In this issue of Biological Psychiatry, Grothe et al. (1) report that the atrophy of basal forebrain cholinergic nuclei associated with aging, mild cognitive impairment (MCI), and Alzheimer’s disease (AD) can be visualized in vivo with innovative imaging methods. There are plenty of reasons why such investigations deserve to be pursued. It was the discovery of the cholinergic lesion in the 1970s that shifted the focus of research in AD from descriptive morphology to systems neuroscience. It is now well known that the cerebral cortex receives a massive cholinergic innervation, that this innervation arises from the nucleus basalis of Meynert (Ch4), that it is highly vulnerable to progressive degeneration in AD, and that the discovery of this vulnerability has introduced the first rational and effective treatment for AD. However, it is equally common knowledge that the cholinergic lesion of AD does not arise in isolation, that its exact contribution to the cognitive impairment remains elusive, and that cholinergic therapies cannot reverse or slow disease progression (2).
The vulnerability of cortical cholinergic innervation in AD reflects the anatomy rather than neurochemistry of this pathway. Cortical cholinergic axons arise from the Ch4 nucleus, a component of the basolateral cortical strip. In addition to Ch4, this strip includes the pyriform cortex, amygdala, hippocampus, and entorhinal cortex. This strip is the site at which intraneuronal neurofibrillary tangles first emerge during normal aging and at which they reach their highest concentrations in MCI and AD (3). Neurofibrillary tangles eventually lead to the death of the Ch4 neurons within which they form, giving rise to the atrophy that can now be visualized in vivo as reported by Grothe et al. (1). The neurofibrillary degeneration within cell bodies of Ch4 eventually triggers a loss of cholinergic axons directed to the cerebral cortex and of their presynaptic cholinergic terminals within the cerebral cortex (Figure 1). Cholinergic pathways arising outside of this basotemporal strip, such as the reticulothalamic and intrastriatal, are spared in AD and show no major age-related changes. The special vulnerability to neurofibrillary degeneration in AD is thus focused on the basotemporal strip rather than on cholinergic neurotransmission. By the time the neurofibrillary tangles have become established within the cholinergic Ch4 neurons, they have also invaded the noncholinergic neurons of the amygdalo-hippocampo-entorhinal complex, explaining why even optimal cholinergic therapies can be at best only partially effective.
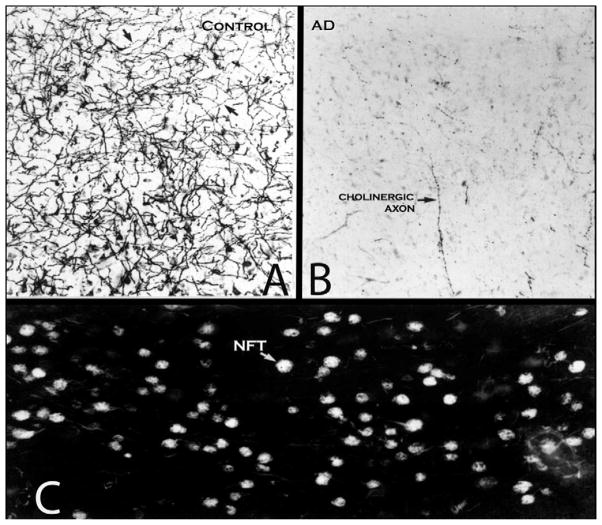
Figure 1.
