Abstract
Methamphetamine is thought to produce its behavioral effects by releasing dopamine (DA), serotonin (5-HT) and norepinephrine. Results from animal studies support this notion, while results from human laboratory studies have not consistently demonstrated the importance of monoamine systems in the behavioral effects of methamphetamine. Human laboratory procedures of drug-discrimination are well suited to assess neuropharmacological mechanisms of the training drug by studying pharmacological manipulation. In this human laboratory study, six participants with a history of recreational stimulant use learned to discriminate 10 mg oral methamphetamine. After acquiring the discrimination (i.e., ≥80% correct responding on 4 consecutive sessions), the effects of a range of doses of methamphetamine (0, 2.5, 5, 10 and 15 mg), alone and in combination with 0 and 20 mg aripiprazole (a partial agonist at D2 and 5-HT1A receptors), were assessed. Methamphetamine alone functioned as a discriminative stimulus, produced prototypical stimulant-like subject-rated drug effects (e.g., increased ratings of Good Effects, Talkative-Friendly, and Willing to Pay For) and elevated cardiovascular indices. These effects were generally a function of dose. Aripiprazole alone did not occasion methamphetamine-appropriate responding or produce subject-rated effects, but modestly impaired performance. Administration of aripiprazole significantly attenuated the discriminative-stimulus and cardiovascular effects of methamphetamine, as well as some of the subject-rated drug effects. These results indicate that monoamine systems likely play a role in the behavioral effects of methamphetamine in humans. Moreover, given the concordance between past results with d-amphetamine and the present findings, d-amphetamine can likely serve as a model for the pharmacological effects of methamphetamine.
Keywords: methamphetamine, aripiprazole, drug-discrimination, stimulant abuse
INTRODUCTION
Methamphetamine abuse is a major public health concern. In 2009, 502,000 individuals aged 12 or older had used methamphetamine in the past month.1 Total costs for amphetamine abuse in the US were estimated at $23.4 billion in 2005, including premature death, crime, lost productivity, and medical conditions, such as cardiovascular insults, cognitive dysfunction and infectious disease.2,3,4 These epidemiological, economical and clinical findings highlight the need for a better understanding of the mechanisms contributing to the abuse of methamphetamine in humans.
The abuse-related behavioral effects of methamphetamine are mediated, at least in part, by promoting the release of biogenic amines (e.g., dopamine [DA] and serotonin [5-HT]) to the synaptic cleft. Indeed, several in vitro and in vivo studies have demonstrated that monoaminergic neurotransmission underlies the behavioral effects of amphetamines. For example, a seminal preclinical study showed that dose-dependent enhancements in synaptic levels of DA and 5-HT were directly related to the behavioral responses to amphetamine.5 In agreement with this finding, several preclinical drug-discrimination studies have implicated both central DA and 5-HT systems in mediating the behavioral effects of methamphetamine.6,7,8 In one study, for example, 10 squirrel monkeys were trained to discriminate methamphetamine (0.3 mg/kg) from saline.9 A D2 receptor agonist dose-dependently increased methamphetamine-appropriate responding, whereas pretreatment with remoxipride, a D2 antagonist, attenuated the discriminative-stimulus effect of methamphetamine. The results of two other studies suggest that 5-HT release also contributes to the discriminative-stimulus effects of methamphetamine.7,10 Together, results from animal studies indicate that DA and 5-HT mechanisms contribute to the discriminative stimulus effects of methamphetamine. Whether these findings generalize to humans is unknown.
Several human laboratory studies have evaluated the involvement of monoamine neurotransmission in the behavioral effects of amphetamines using subjective drug-effect questionnaires.11,12,13 In these studies, participants received a range of doses of amphetamine alone and following pretreatment with a DA antagonist. Inferences regarding the neuropharmacological mechanisms mediating the effects of amphetamine were made depending on the pretreatment drugs that alter the subjective drug effects. For example, in a series of previous studies the subjective effects of d-amphetamine (10-20 mg) were assessed following pretreatment with the DA antagonists pimozide (1-8 mg) and fluphenazine (3-6 mg).11,12,13 d-Amphetamine produced prototypical positive subject-rated effects (e.g., Good Effects, Like Drug), and the DA antagonists did not modify these effects of d-amphetamine. In a subsequent study, the subject-rated effects of methamphetamine (0 or 20 mg) were assessed following pretreatment with haloperidol (0 or 3 mg), a D2 antagonist, or risperidone (0 or 0.75 mg), an atypical antipsychotic that is a mixed DA/5-HT antagonist.14 Neither haloperidol nor risperidone significantly altered the stimulant-like subject-rated effects of methamphetamine in this study. Together, the human laboratory studies that used only subjective drug-effect questionnaires to assess neuropharmacological mechanisms of amphetamines have not convincingly demonstrated the involvement of brain monoamine systems in mediating the behavioral effects of amphetamines.
The extant literature, however, suggests that the concomitant use of a drug-discrimination procedure and subject-rated questionnaires produce results that are consistent with the notion that central monoamine systems, namely DA and 5-HT, mediate the behavioral effects of amphetamines in humans.15,16 For example, in a previous study conducted in our laboratory, risperidone, an atypical antipsychotic with antagonist actions at D2 and 5-HT2 receptors, significantly attenuated the discriminative stimulus and some positive subject-rated effects of d-amphetamine, suggesting contribution of DA and 5-HT transmission to the behavioral effects of d-amphetamine in humans.17 In addition, another study from our laboratory showed that 20 mg aripiprazole, an atypical antipsychotic with partial agonist actions at D2 and 5-HT1A receptors, attenuated the discriminative stimulus and positive subject-rated effects of d-amphetamine.18 These studies highlight the utility of drug-discrimination and subject-rated measures in delineating neuropharmacological mechanisms of d-amphetamine in humans. However, no study thus far has concomitantly used drug-discrimination and subject-rated effects measures to determine the roles of DA and 5-HT receptors in mediating the behavioral effects of methamphetamine.
Worth noting is that although behavioral and pharmacological effects of d-amphetamine and methamphetamine overlap extensively19,20, there are meaningful neuropharmacological differences between various amphetamine analogues. For example, d-amphetamine and methamphetamine both increase DA levels in rat caudate to a comparable degree, whereas 5-HT levels are significantly higher following the administration of methamphetamine.21 It is likely, then, that the relative contribution of 5-HT1A/2A receptors would differ in mediating discriminative stimulus effects of d-amphetamine and methamphetamine, and that drugs acting on 5-HT1A/2A receptors (e.g., aripiprazole) could differentially modify the discriminative-stimulus effects of d-amphetamine and methamphetamine. Moreover, these and other neuropharmacological differences between these two drugs could potentially underlie the differences in abuse of these drugs. Indeed, clinical studies indicate that methamphetamine is perceived to pose a greater risk of abuse and dependence than does d-amphetamine.22 The present study, therefore, was conducted to determine whether aripiprazole, the drug that attenuates the discriminative stimulus effects of d-amphetamine18, also attenuates the discriminative-stimulus effects of methamphetamine in humans.
The present study first replicated the previous findings by determining whether methamphetamine functions as a discriminative stimulus, and then assessed pharmacological modification of these effects by aripiprazole. To more fully characterize the effects of these drugs and drug combinations, a battery of self-reported drug-effect questionnaires, a performance task and physiological indices were also included. We hypothesized that, when administered concurrently, 20 mg aripiprazole would act as an antagonist and result in rightward shifts in the methamphetamine dose-effect curves.
MATERIALS AND METHODS
Participants
Twelve healthy adult humans were recruited from the local community via newspaper advertisements, flyers and word of mouth to participate in this experiment. Four of these participants were discharged from the study because they were unable to acquire the discrimination, one was discharged due to having vital signs exceeding our safety limits following 10 mg methamphetamine administration in the sampling phase and one was lost to follow-up. Data from these participants were not included in the analyses. Six (3 males, 3 females; 5 White, 1 Hispanic) participants completed this experiment.
The participants earned $40 for completing each laboratory session. The participants received this $40 at the end of each session they completed. Participants who finished all of their scheduled sessions were also paid a completion allowance of up to $40 per session based on their performance on the point-distribution task. Thus, a total amount the participants earned per session was up to $80. The completion allowance was given to the participants when they finished the entire experiment.
