Abstract
Reaction of 10-deacetylbaccatin III (III) and its 7-TES derivative (IV) with DAST under various conditions resulted in the formation of an array of new fluorinated and non-fluorinated 13-keto taxoid compounds (2a–4a) through a vinylogous pinacol-pinacolone rearrangement. Further fluorination of some of these products (2a, 3a) with NFSi or Selectfluor gave additional derivatives. Sodium borohydride reduction of the 13-keto group of these products (2a, 2b, 3a, 3b, 4a, 8, 9, 11–14) led to a series of 9α-hydroxy taxoid derivatives, which were esterified using the docetaxel side chain employing the corresponding protected β-lactam, followed by deprotection to furnish a library of docetaxol analogs and related compounds. A selected number of synthesized compounds (7, 10, 19a, 19b, 21a, 21b, 23, 27, 29, 34–36) were submitted to the National Cancer Institute (NCI) 60 cell line screening program and tested for cytotoxic properties. Taxoids 19a, 19b, 21a, 21b, 23, 27, 29, 34 and 35 were found to exhibit significant anticancer activity against various cancerous cell lines with 23, 27, and 29 being the most potent compounds, demonstrating GI50 values of ≤ 5 nM in several assays.
Keywords: 10-deacetylbaccatin III, docetaxel analogs, paclitaxel, fluorination, natural products, anticancer agents
INTRODUCTION
Cancer remains as one of the most devastating diseases of our times with millions of patients suffering from it around the world.1–6 Moreover, the disease is on the rise and no organ of the human body is immune to its intrusion. Despite the plethora of anticancer drugs currently available for combating cancer, serious deficiencies still prevail, particularly with regards to their efficacy and safety. These chemotherapeutic agents range from small organic molecules and natural products and their analogs, to antibodies and antibody-drug conjugates.7,8
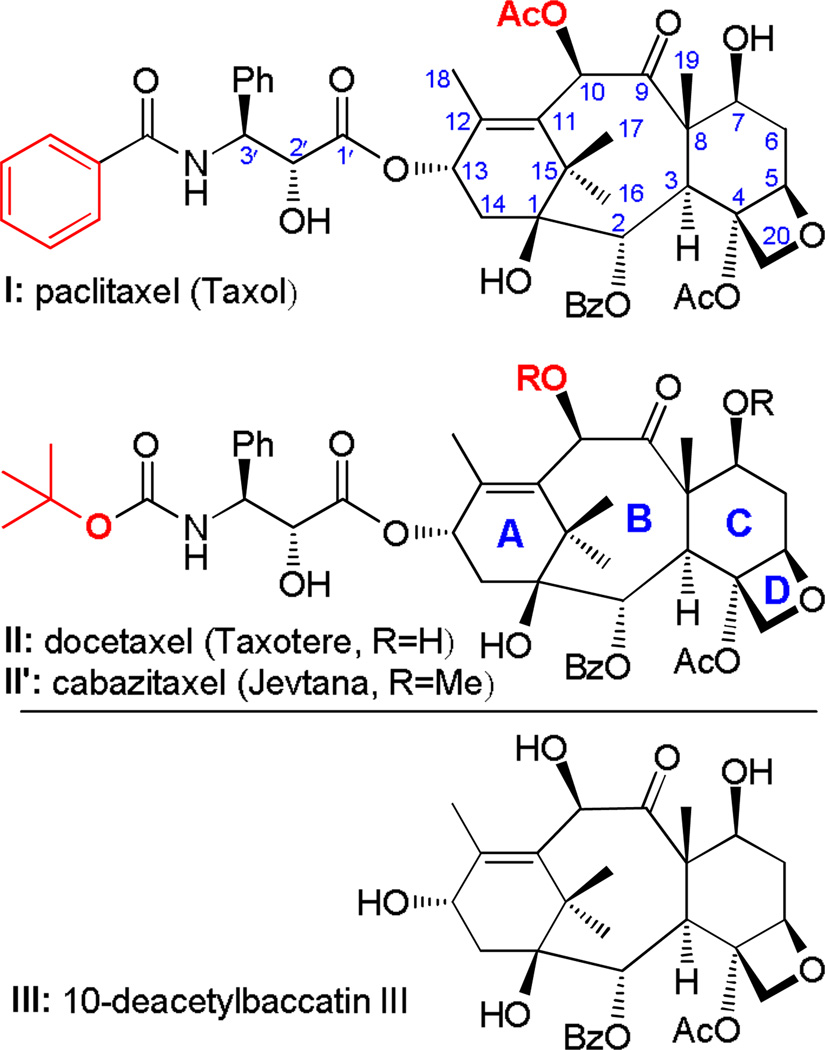
Among the natural products and their semi-synthetic analogs, paclitaxel (I, Fig. 1) and docetaxel (II, Fig. 1) are perhaps the most well-known and frequently employed due to their potency, selectivity and novel mechanism of action.9–11 Paclitaxel is clinically used in the treatment of breast cancer, advanced ovarian cancer, non-small cell lung cancer, and advanced forms of Kaposi's sarcoma,12 whereas docetaxel is employed against breast cancer, non-small cell lung cancer, gastric cancer, prostate cancer, and squamous cell carcinoma of the head and neck.13–15 Cabazitaxel (Jevtana, II′), a recently introduced derivative of docetaxel (docetaxel methylated at C7 and C10 hydroxyl groups), is used to treat hormone-refractory prostate cancer. The latter success underscores the importance of fine-tuning the structures of these agents in order to amplify their selectivity towards specific cancers, a theme that is currently attracting special attention in view of personalized medicine.
Fig. 1.
Structures of paclitaxel (I), docetaxel (II), cabazitaxel (II′), and 10-deacetylbaccatin III (III). Bz = benzoyl; Ac = acetyl; Ph = phenyl.
A large number of paclitaxel and docetaxel analogs became available over the last three decades leading to a useful body of structure-activity relationships (SARs).16–38 Enabled by synthesis, these studies were facilitated by the ready availability of paclitaxel and its precursor 10-deacetylbaccatin III (III, Fig. 1). Motivated by the approval of cabazitaxel and the successes in medicinal chemistry resulting from the introduction of fluorine residues into small molecules,39 we initiated a research program directed towards the synthesis of fluorinated and other novel taxoids starting from 10-deacetylbaccatin III (III, Scheme 1) and its 7-OTES derivative (IV, Scheme 1). The virtues of introducing fluorine atoms into organic molecules have been widely recognized and reported;39–44 they include increased metabolic stability (resistance to biochemical degradation), enhanced lipophilicity (logP), and stronger binding affinities, often leading to improved pharmacological properties. It is notable that the current number of clinically used drugs containing one or more fluorine atoms stands at 20–30% and is steadily increasing.40, 42
Scheme 1.
