Abstract
Background.
Exercise intolerance is the primary chronic symptom in patients with heart failure and preserved ejection fraction (HFPEF), the most common form of heart failure in older persons, and can result from abnormalities in cardiac, vascular, and skeletal muscle, which can be further worsened by physical deconditioning. However, it is unknown whether skeletal muscle abnormalities contribute to exercise intolerance in HFPEF patients.
Methods.
This study evaluated lean body mass, peak exercise oxygen consumption (VO2), and the short physical performance battery in 60 older (69±7 years) HFPEF patients and 40 age-matched healthy controls.
Results.
In HFPEF versus healthy controls, peak percent total lean mass (60.1±0.8% vs. 66.6±1.0%, p < .0001) and leg lean mass (57.9±0.9% vs. 63.7±1.1%, p = .0001) were significantly reduced. Peak VO2 was severely reduced including when indexed to leg lean mass (79.3±18.5 vs. 104.3±20.4ml/kg/min, p < .0001). Peak VO2 was correlated with percent total (r = .51) and leg lean mass (.52, both p < .0001). The slope of the relationship of peak VO2 with percent leg lean mass was markedly reduced in HFPEF (11±5ml/min) versus healthy controls (36±5ml/min; p < .001). Short physical performance battery was reduced (9.9±1.4 vs. 11.3±0.8) and correlated with peak VO2 and total and leg lean mass (all p < .001).
Conclusion.
Older HFPEF patients have significantly reduced percent total and leg lean mass and physical functional performance compared with healthy controls. The markedly decreased peak VO2 indexed to lean body mass in HFPEF versus healthy controls suggests that abnormalities in skeletal muscle perfusion and/or metabolism contribute to the severe exercise intolerance in older HFPEF patients.
Key Words: Exercise, Cardiovascular, Functional performance, Physical function, Sarcopenia, Muscle
Approximately 50% or more of heart failure (HF) patients have preserved left ventricular ejection fraction, and the proportion is greater among women and the elderly adults (1,2). The primary symptom in patients with chronic heart failure and preserved ejection fraction (HFPEF), even when well compensated, is severe exercise intolerance, which can be measured objectively as decreased peak exercise pulmonary oxygen uptake (peak VO2), and is associated with their reduced quality of life (3–10). However, the pathophysiology of exercise intolerance in HFPEF is not well understood. We recently reported that although peak exercise cardiac output and arteriovenous oxygen difference (A-VO2 Diff) were reduced in older HFPEF patients compared with age-matched healthy controls (HC) (5), the change in A-VO2 Diff from rest to peak exercise was the strongest independent predictor of the reduced peak VO2 in HFPEF patients (5). Furthermore, improved A-VO2 Diff accounted for the most of the improvement in peak VO2 following exercise training (11). These findings regarding A-VO2 Diff suggest that skeletal muscle hypoperfusion, skeletal atrophy, and/or abnormal muscle metabolism play an important role in the severe exercise intolerance experienced by HFPEF patients (12).
Lower extremity skeletal muscle mass is also an important predictor of impaired physical functional performance (13). Furthermore, previous studies in middle-aged patients with HF and reduced ejection fraction have reported that these patients have skeletal muscle atrophy compared with healthy age-matched controls (HC) and reduced lower extremity muscle mass that was related to their reduced peak VO2 (14–18). Currently, however, it is unknown whether HFPEF patients have reduced skeletal muscle mass beyond that which occurs with normal aging and whether skeletal muscle atrophy contributes to reduced exercise capacity and physical functioning in this common and important disorder in older persons. Therefore, the purpose of this study was to determine whether older HFPEF patients have reduced total and leg lean mass and if so whether this contributes to their severely reduced exercise capacity and physical functional performance. This information has therapeutic implications.
Methods
Participants
As previously described in studies from our laboratory (4,5,11,19), HFPEF patients had symptoms and signs of HF as defined by National Health and Nutrition Examination Survey HF clinical score of ≥3 and the criteria of Rich and coworkers (20,21) and had preserved resting systolic function (ejection fraction ≥50% and no segmental wall motion abnormalities), and no significant ischemic or valvular heart disease, pulmonary disease, anemia, or other disorder that could explain the patients’ symptoms (4,5,19).