Participants ranged in age from 19-24 years (mean = 22), in education from 13-16 years (mean = 14) and in weight from 54-77 kg (mean = 65). All participants reported recreational stimulant use within the past year, but they did not meet DSM-IV criteria for stimulant dependence. Four participants reported recreational amphetamine (Adderall®) use, one reported recreational methylphenidate (Concerta®) use and one reported recreational amphetamine (Adderall®) and cocaine use in the year prior to screening.
Prior to enrollment, all potential participants completed standard comprehensive medical, physical and psychological screens, including routine clinical laboratory blood and urine chemistry tests as well as an electrocardiogram. Potential participants with histories of serious physical disease, current physical disease (e.g., impaired cardiovascular functioning, chronic obstructive pulmonary disease, etc.), seizure, head trauma or CNS tumors, or current or past histories of serious psychiatric disorder (i.e., Axis I, DSM-IV), including ADHD and substance dependence disorders, were excluded from participation. All participants were in good health with no contraindications to stimulant or antipsychotic medications. Female participants had to report using an effective form of birth control in order to participate and must not have been pregnant. Female participants were also screened for pregnancy (urine HCG; Mainline Technology, Ann Arbor, MI) prior to each session to ensure that they did not continue in the study if pregnant. None of the female participants tested positive for pregnancy throughout the experimental protocol. The Institutional Review Board of the University of Kentucky Chandler Medical Center approved this study and the informed consent document. Participants signed the informed consent after passing appropriate sobriety tests and prior to enrolling in the study.
General Procedures
Participants were enrolled as outpatients at the Laboratory of Human Behavioral Pharmacology at the University of Kentucky Chandler Medical Center. Participants completed one “practice” session to familiarize them with the behavioral measures and daily laboratory routine. Experimental drugs were not administered on this day. Participants then completed 22-30 (mean=26) experimental sessions.
Participants were informed that during their participation they would receive various drugs and that these could include placebo, methamphetamine and aripiprazole. Other than this general information, participants were blind to the type of drug administered. Participants were told that the purpose of the study was to see how different drugs affect mood and behavior and whether people are able to detect the presence of a drug. Participants were given no instruction of what they were “supposed” to do or of what outcomes might be expected. Participants were asked to abstain from any illicit drug use for the duration of the experiment. In addition, participants were also asked not to ingest food or caffeine for 4 hours prior to each experimental session, and alcohol for 12 hours prior to and following each experimental session.
Experimental sessions were generally conducted daily Monday through Friday. The time of day at which each session began ranged from 8:00 to 10:00 AM, but was generally held constant for individual participants. On experimental session days, participants followed a daily routine. Each experimental session day participants were first provided a light breakfast. Participants then provided an expired air sample, which was assayed for the presence of alcohol using an Alco-Sensor breathalyzer (Intoximeters, St. Louis, MO). Participants also underwent a field sobriety test. Participants had to provide a breath sample negative for alcohol and pass the field sobriety test to continue with the scheduled experimental session. At the beginning of each session, participants provided a urine sample that was screened for the presence of amphetamines, barbiturates, benzodiazepines, cocaine, opioids and THC. If a urine sample was positive for any drug, other than THC or compounds administered experimentally, the session was canceled until the participant provided a drug-free urine sample. No participants tested positive for the presence of drugs other than those administered experimentally or THC throughout the experimental protocol. Participants who reported the use of tobacco were permitted to smoke one tobacco cigarette midway through the experimental session. Participants were not able to smoke again until the experimental testing was completed.
On experimental session days, participants completed the drug-effect questionnaires and a performance task approximately 30 min before drug administration and then completed the drug-discrimination, drug-effect questionnaires and performance task 1, 2, 3, 4 and 5 hr after drug administration.
Drug-Discrimination Measure
This experiment consisted of three phases, which were completed in a fixed order: 1) sampling phase, 2) acquisition phase and 3) test phase.
Sampling Phase
All participants completed two sampling sessions to acquaint them with the drug effects. During each sampling session, participants ingested three capsules that contained a total of 10 mg methamphetamine. Methamphetamine was identified by letter code (e.g., Drug A), but the participants were not explicitly informed of the contents of the capsules. Methamphetamine (10 mg) is identified as Drug A here for illustrative purposes only. A unique letter code was used for each participant. Participants read the following set of instructions prior to receiving the capsules and a research assistant also read them aloud.
Instructions (Sampling Sessions). This is Drug A. When you think you received Drug A, and in fact you did receive Drug A, you can earn extra money by responding on the button labeled Drug A. During this session you should pay close attention to how Drug A makes you feel, because in the future we will not tell you if you received Drug A. Instead, you will have to decide whether or not you received Drug A. In these future sessions, if you think you received Drug A, and in fact you did receive Drug A, you can earn extra money by responding on the button labeled Drug A.
Whenever you do not think you received Drug A, and in fact you did not receive Drug A, you can earn extra money by responding on the button labeled Not Drug A.
Acquisition Phase
After the sampling phase, an acquisition phase was conducted to determine whether participants could discriminate 10 mg methamphetamine. During this phase, participants ingested capsules under double-blind conditions, but they were not told whether the capsules contained 10 mg methamphetamine (e.g., Drug A) or placebo (e.g., Not Drug A). Participants were not explicitly instructed that they would be attempting to acquire a drug versus placebo discrimination. Participants read the following set of instructions prior to receiving the capsules and a research assistant also read them aloud.
Instructions (Acquisition and Test Phases). Today we will not tell you whether you received Drug A or Not Drug A. Instead, you will have to decide whether you received Drug A or Not Drug A. If you think you received Drug A, and in fact you did receive Drug A, you can earn extra money by responding on the button labeled Drug A. If you do not think you received Drug A, and in fact you did not receive Drug A, you can earn extra money by responding on the button labeled Not Drug A. For example, if you feel that you did not receive any drug today, you should respond on the button labeled Not Drug A. Similarly, if you think that you received a drug, but it feels different than Drug A, you should respond on the button labeled Not Drug A. You can change your drug identifications throughout today’s session based on what you think at the time.
At the end of today’s session, you will be given an envelope that will tell you if you received Drug A or Not Drug A. The number of points that you accumulated on the correct button will then be converted to money and you will be told how much bonus money you earned during today’s session. At the end of some sessions, we may not be able to tell you whether you received Drug A or Not Drug A. On the days that we cannot tell whether you received Drug A or Not Drug A, your bonus earnings will be the greatest amount of money that you earned on either the Drug A or the Not Drug A button.
At the end of each experimental session, participants opened a sealed envelope that informed them and the research assistant of the identity of the drug administered (e.g., Drug A or Not Drug A). The points that participants allocated to the correct option were converted to bonus money at the rate of $0.08/point, which they received at the end of the entire experiment. Participants were considered to have acquired the discrimination if they allocated 80% or more points to the correct option on the drug-discrimination task across four consecutive sessions. Participants were discharged if they did not meet the discrimination criterion within 12 sessions. The order of drug administration during the acquisition phase was random except that each participant received each training condition, 10 mg methamphetamine and placebo, at least twice.
Test Phase
After successfully completing the acquisition phase, participants entered a test phase. The test phase consisted of test days interspersed with acquisition sessions. The test sessions were identical to the acquisition sessions except that participants did not receive any feedback concerning their drug-discrimination performance. On the test sessions, participants earned the bonus money allocated to the Drug A or Not Drug A option, whichever was greater. Participants were not told the purpose of these test sessions, nor did they know when they were scheduled until after they opened the sealed envelope.
Additional acquisition sessions were interspersed among the test days to ensure participants continued to accurately discriminate 10 mg methamphetamine. These acquisition sessions were identical to those in the acquisition phase (i.e., 10 mg methamphetamine or placebo). If a participant responded incorrectly on an acquisition session (i.e., < 80% correct), additional acquisition sessions were scheduled. These additional acquisition sessions continued until the participant correctly identified both of the training conditions once each.
