Abstract
Age-related impairment of angiogenesis is likely to play a central role in cerebromicrovascular rarefaction and development of vascular cognitive impairment, but the underlying mechanisms remain elusive. To test the hypothesis that dysregulation of Dicer1 (ribonuclease III, a key enzyme of the microRNA [miRNA] machinery) impairs endothelial angiogenic capacity in aging, primary cerebromicrovascular endothelial cells (CMVECs) were isolated from young (3 months old) and aged (24 months old) Fischer 344 × Brown Norway rats. We found an age-related downregulation of Dicer1 expression both in CMVECs and in small cerebral vessels isolated from aged rats. In aged CMVECs, Dicer1 expression was increased by treatment with polyethylene glycol–catalase. Compared with young cells, aged CMVECs exhibited altered miRNA expression profile, which was associated with impaired proliferation, adhesion to vitronectin, collagen and fibronectin, cellular migration (measured by a wound-healing assay using electric cell–substrate impedance sensing technology), and impaired ability to form capillary-like structures. Overexpression of Dicer1 in aged CMVECs partially restored miRNA expression profile and significantly improved angiogenic processes. In young CMVECs, downregulation of Dicer1 (siRNA) resulted in altered miRNA expression profile associated with impaired proliferation, adhesion, migration, and tube formation, mimicking the aging phenotype. Collectively, we found that Dicer1 is essential for normal endothelial angiogenic processes, suggesting that age-related dysregulation of Dicer1-dependent miRNA expression may be a potential mechanism underlying impaired angiogenesis and cerebromicrovascular rarefaction in aging.
Key Words: Angiogenesis, Capillary density, Vascular aging, Cerebrovascular, Cardiovascular aging, Epigenetics.
VASCULAR cognitive impairment (VCI) is the second most prevalent type of age-associated cognitive dysfunction in the United States today (1). Numerous clinical and experimental studies demonstrate that a large portion of the clinical picture of VCI is due to pathological microvascular alterations (2). Importantly, there is convincing evidence that aging is associated with cerebromicrovascular rarefaction and that decreases in cerebromicrovascular density contribute to the age-related decline in cerebral blood flow (3–16). The resulting mismatch between metabolic demand and blood flow has been shown to be important contributing factors to aging-induced cognitive impairment in the absence of or preceding neurodegeneration in the elderly individuals (1,4,7,17,18).
The process of angiogenesis, new capillary formation from existing blood vessels, is critical for maintenance of the cerebromicrovasculature. Previous studies in laboratory rodents demonstrate that aging is associated with a progressive deterioration of microvascular homeostasis in many organs due to age-related impairment of angiogenic processes (19–23). It is assumed that these changes have a key role in age-related microvascular rarefaction (24), decreasing tissue blood supply, and impairing adaptation to hypoxia (25–27). Yet, the underlying mechanisms by which aging impairs endothelial angiogenic processes remain elusive.
MicroRNAs (miRNAs) are short, endogenous, noncoding transcripts that negatively regulate the expression of specific mRNA targets. Recent studies demonstrate that angiogenesis is regulated by miRNAs (28–30). Dicer1 (ribonuclease III) is a key enzyme of the miRNA machinery, which is responsible for synthesis of mature functional miRNAs. There is evidence that Dicer1 in endothelial cells may play a role in regulation of angiogenic processes (30–33). miRNAs control life span and the pace of aging in model organisms (34–36), and there is preliminary evidence that miRNA expression and Dicer1 expression in the liver may be altered also in mammalian aging (37,38). However, no studies have investigated age-related alterations in Dicer1 and miRNA expression profile in cerebromicrovascular endothelial cells (CMVECs). Furthermore, there are no studies to our knowledge investigating the role of Dicer1-dependent pathways in impaired endothelial angiogenic capacity in aging.
This study was designed to elucidate how aging and reactive oxygen species (ROS) alter Dicer1 expression, how dysregulation of Dicer1 in aging affects the angiogenic miRNA signature and what is the causal role of dysregulation of Dicer1 in age-related impairment of angiogenesis. Using CMVECs isolated from young and aged Fischer 344 × Brown Norway (F344 × BN) rats, we tested the hypothesis that overexpression of Dicer1 in aged endothelial cells improves angiogenic capacity, including proliferation, adhesion, migration, and ability to form capillary-like structures. We also determined whether siRNA knockdown of Dicer1 impairs angiogenic processes in young CMVECs, mimicking the cerebromicrovascular aging phenotype.
Materials and Methods
Animals and Vessel Isolation
F344 × BN rats were used as a model of aging, as this strain has a lower incidence of age-specific pathology than other rat strains. Thus, in F344 × BN rats, the primary effects of aging can be studied uncomplicated by compensatory effects caused by age-related pathology. Male F344 × BN rats (3 and 24 months old; n = 15 in each group) were obtained from the National Institute on Aging. All animals were disease free with no signs of systemic inflammation and/or neoplastic diseases. The rats were housed in an environmentally controlled vivarium under pathogen-free conditions with unlimited access to food and water and a controlled photoperiod (12h light; 12h dark). All rats were maintained according to National Institutes of Health guidelines, and all animal use protocols were approved by the Institutional Animal Care and Use Committees of the participating institutions. The animals were euthanized with CO2. From the first cohort of animals, branches of the middle cerebral arteries and the hippocampi were isolated using sterile microsurgery instruments. From the second cohort of animals, the brains were rapidly dissected to establish primary CMVEC cultures.
Establishment and Characterization of Primary CMVEC Cultures
Brains were removed aseptically, rinsed in ice-cold phosphate-buffered saline (PBS) and minced into ≈1mm squares. The tissue was washed twice in ice-cold 1× PBS by low-speed centrifugation (50g, 2–3 minutes). The diced tissue was digested in a solution of collagenase (800U/g tissue), hyaluronidase (2.5U/g tissue), and elastase (3U/g tissue) in 1mL PBS/100mg tissue for 45 minutes at 37°C in rotating humid incubator. The digested tissue was passed through a 100-µm cell strainer to remove undigested blocks. The single-cell lysate was centrifuged for 2 minutes at 70g. After removing the supernatant carefully, the pellet was washed twice in cold PBS supplemented with 2.5% fetal calf serum, and the suspension was centrifuged at 300g, for 5 minutes at 4°C.
To create an endothelial cell enriched fraction, the cell suspension was gradient centrifuged using OptiPrep solution (Axi-Shield, PoC, Norway). Briefly, the cell pellet was resuspended in Hanks’ balanced salt solution (HBSS) and mixed with 40% iodixanol thoroughly (final concentration: 17% (w/v) iodixanol solution; ρ = 1.096g/mL). Two milliliters of HBSS were layered on top and centrifuged at 400g for 15 minutes at 20°C. Endothelial cells, which banded at the interface between HBSS and the 17% iodixanol layer, were collected. The endothelial cell–enriched fraction was incubated for 30 minutes at 4°C in dark with anti-CD31/PE (BD BD Biosciences, San Jose, CA) and anti-MCAM/fluorescein isothiocyanate (FITC) (BD Biosciences). After washing the cells twice with MACS Buffer (Milltenyi Biotech, Cambridge, MA, USA), anti-FITC magnetic bead–labeled and anti-PE magnetic bead–labeled secondary antibodies were used for 15 minutes at room temperature. Endothelial cells were collected by magnetic separation using the MACS LD magnetic separation columns according to the manufacturer’s guidelines (Milltenyi Biotech). The endothelial fraction was cultured on fibronectin coated plates in Endothelial Growth Medium (Cell Application, San Diego, CA) for 10 days.
Endothelial cells were phenotypically characterized by flow cytometry (GUAVA 8HT, Merck Millipore, Billerica, MA). Briefly, antibodies against five different endothelial-specific markers were used (anti-CD31-PE, anti-erythropoietin receptor-APC, anti-vascular endothelial growth factor (VEGF) R2-PerCP, anti-intercellular adhesion molecule-fluorescein, and anti-CD146-PE) and isotype-specific antibody–labeled fractions served as negative controls. All antibodies were purchased from R&D Systems (Minneapolis, MN).
Western Blotting
To analyze protein expression of Dicer1 in homogenates of young and aged CMVECs, Western blotting was performed as described (39), using the following primary antibody: anti-Dicer1, #3363S Cell Signaling Technology (Beverly, MA; 1:2000 in 5% milk). All polyvinylidene fluoride membranes were incubated in primary antibody overnight at 4°C. A donkey anti-rabbit polyclonal secondary antibody was used (Abcam, ab16284; 1:2000 in 5% milk). Equal amounts of protein were loaded in each lane. Because among the housekeeping genes tested the relative mRNA expression of β-actin was not statistically different in young and aged CMVECs (β-actin:hypoxanthine phosphoribosyltransferase [HPRT] ratio; young: 1±0.16, aged: 0.73±0.02, ns), for normalization purposes we used mouse anti-β-actin (Abcam, ab6276, 1:10,000 in 5% milk) with a sheep anti-mouse IgG horseradish peroxidase–linked secondary antibody (NA931V GE Healthcare UK, 1:10,000).
Measurement of Cellular ROS Production
To assess cellular peroxide production, we used the cell-permeant oxidative fluorescent indicator dye CM-H2DCFDA (5 [and 6]-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate-acetyl ester, Invitrogen, Carlsbad, CA) as we previously reported (40,41). In brief, cells were washed with warm PBS and incubated with CM-H2DCFDA (10 µM, at 37°C, for 30 minutes). CM-H2DCFDA fluorescence was assessed by flow cytometry (40,41).
Assessment of the Effects of Oxidative Stressors and Antioxidants on Dicer1 Expression in CMVECs
To assess the effect of scavenging of H2O2 on Dicer1 expression, we treated young and aged CMVECs with polyethylene glycol–catalase (200U/mL for 24h). To assess the effects of oxidative stressors on Dicer1 expression, young CMVECs were treated with H2O2 (0.1–10 µmol/L, for 24h), high glucose (30 mmol/L, for 24h), and TNFα (10ng/mL, for 24h). To assess the effects of activators of Nrf2 signaling, aged CMVECs were treated with two structurally different potent inducers of Nrf2: resveratrol (10−7 to 10−5 mol/L, for 24h) and sulforaphane (10−6 mol/L, for 24h). Expression of Dicer1 mRNA was assessed by quantitative real-time RT-PCR (described in the following section).
miRNA Expression Profiling
The expression profile of 373 unique rat miRNAs in hippocampi of aged and young rats and CMVECs derived from young and aged rats was analyzed using the TaqMan Array Rodent MicroRNA A+B Cards Set v3.0 (Applied Biosystems, Life Technologies, Carlsbad, CA).
Knockdown and Overexpression of Dicer1
To disrupt Dicer1 signaling in young CMVECs, Dicer1 was downregulated (by ~50%) by RNA interference using four proprietary shRNA sequences (OriGene Technologies, Rockville, MD) and the electroporation-based Amaxa Nucleofector technology (Amaxa, Gaithersburg, MD), as we have previously reported (42–45). Experiments were performed on Day 2 after the transfection, when gene silencing was optimal. Overexpression of Dicer1 (approximately twofold) was achieved in CMVECs by transfection with a Dicer1 full-length cDNA encoding plasmid, pDESTmycDICER (Addgene plasmid 19873, Addgene, www.addgene.org, provided by the laboratory of Dr. Thomas Tuschl (46)). Overexpression and knockdown of Dicer1 were confirmed by Western blotting. The negative controls were vector only and scrambled shRNA (Origen), respectively. To induce angiogenic processes, CMVECs were treated with recombinant human (VEGF, 100ng/mL; R&D systems).