Age-matched, sedentary HC participants were recruited and screened and excluded if they had any chronic medical illness, were on any chronic medication, had current complaints or an abnormal physical examination (including blood pressure ≥ 140/90 mmHg), had abnormal results on the screening tests (electrocardiogram, cardiopulmonary exercise, and spirometry), or were regularly exercising.
Echocardiography
As previously described (4,5,19), resting two-dimensional echocardiography, Doppler, and tissue Doppler were performed by an experienced, registered echosonographer using a Philips ultrasound imaging system fitted with multifrequency transducer. Standard two-dimensional images were obtained in the parasternal long-axis and short-axis views and apical four- and two-chamber views.
Exercise Testing
Exercise testing was performed as previously described in the upright position on an electronically braked bicycle using a staged protocol starting at 12.5W for 2 minutes, increasing to 25W for 3 minutes, and with 25W per 3-minute increments thereafter to exhaustion (4,5,11,19,22–24). Expired gas analysis was conducted using a commercially available system (CPX-2000; MedGraphics, Minneapolis, MN) that was calibrated before each test with a standard gas of known concentration and volume. Breath-by-breath gas exchange data were measured continuously during exercise and averaged every 15 seconds, and peak values were averaged from the last two 15-second intervals during peak exercise (4,5,11,19,22–24).
Body Composition
Total lean mass (total and leg) and total mass were measured by dual energy X-ray absorptiometry (DXA, Hologic Delphi QDR, Bedford, MA, software Version 12.3). Leg lean mass was calculated as the sum of nonbone lean mass in both legs (25,26). DXA measurements and region of interest identification were performed by the same technician according to standardized protocols. Lean mass calibration was verified with twice weekly scans of a whole-body phantom. Sarcopenia and sarcopenic obesity were determined based on the criteria from Newman and coworkers from the health ABC study (27).
Physical Functional Performance
Physical functional performance was assessed using the short physical performance battery (SPPB) that consists of three subtasks: standing balance, walking speed, and time to rise from a chair five times (28). Each test was scored from 0 (unable to complete the test) to 4 (highest level of performance on a test), which is used to derive a summary performance score (range: 0–12) (28).
Statistical Methods
Descriptive statistics of the population for the variables of interest are reported as means and standard deviations for continuous variables and n (the number in the category) and percent for categorical variables. Simple comparison between the unadjusted means between two groups was performed by the two-sample t-test for continuous data, by Fisher’s exact test for binomial variables, and by Chi-square tests for general categorical variables. Although the HC group was selected to approximately match the distribution of gender and age of the HFPEF group, comparisons of variables between the HFPEF and HC were adjusted for gender and age by analysis of covariance. Adjustment for physical variables was performed by adding these as additional covariates. A mediation analysis of the main effect of HFPEF versus HC after adjusting for gender and age was performed, and Goodman’s unbiased estimate of the mediation effect was used. Possible interactions between the effect of a significant predictor and the HFPEF indicator variable were also tested. Analysis on all outcomes, and the slope of the peak VO2 and percent lean mass relationship were also stratified by gender. The interaction effects and the main effects were made at the 5% two-sided level of significance.
Results
Participant Characteristics
Characteristics of the HFPEF and HC are shown in Table 1. The HFPEF and HC groups were well matched for age; however, body mass was higher for HFPEF than HC. The HFPEF patients had similar characteristics, including BMI, to those reported from population studies and from previous reports from our laboratory (4,5,19). HFPEF patients were stable, New York Heart Association (NYHA) class II (80%) and III (20%). The patients had typical echo-Doppler characteristics of HFPEF, including normal left ventricular ejection fraction, significant left ventricular hypertrophy, concentric left ventricular remodelling, altered diastolic filling pattern, reduced mitral annulus velocity, and increased early filling to annulus velocity ratio compared with HC (Table 1). Chronic systemic hypertension was present in 73% of HFPEF patients, and diabetes was present in 12%.
Table 1.