Ten methamphetamine-aripiprazole conditions were studied during the test phase: (1) 0 mg methamphetamine plus 0 mg aripiprazole; (2) 2.5 mg methamphetamine plus 0 mg aripiprazole; (3) 5 mg methamphetamine plus 0 mg aripiprazole; (4) 10 mg methamphetamine plus 0 mg aripiprazole; 5) 15 mg methamphetamine plus 0 mg aripiprazole; (6) 0 mg methamphetamine plus 20 mg aripiprazole; (7) 2.5 methamphetamine plus 20 mg aripiprazole; 8) 5 mg methamphetamine plus 20 mg aripiprazole; (9) 10 mg methamphetamine plus 20 mg aripiprazole; and (10) 15 mg methamphetamine plus 20 mg aripiprazole. The order of drug administration during this phase of the experiment was random, except that an active drug dose was never administered on more than three consecutive sessions.
Drug-Discrimination Task
A point-distribution drug-discrimination task was completed 1, 2, 3, 4 and 5 hours after oral drug administration on an Apple Macintosh computer (Apple Computer, Inc., Cupertino, CA). In this procedure, participants were required to distribute 100 points between two options (i.e., Drug A or Not Drug A).17 During sampling and acquisition sessions, points accumulated on the correct option were exchangeable for money at a rate of $0.08/point. During test sessions, participants were credited with the greater number of points allocated to the Drug A or Not Drug A option, which were exchangeable at the same rate. Thus, participants were able to earn a maximum of $40/session on this task. The dependent measure in this procedure was percent methamphetamine-appropriate responding.
Self-Report Questionnaires, Performance Task, Cardiovascular Measures
Three self-reported drug-effect questionnaires and a performance task were administered on an Apple Macintosh computer and were completed in fixed order. These questionnaires were completed approximately 30 min before drug administration, and 1, 2, 3, 4, and 5 h after drug administration.
Addiction Research Center Inventory (ARCI)
The short form of the ARCI consisted of 49 true/false questions and contained five major subscales: the morphine-benzedrine group (MBG; a measure of euphoria), the pentobarbital, chlorpromazine, alcohol group (PCAG; a measure of sedation), the lysergic acid diethylamide (LSD; a measure of dysphoria), and the benzedrine group and amphetamine scales (BG and A, respectively; Stimulant-Sensitive Scales).19,23
Adjective Rating Scale
The Adjective-Rating Scale consisted of 32 items and contained two subscales: Sedative and Stimulant. These subscales are sensitive to the acute effects of orally administered sedative and stimulant drugs.24 Participants rated each item using the computer mouse to point to and select among one of five response options: Not at All, A Little Bit, Moderately, Quite a Bit, and Extremely (scored numerically from 0 to 4, respectively).
Visual-Analog Scale Drug-Effect Questionnaire
The Drug-Effect Questionnaire consisted of 20 items. This questionnaire is sensitive to the acute effects of orally administered stimulants.17,25 In all, 20 items were presented on the video screen, one at a time. Participants rated each adjective on a 0-100 scale. The individual items are reported in our previous study.17
Digit-Symbol-Substitution Test (DSST)
A computerized version of the DSST, which has been described previously, was used in this experiment.26 This measure is sensitive to the effects of orally administered sedative and stimulant drugs.17 Briefly, participants used a numeric keypad to enter a geometric pattern associated with one of nine digits displayed on a video screen. Participants had 90 s to enter as many geometric patterns as possible. The dependent measure was the number of patterns the participant completed (i.e., trials completed) and the number of patterns the participant entered correctly (i.e., trials correct).
Heart rate and blood pressure
Heart rate and blood pressure were recorded using an automated blood-pressure monitor (Spot Vital Signs LXi, Welch Allyn, Skaneateles Falls, NY). Heart rate and blood pressure were monitored for approximately 30 min before drug administration and at hourly intervals for 5 h afterwards. Heart rate and blood pressure were recorded immediately before participants completed the drug-discrimination, self-reported drug-effect questionnaires, and performance task.
Drug Administration
All drug conditions were administered in a double-blind fashion under medical supervision. Commercially available tablets (2.5 or 5 mg) were encapsulated in a size 0 capsule to prepare the methamphetamine doses (Desoxyn®, Abbott Laboratories, Chicago, IL). Commercially available tablets (20 mg) were encapsulated in a size 0 capsule to prepare the aripiprazole doses (Abilify®, Bristol Myers Squibb, New York, NY). Cornstarch was used to fill the remainder of all the capsules. Placebo capsules contained only cornstarch. Methamphetamine, aripiprazole and placebo capsules were identical in appearance. The University of Kentucky Chandler Medical Center Investigational Pharmacy prepared the capsules.
During each experimental session, participants ingested four capsules (i.e., three methamphetamine- or placebo-containing capsules, and one aripiprazole or placebo-containing capsule). Administering the appropriate number of drug- or placebo-containing capsules varied the dose. Capsules were taken orally with water. Drug administration procedures were designed to ensure that participants swallowed the capsules and did not open them in their mouths to taste the contents.27 Drug administration sessions were separated at least by 24 hours. Generally, participants completed 3-4 sessions per week. An active drug dose was never administered on more than 3 consecutive days.
Methamphetamine and aripiprazole doses were chosen based on the results from previous human behavioral pharmacology research.18,20,28 The behavioral effects of methamphetamine peak approximately 2-3 h after oral administration.20 Peak aripiprazole plasma concentrations occur approximately 3-4 h after oral administration.29 Based on these pharmacokinetic data, methamphetamine and aripiprazole were administered simultaneously to assess behavioral effects across peak effects of both drugs.
References below to placebo pertain to sessions in which placebo doses of both methamphetamine (i.e., 0 mg) and aripiprazole (i.e., 0 mg) were administered. References to methamphetamine alone pertain to sessions in which an active dose of methamphetamine was administered in combination with 0 mg aripiprazole. References to aripiprazole alone pertain to sessions in which the active dose of aripiprazole was administered in combination with 0 mg methamphetamine.
Data Analysis
Statistical analyses of group data were conducted to examine drug effects on the drug-discrimination task, self-reported drug-effect questionnaires, DSST, heart rate and blood pressure. Data were analyzed statistically as raw scores. For all measures, effects were considered significant at p ≤ 0.05.
For the 10 mg methamphetamine alone and placebo conditions, data were averaged across the four sessions of the acquisition phase in which the participant met the discrimination criterion as well as all exposures to these conditions in the test phase. Drug-discrimination, subject-rated, performance and cardiovascular data were analyzed statistically as area-under-the-time-action curve (AUC), which was calculated for each of the participants using the trapezoidal method. Data were analyzed by two-factor repeated-measure analysis of variance (ANOVA) with methamphetamine (0, 2.5, 5, 10, and 15 mg) and aripiprazole (0 and 20 mg) as factors (StatView 5.0.1, SAS Institute Inc., Cary, NC). Post-hoc tests were conducted if a significant effect of methamphetamine or aripiprazole, or an interaction of these two factors, was detected. Post-hoc tests were first conducted to compare each of the nine active drug conditions with placebo. Next, if a dose of methamphetamine alone increased responding significantly above placebo, post-hoc tests were conducted to compare the effects of these doses of methamphetamine alone and in combination with 20 mg aripiprazole.