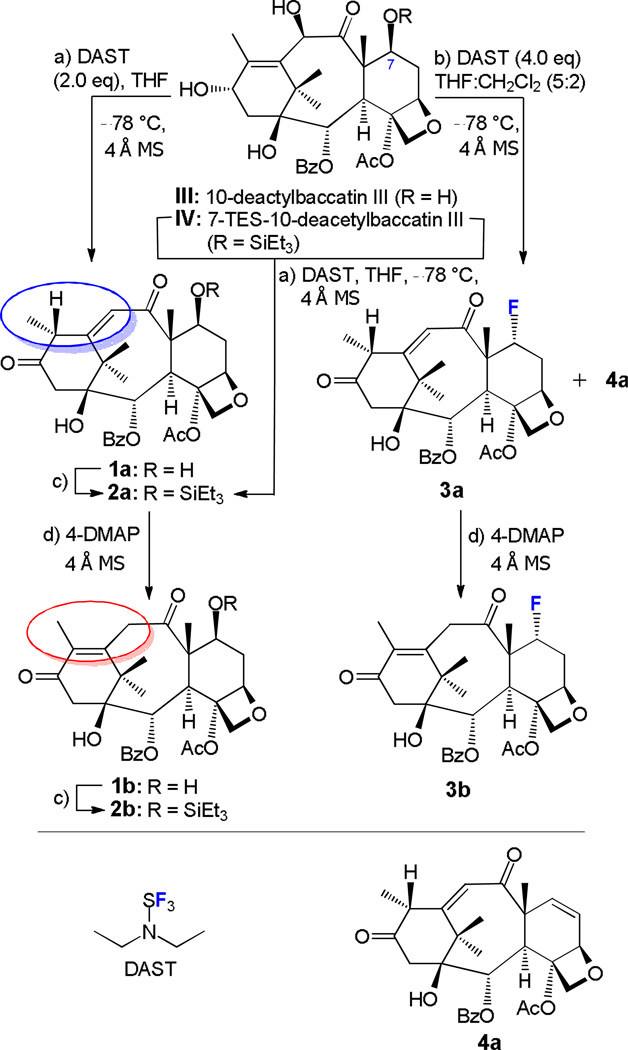
Reaction of 10-deacetylbaccatin III (III) with DAST. Synthesis of compounds 1a–4a and 1b–3b. Reagents and conditions: a) DAST (2.0 equiv), THF, 4 Å MS, −78 °C, 3 h, III → 1a (93%); IV → 2a (70%); b) DAST (4.0 equiv), THF:CH2Cl2 (5:2), 4 Å MS, −78 → 25 °C, 24 h, III → 3a (63%), plus 4a (≤ 20%); c) Et3SiCl (5.0 equiv), Et3N (5.0 equiv), 4-DMAP (0.5 equiv), THF, 0 °C, 1.5 h, 1a → 2a (74%); 1b → 2b (80%); d) 4-DMAP (2.5 equiv), THF, 4 Å MS, 25 °C, 20–24 h, 1a → 1b (98%); 2a → 2b (96%); 3a → 3b (78%). DAST = (diethylamino)sulfurtrifluoride; TES = triethylsilyl; MS = molecular sieves; THF = tetrahydrofuran; 4-DMAP = 4-(dimethylamino)pyridine.
In this article, we report the results of our investigations on the synthesis and biological evaluation of new taxoids, including a number of fluorinated docetaxel analogs.45
RESULTS AND DISCUSSION
Reaction of 10-deacetylbaccatin III (III) with DAST
The present study began with 10-deacetylbaccatin III (III) and its reaction with the common fluorinating agent (diethylamino)sulfur trifluoride (DAST).46 Our intention was to obtain as many fluorinated species as possible for the purposes of converting them, through side chain attachment, to full docetaxel structures for biological evaluation. The top priority at the initial stage was, therefore, structural elucidation of products rather than yield optimization, the latter being postponed until the properties of the molecule would dictate it.
Thus, and as shown in Scheme 1, reaction of III with 2.0 equiv of DAST in THF in the presence of 4 Å MS at −78 °C over 3 h [conditions (a), Scheme 1] furnished enone 1a in 93% yield.47 Product 1a was found to be labile and easily transformed to its apparently more stable isomeric paclitaxel-related form 1b (red ellipsoid, Scheme 1) on silica gel. This conversion could also be induced by 4-DMAP in THF at ambient temperature in 98% yield [conditions (d)]. For the purposes of further manipulations, compound 1a was also converted to its 7-OTES derivative 2a under conditions (c) [Et3SiCl (5.0 equiv), Et3N (5.0 equiv), 4-DMAP (0.5 equiv), THF, 0 °C, 1.5 h; in 74% yield] and thence to the isomerizedtaxoid 2b under basic conditions [conditions (d), 96% yield]. The latter was also obtained from the previously prepared taxoid 1b upon silylation in 80% yield [conditions (c)]. Importantly, enone 2a could also be prepared directly from 7-trimethylsilyl-10-deacetylbaccatin III48 (IV) (Scheme 1) in 70% yield by exposure of the latter to DAST [2.0–4.0 equiv, THF, 4 Å MS, −78 °C, 1–3 h, conditions (a)].
Interestingly, 10-deacetylbaccatin III (III) reacted differently with DAST under modified conditions (b) (Scheme 1). Thus, treatment of III with 4.0 equiv of DAST in THF:CH2Cl2 (5:2), in the presence of 4 Å MS, at −78 to 25 °C, over 24 h led to fluorinated enone 3a in 63% yield accompanied by varying amounts (≤ 20%) of elimination product 4a, inseparable by silica gel chromatography. The formation of product 4a could be suppressed by diluting the reaction mixture with further amounts of CH2Cl2 after the addition of the first 2.0 equiv of DAST and carefully controlling the temperature during the reaction (see ESI for experimental details). Similarly to 1a, enone 3a isomerizes to taxoid 3b in 78% yield under conditions (d) (Scheme 1).