Participant Characteristics
| HFPEF (n = 60) | HC (n = 40) | p Value | |
|---|---|---|---|
| Age | 69.8±7.3 | 69.4±7.1 | .78 |
| Women | 41 (68) | 20 (50) | .09 |
| Weight (kg) | 81.1±15.0 | 75.9±14.1 | .09 |
| Body mass index (kg/m2) | 29.9±4.3 | 25.8±4.0 | <.0001 |
| Body surface area (m2) | 1.88±0.21 | 1.88±0.19 | .90 |
| Resting systolic blood pressure (mmHg) | 140±18 | 124±12 | <.0001 |
| Resting diastolic blood pressure (mmHg) | 77±12 | 75±7 | .55 |
| Brain natriuretic peptide | 81±71 | 34±13 | <.0001 |
| LV mass (g) | 225±71 | 137±35 | <.0001 |
| LV mass/EDV ratio | 3.4±1.9 | 1.7±0.4 | <.0001 |
| Ejection fraction | 65±7 | 63±7 | .19 |
| Left atrium area (cm2) | 15.0±4.9 | 14.7±3.3 | .79 |
| Left atrium volume (cm3) | 39.2±16.5 | 40.9±14.3 | .61 |
| Lateral eʹ (cm/s) | 8.2±2.2 | 9.4±1.9 | .004 |
| E/eʹ | 9.5±3.5 | 7.5±2.0 | .001 |
| Doppler diastolic function pattern | |||
| Normal | 4 (8) | 31 (78) | <.0001 |
| Abnormal relaxation | 39 (80) | 9 (22) | |
| Pseudonormal | 6 (12) | 0 (0) | |
| Hemoglobin (g/dL) | 13.3±1.5 | 13.4±1.1 | .73 |
| Peak workload (W) | 72±25 | 116±39 | <.0001 |
| Peak heart rate (bpm) | 130±21 | 147±16 | <.0001 |
| Peak respiratory exchange ratio | 1.12±0.08 | 1.17±0.09 | .01 |
| Diabetes mellitus | 7 (12) | — | |
| History of hypertension | 44 (73) | — | |
| New York Heart Association Class | |||
| II | 48 (80) | — | |
| III | 12 (20) | — | |
| Medications | |||
| Angiotensin converting enzyme inhibitors | 4 (7) | ||
| Digoxin | 1 (2) | — | |
| Diuretics | 40 (67) | — | |
| β-blockers | 25 (42) | — | |
| Calcium channel blockers | 21 (35) | — | |
| Nitrates | 3 (5) | — | |
Notes: HFPEF = heart failure and preserved ejection fraction; HC = healthy age-matched controls; LV = left ventricular; EDV = end-diastolic volume; eʹ = early mitral annulus filling velocity; E = early filling velocity. Values are mean (SD) or number (percent).
Exercise Capacity
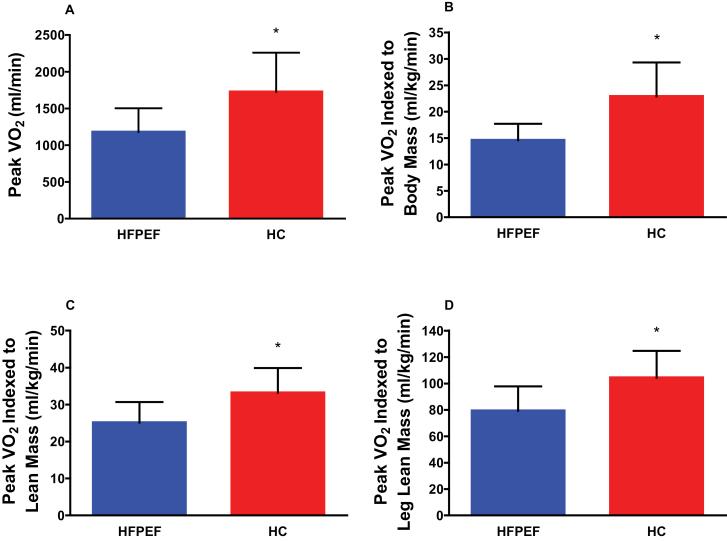
Peak pulmonary oxygen uptake measured in milliliter per minute (1180±323 vs. 1727±533, p < .0001) or indexed to body mass (14.6±3.1 vs. 22.9±6.4ml/kg/min, p < .0001), total lean mass (25.1±5.6 vs. 33.2±6.7ml/kg/min, p < .0001), or leg lean mass (79.3±18.5 vs. 104.3±20.4ml/kg/min, p < .0001) was significantly reduced in HFPEF patients versus HC (Figure 1). Peak exercise power output (HFPEF: 72±25 vs. HC: 116±39W, p < .0001), heart rate (HFPEF: 130±21 vs. HC: 147±16 bpm, p < .0001), and respiratory exchange ratio (HFPEF: 1.12±0.08 vs. HC: 1.17±0.09, p = .01) were reduced in HFPEF patients compared with HC (Table 1).