Quantitative Real-Time RT-PCR
A quantitative real-time RT-PCR technique was used to analyze mRNA expression of Dicer1 and selected miRNAs, as previously reported (39,47–51). In brief, total RNA was isolated with a Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed using Superscript III RT (Invitrogen). A real-time RT-PCR technique was used to analyze mRNA expression using a Strategen MX3000, as reported (39,47–51). Amplification efficiencies were determined using a dilution series of a standard vascular sample. Quantification was performed using the efficiency-corrected ΔΔCq method. The relative quantities of the reference genes HPRT, GAPDH, and ACTB were determined, and a normalization factor was calculated based on the geometric mean for internal normalization. Oligonucleotides used for quantitative real-time RT-PCR are listed in Table 1. To assess expression of MIR193-b, MIR214, MIR574, MIR744, MIR532, MIR672, MIR145, and MIR146, primer sequences proprietary to Applied Biosystems were used.
Table 1.
Oligonucleotides for Real-Time RT-PCR
| mRNA or miRNA Targets | Description | Sense | Antisense |
|---|---|---|---|
| Dicer1 | Ribonuclease type III | CACTACAACACTATTACTGATT | GTGCTTGGTTATGAGGTA |
| Hprt | Hypoxanthine phosphoribosyltransferase 1 | AAGACAGCGGCAAGTTGAATC | AAGGGACGCAGCAACAGAC |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | CCAAGGAGTAAGAAACCC | TTGATGGTATTCGAGAGAAGG |
| Actb | β-Actin | GAAGTGTGACGTTGACAT | ACATCTGCTGGAAGGTG |
Note. RT-PCR = reverse transcription-polymerase chain reaction.
Cell Adhesion Assays
Angiogenesis is a multistep process involving cell adhesion, proliferation, migration, and morphogenesis (52). To determine the effects of Dicer1 in regulation of the adhesion capacity of CMVECs, cells were transfected with control plasmid, Dicer1 siRNA, or Dicer1 cDNA. After 48 hours, they were collected, washed, counted, and labeled with the fluorescent CyQuant dye (Invitrogen; incubation time: 60 minutes at 37°C). Equal amounts of cells, stimulated with VEGF (100ng/mL), were seeded in 96-well plates previously coated with 50 µL of either vitronectin (1.6 µg/mL), collagen (50 µg/mL), fibronectin (50 µg/mL), laminin (50 µg/mL), or bovine serum albumin (BSA, 12 µg/ml), which was used as the negative control. After 3 hours of incubation at 37°C, unattached cells were removed by rinsing the wells three times with warm PBS. The ratio of adhering cells was quantified by assessing the background-corrected fluorescence (excitation/emission: 508/527nm, respectively) using an Infinite M200 plate reader (Tecan, Research Triangle Park, NC).
As an additional measurement, we used electric cell–substrate impedance sensing (ECIS) technology (Applied Biophysics, Troy, NY) to monitor adhesion of CMVECs to collagen. Briefly, VEGF (100ng/mL) stimulated cells were seeded in collagen coated 96-well array culture dishes containing gold film surface electrodes (ECIS 96W1E). The same numbers of cells were added to each well in complete cell culture medium (2.5 × 105 cells/well). The arrays were placed in an incubator and the time course for changes of capacitance (at 60kHz) due to the adhesion of cells to the active electrode was obtained. The inverse of the time to reach 50% cell adhesion (100% change corresponds to the maximum level of cell coverage reached on the active electrode) was used as an index of adhesiveness.
Cell Proliferation Assay
Cell proliferation capacity was assessed in CMVECs transfected with Dicer1 siRNA, Dicer1 full-length cDNA encoding plasmid, or the respective scrambled control plasmids using the flow cytometry-based Guava CellGrowth assay (Guava Technologies, Hayward, CA) as recently reported (51). Briefly, cells were collected, resuspended in PBS containing 0.1% BSA, and stained with 16 µmol/L carboxyfluorescein diacetate succinimidyl ester (CFSE) for 15 minutes at 37°C. This dye diffuses into cells and is cleaved by intracellular esterases to form an amine-reactive product that produces a detectable fluorescence and binds covalently to intracellular lysine residues and other amine sources. Upon cell division, CFSE divides equally into the daughter cells halving the CFSE concentration of the mother cell; therefore, there is an inverse correlation between the fluorescence intensity and the proliferation capacity of the cells. After incubation, unbound dye was quenched with serum-containing medium. Then, cells were washed three times and incubated for 24 hours with 100ng/mL VEGF. Finally, cells were collected, washed, stained with propidium iodide (to gate out dead cells), and analyzed with a flow cytometer (Guava EasyCyte 8HT; Millipore, Billerica, MA). The inverse of the fluorescence intensity was used as an index of proliferation.
Assessment of Cell Migration by ECIS-Based Wound-Healing Assay
The ECIS technology was used to monitor migration of CMVECs in a wound-healing assay as reported (51). Briefly, CMVECs (2.5 × 105 cells/well) were seeded in 96-well array culture dishes (ECIS 96W1E), placed in an incubator (37°C), and changes in resistance and impedance were continuously monitored. When impedance reached a plateau, cells in each well were subjected to an elevated field pulse (“wounding”) of 5 mA applied for 20 seconds at 100kHz, which killed the cells present on the small active electrode due to severe electroporation. The detachment of the dead cells was immediately evident as a sudden drop in resistance (monitored at 4,000 Hz) and a parallel increase in conductance. VEGF (100ng/mL) was immediately added to each well. CMVECs surrounding the active electrode that had not been subjected to the wounding then migrated inward to replace the detached dead cells resulting in resistance recovery (continuously monitored at 4,000 Hz for up to 24 hours). Time to reach 50% resistance recovery (corresponding to 50% confluence on the active electrode) was determined for cells in each experimental group and this parameter, and the known physical dimensions of the electrode were used to calculate the migration rate (expressed as µm/h).
Tube Formation Assay
To investigate the influence of age and Dicer1 on tube formation ability, 24 hours after transfection with Dicer1 siRNA or Dicer1 full-length cDNA encoding plasmid or the respective scrambled control plasmids, young and aged CMVECs were plated on Geltrex Reduced Growth Factor Basement Membrane Matrix (Invitrogen) in Medium 200PRF (Invitrogen). Briefly, 150 μl/well of Geltrex was distributed in ice-cold 24-well plates. The gel was allowed to solidify while incubating the plates for 30 minutes at 37°C. CMVECs were then seeded at a density of 5 × 104 cells/well and placed in the incubator for 24 hours. Microscopic images were captured using a Nikon Eclipse Ti microscope equipped with a ×10 phase-contrast objective (Nikon Instruments, Melville, NY). The extent of tube formation was quantified by measuring total tube length in five random fields per well using NIS-Elements microscope imaging software (Nikon Instruments), as recently reported (51). The mean of the total tube length per total area imaged (µm tube/mm2) was calculated for each well. Experiments were run in quadruplicates. The experimenter was blinded to the groups throughout the period of analysis.
Data Analysis
Statistical analyses were performed using one-way analysis of variance. p < .05 was considered statistically significant. Data are expressed as means ± SEM.
Results
Age-Related Decline in Dicer1 Expression in the Rat Cerebrovasculature
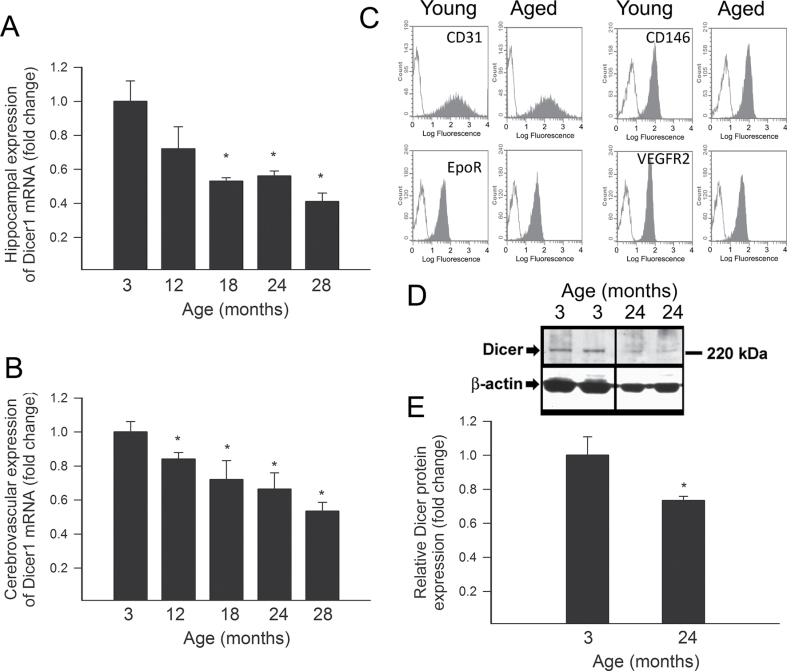
A quantitative real-time RT-PCR technique was used to analyze the effect of aging on Dicer1 expression in the hippocampus and in microdissected branches of the middle cerebral arteries of F344 × BN rats. We found that both hippocampal (Figure 1A) and cerebrovascular (Figure 1B) Dicer1 expression progressively declined with age. To analyze Dicer1 expression specifically in CMVECs, we isolated primary CMVECs from young and aged rats using an immunomagnetic isolation technique. To evaluate the specificity of immunomagnetic isolation technique, flow cytometry was performed in each cell strain (five independent strains were individual isolates from five different brains per age group). Flow cytometric analysis showed that after the third cycle of immunomagnetic selection, there were virtually no CD31−, CD146−, EpoR−, and VEGFR2− cells in the resultant cell populations (Figure 1B). We found that both young and aged CMVECs showed comparable and abundant expression of CD31, CD146, EpoR, and VEGFR2 (Figure 1B). Western blotting showed that CMVECs derived from aged rats exhibit a significantly decreased expression of Dicer1 (Figure 1C and D).
Figure 1.
(A) Quantitative real-time reverse transcription-polymerase chain reaction data showing age-related downregulation of mRNA expression of Dicer in microdissected small branches of the middle cerebral arteries in F344 × BN rats. Data are mean ± SEM (n = 5–7). *p< .05 vs 3-month-old controls. (B) Flow cytometric analysis of endothelial markers in primary cerebromicrovascular endothelial cells (CMVECs) derived from young (3 months old) and aged (24 months old) F344 × BN rats. Analysis of anti-CD31, anti-CD146, anti-EpoR, and anti-VEGFR2 reactivity (filled areas) shows the high purity of endothelial cell cultures. Appropriate isotype controls (blank areas) are also shown. (C) Original Western blots showing protein expression of Dicer in CMVECs isolated from 3- and 24-month-old F344 × BN rats. β-Actin was used for normalization purposes. Bar graphs (Panel D) are summary densitometric values. Data are mean ± SEM. *p < .05 vs 3-month-old control.