Loss of cortical cholinergic innervation in Alzheimer’s disease (AD). (A) Acetylcholinesterase histochemistry was used to stain cholinergic axons (arrows) in the middle temporal gyrus. The autopsy specimen was from a woman who died at the age of 89 years with no dementia. The density of cholinergic fibers is high despite old age. In younger adults an approximately 20% higher density would be expected. (B) Same staining procedure and same part of the brain in a woman who died at the age of 84 years with a severe dementia and the neuropathology of AD. The cortex of the middle temporal gyrus has lost almost all of its cholinergic axons. Only a few isolated axons (arrow) remain. (C) Same subject as in B. The intermediate sector of Ch4 has been stained with thioflavin S, which binds to the neurofibrillary tangles (NFT). This is the part of Ch4 that is the most likely source of projections to the middle temporal gyrus. The majority of nucleus basalis neurons contained tangles (arrow). The data in B and C collectively support the conclusion that neurofibrillary degeneration in Ch4 leads to the destruction of the axons traveling from this nucleus to the cortical surface. The role of amyloid in this process remains uncertain. Magnification in all three photomicrographs is ×100. Ch, nucleus basalis of Meynert.
Those of us who have studied the neuroanatomy of this pathway have marveled at the dense thicket of cholinergic axons and varicosities that reach every part of the cortical surface. The density of these axons is much higher than the density of dopaminergic, serotonergic, or noradrenergic afferents of the cerebral cortex. Cortical cholinergic innervation, especially at muscarinic m1 receptors, is said to have a modulatory influence on cholinoceptive neurons. This effect, mediated through a prolonged inhibition of potassium conductance, has the net effect of enhancing the impact of other excitatory inputs that impinge on the cholinoceptive neuron. As carriers of modulatory signals, cortical cholinergic pathways do not encode the content of experiences but may instead influence their relative attentional salience, motivational valence, and memorability. The anatomical details fit this pattern of functionality. Axonally transported tracers in the primate laboratory have shown that although Ch4 neurons innervate every corner of the cerebral cortex, they receive major cortical inputs only from limbic and paralimbic areas (4). The Ch4 nucleus is therefore positioned to function as a cholinergic amplifier that can influence the function of the entire cortical surface according to the prevailing state of the limbic system. Neurodegeneration of even a small number of Ch4 neurons can thus disrupt synaptic activity throughout the cortical surface and cause widespread perturbations of attentional modulation, motivational activation, and efficiency of memory function.
The physiologic action of acetylcholine at muscarinic and nicotinic receptors is relatively well understood. A less widely appreciated effect of acetylcholine is related to its influence on structural neuroplasticity. In one experiment on rats, all whiskers except those connected with the D2 and D3 barrels of somatosensory cortex were trimmed. This intervention altered the associative linkage between D2 and D3 so that the D2 neurons started to show a greater coupling with those of D3 than with those of the adjacent D1, whose whisker had been trimmed. This altered pairing, indicative of experience-induced synaptic plasticity, could not be obtained in rats with selective immunotoxic lesions of Ch4 neurons (5). In another experiment, pairing auditory stimuli with the electrical stimulation of the Ch4 in adult rats caused a long-lasting reorganization of primary auditory cortex so that the area optimally responsive to the paired tone expanded substantially. This plasticity was not observed following the selective immunotoxic destruction of the cholinergic Ch4 neurons (6). In an ex vivo experiment, brief muscarinic activation caused the emergence of fine filopodia from spine heads of hippocampal neurons, an effect that was inhibited by the cholinergic blocker atropine (7). It appears, therefore, that cortical cholinergic denervation can undermine not only the physiological integrity of synaptic transmission but also the capacity for structural neuroplasticity. A loss of this capacity would be expected to interfere with the acquisition of new knowledge and also with the adaptive response to attrition and injury.