Figure 1.
Peak VO2 (absolute and indexed to body mass, total lean body mass, and leg lean mass) in HFPEF and HC. Values are mean ± SD; *p < .001 versus HC, adjusted for age and gender.
Body Composition
No significant difference was found between groups for total lean mass or leg lean mass; however, the percent total lean mass and percent leg lean mass were significantly reduced in HFPEF versus HC (Table 2). Total and percent fat mass and total and percent leg fat mass were increased in HFPEF versus HC (Table 2). Sarcopenia was present in 42% of HFPEF compared with 28% of HC (p = .20), whereas sarcopenic obesity was present in 25% of HFPEF compared with 5% of HC (p = .01).
Table 2.
Body Composition in HFPEF Patients and HC
| HFPEF | HC | HFPEF | HC | ||
|---|---|---|---|---|---|
| Raw Data | Adjusted Data* | p Value | |||
| Total mass (kg) | 79.9±14.4 | 76.9±14.1 | 80.8±1.7 | 75.5±2.1 | .06 |
| Total lean (kg) | 47.3±9.5 | 51.7±10.2 | 48.3±0.9 | 50.1±1.1 | .22 |
| Total lean (%) | 59.4±7.3 | 67.6±7.8 | 60.1±0.8 | 66.6±1.0 | <.0001 |
| Total fat (kg) | 30.5±8.9 | 22.8±8.3 | 30.3±1.1 | 23.1±1.4 | .001 |
| Total fat (%) | 37.9±7.6 | 29.3±8.2 | 37.3±0.9 | 30.3±1.0 | <.0001 |
| Leg mass (kg) | 26.5±4.7 | 25.5±4.6 | 26.7±0.6 | 25.2±0.7 | .12 |
| Leg lean (kg) | 15.1±3.4 | 16.5±3.5 | 15.4±0.3 | 16.0±0.4 | .30 |
| Leg lean (%) | 57.0±8.8 | 65.2±10.1 | 57.9±0.9 | 63.7±1.1 | .0001 |
| Leg fat (kg) | 10.7±3.4 | 8.0±3.4 | 10.5±0.4 | 8.4±0.5 | .001 |
| Leg fat (%) | 40.1±9.3 | 31.3±10.6 | 39.1±0.9 | 32.8±1.2 | <.0001 |
Notes: HFPEF = heart failure and preserved ejection fraction; HC = healthy age-matched control. Raw data are presented as mean ± SD; *adjusted for age and gender and presented as least square means ± standard error. p value corresponds to the adjusted data.
Physical Functional Performance
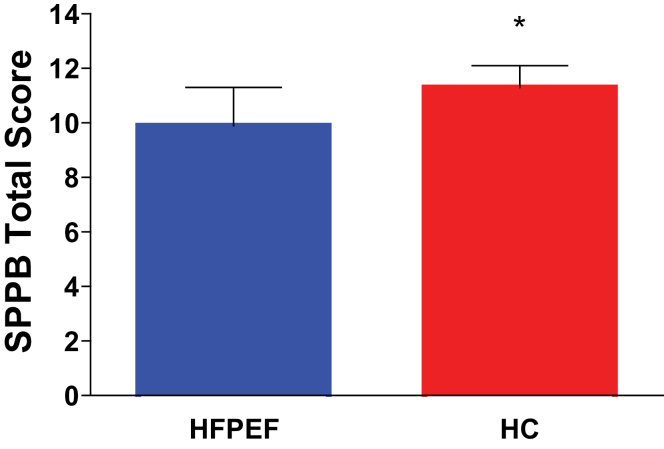
The SPPB total score was significantly reduced in HFPEF compared with HC (9.9±1.4 vs. 11.3±0.8, p < .0001; Figure 2). The chair stand time was greater, whereas the chair stand score was reduced in HFPEF versus HC (both p values <.0001; Table 3). No significant difference was found between groups for walking speed, gait speed score, or balance score (Table 3).