Age-Associated Oxidative Stress Is Associated With Downregulation of Dicer1 in CMVECs
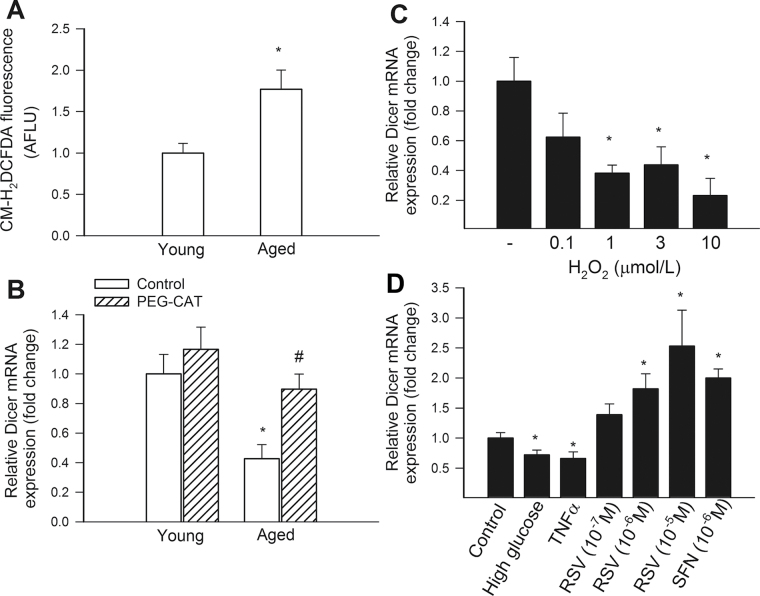
Using a CM-H2DCFDA fluorescence-based method, we demonstrated that aging results in increased H2O2 production in CMVECs (Figure 2A). The finding that scavenging of H2O2 significantly increases Dicer1 expression in aged CMVECs suggests that age-related oxidative stress and dysregulation of Dicer1 are causally related (Figure 2B). Further support for this concept is provided by the experiments showing that administration of exogenous H2O2 results in significant downregulation of Dicer1 expression in young CMVECs (Figure 2C), mimicking the aging phenotype. We found that experimental hyperglycemia and treatment with TNFα (for 24 hours), which are also known to increase cellular ROS production (53), also result in significant downregulation of Dicer1 in young CMVECs (Figure 2D). Interestingly, short-term exposure (2–4 hours) to high glucose resulted in an upregulation of Dicer1 (data not shown).
Figure 2.
(A) Flow cytometry data showing that compared with young cells, aged cerebromicrovascular endothelial cells (CMVECs) exhibit increased peroxide production, as indicated by the significantly increased CM-H2DCFDA fluorescent signal. Data were obtained using n = 5 cell strains (samples run in triplicates) in each group. Data are mean ± SEM. *p < .05 vs 3-month-old control. (B) Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) data showing the effect of treatment with polyethylene glycol–catalase on mRNA expression of Dicer1 in CMVECs isolated from young and aged F344 × BN rats. Data are mean ± SEM (n = 5 in each group). *p < .05 vs young controls; # p < .05 vs untreated aged CMVECs. (C and D) Quantitative real-time RT-PCR data showing the effect of treatment with the oxidative stressors H2O2 (Panel C), TNFα and high glucose (Panel D) and the Nrf2 activators resveratrol and sulforaphane (Panel D) on mRNA expression of Dicer1 in young CMVECs. Data are mean ± SEM (n = 5 in each group). *p < .05 vs untreated controls.
Treatment of CMVECs with two structurally different inducers of Nrf2 activation, resveratrol (44), and sulforaphane, resulted in a significant upregulation of Dicer1 (Figure 2D). Yet, presently, it is unclear whether the upregulation of Dicer1 expression in CMVECs upon resveratrol and sulforaphane treatment is mediated directly by Nrf2 binding to the Dicer1 promoter. We used rVISTA (http://rvista.dcode.org/), a tool that combines transcription factor-binding sites database search with a comparative sequence analysis (54), to confirm the presence of an ARE consensus sequence in the 5′ flanking region of the human Dicer1 gene (ATGACTGAGCA) and the mouse Dicer1 gene (TGCTGGATCAC). However, we could not identify a conserved ARE consensus sequence in the promoter region of the rat Dicer1 gene.
Downregulation of Dicer1 Is Associated With Alterations in miRNA Expression Profile of CMVECs
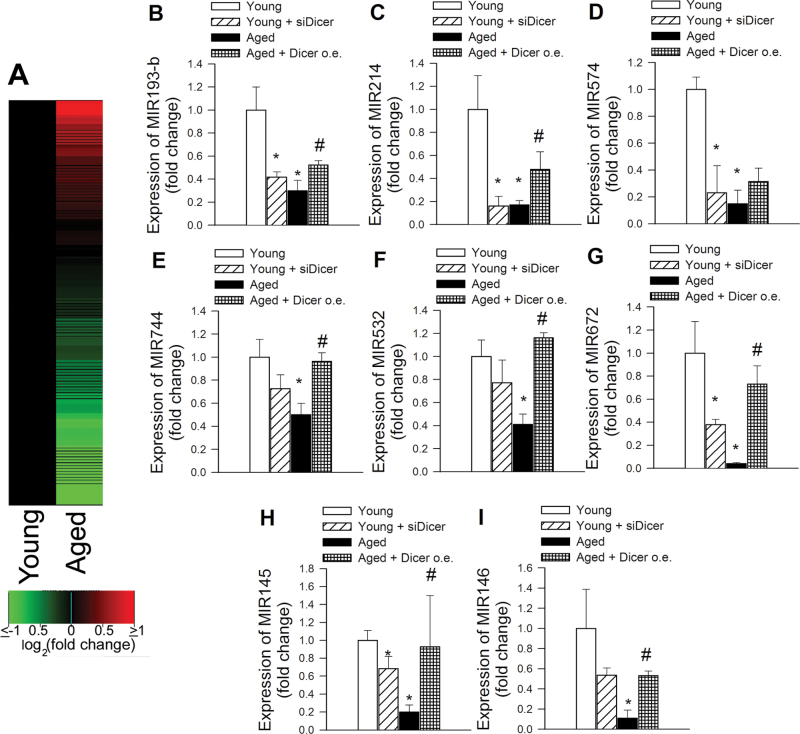
To determine whether age-related downregulation of Dicer1 is associated with alterations in miRNA expression in CMVECs, we first determined global miRNA expression profiles of five independent strains of young CMVECs and five independent strains of aged CMVECs using microfluidic cards containing TaqMan primers and probes for mature rodent miRNAs. Using the Rodent MicroRNA A+B Cards Set v3.0 TaqMan Array, we detected the expression of 148 miRNAs in young and aged CMVECs. We found that the majority (89%) of miRNAs that exhibited significant age-related changes in their expression level were downregulated (Figure 3A). To assess the role of Dicer1 in age-related alterations in miRNA expression patterns, we chose a subset of miRNAs that were underexpressed by more than 50% in aged CMVECs. This subset of miRNAs is known to be expressed in various endothelial cell strains and are thought regulate the expression of a number of genes regulating vital cell cycle processes, such as apoptosis, proliferation, and angiogenesis. The expression level of these miRNAs was further analyzed in aged CMVECs with overexpression of Dicer1 and in young CMVECs with siRNA knockdown of Dicer1. We found that increasing Dicer1 expression in aged CMVECs significantly increased expression miR-193-b, miR-214, miR-744, miR-532, miR-672, miR-145, and miR-146 and tended to increase expression of miR-574 (Figure 3B–I). In contrast, expression of these miRNAs was decreased by siRNA knockdown of Dicer1 in young CMVECs, mimicking the aging phenotype.
Figure 3.
Effects of aging and downregulation of Dicer on miRNA expression in cerebromicrovascular endothelial cells (CMVECs). (A) miRNA expression patterns of five young and five aged CMVEC strains were examined using microfluidic cards containing TaqMan probes and primer pairs for mature rodent miRNAs. A total of 138 miRNAs were expressed at a significant level in CMVECs. The two columns correspond to the expression profiles of young and aged CMVECs, and each row corresponds to a miRNA. The color in each cell reflects the mean level of expression of the corresponding miRNA in the cells, relative to the mean level of expression in young CMVECs. The increasing intensities of green mean that a specific miRNA has a lower mean expression in aged CMVECs and the increasing intensities of red mean that this miRNA has a higher expression in aged CMVECs. The scale reflects mean miRNA abundance ratio relative to the mean level in young CMVECs. Panels B–I: Quantitative real-time reverse transcription-polymerase chain reaction data showing expression of MIR193-b, MIR214, MIR574, MIR744, MIR532, MIR672, MIR145, and MIR146 in young CMVECs, young CMVECs with siRNA knockdown of Dicer (siDicer), aged CMVECs, and aged CMVECs with overexpression (o.e.) of Dicer. Data are means ± SEM (n = 5 in each group). *p < .05 vs young control CMVECs, # p < .05 vs untreated aged CMVECs.