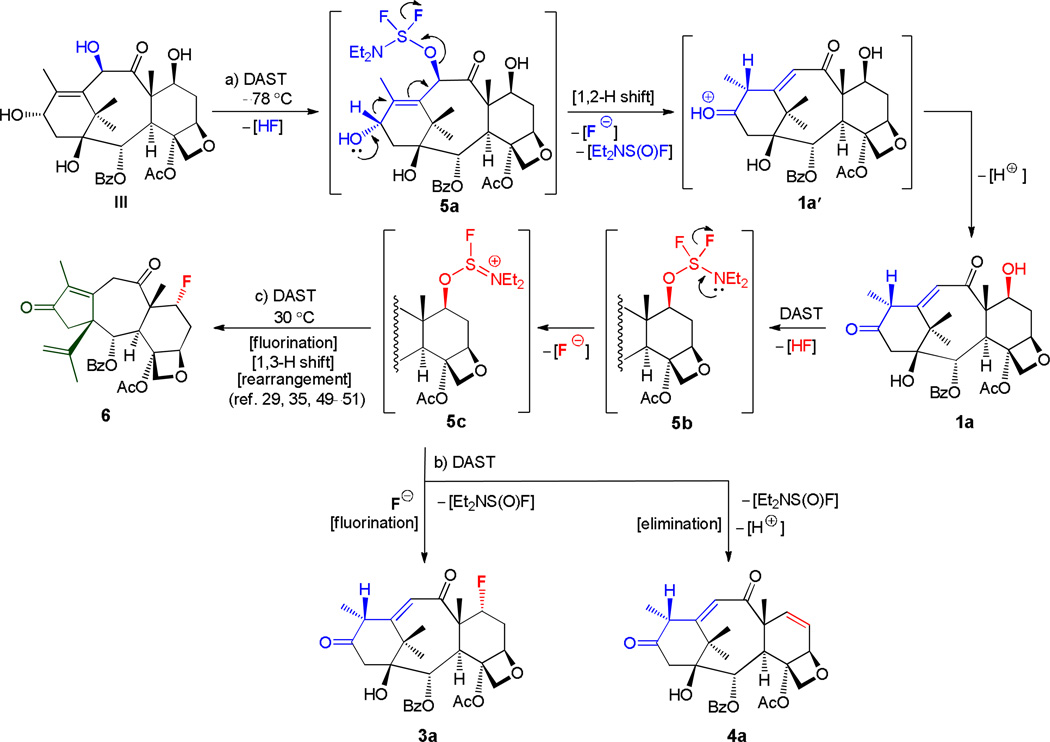
Plausible mechanistic explanations for the reactions mentioned in Scheme 1 are summarized in Scheme 2. Thus, initial reaction of III with DAST may produce intermediate 5a with elimination of HF. This intermediate may then undergo concerted 1,2-H-shift and elimination of Et2NS(O)F and F− to afford protonated species 1a′, from which observed product 1a may be formed through the loss of an H+. A stepwise process involving the formation of an allylic carbocation for this vinylogous pinacol-pinacolone rearrangement (5a → 1a) may also be envisioned. Reaction of hydroxyenone 1a with further amounts of DAST may then lead, initially to 5b, and thence to intermediate 5c, from which all observed products (i.e. 3a, 4a, and 6) may be generated49–52 as shown in Scheme 2.
Scheme 2.
Preparation of compounds 3a, 4a, and 6 and proposed mechanism for their formation. Reagents and conditions: see Scheme 1 for a) and b).
Reaction of enones 2a and 3a with NFSi and Selectfluor
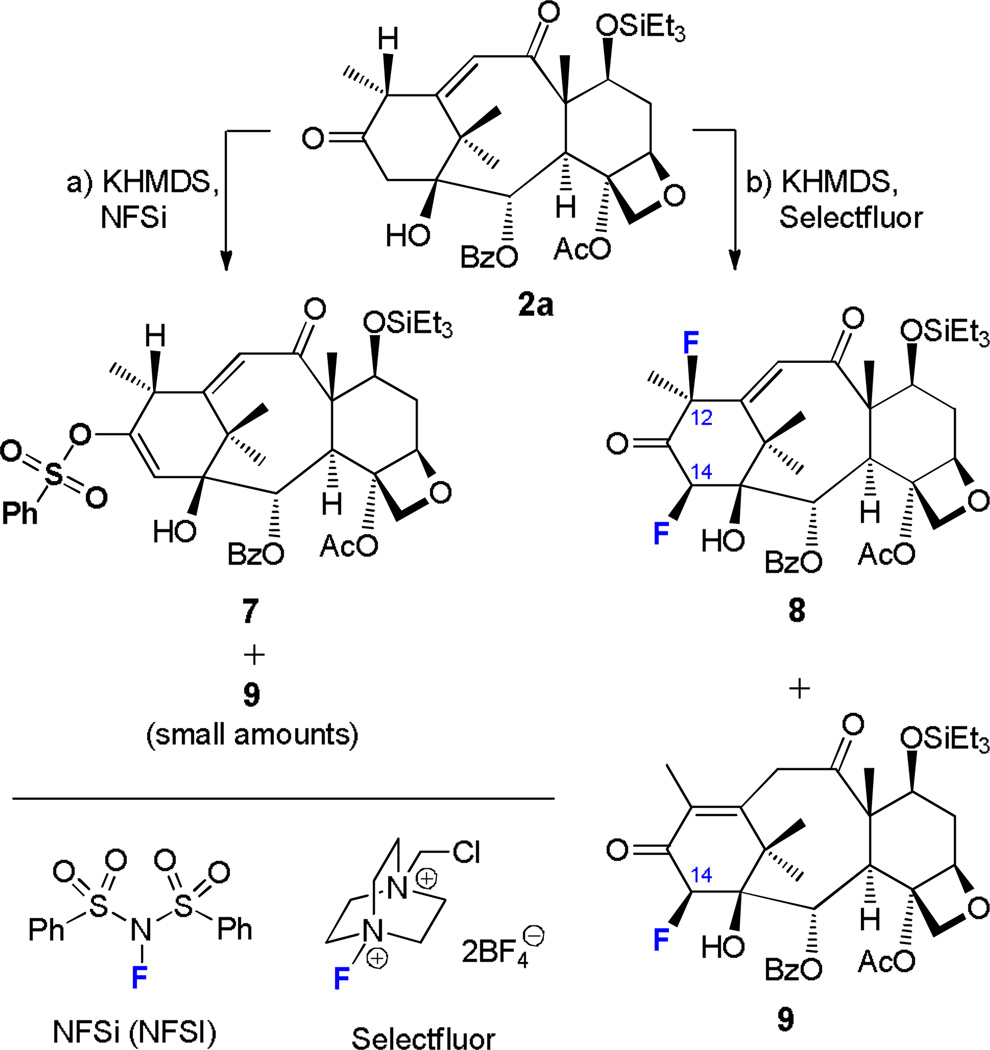
Having gained access to the new taxoid scaffolds mentioned above, we proceeded to explore their chemistry with additional fluorinating agents. Enones 2a and 3a proved to be the most fertile in these pursuits as shown in Schemes 3 and 4, respectively. Thus, treatment of compound 2a with N-fluoro-N-(phenylsulfonyl)benzenesulfonimide (NFSi)53–56 and KHMDS in THF in the presence of 4 Å MS at −78 °C [conditions (a), Scheme 3] led to a complex mixture of products from which rather unexpected sulfonate 7 was isolated as a major product (33% yield), while the expected 14β-fluorotaxoid 9 was obtained as a minor product (≤ 5% yield). In contrast to these results, the reaction of 2a with 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) (F-TEDA-BF4, or Selectfluor)57 under conditions (b) (Scheme 3) [KHMDS (2.0 equiv), Selectfluor (10.0 equiv), THF:DMF (10:1), 4 Å MS, −78 °C, 1–2 h and 25 °C, 1 h] furnished 12β,14β-bis-fluorinated product 9 (24% yield) and varying amounts of 14β-fluorinated compound 9 (≤ 20%) depending on stoichiometry and reaction time.58 The latter product is presumed to arise from 2a via mono-fluorination and enone transposition.