RESULTS
Drug Discrimination
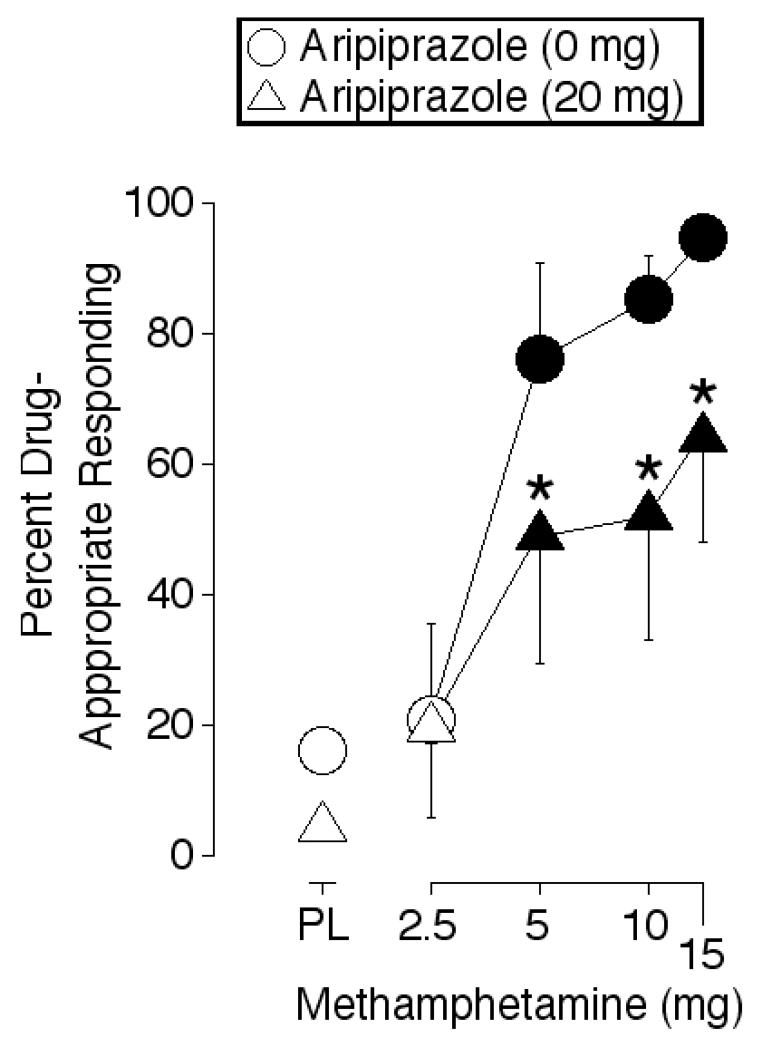
The six participants met the discrimination criterion in an average of 5.7 sessions (range = 4-9). ANOVA revealed a significant main effect of methamphetamine and aripiprazole (Table 1). Methamphetamine (5, 10 and 15 mg) increased drug-appropriate responding above placebo levels, alone and following pretreatment with 20 mg aripiprazole (Figure 1). Percent drug-appropriate responding was significantly lower after the administration of these methamphetamine doses in combination with 20 mg aripiprazole relative to when these methamphetamine doses were administered alone (Table 1).
Table 1. Summary of F statistics and Mean Values.
| F statistics |
Means |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aripiprazole | Methamphetamine | Methamphetamine × Aripiprazole |
Aripipriazole (0 mg) and Methamphetamine |
Aripipriazole (20 mg) and Methamphetamine |
|||||||||
| Measure | (df1,5) | (df4,20) | (df4,20) | 0 mg | 2.5 mg | 5 mg | 10 mg | 15 mg | 0 mg | 2.5 mg | 5 mg | 10 mg | 15 mg |
| Discrimination Performance | |||||||||||||
| Percent Drug-Appropriate Responiding | 12.5 | 10.5 | 0.8 | 16.1 | 20.8 | 76.0 | 85.3 | 94.8 | 5.2 | 20.6 | 50.0* | 53.1* | 65.0* |
| Inventory | |||||||||||||
| A | 8.4 | 2.6 | 0.9 | 1.3 | 1.9 | 2.4 | 3.2 | 3.8 | 0.8 | 1.1 | 1.3* | 3.1 | 2.4* |
| BG | 21.3 | 2.0 | 0.9 | 4.9 | 5.1 | 5.9 | 6.6 | 7.0 | 4.5 | 4.0 | 4.9* | 6.5 | 6.1* |
| Adjective-Rating Scale | |||||||||||||
| Stimulant Scale | 11.2 | 4.6 | 3.1 | 3.3 | 4.3 | 5.8 | 8.8 | 12.4 | 3.1 | 3.3 | 4.3 | 7.2 | 7.4* |
| Sedative Scale | 18.5 | 1.9 | 0.8 | 3.6 | 4.2 | 1.3 | 1.2 | 0.8 | 4.0 | 5.0 | 3.0* | 3.1* | 2.0* |
| Drug-Effect Questionnaire | |||||||||||||
| High | 3.4 | 3.2 | 3.7 | 0.9 | 0.5 | 2.5 | 3.3 | 5.2 | 1.0 | 0.9 | 2.0 | 1.1* | 1.5* |
| Irregular-Racing Heartbeat | 4.2 | 2.9 | 3.5 | 1.1 | 0.8 | 3.3 | 7.9 | 8.8 | 0.8 | 1.7 | 3.1 | 2.0* | 6.9 |
| Talkative-Friendly | 8.8 | 3.3 | 1.9 | 1.0 | 2.2 | 10.1 | 16.0 | 22.6 | 0.9 | 1.3 | 2.6* | 10.9* | 12.8* |
| Any Effect | 3.7 | 4.2 | 0.2 | 8.8 | 12.5 | 19.5 | 26.2 | 33.0 | 2.8 | 8.1 | 10.6 | 20.3 | 20.8* |
| Good Effects | 5.7 | 3.5 | 0.6 | 2.1 | 6.5 | 17.7 | 23.0 | 30.5 | 1.1 | 2.3 | 5.9* | 19.3 | 19.0* |
| Willing to Pay For | 3.7 | 2.9 | 0.9 | 9.8 | 9.5 | 25.6 | 27.1 | 34.0 | 1.9 | 9.5 | 4.1* | 23.3 | 23.9 |
| Restless | 0.0 | 3.2 | 1.2 | 1.0 | 2.3 | 4.0 | 10.2 | 16.7 | 3.5 | 5.4 | 4.3 | 13.1 | 6.8* |
| Rush | 3.9 | 2.9 | 1.8 | 1.0 | 3.9 | 9.4 | 8.8 | 14.2 | 2.5 | 1.9 | 3.4* | 11.4 | 6.8* |
| Active-Alert-Energetic | 11.8 | 2.5 | 0.8 | 1.9 | 3.0 | 14.8 | 19.7 | 25.9 | 1.0 | 1.9 | 3.6* | 18.3 | 16.6* |
| Digit-Symbol-Substitution-Task | |||||||||||||
| Number of Trials Attempted | 9.2 | 6.6 | 0.6 | 68.8 | 69.1 | 71.5 | 71.9 | 73.3 | 64.7* | 67.4 | 67.8* | 70.8 | 70.1* |
| Number of Trials Correct | 21.0 | 8.6 | 0.8 | 66.2 | 66.3 | 68.6 | 69.3 | 70.2 | 60.3* | 63.8 | 64.4* | 66.9 | 67.9 |
| Cardiovascular Measures | |||||||||||||
| Systolic Pressure | 3.4 | 10.4 | 2.1 | 114.3 | 115.4 | 121.8 | 124.1 | 127.0 | 117.7 | 116.4 | 117.0* | 120.4* | 124.7 |
| Diastolic Pressure | 8.3 | 5.9 | 2.5 | 70.0 | 71.5 | 73.1 | 74.7 | 77.7 | 71.9 | 71.5 | 70.8* | 71.3* | 74.2* |
| Heart Rate | 3.0 | 2.8 | 4.0 | 72.5 | 72.1 | 77.9 | 78.4 | 83.5 | 78.6* | 80.4* | 80.3 | 78.2 | 78.8* |
Summary statistics are not included for a particular measure if the two-factor ANOVA failed to reveal a significant effect of Aripiprazole, Methamphetamine or interaction of Aripiprazole and Methamphetamine. Discrimination data were analyzed as the total percent of points allocated to the drug option across the five hour session. All other data were analyzed as area-under-the-time-action curve (AUC). A = Amphetamine; BG = Benzedrine Group. Bold and underlined F values are statistically significant (p ≤ .05). Bold mean values are significantly different from the corresponding placebo values (i.e., 0 mg methamphetamine). An asterisk indicates a significant difference between the values under 0 mg and 20 mg aripiprazole conditions at the indicated methamphetamine dose
Figure 1.