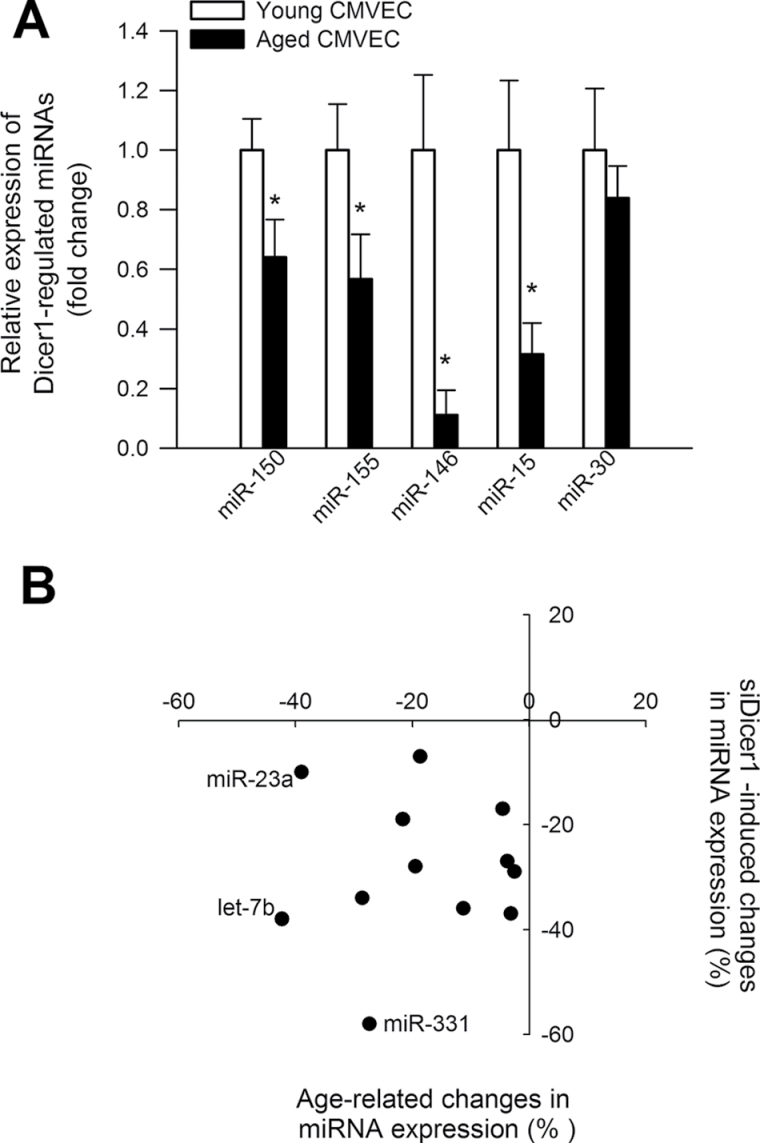
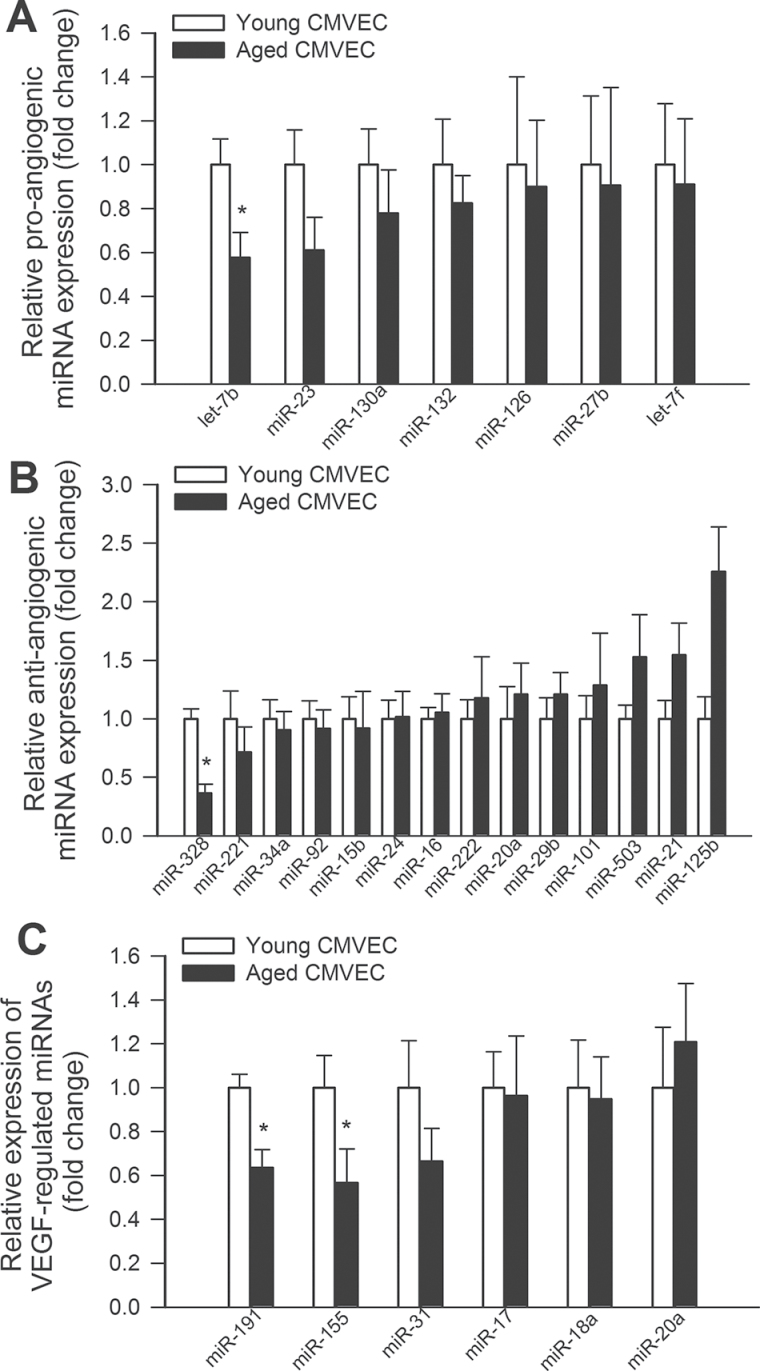
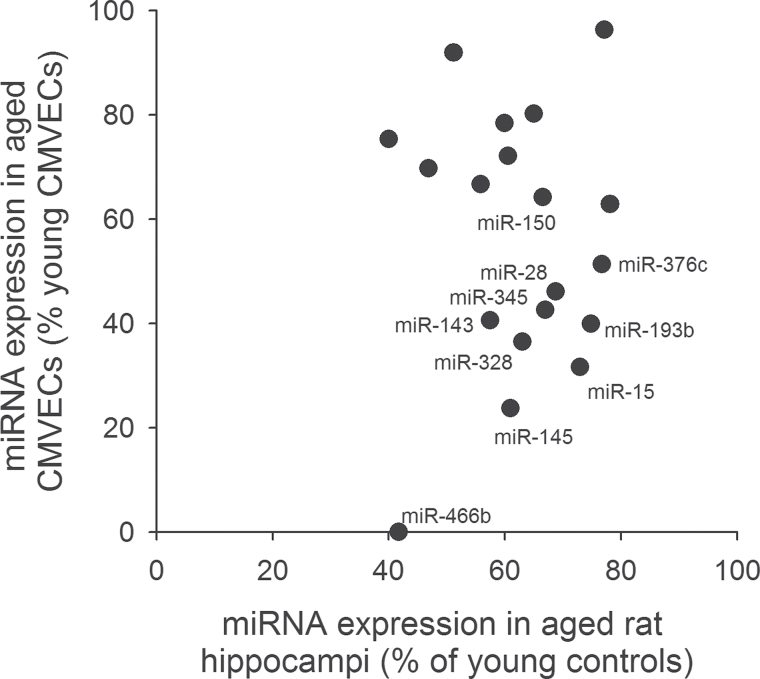
We also analyzed the expression levels of known Dicer1-dependent miRNAs in CMVECs derived from young and aged rats. Figure 4A depicts expression of miRNA species, which were demonstrated in previous studies to be significantly downregulated in tissues of Dicer fl/fl Tie2cre + mice compared with that in Dicer fl/fl Tie2cre − control mice (55). We found that several Dicer1-regulated genes were underexpressed in aged CMVECs, including miR-150, a known regulator of endothelial cell migration (56) (Figure 4A). Panel B depicts expression of miRNA species, which were demonstrated in previous studies to be significantly downregulated in human endothelial cells after siRNA knockdown of Dicer1 (30). We found that expression of miRNAs, which are downregulated by siDicer1 treatment, also exhibits age-related declines (Figure 4B). We also analyzed the expression levels of known proangiogenic and antiangiogenic miRNAs (57), respectively, in CMVECs derived from young and aged animals (Figure 5A and B). We found that expression of let-7b significantly decreased in CMVECs derived from aged animals (Figure 5A), whereas age-related decline in expression of other proangiogenic miRNAs did not reach statistical significance. Previous studies identified VEGF-regulated endothelial miRNAs (32), which likely play a role in regulation of VEGF signaling and angiogenesis. We found that expression levels miR191 and miR155 were significantly decreased in CMVECs derived from aged animals (Figure 5C). Expression of miR31 also tended to decrease in aged CMVECs but the difference did not reach statistical significance. To further substantiate the in vivo significance of our findings, we have analyzed miRNA expression profile in the hippocampi of young and aged rats. We have identified a subset of miRNAs that were both significantly downregulated in the hippocampi of aged rats and the expression of which could be detected in cultured CMVECs. Figure 6 shows the expression of miRNAs, which are downregulated in aged hippocampi and in aged CMVECs.
Figure 4.
Expression levels of known Dicer1-dependent miRNAs in cerebromicrovascular endothelial cells (CMVECs) derived from young and aged rats. Panel A depicts expression of miRNA species, which were demonstrated in previous studies to be significantly downregulated in tissues of Dicer fl/fl Tie2cre + mice compared with that in Dicer fl/fl Tie2cre − control mice (55). *p < .05 vs young CMVECs. Panel B depicts expression of miRNA species, which were demonstrated in previous studies to be significantly downregulated in human umbilical vein endothelial cells after siRNA knockdown of Dicer1 (30). Note that direction of siDicer1-induced changes and age-related changes in the endothelial expression of each miRNA is similar.
Figure 5.
Panels A and B depict the expression levels of known proangiogenic and antiangiogenic miRNAs (57), respectively, in cerebromicrovascular endothelial cells (CMVECs) derived from young and aged animals. *p < .05 vs young CMVECs. Panel C: Expression levels of known vascular endothelial growth factor (VEGF) regulated miRNAs in CMVECs derived from young and aged animals. *p < .05 vs young CMVECs. List of VEGF-regulated endothelial miRNAs is taken from reference (32).
Figure 6.
(A) Expression of miRNAs, which are downregulated in hippocampi of aged rats and in aged cerebromicrovascular endothelial cells.