Scheme 3.
Fluorination of 2a with NFSi and Selectfluor. Synthesis of compounds 7–9. Reagents and conditions: a) KHMDS (2.2 equiv), NFSi (3.0 equiv), THF, 4 Å MS, −78 °C, 1 h and 25 °C, 1 h, 2a → 7 (33%), plus 9 (≤ 5%); b) KHMDS (2.0 equiv), Selectfluor (10.0 equiv), THF:DMF (10:1), 4 Å MS, −78 °C, 1–2 h and 25 °C, 1 h, 2a → 9 (24%), plus 9 (≤ 20%). KHMDS = potassium bis(trimethylsilyl)amide; NFSi = N-fluorobenzenesulfonimide; Selectfluor = 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate); DMF = N,N-dimethylformamide.
Scheme 4.
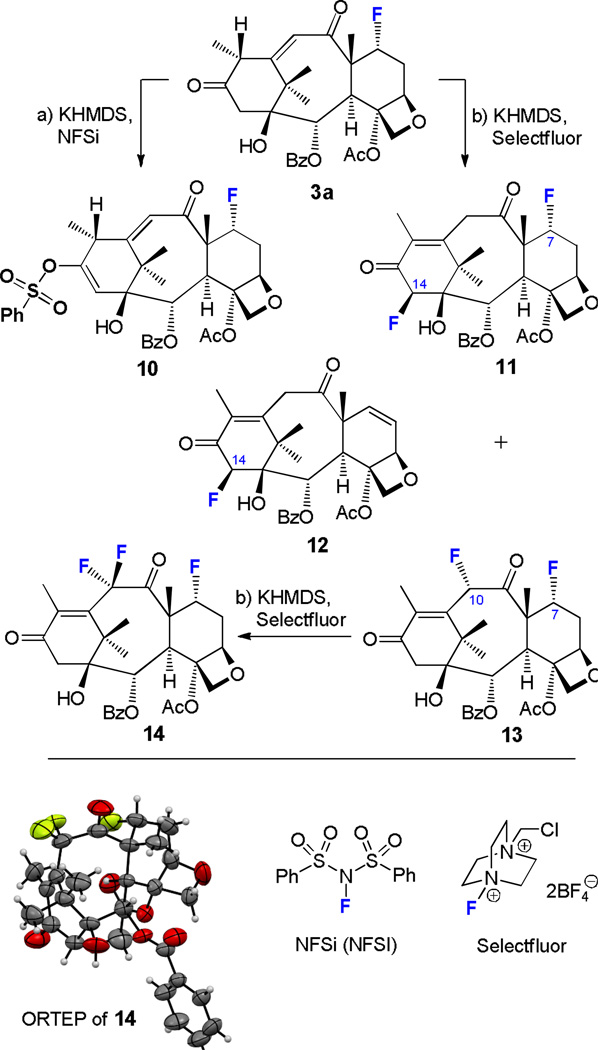
Fluorination of 3a with NFSi and Selectfluor. Synthesis of compounds 10–14. Reagents and conditions: a) KHMDS (2.2 equiv), NFSi (3.0 equiv), THF, 4 Å MS, −78 °C, 1 h and 25 °C, 1 h, 3a → 10 (24%); b) KHMDS (2.0 equiv), Selectfluor (10.0 equiv), THF:DMF (10:1), 4 Å MS, −78 °C, 1–2 h and 25 °C, 1 h, 3a → 11 (≤ 5%), plus 12 (≤ 2%), plus 13 (17%); 13 → 14 (97%).
7α-Fluoroenone 3a also entered a number of interesting reactions with NFSi and Selectfluor (Scheme 4). Thus, treatment of 3a with NFSi under conditions (a) [KHMDS (2.2 equiv), NFSi (3.0 equiv), THF, 4 Å MS, −78 °C, 1 h and 25 °C, 1 h] furnished 7α-fluoro vinyl sulfonate 10 (24% yield) mirroring the reaction of its 7-OTES counterpart 2a (Scheme 3). On the other hand, reaction of 3a with Selectfluor under conditions (b) [KHMDS (2.0 equiv), Selectfluor (10.0 equiv), THF:DMF (10:1), 4 Å MS, −78 °C, 1–2 h and 25 °C, 1 h] led to 7α,14β-difluoroenone 11 (≤ 5% yield) as a minor product (plus trace amounts of compound 12 (≤ 2%), presumably arising from contaminant 4a present in starting material 3a, see Scheme 1), and 7α,10α-difluoroenone 13 (17% yield). Further treatment of the latter with excess of Selectfluor under the same set of conditions led to trifluoro enone 14 (mp = 229–230 °C, CH3Cl/hexanes/EtOAc 10:5:1) in 97% yield. The structure of 14 was unambiguously established by 2D NMR spectroscopic techniques and X-ray crystallographic analysis (see ORTEP drawing, Scheme 4).
Having a variety of rearranged (2a, 2b, 4a) and fluorinated (3a, 3b, 8, 9, 11–14) taxoids in hand, we pursued their conversion to docetaxel analogs through reduction of their C-13 carbonyl moieties and attachment of the side chain.