Percent drug-appropriate appropriate responding maintained by methamphetamine alone, aripiprazole alone, methamphetamine-aripiprazole combinations, and placebo. Data are expressed as area-under-the-time-action curve (AUC). X-axes: methamphetamine dose. Data points above PL represent values when the doses of aripiprazole were administered in combination with 0 mg methamphetamine. Data points above 2.5, 5, 10 and 15 represent the effects of the methamphetamine dose administered in combination with 0 mg (circles) or 20 mg (triangles) aripiprazole. Data points show means of six participants. Filled symbols indicate those values that are significantly different from the placebo-placebo condition (i.e., circle above PL). An asterisk indicates a significant difference between the 0 and 20 mg aripiprazole conditions at the indicated methamphetamine dose.
Addiction Research Center Inventory (ARCI)
ANOVA revealed a significant main effect of aripiprazole on scores on the A and BG scales of the ARCI (Table 1). Methamphetamine (5, 10 and 15 mg) alone increased scores on the A scale responding above placebo levels, while only the two higher doses did so following aripiprazole pretreatment (Figure 2). Scores on the A scale were significantly lower after the administration of 5 and 15 mg methamphetamine in combination with 20 mg aripiprazole relative to when these methamphetamine doses were administered alone. Methamphetamine (5, 10 and 15 mg) alone increased scores on the BG scale responding above placebo levels, while 10 and 15 mg methamphetamine did so following aripiprazole pretreatment (Table 1). Scores on the BG scale were significantly lower after the administration of 5 and 15 mg methamphetamine in combination with 20 mg aripiprazole relative to when these methamphetamine doses were administered alone (Table 1). The combined effects of 2.5 mg methamphetamine and 20 mg aripiprazole were significantly less than those observed with placebo.
Figure 2.
Effects of methamphetamine alone, aripiprazole alone, methamphetamine-aripiprazole combinations, and placebo on the A scale from the Addiction Research Center Inventory (ARCI) and the Stimulant scale from the Adjective Rating Scale. Data are expressed as area-under-the-time-action curve (AUC). All other details are as in Figure 1.
Adjective-Rating Scale
ANOVA revealed a significant interaction of methamphetamine and aripiprazole on scores on the Stimulant Scale of the Adjective-Rating Scale (Table 1). Methamphetamine (5, 10 and 15 mg) alone increased these scores significantly above placebo levels, whereas only the 10 and 15 mg dose did so when administered in combination with 20 mg aripiprazole (Figure 2). Scores on this scale were significantly lower after administration of 15 mg methamphetamine in combination with 20 mg aripiprazole relative to this dose of methamphetamine alone.
ANOVA revealed a significant main effect of aripiprazole on scores on the Sedative Scale of the Adjective-Rating Scale (Table 1). Methamphetamine (5, 10 and 15 mg) alone decreased these scores significantly below placebo levels (Table 1). Scores on this scale were significantly higher after administration of methamphetamine (5, 10 and 15 mg) in combination with 20 mg aripiprazole relative to these doses of methamphetamine alone. Combining 2.5 mg methamphetamine and 20 mg aripiprazole increased these scores significantly above levels observed with placebo.
Drug Effect Questionnaire
ANOVA revealed a significant interaction of methamphetamine and aripiprazole on two items from the Drug-Effect Questionnaire: High and Irregular-Racing Heartbeat (Table 1). Methamphetamine (5, 10 and 15 mg) alone increased ratings of High significantly above placebo levels, whereas none of these doses did so when administered in combination with 20 mg aripiprazole (Table 1). Ratings of High were significantly lower after administration of methamphetamine (10 and 15 mg) in combination with 20 mg aripiprazole relative to these doses of methamphetamine alone. Methamphetamine (5, 10 and 15 mg) alone increased ratings of Irregular-Racing Heartbeat significantly above placebo levels, whereas only the highest dose did so when administered in combination with 20 mg aripiprazole. Ratings of Irregular-Racing Heartbeat were significantly lower after administration of 10 mg methamphetamine in combination with 20 mg aripiprazole relative to this dose of methamphetamine alone (Table 1).
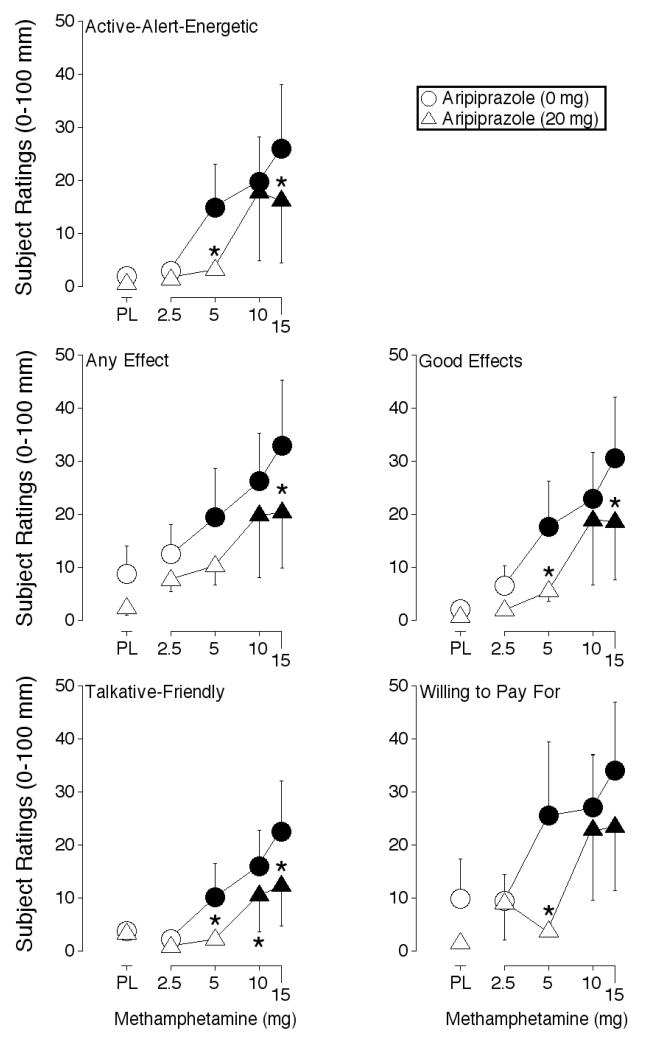
ANOVA revealed a significant main effect of methamphetamine and aripiprazole on ratings of Talkative-Friendly (Table 1). Methamphetamine (5, 10 and 15 mg) alone increased these ratings significantly above placebo levels, whereas only the 10 and 15 mg dose did so when administered in combination with 20 mg aripiprazole (Figure 3). Ratings of Talkative-Friendly were significantly lower after administration of 5, 10 and 15 mg methamphetamine in combination with 20 mg aripiprazole relative to these doses of methamphetamine alone (Table 1).
Figure 3.
Effects of methamphetamine alone, aripiprazole alone, methamphetamine-aripiprazole combinations, and placebo on ratings of Active-Alert-Energetic, Any Effect, Good Effects, Talkative-Friendly, and Willing to Pay For from the Drug-Effect Questionnaire. Data are expressed as area-under-the-time-action curve (AUC). All other details are as in Figure 1.
ANOVA revealed a significant main effect of methamphetamine on five items from the Drug-Effect Questionnaire: Any Effect, Good Effects, Willing to Pay For, Restless and Rush (Table 1). Figure 3 shows the effects of methamphetamine alone and in combination with aripiprazole for three of these items: Any Effect, Good Effects, and Willing to Pay For. Methamphetamine (5, 10 and 15 mg) alone increased these ratings significantly above placebo levels, whereas only the 10 and 15 mg dose did so when methamphetamine was administered in combination with 20 mg aripiprazole. Ratings of Any Effect were significantly lower after administration of 15 mg methamphetamine in combination with 20 mg aripiprazole relative to this dose of methamphetamine alone. Ratings of Good Effects were significantly lower after administration of 5 and 15 mg methamphetamine in combination with 20 mg aripiprazole relative to these doses of methamphetamine alone. Ratings of Willing to Pay For were significantly lower after administration of 5 mg methamphetamine in combination with 20 mg aripiprazole relative to this dose of methamphetamine alone (Table 1).