Downregulation of Dicer1 Is Associated With Impaired Adhesion of CMVECs to Extracellular Matrix Proteins
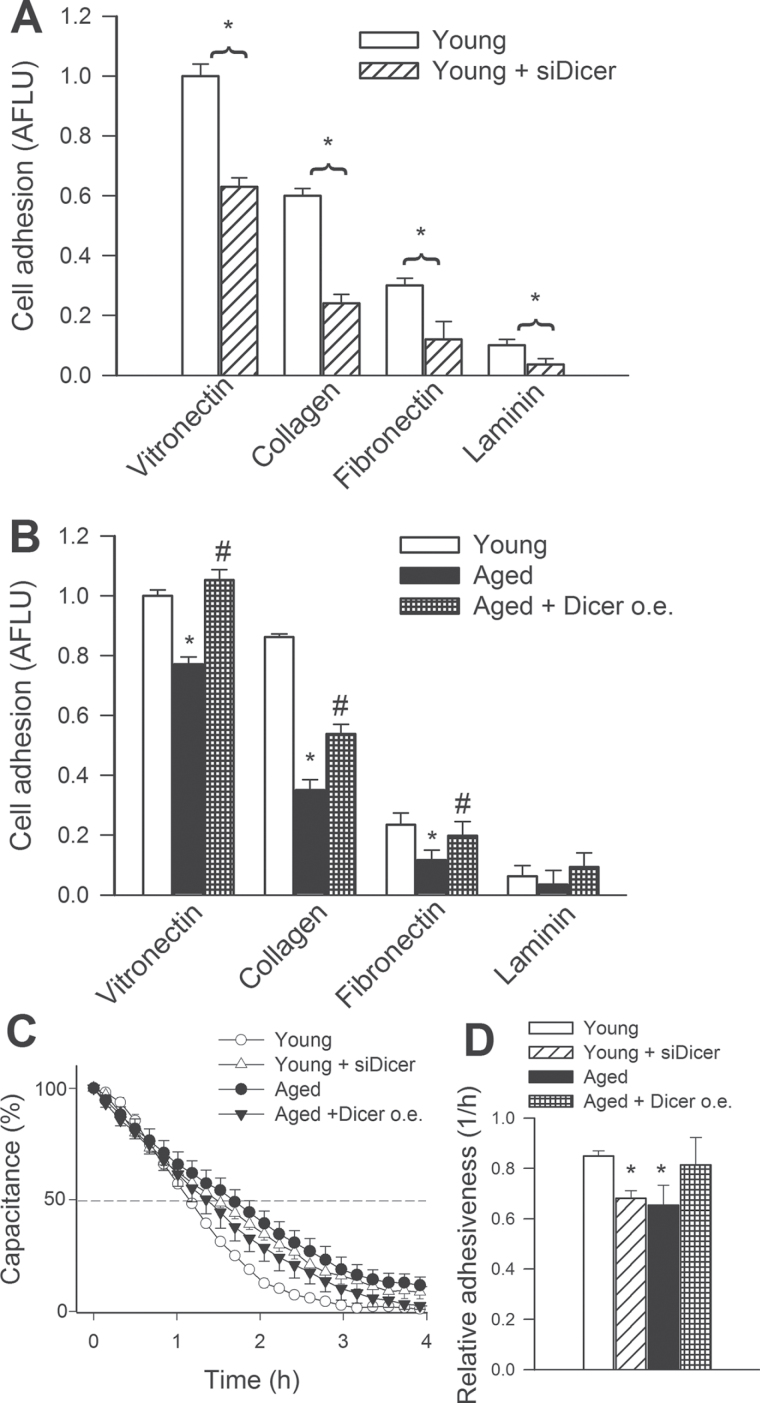
Despite the rapid progress of aging research in the last few years (58–94), there are no studies to our knowledge investigating the role of Dicer1 and miRNAs in age-related endothelial alterations that contribute to the development of VCI. To determine the role of Dicer1 on angiogenic capacity, we overexpressed Dicer1 in aged CMVECs and knocked down Dicer1 using siRNA in young CMVECs. Cell adhesion experiments were performed to investigate whether downregulation of Dicer1 affects VEGF-induced adhesion of CMVECs to different components of the extracellular matrix. We found that disruption of Dicer1 signaling by Dicer1 siRNA treatment impaired the ability of CMVECs to adhere to vitronectin, collagen, fibronectin, and laminin (Figure 7A). Aged CMVECs exhibited impaired adhesion to vitronectin, collagen, and fibronectin (Figure7B). The ability of aged CMVECs to adhere to these extracellular matrix components was significantly improved by overexpression of Dicer1 (Figure 7B).
Figure 7.
Disruption of Dicer-dependent pathways by siRNA knockdown of Dicer (siDicer) significantly impairs adhesion capacity of cerebromicrovascular endothelial cells (CMVECs) derived from young F344 × BN rats. CMVECs, loaded with the fluorescent dye CyQuant and stimulated with vascular endothelial growth factor (VEGF) (100ng/mL) were seeded in vitronectin, collagen, fibronectin, or laminin-coated plates (Methods section). After 3h of incubation, nonadherent cells were washed away, and the ratio of adhering cells was quantified by assessing the background-corrected fluorescence at 508/527nm. Data are expressed as normalized arbitrary fluorescence light units (means ± SEM) (n = 5 in each group), *p < .05 vs young control. (B) Adhesion capacity of CMVECs isolated from aged F344 × BN rats is impaired compared with that of young cells, and it is significantly improved by overexpression (o.e.) of Dicer. Data are means ± SEM (n = 5 in each group), *p < .05 vs young control, # p < .05 vs untreated. (C) Analysis of VEGF (100ng/mL) stimulated cell adhesion by electric cell–substrate impedance sensing technology (Methods section). Panel C: Time course of changes of capacitance (at 60kHz) after addition of CMVECs to collagen-coated wells. 100% change corresponds to the maximum level of cell coverage reached on the active electrode. Time to reach 50% cell adhesion was obtained, and its inverse was used as an index of adhesiveness. Panel D depicts the summary data for relative cell adhesiveness in the four experimental groups. Data are means ± SEM (n = 5 in each group), *p < .05 vs young control.
In other experiments, we used ECIS technology to monitor changes of capacitance (at 60kHz) due to the adhesion of VEGF (100ng/mL) stimulated cells to the collagen-coated active electrode (Figure 7C). Time to reach 50% cell adhesion was used to calculate an index of adhesiveness. We found that aged CMVECs, but not aged CMVECs overexpressing Dicer1, exhibited impaired adhesiveness to collagen (Figure 7D). Further, siRNA knockdown of Dicer1 significantly impaired the ability of VEGF-treated young CMVECs to adhere to collagen, mimicking the aging phenotype (Figure 7D).
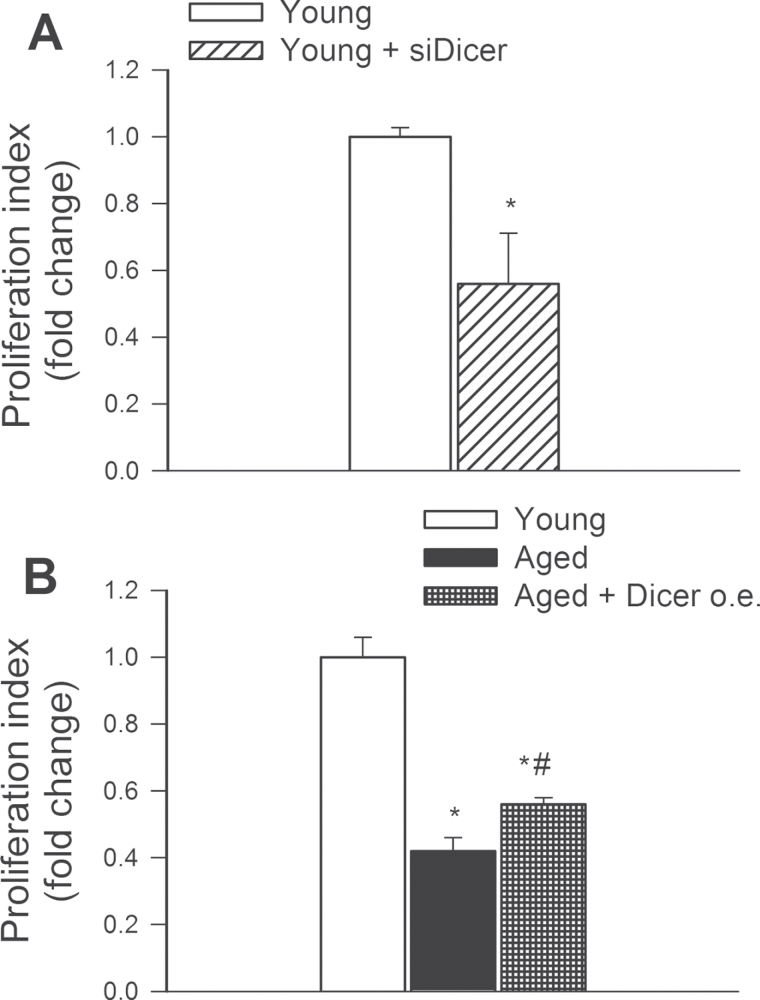
Downregulation of Dicer1 Is Associated With Impaired Proliferative Capacity of CMVECs
Proliferation represents a key step in angiogenesis. Proliferative capacity of young and aged CMVECs was compared after incubation with VEGF for 24 hours. We found that siRNA knockdown of Dicer1 significantly increased CFSE fluorescence in CMVECs, indicating that proliferation capacity is impaired by Dicer1 dysregulation (Figure 8A). Aged CMVECs exhibited impaired proliferative capacity, which was significantly improved by overexpression of Dicer1 (Figure 8B).
Figure 8.
(A) Disruption of Dicer-dependent pathways by siRNA knockdown of Dicer (siDicer) significantly impairs proliferation capacity of cerebromicrovascular endothelial cells (CMVECs) isolated from young F344 × BN rats. Cell proliferation capacity was assessed in CMVECs stimulated with vascular endothelial growth factor (100ng/mL) using the flow cytometry based Guava CellGrowth assay (Methods section). As index of proliferation capacity, the inverse of the mean fluorescence intensity of the indicator dye CFSE was used. Data are means ± SEM (n = 5 in each group), *p < .05 vs control. (B): Proliferation capacity of CMVECs isolated from aged F344 × BN rats is impaired compared with that of young cells and it is significantly improved by overexpression (o.e.) of Dicer. Data are means ± SEM (n = 5 in each group), *p < .05 vs young control, # p < .05 vs untreated.
Downregulation of Dicer1 Is Associated With Impaired Migratory Capability of CMVECs
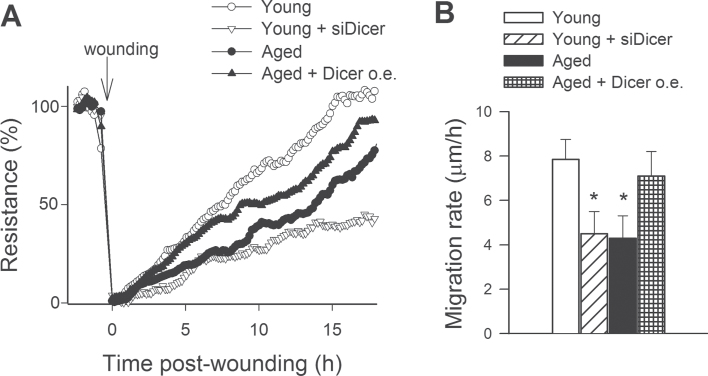
The migratory capability of vascular endothelial cells has a pivotal role in the maintenance of microvascular integrity and angiogenesis. An ECIS-based wound-healing assay was used to assess the effect of downregulation of Dicer1 on migratory capability of VEGF-treated CMVECs (Figure 9A). We found that aged CMVECs exhibited impaired migratory capability compared with young CMVECs (Figure 9B). In contrast, the calculated migration rate of aged CMVECs with overexpression of Dicer1 did not differ from that of young CMVECs (Figure 9B). In young CMVECs, siRNA knockdown of Dicer1 significantly decreased migration rate, mimicking the aging phenotype (Figure 9B).
Figure 9.
Migration capacity of cerebromicrovascular endothelial cells (CMVECs) isolated from aged F344 × BN rats is impaired compared with that of cells isolated from young F344 × BN rats, and it is significantly improved by overexpression (o.e.) of Dicer. In contrast, disruption of Dicer-dependent pathways by siRNA knockdown of Dicer (siDicer) significantly impairs migration capacity of young CMVECs, mimicking the aging phenotype. Vascular endothelial growth factor (100ng/mL) stimulated cell migration was monitored by electric cell-substrate impedance sensing technology in a wound-healing assay (Methods section). Panel A: Time course of resistance recovery after wounding (electric pulse of 5 mA for 20 s at 60kHz; 100% represents prewounding levels). Resistance (at 4,000 Hz) was monitored in every 160 s. Data are mean ± SEM (n = 5 in each group). Time to reach 50% resistance recovery (corresponding to 50% confluence on the active electrode) was determined for each group, and this parameter and the known physical dimensions of the electrode were used to calculate the migration rate (expressed as µm/h). Panel B depicts the summary data for migration rate in each group. Data are means ± SEM (n = 5 in each group), *p < .05 vs young control.