Figure 2.
Physical functional performance in HFPEF and HC. Values are mean ± SD; *p < .001 versus HC, adjusted for age and gender.
Table 3.
Physical Functional Performance in HFPEF and HC
| HFPEF | HC | HFPEF | HC | ||
|---|---|---|---|---|---|
| Raw Data | Adjusted Data* | p Value | |||
| Walk speed (m/s) | 1.17±0.22 | 1.23±0.17 | 1.16±0.03 | 1.24±0.03 | .08 |
| Chair stand time (s) | 14.8±3.6 | 10.9±2.7 | 14.7±0.4 | 11.0±0.5 | <.0001 |
| Balance score | 3.7±0.8 | 4.0±0.2 | 3.7±0.1 | 3.9±0.1 | .054 |
| Gait speed score | 3.9±0.2 | 4.0±0.0 | 3.95±0.02 | 4.00±0.03 | .14 |
| Chair stand score | 2.3±1.0 | 3.4±0.7 | 2.3±0.1 | 3.4±0.1 | <.0001 |
Notes: HFPEF = heart failure and preserved ejection fraction; HC = healthy age-matched control. Raw data are presented as mean ± SD; *adjusted for age and gender and presented as least square means ± standard error. p value corresponds to the adjusted data.
Relationships of Lean Mass With Physical Functional Performance and Exercise Capacity
The SPPB score was positively correlated with peak VO2, expressed in milliliter per minute and milliliter per kilogram per minute (r = .5 and .6, respectively, p < .001 for both). The SPPB score was positively correlated with percent total and leg lean mass (r = .4 and .3, respectively, p < .001 for both).
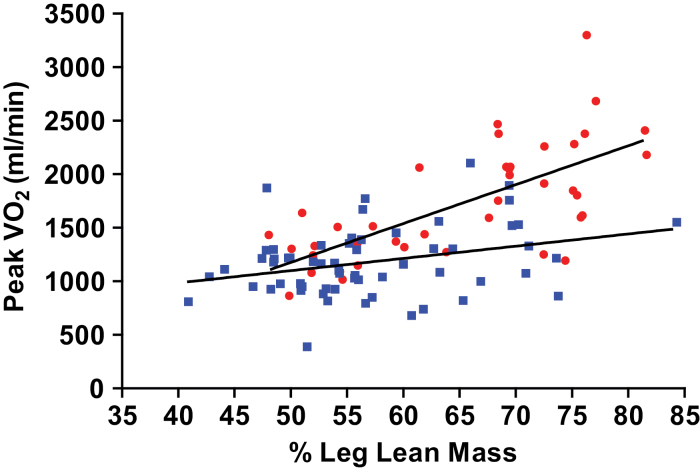
Peak exercise VO2 (ml/min) was positively correlated with percent total lean mass and with percent leg lean mass (r = .51 and .52, p < .0001 for both). There was a significant group interaction in the relationship of peak VO2 with both percent total lean and percent leg lean mass (Figure 3). The increase in peak VO2 with increasing percent leg lean mass was markedly reduced in HFPEF (slope = 11±5ml/min) compared with HC (slope = 36±5ml/min; p < .001). Across the range of observed percent lean leg mass (48%–70%), this interaction resulted in intergroup differences that were relatively large. For instance, for 70% leg lean mass, a HFPEF patient’s peak VO2 was 574ml/min lower than an age-matched HC participant (HFPEF: 1,327 vs. HC: 1,901ml/min) (Figure 3).
Figure 3.
Relationship between peak VO2 (ml/min) and percent leg lean mass in HFPEF and HC. HFPEF (filled squares) and HC (filled circles).