Reduction of diketones 2a, 3a, and 4, and preparation of docetaxel analogs 19a, 19b, 21a, 21b and 23
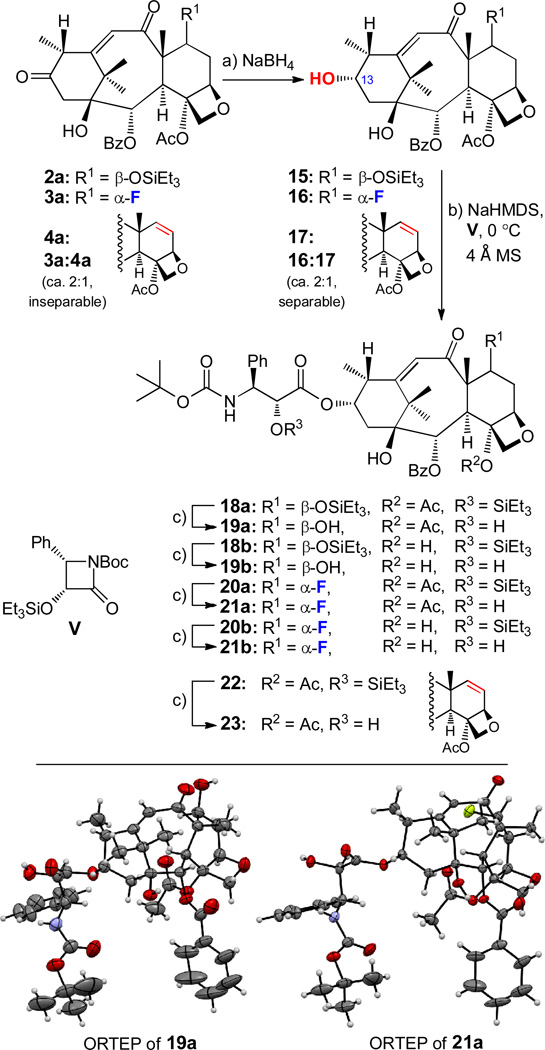
As a prelude to the preparation of docetaxel analogs from the dicarbonyl compounds 2a, 3a, and 4a, we subjected the latter to NaBH4 reduction in the hope that the less hindered 13-carbonyl moiety would be preferentially and selectively reduced to the corresponding 13-hydroxy precursors. Indeed, exposure of 2a, 3a and 4a (as a pure compound or a ca. 2:1 of 3a:4a inseparable mixture) to NaBH4 [2.5 equiv, THF:MeOH (1:2), 0 °C, procedure (a), Scheme 5] led to alcohols 15 (94%), 16 (71%), and 17 [30% yield after chromatographic separation from the concomitantly formed 16 (66% yield)], respectively, all possessing the desired 13α configuration as shown in Scheme 5. Each of these hydroxy compounds was acylated using procedure (b) (Scheme 5): NaHMDS (2.5 equiv) and β-lactam side chain equivalent (3R,4S)-3-triethylsilanyloxy-4-phenyl-N-Boc-2-azetidinone (V) (5.0–10.0 equiv) to afford 7,2'-bis-silylated-4-acetoxy products 18a (53% yield), 20a (53% yield), 2′-silylated-4-acetoxy-Δ7,8 product 22 (31% yield) or 7,2′-bis-silylated-4-deacetyl products 18b (60% yield), and 20b (52% yield), depending on the conditions employed (see ESI). These compounds were then desilylated by exposure to conditions (c) (Scheme 5): aqueous HCl, THF:MeOH (1:2), 0 °C, leading to docetaxel analogs 19a (83% yield), 19b (89% yield), 21a (96% yield), 21b (73% yield), and 23 (94% yield). The absolute stereochemical configurations of 19a (mp = 178–181 °C, CH3CN) and 21a (mp = 233–235 °C, CH3CN/hexanes/EtOH 10:2:5) were confirmed by X-ray crystallographic analysis (see ORTEP representations of 19a and 21a, Scheme 5).
Scheme 5.
Synthesis of compounds 15–17, 18a–21a, 18b–21b, 22 and 23. Reagents and conditions: a) NaBH4 (2.5 equiv), THF:MeOH (1:2), 0 °C, 0.5–3 h, 2a → 15 (94%); 3a → 16 (71%); 3a:4a (ca. 2:1 by 1H NMR) → 16 (66%), plus 17 (30%); b) NaHMDS (2.5 equiv), V (5.0–10.0 equiv), THF, 4 Å MS, 0 °C, 40 min (or −78 → 0 °C, 40 min see ESI for detailed procedure B1, B2, and B3), 15 → 18a (53%); 15 → 18b (60%); 16 → 20a (53%); 16 → 20b (52%); 17 → 22 (31%); c) aq. HCl (1.0 M) (excess), THF:MeOH (1:2), 0 °C, 1–3 h, 18a → 19a (83%); 18b → 19b (89%); 20a → 21a (96%); 20b → 21b (73%); 22 → 23 (94%). NaHMDS = sodium bis(trimethylsilyl)amide; V = (3R,4S)-3-triethylsilanyloxy-4-phenyl-N-Boc-2-azetidinone.
