] [PubMed] [Google Scholar]
- 18.Abbaticchio G, de Fini M, Giagulli VA, Santoro G, Vendola G, Giorgino R. Circannual rhythms in reproductive functions of human males, correlations among hormones and hormone-dependent parameters. Andrologia. 1987;19:353–361. doi: 10.1111/j.1439-0272.1987.tb02314.x. [DOI] [PubMed] [Google Scholar]
- 19.Maes M, Mommen K, Hendrickx D, Peeters D, D'Hondt P, Ranjan R, De Meyer F, Scharpe S. Components of biological variation, including seasonality, in blood concentrations of TSH, TT3, FT4, PRL, cortisol and testosterone in healthy volunteers. Clin Endocrinol (Oxf) 1997;46:587–598. doi: 10.1046/j.1365-2265.1997.1881002.x. [DOI] [PubMed] [Google Scholar]
- 20.Martikainen H, Tapanainen J, Vakkuri O, Leppaluoto J, Huhtaniemi I. Circannual concentrations of melatonin, gonadotrophins, prolactin and gonadal steroids in males in a geographical area with a large annual variation in daylight. Acta Endocrinol (Copenh) 1985;109:446–450. doi: 10.1530/acta.0.1090446. [DOI] [PubMed] [Google Scholar]
- 21.Ren L, Weng Q, Kishimoto M, Watanabe G, Jaroenporn S, Taya K. Effect of short period vasectomy on FSH, LH, inhibin and testosterone secretions, and sperm motility in adult male rats. Exp Anim. 60:47–56. doi: 10.1538/expanim.60.47. [DOI] [PubMed] [Google Scholar]
- 22.Mo ZN, Huang X, Zhang SC, Yang JR. Early and late long-term effects of vasectomy on serum testosterone, dihydrotestosterone, luteinizing hormone and follicle-stimulating hormone levels. J Urol. 1995;154:2065–2069. [PubMed] [Google Scholar]
- 23.Hsing AW, Stanczyk FZ, Belanger A, Schroeder P, Chang L, Falk RT, Fears TR. Reproducibility of serum sex steroid assays in men by RIA and mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2007;16:1004–1008. doi: 10.1158/1055-9965.EPI-06-0792. [DOI] [PubMed] [Google Scholar]