Relationships of Diastolic Function With Exercise Capacity
Among HC, lateral eʹ (r = .39; p = .02) and E/eʹ (r = .39; p = .01) were significantly related to peak VO2. However, among the HFPEF patients, there were no significant relationships between diastolic function grade, lateral eʹ, or E/eʹ and peak VO2, whether expressed in ml/min or indexed to body mass, lean body mass, and leg lean mass (r values all <.15, p values all >.33).
Effect of Gender on Exercise Capacity, Body Composition, and Physical Functional Performance
Given the known effect of gender on exercise capacity, body composition, and physical function (29), in addition to adjusting for gender in the primary analyses, we performed further analysis stratifying outcomes based on gender. Similar to our findings above, total fat, percent total fat, and percent leg fat were significantly higher, whereas percent total lean and percent total leg lean mass were significantly lower in older male and female HFPEF patients versus gender-matched HC. Peak VO2 (ml/min or indexed to body mass, body mass, total lean, or leg lean mass), chair stand time, chair stand score, and total SPPB score were significantly lower in male and female HFPEF patients versus gender-matched HC. Finally, the slope of the relationship between peak VO2 and percent leg lean mass was not dependent on gender (p = .22).
Discussion
Severe exercise intolerance is the primary symptom in patients with chronic HFPEF, even when stable and well compensated (3–10). Previous studies have demonstrated that skeletal muscle atrophy contributes to exercise intolerance in patients, primarily middle-aged men, with HF and reduced ejection fraction. However, it has been unknown whether older HFPEF patients have reduced skeletal muscle mass beyond that which occurs with normal aging and if so whether this contributes to reduced exercise capacity and physical functional performance. The novel finding of this study is that the percent total and leg lean mass, physical functional performance, and peak VO2 were significantly reduced in HFPEF patients compared with age-matched sedentary HC. The SPPB score was positively correlated with peak VO2 (expressed as either ml/min or ml/kg/min), and both SPPB and peak VO2 were correlated with percent total and leg lean mass. Our results also indicate that the increase in peak VO2 for a similar percent increase in total or leg lean mass is markedly lower in HFPEF patients compared with HC. This suggests that skeletal muscle hypoperfusion or impaired oxygen utilization by the active skeletal muscles may play an important role in limiting exercise performance in elderly HFPEF patients. These findings have important therapeutic implications.
Several lines of evidence suggest that peripheral “noncardiac” factors are important contributors to exercise intolerance in elderly patients with HFPEF (5–8). We previously reported that the strongest determinant of the severely reduced exercise capacity in elderly HFPEF patients compared with age-matched healthy volunteers was reduced peak A-VO2 Diff, an observation recently confirmed by Bhella and coworkers (8). We also recently demonstrated that the improvement in peak VO2 after 4 months of endurance exercise training in elderly, stable, compensated HFPEF patients was due primarily to increased peak A-VO2 Diff (11). These findings suggested that abnormalities in the quality or quantity of skeletal muscle may contribute to reduced peak VO2 in older HFPEF patients, and their improvement may contribute to the improved exercise performance with training.
We believe this is the first reported study to examine lean mass and its relationship to physical function in older HFPEF patients. However, our findings are supported by several prior reports from other investigators showing that HF and reduced ejection fraction patients have significant skeletal muscle abnormalities, including skeletal muscle atrophy, decreased oxidative fibers and enzymes, capillarity, and mitochondrial volume density, and that these contribute to their reduced peak VO2 (14–18,30). Koster and coworkers recently reported that healthy older adults with greater fat mass also had more leg lean mass (31). Based on these results, we would have expected the older HFPEF patients to have increased leg lean mass compared with HC given the larger fat mass in the former group. Moreover, 25% of the older HFPEF patients had sarcopenic obesity compared with 5% of HC. Accordingly, this study expands upon prior studies significantly by demonstrating that older HFPEF patients have reduced percent total and leg lean mass and increased total and percent fat and leg fat mass. Moreover, our observation that the peak VO2 indexed to total lean mass or leg lean mass is significantly reduced in HFPEF patients compared with HC, coupled with our finding that the increase in peak VO2 with increasing percent total lean mass is markedly lower for HFPEF versus HC, suggests that impaired diffusive oxygen transport secondary to increased intramuscular fat (32) or oxygen utilization by the active muscles may contribute the reduced exercise capacity in elderly HFPEF patients (12). Indeed, recent preliminary data from Bhella and coworkers using 31Phosphate magnetic resonance spectroscopy during and after performing static leg lifts suggested that skeletal muscle oxidative metabolism is impaired in older HFPEF patients (8).