Downregulation of Dicer1 Is Associated With Impaired Formation of Capillary-Like Structures by CMVECs
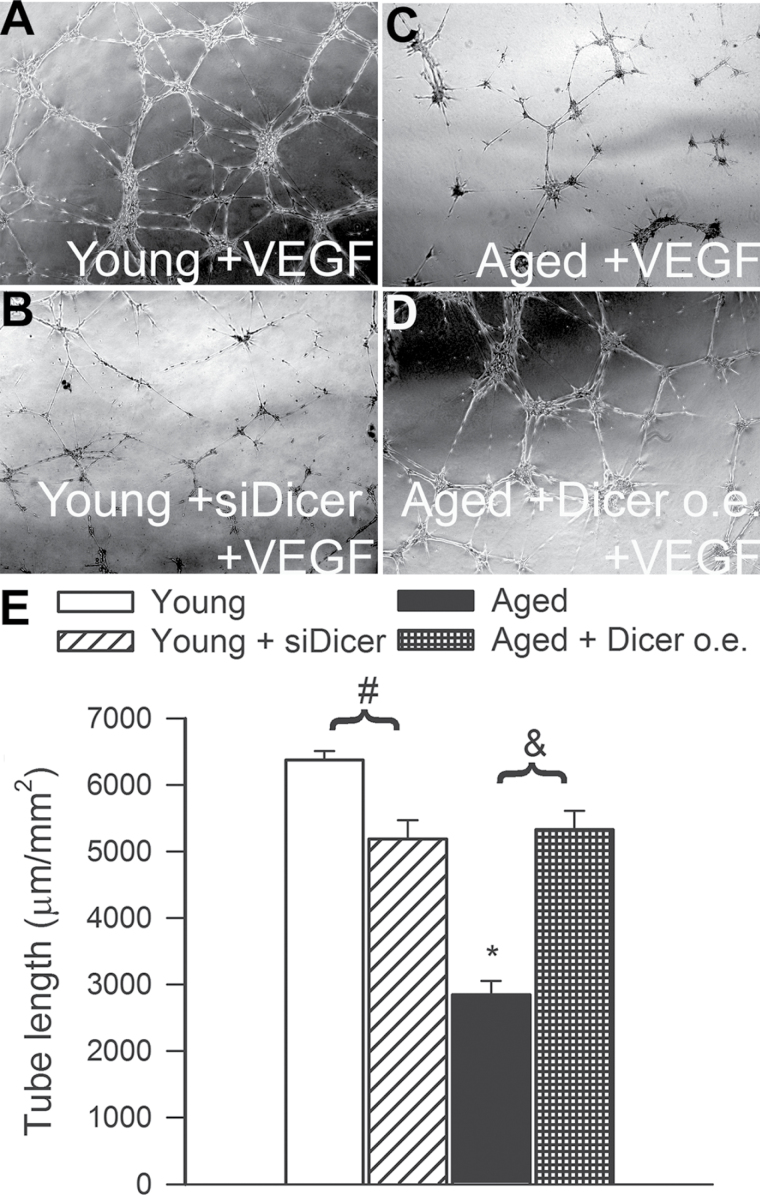
When seeded onto Geltrex matrices, young CMVECs formed elaborated capillary networks in the presence of VEGF (Figure 10A). We found that siRNA knockdown of Dicer1 significantly inhibited the formation of capillary-like structures by young CMVECs (Figure 10B and E). Compared with young cells in aged CMVECs, formation of capillary-like structures was significantly impaired (Figure 10C and E). The finding that overexpression of Dicer1 significantly improved formation of capillary-like structures by aged CMVECs (Figure 10D and E) suggests that age-related downregulation of Dicer1 is causally linked to the impaired angiogenic capacity of aged endothelial cells.
Figure 10.
Tube-forming ability of cerebromicrovascular endothelial cells (CMVECs) isolated from aged F344 × BN rats is impaired compared with that of cells isolated from young F344 × BN rats, and it is significantly improved by overexpression (o.e.) of Dicer. In contrast, disruption of Dicer-dependent pathways by siRNA knockdown of Dicer (siDicer) significantly impairs the ability of young CMVECs to form capillary-like structures, mimicking the aging phenotype. CMVECs were plated on Geltrex matrix-coated wells, and tube formation was induced by treating cells with vascular endothelial growth factor (100ng/mL, for 24h). Representative examples of capillary-like structures are shown in Panel A. Summary data, expressed as total tube length per total area scanned (µm tube/mm2), are shown in Panel B. Data are means ± SEM (n = 5 in each group), *p < .05 vs control.
Discussion
The principal new findings of this study are that (1) age-related dysregulation of Dicer1-dependent miRNA expression is associated with impaired angiogenic response in aged rat CMVECs, that (2) a functional Dicer1-dependent pathway is essential for a healthy angiogenic response of CMVECs, and that (3) upregulation of Dicer1 in aged CMVECs confers proangiogenic effects, counteracting, at least in part, the adverse effects of aging.
To our knowledge, this is the first study demonstrating that expression of Dicer1 significantly declines with age in the cerebral circulation (Figure 1). Demonstration of age-related downregulation of Dicer1 in the CMVECs (Figure 1E) is particularly important, as Dicer1-dependent pathways have been shown to regulate, in addition to angiogenesis (30–33), multiple aspects of cellular physiology relevant for vascular aging, including replicative senescence (95,96), mechanotransduction (97), NO production (31,97), endothelial apoptosis (98), and inflammation (31). Age-related alterations in Dicer1 expression in other organs have not yet been documented, but previous studies have linked decreased Dicer1 expression to induction of a premature senescence cellular phenotype both in vivo and in vitro (99).
Previous studies demonstrate that oxidative stress contributes to age-related microvascular alterations in aging (100) and that age-related oxidative stress is associated with a decreased angiogenic capacity of endothelial cells (20,21,101,102). Our studies demonstrate that increased H2O2 levels promote downregulation of Dicer1 expression in aged CMVECs (Figure 2), suggesting a novel mechanism linking age-related oxidative stress to microvascular impairment. The mechanisms involved in H2O2-dependent transcriptional regulation of Dicer1 in endothelial cells are presently unknown and need to be elucidated in future studies. There are data suggesting that redox signaling in endothelial cells is subject to regulation by miRNAs (103); thus, it is likely that this pathway is regulated by a series of complex feedback loops. Our findings showing upregulation of Dicer1 by resveratrol (Figure 2D) are interesting, as resveratrol treatment was shown to increase capillary density in the brain of aged mice (104). Our previous studies also demonstrated that resveratrol treatment attenuates oxidative stress in vascular endothelial cells in aged mice (105).
We have identified a number of miRNAs that are downregulated in CMVECs during physiological aging (Figures 3–5). We posit that age-related oxidative stress and downregulation of Dicer1 are causally involved in dysregulation of miRNAs in aged CMVECs (Figure 3). This concept is supported by the findings that overexpression of Dicer1 in aged CMVECs increased expression of age-sensitive miRNAs (Figure 3). Furthermore, knockdown of Dicer1 resulted in downregulation of the same miRNAs, mimicking the aging phenotype (Figure 3). Previous studies using Dicer fl/fl Tie2cre + mice (55) and cultured human umbilical vein endothelial cells with siRNA knockdown of Dicer1 (30) have identified a group of miRNAs the production of which is Dicer1-dependent. We found age-related changes in the expression levels of these known Dicer1-dependent miRNAs are consistent with the diminished expression of Dicer1 in aged CMVECs (Figure 4). It should be noted that although the majority of miRNAs that exhibited significant age-related changes in their expression level were downregulated, there were also certain miRNAs that were upregulated in aged endothelial cells. These alterations cannot be explained by age-related downregulation of Dicer1. Analysis of age-related changes in miRNA profiles and expression of factors involved in miRNA processing in other organs (106), including the heart (107), raises the possibility that age-related changes in the expression of additional factors involved in miRNA regulation (e.g., Ago1 and Ago2 proteins (107)) may also contribute to dysregulation of miRNA expression in aged CMVECs.
Interestingly, we found that expressions of most miRNAs, which are downregulated in aged CMVECs, are also downregulated in hippocampi of aged rats (Figure 6). However, we cannot conclude the aging results in similar changes in miRNA expression profile in every tissue. A recent analysis of miRNA expression profile in aortas of 18 months old mice showed a significant age-related decline (>1.5-fold) in the expression of 14 miRNAs (108). Yet, we could detect the expression of only four of these miRNAs in CMVECs, and only the expression of miR-31 exhibited an age-related decline in these cells (by 35% vs a 41% decline in aged mouse aortas). Recent in vitro studies identified changes in expression of various miRNAs (95,96) with increased number of passages, which have been implicated in governing senescence in human umbilical vein endothelial cells. We could not demonstrate consistent changes in these miRNAs in our study. Thus, further studies are warranted to analyze the overlap between age-related and in vitro and senescence-related changes in endothelial miRNA expression profile and to elucidate the precise functions of these miRNAs in regulating microvascular aging. It will also be important to identify miRNAs, the endothelial expression of which shows similar age-related changes across different species.
There is increasing experimental evidence for the involvement of miRNAs in the regulation of the angiogenic process (31,32,57). Here, we report that age-related dysregulation of Dicer1 is associated with significant changes in various pro- and antiangiogenic miRNAs and VEGF-regulated miRNAs (Figure 5). Our studies provide strong evidence that a functional Dicer1-dependent pathway is essential for a healthy endothelial angiogenic response in the cerebromicrovasculature because all the major steps of the angiogenic process, including adhesion (Figure 7), proliferation (Figure 8), migration (Figure 9), and formation of capillary-like structures (Figure 10), are compromised by disruption of Dicer1 signaling in CMVECs, extending previous findings in different cell types (30–33). Because overexpression of Dicer1 in aged CMVECs exerts proangiogenic effects, improving cell adhesion (Figure 7), proliferation (Figure 8), migration (Figure 9), and endothelial tube formation (Figure 10), it is likely that age-related decline in Dicer1 expression contributes to the aging-induced impairment of endothelial angiogenic capacity. Other mechanisms, which likely contribute to the induction of the antiangiogenic phenotype in CMVECs include circulating and/or paracrine IGF-1 deficiency (7,24,109), age-related Nrf2 dysfunction (51), and alterations in expression of pro- and anti-inflammatory factors in the brain (110).
The pathophysiological consequences of impaired angiogenesis associated with dysregulation of Dicer1-dependent pathways are likely multiple. Dicer1 depletion may decrease capillary density in the brain and negatively affect cerebral angiogenesis and/or collateral formation induced by physiological (e.g., exercise, local ischemia) or pharmacological stimuli. Indeed, aging results in cerebromicrovascular rarefaction (4,7) and impaired cerebral angiogenesis in response to hypoxia or VEGF administration (26,111). It is thought that microvascular rarefaction and impairment of compensatory proliferation of the cerebral resistance vessels and the capillary network play a prominent role in impairment of regional cerebral blood flow and the occurrence of VCI with age. Because the role of miRNA regulation and function in the aging cardiovascular system is a new emerging area, additional investigations are needed to study the contribution of individual miRNAs or miRNA clusters in controlling gene expression that underlie microvascular aging.