Reduction of diketones 2b and 3b and preparation of docetaxel analogs 27 and 29
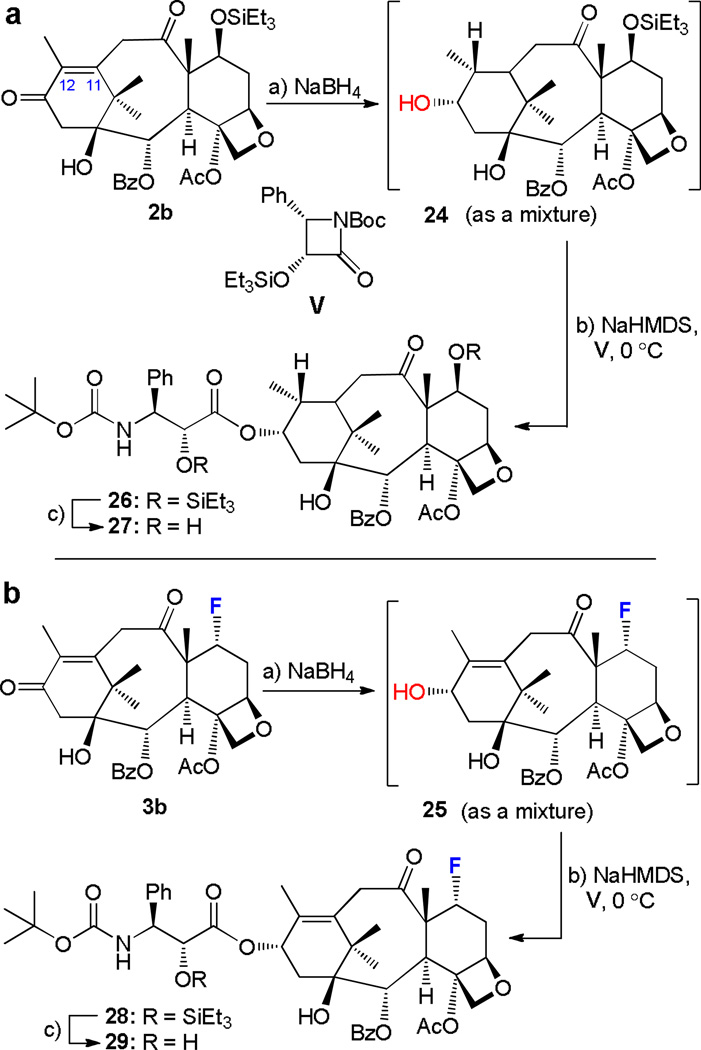
In contrast to the smooth reduction of diketones 2a, 3a, and 4a discussed above, the reaction of compounds 2b and 3b with NaBH4 proved to be complicated and low yielding with regards to the desired 13α-hydroxy products. Thus, and as shown in Scheme 6a, treatment of 2b with a large excess of NaBH4 resulted, through a rather sluggish reaction, in the formation of a complex mixture, in which the over-reduced product 24 was detected as the major compound. The same complications arose in the case of fluorinated diketone 3b (Scheme 6b) which led, upon treatment with excess of NaBH4, to a complex mixture, in which the desired alcohol 25 (note the retention at the Δ11,12 olefinic bond as contrasted with 24) was determined to be the predominant product. Unfortunately, neither product (i.e. 24 and 25) could be isolated in pure form by flash chromatography (silica gel), forcing us to employ the crude mixtures in the side chain attachment step. Thus, treatment of the mixtures containing 24 or 25 (see Scheme 6) with NaHMDS and β-lactam V at 0 °C as described above [conditions (b)] furnished pure coupling products 26 (24% yield) or 28 (30% yield), respectively, in pure form after chromatographic separation. Desilylation of products 26 and 28 under conditions (c), (Scheme 6) [aq. HCl, THF:MeOH (1:2), 0 °C] led to docetaxel analogs 27 (58% yield) and 29 (89% yield), respectively.
Scheme 6.
Stereo- and regioselectivity of the reduction of ketones 2b and 3b. Synthesis of compounds 24–29. Reagents and conditions: a) NaBH4 (2.5 equiv → large excess), THF:MeOH (1:2), 0 °C, 1–3 h; b) NaHMDS (2.5 equiv), V (5.0–10.0 equiv), THF, 4 Å MS, 0 °C, 40 min, 24 (mixture) → 26 (24%); 25 (mixture) → 28 (30%); c) aq. HCl (1.0 M), THF:MeOH (1:2), 0 °C, 1–3 h, 26 → 27 (58%); 28 → 29 (89%).
Reduction of diketones 8, 13 and 14
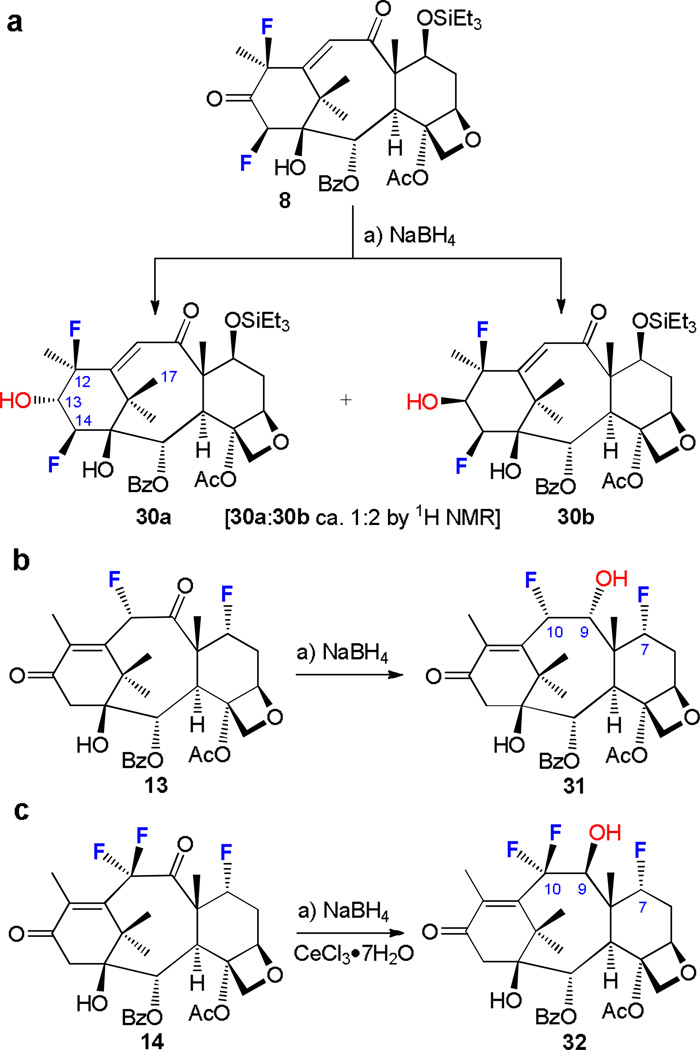
Fluorinated diketones 8, 13 and 14 exhibited interesting reactivity towards NaBH4 reduction as shown in Scheme 7. Thus, treatment of 12β,14β-difluorodiketone 9 with a large excess of NaBH4 [THF:MeOH = 1:2, conditions (a)] resulted in the formation of a mixture of 13α- and 13β-hydroxyenones 30a (44% yield) and 30b (39% yield) (Scheme 7a). Apparently the replacement of the hydrogen atoms at the C-12 and C-14 positions with fluorines influences the stereoselectivity of this reduction (compare 2a → 15, Scheme 5). Even more interesting was the NaBH4-mediated reduction of 7α,10α-difluorodiketone 13 (Scheme 7b) which led to 9α-hydroxyenone 31 (58% yield). In contrast, the reduction of the trifluoro diketone 14 (Scheme 7c) under the same conditions (a) proved sluggish and required Luche conditions (NaBH4–CeCl3•7H2O), leading to 9β-hydroxyenone 32 (95% yield). The observed reactivity enhancement of the C-9 carbonyl moiety over the C-13 carbonyl group within diketones 13 and 14 may be attributed to the electron-withdrawing properties of the fluorine substituents at C-7 and C-10 carbons adjacent to the former functional group. The opposite stereochemical outcome of this reduction in the case of 14, as compared to that of diketone 13 (Scheme 7b), is presumably due to the steric effect of the 10β-fluorine residue in the latter, an outcome observed also in the reduction of 8, which led partially to reversal of stereoselectivity (see 8 → 30a + 30b, Scheme 7a).
Scheme 7.