A recent review of studies that assessed exercise performance in HFPEF patients reported that peak VO2 was below the minimal level required for full and independent living, and as a result, many HFPEF patients are at increased risk for functional dependence (12). Consistent with this observation, we found that physical functional performance by the SPPB score was significantly reduced in older HFPEF patients compared with age-matched HC. Moreover, our findings that a lower SPPB score was associated with a lower peak VO2 (ml/min or ml/kg/min) and with decreased percent total or leg lean mass suggest that exercise interventions that increase muscle mass and strength and aerobic capacity, such as combined resistance and aerobic exercise training, may be optimal for improving physical functional performance, muscle strength and mass, and peak VO2 in elderly HFPEF patients.
Limitations
We did not measure intramuscular fat with novel imaging modalities (eg, magnetic resonance imaging) or skeletal muscle perfusion or morphology (eg, fiber composition, oxidative enzymes, mitochondrial density); therefore, the role that increased intramuscular fat, skeletal muscle hypoperfusion, or abnormal metabolism plays in limiting peak VO2 requires further study. Also, we did not assess lower extremity maximal muscular strength; thus, the contribution that this has on our observed decline in physical functional performance also requires further study.
The DXA-based lean body mass determinations assume a fixed degree of tissue hydration, and lean body mass determinations can be sensitive to extravascular fluid. Although there is no evidence that tissue hydration differs between HFPEF patients and controls, and the HFPEF patients in this study were stable, well compensated, and clinically euvolumic, this is a potential source of error that should be considered (33).
There were more women than men in the HFPEF group, in accord with population-based studies, and this created a modest, nonsignificant gender imbalance compared with HF. However, we believe this did not influence our key findings because we adjusted for gender in the primary analyses and performed additional analyses stratified by gender and because the relationship between peak VO2 and leg lean mass was not dependent on gender.
A final limitation of this study is that we did not have a control group of older individuals with hypertension (without HF); therefore, future studies are required to examine exercise capacity, body composition, and physical functioning across the HFPEF continuum (eg, healthy older controls, older controls with obesity, hypertension and concentric ventricular hypertrophy or remodeling without signs or symptoms of heart failure, and age-matched HFPEF patients) to determine the role that comorbidities have on these outcomes.
Summary
Older HFPEF patients have significantly reduced percent total and leg lean mass compared with age-matched HC. This is associated with their severely reduced peak VO2 (expressed as absolute or indexed to body mass, total lean mass, or leg lean mass) and physical functional performance compared with HC. Sarcopenic obesity was present in 25% of HFPEF patients compared with 5% of HF. Our finding that the increase in peak VO2 for the same percent change in lean mass is markedly reduced in HFPEF patients than HC suggests that impaired skeletal muscle metabolism or perfusion may contribute to exercise intolerance in HFPEF patients.
Funding
This study was supported by the following research grants: NIH Grant R37-AG18915 and The Claude D. Pepper Older Americans Independence Center of Wake Forest University NIH Grant P30-AG21332.