Funding
This work was supported by grants from the American Federation for Aging Research (to A.C.), the Oklahoma Center for the Advancement of Science and Technology (to A.C., Z.U., and W.E.S.), the American Heart Association (to A.C., P.T., and Z.U.), the National Institutes of Health (AG031085 to A.C.; AT006526 to Z.U.; AG038747, NS056218, and P01 AG11370 to W.E.S.), the Ellison Medical Foundation (to W.E.S.); the Arkansas Claude Pepper Older Americans Independence Center at University of Arkansas Medical Center (to A.C.); the Hungarian National Development Agency (TÁMOP/SROP-4.2.1/b-10/2/KONV-2010-0012, to ZU); and Hungarian National Science Research Funds (OTKA K71591).
Acknowledgments
The authors would like to express their gratitude for the support of the Donald W. Reynolds Foundation, which funds aging research at the University of Oklahoma Health Sciences Center under its Aging and Quality of Life Program. We would like to gratefully acknowledge Prof. Akos Koller (University of Pecs, Pecs, Hungary) for providing valuable comments on the manuscript.
References
- 1. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42: 2672–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011; 12: 723–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mitschelen M, Garteiser P, Carnes BA, et al. Basal and hypercapnia-altered cerebrovascular perfusion predict mild cognitive impairment in aging rodents. Neuroscience. 2009; 164: 918–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev. 2003; 2: 149–168 [DOI] [PubMed] [Google Scholar]
- 5. Khan AS, Lynch CD, Sane DC, Willingham MC, Sonntag WE. Growth hormone increases regional coronary blood flow and capillary density in aged rats. J Gerontol A Biol Sci Med Sci. 2001; 56: B364–B371 [DOI] [PubMed] [Google Scholar]
- 6. Lynch CD, Cooney PT, Bennett SA, et al. Effects of moderate caloric restriction on cortical microvascular density and local cerebral blood flow in aged rats. Neurobiol Aging. 1999; 20: 191–200 [DOI] [PubMed] [Google Scholar]
- 7. Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997; 138: 3515–3520 [DOI] [PubMed] [Google Scholar]
- 8. Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab. 1991; 11: 684–689 [DOI] [PubMed] [Google Scholar]
- 9. Moeller JR, Ishikawa T, Dhawan V, et al. The metabolic topography of normal aging. J Cereb Blood Flow Metab. 1996; 16: 385–398 [DOI] [PubMed] [Google Scholar]
- 10. Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001; 64: 575–611 [DOI] [PubMed] [Google Scholar]
- 11. Kawamura J, Terayama Y, Takashima S, et al. Leuko-araiosis and cerebral perfusion in normal aging. Exp Aging Res. 1993; 19: 225–240 [DOI] [PubMed] [Google Scholar]
- 12. Krejza J, Mariak Z, Walecki J, Szydlik P, Lewko J, Ustymowicz A. Transcranial color Doppler sonography of basal cerebral arteries in 182 healthy subjects: age and sex variability and normal reference values for blood flow parameters. AJR Am J Roentgenol. 1999; 172: 213–218 [DOI] [PubMed] [Google Scholar]
- 13. Schultz SK, O’Leary DS, Boles Ponto LL, Watkins GL, Hichwa RD, Andreasen NC. Age-related changes in regional cerebral blood flow among young to mid-life adults. Neuroreport. 1999; 10: 2493–2496 [DOI] [PubMed] [Google Scholar]
- 14. Bentourkia M, Bol A, Ivanoiu A, et al. Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: effect of aging. J Neurol Sci. 2000; 181: 19–28 [DOI] [PubMed] [Google Scholar]
- 15. Hagstadius S, Risberg J. Regional cerebral blood flow characteristics and variations with age in resting normal subjects. Brain Cogn. 1989; 10: 28–43 [DOI] [PubMed] [Google Scholar]
- 16. Pagani M, Salmaso D, Jonsson C, et al. Regional cerebral blood flow as assessed by principal component analysis and (99m)Tc-HMPAO SPET in healthy subjects at rest: normal distribution and effect of age and gender. Eur J Nucl Med Mol Imaging. 2002; 29: 67–75 [DOI] [PubMed] [Google Scholar]
- 17. Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res. 2002; 54: 25–35 [DOI] [PubMed] [Google Scholar]
- 18. Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011; 37: 56–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tarnawski AS, Pai R, Tanigawa T, Matysiak-Budnik T, Ahluwalia A. PTEN silencing reverses aging-related impairment of angiogenesis in microvascular endothelial cells. Biochem Biophys Res Commun. 2010; 394: 291–296 [DOI] [PubMed] [Google Scholar]
- 20. Bach MH, Sadoun E, Reed MJ. Defects in activation of nitric oxide synthases occur during delayed angiogenesis in aging. Mech Ageing Dev. 2005; 126: 467–473 [DOI] [PubMed] [Google Scholar]
- 21. Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem. 2003; 51: 1119–1130 [DOI] [PubMed] [Google Scholar]
- 22. Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010; 65: 1028–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahluwalia A, Tarnawski AS. Activation of the metabolic sensor-AMP activated protein kinase reverses impairment of angiogenesis in aging myocardial microvascular endothelial cells. Implications for the aging heart. J Physiol Pharmacol. 2011; 62: 583–587 [PubMed] [Google Scholar]
- 24. Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances J Gerontol A Biol Sci Med Sci. 2012;67: 599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murugesan N, Demarest TG, Madri JA, Pachter JS. Brain regional angiogenic potential at the neurovascular unit during normal aging. Neurobiol Aging. 2012; 33: 1004.e1–1004.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benderro GF, Lamanna JC. Hypoxia-induced angiogenesis is delayed in aging mouse brain. Brain Res. 2011; 1389: 50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ingraham JP, Forbes ME, Riddle DR, Sonntag WE. Aging reduces hypoxia-induced microvascular growth in the rodent hippocampus. J Gerontol A Biol Sci Med Sci. 2008; 63: 12–20 [DOI] [PubMed] [Google Scholar]
- 28. Bonauer A, Carmona G, Iwasaki M, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009; 324: 1710–1713 [DOI] [PubMed] [Google Scholar]
- 29. Doebele C, Bonauer A, Fischer A, et al. Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood. 2010; 115: 4944–4950 [DOI] [PubMed] [Google Scholar]
- 30. Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007; 101: 59–68 [DOI] [PubMed] [Google Scholar]
- 31. Suárez Y, Fernández-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007; 100: 1164–1173 [DOI] [PubMed] [Google Scholar]
- 32. Suárez Y, Fernández-Hernando C, Yu J, et al. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci USA. 2008; 105: 14082–14087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005; 280: 9330–9335 [DOI] [PubMed] [Google Scholar]
- 34. Boehm M, Slack F. A developmental timing microRNA and its target regulate life span in C. elegans. Science. 2005; 310: 1954–1957 [DOI] [PubMed] [Google Scholar]
- 35. Grillari J, Grillari-Voglauer R. Novel modulators of senescence, aging, and longevity: small non-coding RNAs enter the stage. Exp Gerontol. 2010; 45: 302–311 [DOI] [PubMed] [Google Scholar]
- 36. Ibáñez-Ventoso C, Yang M, Guo S, Robins H, Padgett RW, Driscoll M. Modulated microRNA expression during adult lifespan in Caenorhabditis elegans. Aging Cell. 2006; 5: 235–246 [DOI] [PubMed] [Google Scholar]
- 37. Bates DJ, Li N, Liang R, et al. MicroRNA regulation in Ames dwarf mouse liver may contribute to delayed aging. Aging Cell. 2010; 9: 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maes OC, An J, Sarojini H, Wang E. Murine microRNAs implicated in liver functions and aging process. Mech Ageing Dev. 2008; 129: 534–541 [DOI] [PubMed] [Google Scholar]
- 39. Ungvari Z, Bailey-Downs L, Gautam T, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011; 66: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Csiszar A, Podlutsky A, Podlutskaya N, et al. Testing the oxidative stress hypothesis of aging in primate fibroblasts: Is there a correlation between species longevity and cellular ROS production? J Gerontol A Biol Sci Med Sci. 2012;67:841–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ungvari Z, Sosnowska D, Podlutsky A, Koncz P, Sonntag WE, Csiszar A. Free radical production, antioxidant capacity, and oxidative stress response signatures in fibroblasts from Lewis dwarf rats: effects of life span-extending peripubertal GH treatment J Gerontol A Biol Sci Med Sci. 2011;66:501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Csiszar A, Ahmad M, Smith KE, et al. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol. 2006; 168: 629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Csiszar A, Labinskyy N, Jimenez R, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009; 130: 518–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010; 299: H18–H24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ungvari Z, Bailey-Downs L, Gautam T, et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011; 300: H1133–H1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Landthaler M, Gaidatzis D, Rothballer A, et al. Molecular characterization of human Argonaute-containing ribonucleoprotein complexes and their bound target mRNAs. RNA. 2008; 14: 2580–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bailey-Downs LC, Mitschelen M, Sosnowska D, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging J Gerontol A Biol Sci Med Sci. 2011;67:313–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bailey-Downs LC, Sosnowska D, Toth P, et al. Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012; 67: 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment J Gerontol A Biol Sci Med Sci. 2012; 67:811–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ungvari Z, Gautam T, Koncz P, et al. Vasoprotective effects of life span-extending peripubertal GH replacement in Lewis dwarf rats. J Gerontol A Biol Sci Med Sci. 2010; 65: 1145–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Valcarcel-Ares MN, Gautam T, Warrington JP, et al. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012; 67: 821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clapp C, Thebault S, Jeziorski MC, Martínez De La Escalera G. Peptide hormone regulation of angiogenesis. Physiol Rev. 2009; 89: 1177–1215 [DOI] [PubMed] [Google Scholar]
- 53. Ungvari Z, Bailey-Downs L, Sosnowska D, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011; 301: H363–H372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 2004; 32: W273–W279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou L, Seo KH, He HZ, et al. Tie2cre-induced inactivation of the miRNA-processing enzyme Dicer disrupts invariant NKT cell development. Proc Natl Acad Sci USA. 2009; 106: 10266–10271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hergenreider E, Heydt S, Tréguer K, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012; 14: 249–256 [DOI] [PubMed] [Google Scholar]
- 57. Suárez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res. 2009; 104: 442–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Behrens MI, Silva M, Schmied A, et al. Age-dependent increases in apoptosis/necrosis ratios in human lymphocytes exposed to oxidative stress. J Gerontol A Biol Sci Med Sci. 2011; 66: 732–740 [DOI] [PubMed] [Google Scholar]
- 59. Carnes BA, Riesch R, Schlupp I. The delayed impact of parental age on offspring mortality in mice. J Gerontol A Biol Sci Med Sci. 2012; 67: 351–357 [DOI] [PubMed] [Google Scholar]