ANOVA revealed a significant main effect of aripiprazole on ratings of Active-Alert-Energetic from the Drug-Effect Questionnaire (Table 1). Methamphetamine (5, 10 and 15 mg) alone increased these ratings significantly above placebo levels, whereas only the 10 and 15 mg dose did so when methamphetamine was administered in combination with 20 mg aripiprazole (Figure 3). Ratings of Active-Alert-Energetic were significantly lower after administration of 5 and 15 mg methamphetamine in combination with 20 mg aripiprazole relative to these doses of methamphetamine alone (Table 1).
DSST
ANOVA revealed a significant main effect of methamphetamine and aripiprazole on the number of trials attempted on the DSST (Table 1). Methamphetamine (5, 10 and 15 mg) alone increased the number of trials attempted significantly above placebo levels. Aripiprazole alone decreased the number of trials attempted significantly below placebo levels. The number of trials attempted was significantly lower after administration of 5 and 15 mg methamphetamine in combination with 20 mg aripiprazole relative to these doses of methamphetamine alone. ANOVA revealed a significant main effect of methamphetamine and aripiprazole on the number of trials correct on the DSST (Table 1). Methamphetamine (10 and 15 mg) alone increased the number of trials correct significantly above placebo levels (Table 1). Aripiprazole alone decreased the number of trials correct significantly below placebo levels. The number of trials attempted was significantly lower after administration of 5 mg methamphetamine in combination with 20 mg aripiprazole relative to this dose of methamphetamine alone.
Blood Pressure and Heart Rate
ANOVA revealed a significant main effect of methamphetamine on systolic blood pressure (Table 1). Methamphetamine (5, 10 and 15 mg) alone increased systolic blood pressure above placebo levels, while only the two higher doses of methamphetamine did so following aripiprazole pretreatment (Figure 4). Systolic blood pressure was significantly lower after administration of 5 and 10 mg methamphetamine in combination with 20 mg aripiprazole relative to these doses of methamphetamine alone. ANOVA revealed a significant main effect of methamphetamine and aripiprazole on diastolic blood pressure (Table 1). Methamphetamine (5, 10 and 15 mg) alone increased diastolic blood pressure above placebo levels, while only the highest dose of methamphetamine did so following aripiprazole pretreatment (Figure 4). Diastolic blood pressure was significantly lower after administration of 5, 10 and 15 mg methamphetamine in combination with 20 mg aripiprazole relative to these doses of methamphetamine alone.
Figure 4.
Effects of methamphetamine alone, aripiprazole alone, methamphetamine-aripiprazole combinations, and placebo on systolic and diastolic blood pressure. Data are expressed as area-under-the-time-action curve (AUC). All other details are as in Figure 1.
ANOVA revealed significant interaction of methamphetamine and aripiprazole on heart rate (Table 1). Methamphetamine (5, 10 and 15 mg) alone increased heart above placebo levels, while all doses of methamphetamine did so following aripiprazole pretreatment. Aripiprazole alone increased heart rate significantly above placebo levels. Heart rate was significantly higher after administration of 2.5 mg methamphetamine in combination with 20 mg aripiprazole relative to this dose of methamphetamine alone. Heart rate was significantly lower after administration of 15 mg methamphetamine in combination with 20 mg aripiprazole relative to this dose of methamphetamine alone (Table 1).
DISCUSSION
The present study examined the discriminative stimulus, subject-rated, and cardiovascular effects of a range of doses of methamphetamine combined with placebo or 20 mg aripiprazole. Methamphetamine alone functioned as a discriminative stimulus, produced positive subject-rated effects and elevated cardiovascular indices. Aripiprazole alone did not occasion methamphetamine-appropriate responding or produce subject-rated effects, but modestly impaired performance. Concurrent administration of aripiprazole significantly attenuated the discriminative-stimulus and cardiovascular effects of methamphetamine, as well as some of the positive subject-rated effects of methamphetamine. Both aripiprazole and methamphetamine, alone and in combination, were well tolerated by all participants and no severe or unanticipated adverse events occurred.
The finding that 10 mg oral methamphetamine served as a discriminative stimulus is concordant with the results from two human laboratory studies demonstrating that 10 mg methamphetamine can function as a discriminative stimulus.20,28 One of these previous studies showed that methamphetamine increased drug-appropriate responding, and acute pretreatment with the N-methyl-D-aspartate receptor antagonist memantine did not occasion methamphetamine-like responding.28 The other human-laboratory study showed that methamphetamine functioned as a discriminative-stimulus and d-amphetamine as well as methylphenidate fully substituted for methamphetamine.20 The present study first replicated the previous findings by showing that methamphetamine functions as a discriminative stimulus, and then extended these previous findings by showing that the discriminative stimulus effects of methamphetamine were sensitive to pharmacological manipulation. The present results are also concordant with the findings from the animal studies demonstrating that methamphetamine produced discriminative-stimulus effects in rats, monkeys and pigeons.6,7,10,30 Thus, the present finding support the notion that across a range of species methamphetamine can function as a discriminative stimulus.
Findings from animal studies indicate roles of DA and 5-HT systems in the discriminative stimulus effects of methamphetamine. For example, administration of full agonists at DA D2 receptors (e.g., quinpirole, (+) PHNO) or 5-HT 5-HT1A receptors (e.g., 8-OH-DPAT) substitute for methamphetamine in animals trained to discriminate methamphetamine from vehicle9,10, whereas administration of partial agonists at D2 receptors (e.g., SDZ 208-911) or 5-HT1A receptors (e.g., buspirone) only partially substituted for methamphetamine.7,9 In the present study, aripiprazole, a partial agonist at D2 and 5-HT1A receptors, did not occasion methamphetamine-appropriate responding, suggesting that aripiprazole did not exhibit agonist-like activity to the extent that the D2 and 5-HT1A receptors are involved in the discriminative stimulus effects of methamphetamine.
In agreement with the drug-discrimination data, which suggest that 20 mg aripiprazole was not a discernable interoceptive stimulus, no increases in subject ratings were observed following administration of aripiprazole alone, despite the fact that antipsychotic drugs can produce sedative-like effects. These data are also consistent with previous studies from our laboratory in which the behavioral effects of d-amphetamine were determined in combination with aripiprazole or risperidone.17,18 In those studies, with nearly identical procedures employed, 20 mg of aripiprazole or 1 mg of risperidone did not occasion d-amphetamine-appropriate responding, nor did they increase subject ratings associated with sedation (i.e., PCAG scale of the ARCI; sedative subscale of the Adjective-Rating Scale; Sluggish, Fatigued, Lazy item from the Drug Effect Questionnaire). However, in agreement with the well-documented effects of antipsychotics on behavioral motor control, aripiprazole alone impaired performance in the present study, as measured by a computerized version of the DSST. 17,18 These results are concordant with the previous studies from our laboratory, which reported that aripiprazole and risperidone impaired performance on the DSST.17,18 Interestingly, aripiprazole did not increase subject ratings of Performance Impaired on the Drug Effect Questionnaire in the present study, indicating that although aripiprazole produced decrements in psychomotor performance, participants were unable to perceive this change.
Drug-discrimination procedures are well suited to assess neuropharmacological mechanisms of the training drug by studying pharmacological manipulation.15 In the present experiment, concurrent administration of aripiprazole, a partial agonist at D2/5-HT1A receptors and an antagonist at 5-HT2A receptors, significantly attenuated the discriminative-stimulus effects of the training drug methamphetamine, suggesting a role of D2 and 5-HT1A/2A receptors in mediating the behavioral effects of methamphetamine in humans. These data are concordant with the previous human laboratory studies showing that pretreatment with atypical antipsychotics that are partial agonists or antagonists in DA and 5-HT systems (e.g., aripiprazole, risperidone) attenuate the discriminative stimulus effects of d-amphetamine. 17,18 However, a new contribution of the present study can be highlighted in that this study demonstrated pharmacological modification of the behavioral effects of methamphetamine (which elevates synaptic 5-HT to a greater magnitude than does d-amphetamine) by concomitantly using a drug-discrimination procedure and subject-rated measures. The current results are also in agreement with findings from the animal studies demonstrating that pretreatments with DA or 5-HT receptor antagonists shift methamphetamine dose-response curves rightward.7,8,9,30 Additionally, the present findings support the notion that a partial agonist, such as aripiprazole, would act as an antagonist when there are high levels of neurotransmitter present in the synapse, as would occur following the acute administration of methamphetamine. Thus, in agreement with the previous findings in humans and animals, results from the current study indicate that methamphetamine produces discriminative stimulus effects that are sensitive to a pharmacological modification.