Stereo- and regioselectivity of the reduction of ketones 8, 13, and 14. Synthesis of compounds 30a, 30b, 31, and 32. Reagents and conditions: a) NaBH4 (2.5 equiv → large excess), THF:MeOH (1:2), 0 °C, 0.5–2 h (or NaBH4 (large excess), THF:MeOH (1:2), 0 °C, CeCl3 · 7H2O (cat.), see ESI), 8 → 30a (44%), plus 30b (39%); 13 → 31 (58%); 14 → 32 (95%).
While these results were interesting on their own right, they precluded the attachment of the docetaxel side chain at the proper position of the products in order to obtain useful analogs for biological evaluation.
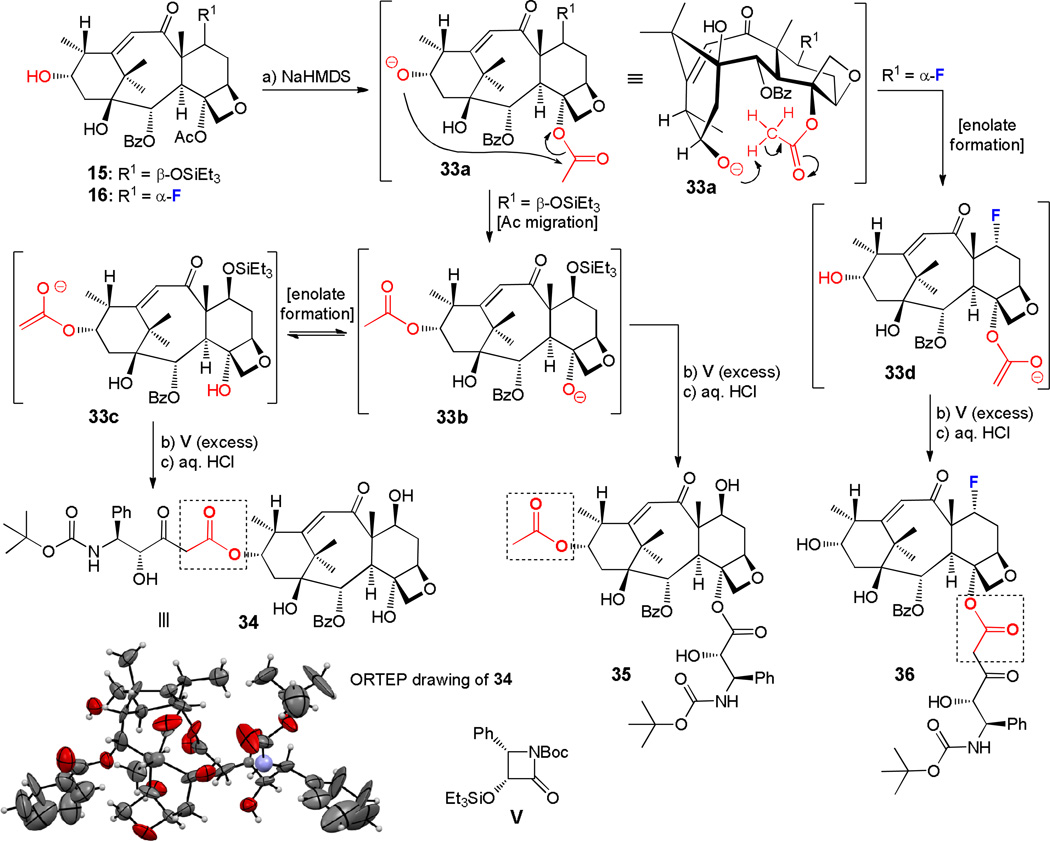
Preparation of unusual taxoids 34–36 from 15 and 16
While optimizing the conditions for the preparation of docetaxel analogs from hydroxy compounds 15 and 16, we encountered a number of unusual side-products, including compounds 34, 35 and 6 (Scheme 8). These compounds were of interest not only from the biological point of view, but also for mechanistic considerations of their formation. Speculative pathways for their generation are outlined in Scheme 8. Thus, under the basic conditions (a) employed for the side chain attachment (i.e. NaHMDS), the C-13-alkoxide (33a) formed from 15 or 16 may undergo intramolecular rearrangement through C-4-acetyl migration as shown in the case of 15 (R1 = β-OTES), or intramolecular enolate formation as shown in the case of 16 (R1 = F) to afford acetate 33b or enolate 33d, respectively. The former may also rearrange to enolate 33c. All three reactive species (i.e. 33c, 33b, and 33d) may then react with β-lactam V to afford the corresponding esters, which upon acid-induced desilylation lead to compounds 34 (39% overall yield from 15), 35 (12% overall yield from 15), and 36 (16% overall yield from 16), respectively as shown in Scheme 8. The absolute configuration of 34 (mp = 161–163 °C, hexanes/EtOAc 10:1) was confirmed by X-ray crystallographic analysis (see ORTEP representation, Scheme 8).
Scheme 8.
Generation of compounds 34–36 and postulated mechanism for their formation from 15, 16 and V. Reagents and conditions: a) NaHMDS (2.0–3.0 equiv), −78 °C, 1 h, then b) V (5.0–10.0 equiv), THF, −78 °C, 0.5 h and 0 °C, 0.5 h, see ESI for detailed procedures B2 and B3; then PTLC, followed by c) aq. HCl (1.0 M) (excess), THF:MeOH (1:2), 0 °C, 1–3 h, (yields reported over 3 steps), 15 → 34 (39%), plus 35 (12%); 16 → 36 (16%). PTLC = preparative thin-layer chromatography.