References
- 1. Kitzman DW, Gardin JM, Gottdiener JS, et al. Cardiovascular Health Study Research Group Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001; 87: 413–419 [DOI] [PubMed] [Google Scholar]
- 2. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006; 355: 251–259 [DOI] [PubMed] [Google Scholar]
- 3. Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991; 17: 1065–1072 [DOI] [PubMed] [Google Scholar]
- 4. Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002; 288: 2144–2150 [DOI] [PubMed] [Google Scholar]
- 5. Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011; 58: 265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006; 114: 2138–2147 [DOI] [PubMed] [Google Scholar]
- 7. Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010; 56: 845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhella PS, Prasad A, Heinicke K, et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011; 13: 1296–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010; 56: 855–863 [DOI] [PubMed] [Google Scholar]
- 10. Katz SD, Maskin C, Jondeau G, Cocke T, Berkowitz R, LeJemtel T. Near-maximal fractional oxygen extraction by active skeletal muscle in patients with chronic heart failure. J Appl Physiol. 2000; 88: 2138–2142 [DOI] [PubMed] [Google Scholar]
- 11. Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012; 60: 120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haykowsky M, Brubaker P, Kitzman D. Role of physical training in heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2012; 9: 101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buford TW, Lott DJ, Marzetti E, et al. Age-related differences in lower extremity tissue compartments and associations with physical function in older adults. Exp Gerontol. 2012; 47: 38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lang CC, Chomsky DB, Rayos G, Yeoh TK, Wilson JR. Skeletal muscle mass and exercise performance in stable ambulatory patients with heart failure. J Appl Physiol. 1997; 82: 257–261 [DOI] [PubMed] [Google Scholar]
- 15. Mancini DM, Walter G, Reichek N, et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992; 85: 1364–1373 [DOI] [PubMed] [Google Scholar]
- 16. Minotti JR, Pillay P, Oka R, Wells L, Christoph I, Massie BM. Skeletal muscle size: relationship to muscle function in heart failure. J Appl Physiol. 1993; 75: 373–381 [DOI] [PubMed] [Google Scholar]
- 17. Cicoira M, Zanolla L, Franceschini L, et al. Skeletal muscle mass independently predicts peak oxygen consumption and ventilatory response during exercise in noncachectic patients with chronic heart failure. J Am Coll Cardiol. 2001; 37: 2080–2085 [DOI] [PubMed] [Google Scholar]
- 18. Harrington D, Anker SD, Chua TP, et al. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997; 30: 1758–1764 [DOI] [PubMed] [Google Scholar]
- 19. Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010; 3: 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995; 333: 1190–1195 [DOI] [PubMed] [Google Scholar]
- 21. Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992; 20: 301–306 [DOI] [PubMed] [Google Scholar]
- 22. John JM, Haykowsky M, Brubaker P, Stewart K, Kitzman DW. Decreased left ventricular distensibility in response to postural change in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2010; 299: H883–H889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haykowsky MJ, Herrington DM, Brubaker PH, Morgan TM, Hundley WG, Kitzman DW. Relationship of flow-mediated arterial dilation and exercise capacity in older patients with heart failure and preserved ejection fraction. J Gerontol A Biol Sci Med Sci. 2013; 68: 161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marburger CT, Brubaker PH, Pollock WE, Morgan TM, Kitzman DW. Reproducibility of cardiopulmonary exercise testing in elderly patients with congestive heart failure. Am J Cardiol. 1998; 82: 905–909 [DOI] [PubMed] [Google Scholar]
- 25. Chen Z, Wang Z, Lohman T, et al. Dual-energy X-ray absorptiometry is a valid tool for assessing skeletal muscle mass in older women. J Nutr. 2007; 137: 2775–2780 [DOI] [PubMed] [Google Scholar]
- 26. Schoeller DA, Tylavsky FA, Baer DJ, et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005; 81: 1018–1025 [DOI] [PubMed] [Google Scholar]
- 27. Newman AB, Kupelian V, Visser M, et al. Health ABC Study Investigators Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003; 51: 1602–1609 [DOI] [PubMed] [Google Scholar]
- 28. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994; 49: M85–M94 [DOI] [PubMed] [Google Scholar]
- 29. Ogawa T, Spina RJ, Martin WH, 3rd, et al. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992; 86: 494–503 [DOI] [PubMed] [Google Scholar]
- 30. Drexler H, Riede U, Münzel T, König H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992; 85: 1751–1759 [DOI] [PubMed] [Google Scholar]
- 31. Koster A, Ding J, Stenholm S, et al. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci. 2011; 66: 888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002; 50: 897–904 [DOI] [PubMed] [Google Scholar]
- 33. Sergi G, Lupoli L, Volpato S, et al. Body fluid distribution in elderly subjects with congestive heart failure. Ann Clin Lab Sci. 2004; 34: 416–422 [PubMed] [Google Scholar]