Although the behavioral and pharmacological effects of d-amphetamine and methamphetamine overlap, there are several neurochemical differences between these amphetamine analogues. For example, although both drugs increase extracellular DA levels to a comparable magnitude, 5-HT levels are significantly higher following the administration of methamphetamine.21 It is likely, then, the relative contribution of 5-HT1A/2A receptors would differ in mediating discriminative stimulus effects of d-amphetamine and methamphetamine, and that medications acting on 5-HT1A/2A receptors (e.g., aripiprazole) could differentially modify the discriminative-stimulus effects of d-amphetamine and methamphetamine. However, the current findings are in agreement with the previous finding from our laboratory showing that pretreatment with aripiprazole attenuated the discriminative stimulus effects of d-amphetamine.18 Concurrent administration of aripiprazole also significantly attenuated nearly all of the subject-rated effects (e.g., Good Effects, Talkative-Friendly, Active-Alert-Energetic) for which there was an effect of methamphetamine in the current study. Thus, across the present and a previous study from our laboratory18, aripiprazole showed similar potency in reducing the discriminative stimulus and several subject-rated effects of d-amphetamine and methamphetamine. These results indicate that despite the subtle difference between the serotonergic mechanisms of d-amphetamine and methamphetamine, aripiprazole reduced the behavioral effects of both these drugs. The consistent effectiveness of aripiprazole in reducing the behavioral effects of methamphetamine and d-amphetamine18,31 suggest that d-amphetamine might serve as a useful pharmacological model to screen medications for methamphetamine dependence.
The present results are discordant with the findings of a previous human laboratory study that showed aripiprazole enhanced the subject-rated effects of methamphetamine.32 The reasons for this discrepancy are unknown, but could be attributable to the differences in the methods used (e.g., drug-discrimination and subject-rated measures versus only subject-rated measures; administering methamphetamine orally versus intravenously; acute versus chronic aripiprazole dosing; different aripiprazole doses; recreational users versus methamphetamine-dependent participants). However, the current findings should be viewed cautiously in the context of the findings from a small clinical trial showing that aripiprazole treatment increased the number of amphetamine-positive urine samples collected relative to placebo treatment.33 Nonetheless, the present findings showing reductions in the discriminative-stimulus effects of methamphetamine by pretreatment with a D2/5-HT1A receptor partial agonist could still provide useful information on the neuropharmacological mechanisms contributing to the behavioral effects of methamphetamine in humans. The present data, hence, provide further support for the utility of drug-discrimination procedures in delineating neuropharmacological mechanisms of stimulant drugs of abuse.
The methamphetamine training dose needs to be considered while interpreting underlying neuropharmacological mechanisms. From an ethical perspective, the minimum discernible dose of the training drug should be used because participants will be exposed to it several times. Lower drug doses are, however, more difficult to discriminate. In the current study, the training dose of methamphetamine may be low, because 4 participants failed to discriminate methamphetamine from placebo. However, in other studies conducted in our laboratory20, a considerably smaller percentage of participants failed to acquire the discrimination (i.e., only 2 participants out of 10 failed to acquire the methamphetamine discrimination). It is possible that the relatively high number of non-discriminators is specific to the present study. Worth noting is that preclinical and human laboratory experiments have shown that the dose-response curve for the training drug is shifted leftward in individuals trained to discriminate lower versus higher drug doses.34,35 In addition, findings from animal studies indicate that the relative contributions of monoamine neurotransmission differ across training doses of stimulants. For example, norepinephrine systems appear to be involved in mediating the discriminative stimulus effects of low cocaine doses (i.e., 3 mg/kg)34, but not higher doses (i.e., 10 mg/kg).36,37 It is likely, therefore, that different results would have been observed if the training dose of methamphetamine were higher. Future studies should determine whether a higher training dose systematically influences the pharmacological sensitivity of the discriminative-stimulus effects of methamphetamine.
There were some limitations to the present study. The doses of methamphetamine used in the study were relatively low. Moreover, the participants in the present study were not methamphetamine-dependent, which would be a more clinically relevant population. Therefore, the small doses of methamphetamine studied in the non-dependent individuals in this study limit the implications of current findings. Another caveat of the current study is that the methamphetamine was administered orally. Administering methamphetamine intranasally, intravenously, and by smoking are most often associated with its abuse, in part because of a rapid onset of stimulant effects. Thus, the present results should be interpreted cautiously, considering the variation in the emergence of the drug effects by different routes. One more potential limitation is that the combination of a stimulant with a drug having sedative-like effects may lead to false positives with respect to the ability of the putative treatment to pharmacologically antagonize the effects of the stimulant. However, in the present study aripiprazole did not produce enhancements in subject-rated sedative effects. Moreover, in a previous study from our laboratory using nearly identical methods, the benzodiazepine oxazepam did not modify the discriminative-stimulus or subject-rated effects of d-amphetamine, but did increase ratings of sedation and impaired psychomotor performance.38 These findings, as well as the notion that the effects of the competitive antagonist aripiprazole could be surmountable, suggest that the present results may represent a pharmacological antagonism of the behavioral effects of methamphetamine by aripiprazole.
In summary, the present study demonstrated that methamphetamine functioned as a discriminative stimulus and produced prototypical stimulant-like subject-rated effects in humans. Acute pretreatment with aripiprazole significantly reduced the discriminative stimulus, some positive subject-rated, and cardiovascular effects of methamphetamine. However, the clinical utility of aripiprazole for managing amphetamine use disorders remains unclear, even though the present results support previous animal laboratory results implicating monoamine systems in the effects of methamphetamine. The consistent effectiveness of aripiprazole for reducing the discriminative stimulus effects of d-amphetamine18 and methamphetamine suggest that the use of d-amphetamine is a valid model for elucidating the behavioral neuropharmacology of methamphetamine in humans.
Acknowledgements
A National Institute on Drug Abuse Grant R01 DA017711 (C.R.R.) supported this research. The manufacturer of aripiprazole (Bristol-Myers Squibb Co., Princeton, NJ) did not sponsor this study.
Abbreviations
- ARCI
Addiction Research Center Inventory
- A
Amphetamine Scale
- ANOVA
analysis of variance
- BG
Benzedrine Group
- DA
dopamine
- DSST
Digit-Symbol-Substitution Test
- DAST
Drug Abuse Screening Test
- LSD
Lysergic Acid Diethylamide
- MAST
Michigan Alcohol Screening Test
- MBG
Morphine-Benzedrine Group
- PCAG
Pentobarbital, Chlorpromazine, Alcohol Group
- 5-HT
serotonin
- THC
tetrahydrocannabinol
Footnotes
The present affiliation of RJS is: Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, University of California at Los Angeles.
The present affiliation of ARV is: Department of Pharmacology and Toxicology, Virginia Commonwealth University.
Conflicts of Interest: The authors report no conflict of interest.