Postmortem examinations show that the degeneration of Ch4 and the resultant cortical cholinergic loss of AD are both profound. Aging and MCI are associated with lesser changes (3). In AD, the major agent of Ch4 pathology and atrophy is the neurofibrillary tangle rather than amyloid deposition. In physiologic aging, neurofibrillary degeneration is common but much more subtle. The age-related Ch4 atrophy detected by Grothe et al. (1) may therefore reflect the presence of additional involutional factors. In aged mice, for example, Ch4 neurons undergo a shrinkage at a time when no similar atrophy can be detected in adjacent cholinergic neurons of the striatum (8). Because mice are not prone to neurofibrillary degeneration, this finding shows that the aging Ch4 is vulnerable to alternative mechanisms of involution. The neurons of Ch4 happen to be dependent for survival on the retrograde transport of nerve growth factor produced in the cerebral cortex. Conceivably, deficient cortical production or retrograde transport of nerve growth factor may be responsible for the age-related atrophy detected in the mouse. Whether such a nonneurofibrillary involution of Ch4 also exists in the aging human brain and the extent to which it affects cortical cholinergic neurotransmission remain to be determined (9). A selective depletion of the calcium-binding protein calbindin has been proposed as an alternative nonneurofibrillary mechanism of age-related involution in Ch4 (10). The resultant perturbation of calcium binding capacity could increase the vulnerability of these neurons to factors that promote atrophy and neurofibrillary degeneration along the age-MCI-AD continuum.
The availability of a quantitative method for the in vivo imaging of Ch4, as reported by Grothe et al. (1), is likely to revive interest in the investigation of cholinergic pathways. Questions that previously required laborious postmortem evaluations can now be approached in vivo in ways that would also allow real-time correlations with cognitive function. In the future, structural imaging of Ch4 could be combined with the imaging of synaptic cholinergic markers in the cerebral cortex. Such investigations would yield critical new information on the functional properties of this unique pathway and further clarify its role in age-related cognitive changes and the pathophysiology of AD.
Acknowledgments
Dr. Mesulam receives grants from the National Institutes of Health, is on several editorial boards of professional journals, and lectures at many universities. Dr. Mesulam also reports support by National Institute on Aging Alzheimer’s Disease Center Grant AG13854.
Footnotes
The author reports no other biomedical financial interests or potential conflicts of interest.
References
- 1.Grothe M, Heinsen H, Teipel SJ. Atrophy of the cholinergic basal forebrain over the adult age range and in early stages of Alzheimer’s disease. Biol Psychiatry. 2012;71:805– 813. doi: 10.1016/j.biopsych.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mesulam M-M. The cholinergic lesion of Alzheimer’s disease: pivotal factor or side show? Learning Memory. 2004;11:43– 49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- 3.Mesulam M-M, Shaw P, Mash D, Weintraub S. Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol. 2004;55:815– 828. doi: 10.1002/ana.20100. [DOI] [PubMed] [Google Scholar]
- 4.Mesulam M-M, Mufson EJ. Neural inputs into the nucleus basalis of the substantia innominata (Ch4) in the rhesus monkey. Brain. 1984;107:253–274. doi: 10.1093/brain/107.1.253. [DOI] [PubMed] [Google Scholar]
- 5.Baskerville KA, Schweitzer JB, Herron P. Effects of cholinergic depletion on experience-dependent plasticity in the cortex of the rat. Neuroscience. 1997;80:1159–1169. doi: 10.1016/s0306-4522(97)00064-x. [DOI] [PubMed] [Google Scholar]
- 6.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 7.Schätzle P, Ster J, Verbich D, McKinney RA, Gerber U, Sonderegger P, et al. Rapid and reversible formation of spine head filopodia in response to muscarinic receptor activation in CA1. J Physiol. 2011;589:4353–4364. doi: 10.1113/jphysiol.2010.204446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesulam MM, Mufson EJ, Rogers J. Age-related shrinkage of cortically projecting cholinergic neurons: a selective effect. Ann Neurol. 1987;22:31–36. doi: 10.1002/ana.410220109. [DOI] [PubMed] [Google Scholar]
- 9.Geula C, Mesulam MM. Cholinergic systems in Alzheimer’s disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer Disease. 2. Philadelphia: Lippincott, Williams & Wilkins; 1999. pp. 269–292. [Google Scholar]
- 10.Geula C, Bu J, Nagykery N, Scinto LFM, Chan J, Joseph J, et al. Loss of calbindin-D28K from aging human cholinergic basal forebrain relation to neuronal loss. J Comp Neurol. 2003;455:249–259. doi: 10.1002/cne.10475. [DOI] [PubMed] [Google Scholar]