- 60. Chandrashekara KT, Shakarad MN. Aloe vera or resveratrol supplementation in larval diet delays adult aging in the fruit fly, Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2011; 66: 965–971 [DOI] [PubMed] [Google Scholar]
- 61. Chung E, Diffee GM. Effect of aging on power output properties in rat skinned cardiac myocytes. J Gerontol A Biol Sci Med Sci. 2011; 66: 1267–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dekker P, van Baalen LM, Dirks RW, et al. Chronic inhibition of the respiratory chain in human fibroblast cultures: differential responses related to subject chronological and biological age. J Gerontol A Biol Sci Med Sci. 2012; 67: 456–464 [DOI] [PubMed] [Google Scholar]
- 63. Ding J, Berryman DE, Jara A, Kopchick JJ. Age- and sex-associated plasma proteomic changes in growth hormone receptor gene-disrupted mice. J Gerontol A Biol Sci Med Sci. 2012; 67: 830–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eghbalieh SD, Chowdhary P, Muto A, et al. Age-related neointimal hyperplasia is associated with monocyte infiltration after balloon angioplasty. J Gerontol A Biol Sci Med Sci. 2012; 67: 109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Florez H, Ma Y, Crandall JP, et al. Parental longevity and diabetes risk in the Diabetes Prevention Program. J Gerontol A Biol Sci Med Sci. 2011; 66: 1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Forman K, Vara E, García C, et al. Effect of a combined treatment with growth hormone and melatonin in the cardiological aging on male SAMP8 mice. J Gerontol A Biol Sci Med Sci. 2011; 66: 823–834 [DOI] [PubMed] [Google Scholar]
- 67. Gesing A, Masternak MM, Wang F, et al. Expression of key regulators of mitochondrial biogenesis in growth hormone receptor knockout (GHRKO) mice is enhanced but is not further improved by other potential life-extending interventions. J Gerontol A Biol Sci Med Sci. 2011; 66: 1062–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gunin AG, Kornilova NK, Vasilieva OV, Petrov VV. Age-related changes in proliferation, the numbers of mast cells, eosinophils, and cd45-positive cells in human dermis. J Gerontol A Biol Sci Med Sci. 2011; 66: 385–392 [DOI] [PubMed] [Google Scholar]
- 69. Hozawa A, Sugawara Y, Tomata Y, et al. Relationship between serum adiponectin levels and disability-free survival among community-dwelling elderly individuals: the Tsurugaya Project. J Gerontol A Biol Sci Med Sci. 2012; 67: 530–536 [DOI] [PubMed] [Google Scholar]
- 70. Inzitari M, Arnold AM, Patel KV, et al. Subclinical vascular disease burden and risk for death and cardiovascular events in older community dwellers. J Gerontol A Biol Sci Med Sci. 2011; 66: 986–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Katsumata Y, Todoriki H, Higashiuesato Y, et al. Metabolic syndrome and cognitive decline among the oldest old in Okinawa: in search of a mechanism. The KOCOA Project. J Gerontol A Biol Sci Med Sci. 2012; 67: 126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Labbé A, Garand C, Cogger VC, et al. Resveratrol improves insulin resistance hyperglycemia and hepatosteatosis but not hypertriglyceridemia, inflammation, and life span in a mouse model for Werner syndrome. J Gerontol A Biol Sci Med Sci. 2011; 66: 264–278 [DOI] [PubMed] [Google Scholar]
- 73. Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor kappaB and forkhead Box O phosphorylation. J Gerontol A Biol Sci Med Sci. 2011; 66: 409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lushchak OV, Gospodaryov DV, Rovenko BM, et al. Balance between macronutrients affects life span and functional senescence in fruit fly Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2012; 67: 118–125 [DOI] [PubMed] [Google Scholar]
- 75. McFarlane D, Wolf RF, McDaniel KA, White GL. Age-associated alteration in innate immune response in captive baboons. J Gerontol A Biol Sci Med Sci. 2011; 66: 1309–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mitterberger MC, Mattesich M, Klaver E, Piza-Katzer H, Zwerschke W. Reduced insulin-like growth factor-I serum levels in formerly obese women subjected to laparoscopic-adjustable gastric banding or diet-induced long-term caloric restriction. J Gerontol A Biol Sci Med Sci. 2011; 66: 1169–1177 [DOI] [PubMed] [Google Scholar]
- 77. Mojtahedi MC, Thorpe MP, Karampinos DC, et al. The effects of a higher protein intake during energy restriction on changes in body composition and physical function in older women. J Gerontol A Biol Sci Med Sci. 2011; 66: 1218–1225 [DOI] [PubMed] [Google Scholar]
- 78. Murias JM, Kowalchuk JM, Ritchie D, Hepple RT, Doherty TJ, Paterson DH. Adaptations in capillarization and citrate synthase activity in response to endurance training in older and young men. J Gerontol A Biol Sci Med Sci. 2011; 66: 957–964 [DOI] [PubMed] [Google Scholar]
- 79. Pérez VI, Cortez LA, Lew CM, et al. Thioredoxin 1 overexpression extends mainly the earlier part of life span in mice. J Gerontol A Biol Sci Med Sci. 2011; 66: 1286–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rochon J, Bales CW, Ravussin E, et al. Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. J Gerontol A Biol Sci Med Sci. 2011; 66: 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tazearslan C, Cho M, Suh Y. Discovery of functional gene variants associated with human longevity: opportunities and challenges. J Gerontol A Biol Sci Med Sci. 2012; 67: 376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yubero-Serrano EM, Gonzalez-Guardia L, Rangel-Zuñiga O, et al. Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress-related genes in elderly men and women. J Gerontol A Biol Sci Med Sci. 2012; 67: 3–10 [DOI] [PubMed] [Google Scholar]
- 83. Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J Gerontol A Biol Sci Med Sci. 2012; 67: 611–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fok WC, Zhang Y, Salmon AB, et al. Short-term treatment with rapamycin and dietary restriction have overlapping and distinctive effects in young mice J Gerontol A Biol Sci Med Sci. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cesari M, Kritchevsky SB, Nicklas B, et al. Oxidative damage, platelet activation, and inflammation to predict mobility disability and mortality in older persons: results from the health aging and body composition study. J Gerontol A Biol Sci Med Sci. 2012; 67: 671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Craft S, Foster TC, Landfield PW, Maier SF, Resnick SM, Yaffe K. Session III: mechanisms of age-related cognitive change and targets for intervention: inflammatory, oxidative, and metabolic processes. J Gerontol A Biol Sci Med Sci. 2012; 67: 754–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Horan MP, Pichaud N, Ballard JW. Review: quantifying mitochondrial dysfunction in complex diseases of aging. J Gerontol A Biol Sci Med Sci. 2012; 67: 1022–1035 [DOI] [PubMed] [Google Scholar]
- 88. Kosik KS, Rapp PR, Raz N, Small SA, Sweatt JD, Tsai LH. Mechanisms of age-related cognitive change and targets for intervention: epigenetics. J Gerontol A Biol Sci Med Sci. 2012; 67: 741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Miller RA. Genes against aging. J Gerontol A Biol Sci Med Sci. 2012; 67: 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pilling LC, Harries LW, Powell J, Llewellyn DJ, Ferrucci L, Melzer D. Genomics and successful aging: grounds for renewed optimism? J Gerontol A Biol Sci Med Sci. 2012; 67: 511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Poon LW, Woodard JL, Stephen Miller L, et al. Understanding dementia prevalence among centenarians. J Gerontol A Biol Sci Med Sci. 2012; 67: 358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Solesio-Jofre E, Lorenzo-López L, Gutiérrez R, López-Frutos JM, Ruiz-Vargas JM, Maestú F. Age-related effects in working memory recognition modulated by retroactive interference. J Gerontol A Biol Sci Med Sci. 2012; 67: 565–572 [DOI] [PubMed] [Google Scholar]
- 93. Taylor CL, Albanese E, Stewart R. The association of dementia with upper arm and waist circumference in seven low- and middle-income countries: the 10/66 cross-sectional surveys. J Gerontol A Biol Sci Med Sci. 2012; 67: 897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zheng JJ, Lord SR, Close JC, et al. Brain white matter hyperintensities, executive dysfunction, instability, and falls in older people: a prospective cohort study. J Gerontol A Biol Sci Med Sci. 2012; 67: 1085–1091 [DOI] [PubMed] [Google Scholar]
- 95. Menghini R, Casagrande V, Cardellini M, et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009; 120: 1524–1532 [DOI] [PubMed] [Google Scholar]
- 96. Vasa-Nicotera M, Chen H, Tucci P, et al. miR-146a is modulated in human endothelial cell with aging. Atherosclerosis. 2011; 217: 326–330 [DOI] [PubMed] [Google Scholar]
- 97. Wu W, Xiao H, Laguna-Fernandez A, et al. Flow-Dependent Regulation of Kruppel-Like Factor 2 Is Mediated by MicroRNA-92a. Circulation. 2011; 124: 633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Asada S, Takahashi T, Isodono K, et al. Downregulation of Dicer expression by serum withdrawal sensitizes human endothelial cells to apoptosis. Am J Physiol Heart Circ Physiol. 2008; 295: H2512–H2521 [DOI] [PubMed] [Google Scholar]
- 99. Mudhasani R, Zhu Z, Hutvagner G, et al. Loss of miRNA biogenesis induces p19Arf-p53 signaling and senescence in primary cells. J Cell Biol. 2008; 181: 1055–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002; 90: 1159–1166 [DOI] [PubMed] [Google Scholar]
- 101. Reed MJ, Bradshaw AD, Shaw M, et al. Enhanced angiogenesis characteristic of SPARC-null mice disappears with age. J Cell Physiol. 2005; 204: 800–807 [DOI] [PubMed] [Google Scholar]
- 102. Koike T, Vernon RB, Gooden MD, Sadoun E, Reed MJ. Inhibited angiogenesis in aging: a role for TIMP-2. J Gerontol A Biol Sci Med Sci. 2003; 58: B798–B805 [DOI] [PubMed] [Google Scholar]
- 103. Shilo S, Roy S, Khanna S, Sen CK. Evidence for the involvement of miRNA in redox regulated angiogenic response of human microvascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008; 28: 471–477 [DOI] [PubMed] [Google Scholar]
- 104. Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci. 2009; 1: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008; 8: 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Smith-Vikos T, Slack FJ. MicroRNAs and their roles in aging. J Cell Sci. 2012; 125: 7–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhang X, Azhar G, Wei JY. The expression of microRNA and microRNA clusters in the aging heart. PLoS ONE. 2012; 7: e34688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Boon RA, Seeger T, Heydt S, et al. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circ Res. 2011; 109: 1115–1119 [DOI] [PubMed] [Google Scholar]
- 109. Sonntag WE, Csiszar A, deCabo R, Ferrucci L, Ungvari Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: progress and controversies. J Gerontol A Biol Sci Med Sci. 2012; 67: 587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Murugesan N, Demarest TG, Madri JA, Pachter JS. Brain regional angiogenic potential at the neurovascular unit during normal aging. Neurobiol Aging. 2012; 33: 1004.e1–1004.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gao P, Shen F, Gabriel RA, et al. Attenuation of brain response to vascular endothelial growth factor-mediated angiogenesis and neurogenesis in aged mice. Stroke. 2009; 40: 3596–3600 [DOI] [PMC free article] [PubMed] [Google Scholar]