BIBLIOGRAPHY
- 1.Substance Abuse and Mental Health Services Administration, Office of Applied Studies . Results from the 2009 National Survey on Drug Use and Health: Detailed tables. Rockville, MD: 2010. [Google Scholar]
- 2.Nicosia N, Reardon E, Lorenz K, et al. The Medicare hospice payment system: a consideration of potential refinements. Health Care Financ Rev. 2009;30:47–59. [PMC free article] [PubMed] [Google Scholar]
- 3.Pasic J, Russo JE, Ries RK, et al. Methamphetamine users in the psychiatric emergency services: a case-control study. Am J Drug Alcohol Abuse. 2007;33:675–86. doi: 10.1080/00952990701522732. [DOI] [PubMed] [Google Scholar]
- 4.Shoptaw S, King WD, Landstrom E, et al. Public Health Issues Surrounding Methamphetamine Dependence. In: Roll JR, Ling W, Rawson RA, Shoptaw S, editors. Methamphetamine Addiction: From Basic Science to Treatment. Guilford Press; New York: 2009. pp. 143–156. [Google Scholar]
- 5.Kuczenski R, Segal D. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czoty PW, Ramanathan CR, Mutschler NH, et al. Drug discrimination in methamphetamine-trained monkeys: effects of monoamine transporter inhibitors. J Pharmacol Exp Ther. 2004;311:720–7. doi: 10.1124/jpet.104.071035. [DOI] [PubMed] [Google Scholar]
- 7.Munzar P, Laufert MD, Kutkat SW, et al. Effects of various serotonin agonists, antagonists, and uptake inhibitors on the discriminative stimulus effects of methamphetamine in rats. J Pharmacol Exp Ther. 1999;291:239–50. [PubMed] [Google Scholar]
- 8.Munzar P, Goldberg SR. Dopaminergic involvement in the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology (Berl) 2000;148:209–216. doi: 10.1007/s002130050044. [DOI] [PubMed] [Google Scholar]
- 9.Tidey JW, Bergman J. Drug discrimination in methamphetamine-trained monkeys: agonist and antagonist effects of dopaminergic drugs. J Pharmacol Exp Ther. 1998;285:1163–74. [PubMed] [Google Scholar]
- 10.Sasaki JE, Tatham TA, Barrett JE. The discriminative stimulus effects of methamphetamine in pigeons. Psychopharmacology (Berl) 1995;120:303–10. doi: 10.1007/BF02311178. [DOI] [PubMed] [Google Scholar]
- 11.Brauer LH, de Wit H. Role of dopamine in d-amphetamine-induced euphoria in normal, healthy volunteers. Exp Clin Psychopharmacol. 1995;3:371–81. [Google Scholar]
- 12.Brauer LH, de Wit H. Subjective responses to d-amphetamine alone and after pimozide pretreatment in normal, healthy volunteers. Biol Psychiatry. 1996;39:26–32. doi: 10.1016/0006-3223(95)00110-7. [DOI] [PubMed] [Google Scholar]
- 13.Brauer LH, de Wit H. High dose pimozide does not block amphetamine-induced euphoria in normal volunteers. Pharmacol Biochem Behav. 1997;56:265–72. doi: 10.1016/s0091-3057(96)00240-7. [DOI] [PubMed] [Google Scholar]
- 14.Wachtel SR, Ortengren A, de Wit H. The effects of acute haloperidol or risperidone on subjective responses to methamphetamine in healthy volunteers. Drug Alcohol Depend. 2002;68:23–33. doi: 10.1016/s0376-8716(02)00104-7. [DOI] [PubMed] [Google Scholar]
- 15.Kelly TH, Stoops WW, Perry AS, et al. Clinical neuropharmacology of drugs of abuse: a comparison of drug-discrimination and subject-report measures. Behav Cogn Neurosci Rev. 2003;2:227–260. doi: 10.1177/1534582303262095. [DOI] [PubMed] [Google Scholar]
- 16.Rush CR, Vansickel AR, Stoops WW. Human Drug Discrimination: Methodological Considerations and Application to Elucidating the Neuropharmacology of Amphetamines. In: Glennon RA, Young R, editors. Drug Discrimination: Applications to Medicinal Chemistry and Drug Studies. Wiley; Hoboken: 2010. [Google Scholar]
- 17.Rush CR, Stoops WW, Hays LR, et al. Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther. 2003;306:195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- 18.Lile JA, Stoops WW, Vansickel AR, et al. Aripiprazole attenuates the discriminative-stimulus and subject-rated effects of D-amphetamine in humans. Neuropsychopharmacology. 2005;30:2103–14. doi: 10.1038/sj.npp.1300803. [DOI] [PubMed] [Google Scholar]
- 19.Martin WR, Sloan JW, Sapira JD, et al. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–58. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- 20.Sevak RJ, Stoops WW, Hays LR, et al. Discriminative stimulus and subject-rated effects of methamphetamine, d-amphetamine, methylphenidate, and triazolam in methamphetamine-trained humans. J Pharmacol Exp Ther. 2009;328:1007–18. doi: 10.1124/jpet.108.147124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczenski R, Segal DS, Cho AK, et al. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15:1308–17. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maxwell JC, Rutkowski BA. The prevalence of methamphetamine and amphetamine abuse in North America: a review of the indicators, 1992-2007. Drug Alcohol Rev. 2008;27:229–35. doi: 10.1080/09595230801919460. [DOI] [PubMed] [Google Scholar]
- 23.Jasinski D. Assessment of the abuse potentiality of morphine-like drugs (methods used in man) In: Martin WR, editor. Drug Addiction I. Springer-Verlag New York Inc.; New York: 1977. pp. 197–258. [Google Scholar]
- 24.Oliveto AH, Bickel WK, Hughes JR, et al. Caffeine drug discrimination in humans: acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther. 1992;261:885–94. [PubMed] [Google Scholar]
- 25.Rush CR, Stoops WW, Wagner FP, et al. Alprazolam attenuates the behavioral effects of d-amphetamine in humans. J Clin Psychopharmacol. 2004;24:410–20. doi: 10.1097/01.jcp.0000130553.55630.ad. [DOI] [PubMed] [Google Scholar]
- 26.McLeod DR, Griffiths RR, Bigelow GE, et al. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instr. 1982;14:463–466. [Google Scholar]
- 27.Abreu ME, Griffiths RR. Drug tasting may confound human drug discrimination studies. Psychopharmacology (Berl) 1996;125:255–7. doi: 10.1007/BF02247336. [DOI] [PubMed] [Google Scholar]
- 28.Hart CL, Haney M, Foltin RW, et al. Effects of the NMDA antagonist memantine on human methamphetamine discrimination. Psychopharmacology (Berl) 2002;164:376–84. doi: 10.1007/s00213-002-1225-9. [DOI] [PubMed] [Google Scholar]
- 29.McGavin JK, Goa KL. Aripiprazole. CNS Drugs. 2002;16:779–86. doi: 10.2165/00023210-200216110-00008. discussion 787-8. [DOI] [PubMed] [Google Scholar]
- 30.Bergman J. Medications for stimulant abuse: agonist-based strategies and preclinical evaluation of the mixed-action D-sub-2 partial agonist aripiprazole (Abilify) Exp Clin Psychopharmacol. 2008;16:475–83. doi: 10.1037/a0014398. [DOI] [PubMed] [Google Scholar]
- 31.Stoops WW, Lile JA, Glaser PE, et al. A low dose of aripiprazole attenuates the subject-rated effects of d-amphetamine. Drug Alcohol Depend. 2006;84:206–9. doi: 10.1016/j.drugalcdep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Newton TF, Reid MS, De La Garza R, et al. Evaluation of subjective effects of aripiprazole and methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol. 2008;11:1037–45. doi: 10.1017/S1461145708009097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiihonen J, Kuoppasalmi K, Fohr J, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164:160–2. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- 34.Terry P, Witkin JM, Katz JL. Pharmacological characterization of the novel discriminative stimulus effects of a low dose of cocaine. J Pharmacol Exp Ther. 1994;270:1041–8. [PubMed] [Google Scholar]
- 35.Kollins SH, Rush CR. Effects of training dose on the relationship between discriminative-stimulus and self-reported drug effects of d-amphetamine in humans. Pharmacol Biochem Behav. 1999;64:319–26. doi: 10.1016/s0091-3057(99)00084-2. [DOI] [PubMed] [Google Scholar]
- 36.Broadbent J, Michael EK, Riddle EE, et al. Involvement of dopamine uptake in the discriminative stimulus effects of cocaine. Behav Pharmacol. 1991;2:187–197. [PubMed] [Google Scholar]
- 37.Cunningham KA, Callahan PM. Monoamine reuptake inhibitors enhance the discriminative state induced by cocaine in the rat. Psychopharmacology (Berl) 1991;104:177–80. doi: 10.1007/BF02244175. [DOI] [PubMed] [Google Scholar]
- 38.Lile JA, Stoops WW, Wagner FP, et al. Oxazepam does not modulate the behavioral effects of d-amphetamine in humans. Pharmacol Biochem Behav. 2005;82:270–9. doi: 10.1016/j.pbb.2005.08.012. [DOI] [PubMed] [Google Scholar]