Biological evaluation of selected compounds
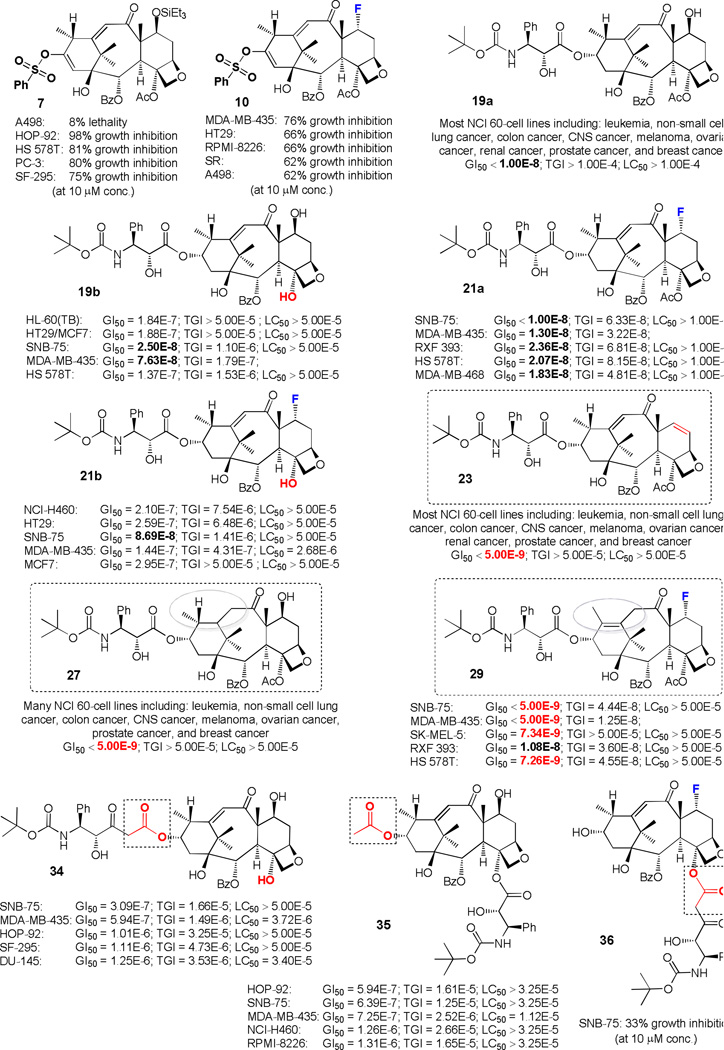
Selected compounds from those synthesized and described above, including sulfonates 7, 10, docetaxel analogs 19a, 19b, 21a, 21b, 23, 27, 29 and side-products 34–36 were submitted to the NCI 60 cell line screening program.45 Initial one-dose screening (at 10 µM conc.) revealed that sulfonates 7, 10 and 7α-fluorinated compound 36 possessed weak cytotoxicity (Fig. 2). Specifically, sulfonate 7 demonstrated moderate results, however, not sufficient for the five-dose screening [98% growth inhibition (GI) for HOP-92 (non-small cell lung cancer) and 8% lethality for A498 (renal cancer)]. 7α-Fluorinated sulfonate 10 was less active [76% growth inhibition (GI) for MDA-MB-435 (melanoma), 66% growth inhibition for HT29 (colon cancer), and RPMI-8226 (leukemia)]. Compound 36 was the least active, suppressing the growth of CNS cancer cell line (SNB-75) by only 33%. While these activities did not warrant further investigation of the compounds mentioned above, those of the remaining did.
Fig. 2.
Cytotoxicities of chosen synthesized taxoids against selected cancer cell lines (NCI). For experimental details and definitions, please use the following weblink: (http://dtp.nci.nih.gov/branches/btb/ivclsp.html). GI50 = growth inhibition of 50%, i.e. drug concentration resulting in a 50% reduction in the net protein increase (as measured by SRB staining) in control cells during the drug incubation; TGI = drug concentration resulting in total growth inhibition; LC50 = concentration of drug resulting in a 50% reduction in the measured protein at the end of the drug treatment as compared to that at the beginning; SRB = sulforhodamine B. Units: Molar. Color code: red (GI50 < 5 nM) and bold black (GI50 = 10–100 nM) numbers indicate high potencies. Average GI50 for Taxol for most cell lines varies from 10–35 nM. For further details on all compounds, see Supplementary Information and http://dtp.nci.nih.gov/ (see Table S1 for compound numbering).
Thus, because of their significant potencies against numerous tumor cell lines, docetaxel analogs 19a, 19b, 21a, 21b, 23, 27, 29, 34, and 35 were advanced to the five-dose screening stage, revealing more details about their cytotoxicities as depicted in Fig. 2 [the most potent compounds are shown in boxes (e.g., 23, 27, and 29); the highest potencies are emphasized in red (less than 5 nM) and bold black (from 10 to 100 nM)]. Specifically, analogs 23, 27, and 29 demonstrated very low GI50 values (less than 5 nM) against most of the 60 cell lines tested: for 23 [for most of the leukemia, non-small cell lung cancer, colon cancer, CNS cancer, melanoma, ovarian cancer, renal cancer, prostate cancer, and breast cancer cell lines GI50 ≤ 5 nM]; for 27 [for many of the leukemia, non-small cell lung cancer, colon cancer, CNS cancer, melanoma, ovarian cancer, prostate cancer, and breast cancer cell lines GI50 ≤ 5 nM]; for 29 [CNS cancer (SNB-75) GI50 ≤ 5 nM; melanoma (MDA-MB-435) GI50 ≤ 5 nM and (SK-MEL-5) GI50 = 7 nM; renal cancer (RXF 393) GI50 = 11 nM; breast cancer (HS 578T) GI50 = 7 nM]. Taxoids 19a, 19b, 21a, and 21b showed moderate cytotoxicity (GI50 values vary from 10 to 100 nM). In particular, for 19a [for most of the leukemia, non-small cell lung cancer, colon cancer, CNS cancer, melanoma, ovarian cancer, renal cancer, prostate cancer, and breast cancer cell lines GI50 ≤ 10 nM)]; for 19b [leukemia (HL-60(TB)) GI50 = 184 nM; colon cancer (HT29) GI50 = 188 nM; CNS cancer (SNB-75) GI50 = 25 nM; melanoma (MDA-MB-435) GI50 = 76 nM; breast cancer (MCF7) GI50 = 188 nM and (HS 578T) GI50 = 137 nM]; for 21a [CNS cancer (SNB-75) GI50 ≤ 10 nM; melanoma (MDA-MB-435) GI50 = 13 nM; renal cancer (RXF 393) GI50 = 24 nM; breast cancer (HS 578T) GI50 = 21 nM and (MDA-MB-468) ] GI50 = 18 nM]; and for 21b [non-small cell lung cancer (NCI-H460) GI50 = 210 nM; colon cancer (HT29) GI50 = 259 nM; CNS cancer (SNB-75) GI50 = 87 nM; melanoma (MDA-MB-435) GI50 = 144 nM; breast cancer (MCF7) GI50 = 295 nM]. Analogs 34 and 35 exhibited low cytotoxicities (GI50 ≥ 500 nM for most of the 60 cell lines) as shown in Fig. 2. For further details of the cytotoxicity data see http://dtp.nci.nih.gov/.
CONCLUSION
An exploration of the reactivity of the readily available taxoid 10-deacetylbaccatin III towards various fluorinating reagents led to a number of novel baccatin III derivatives, some of which were successfully converted to docetaxel analogs through reduction and side chain attachment. Biological evaluation of these analogs at NCI employing their 60 cell line screening assays led to the identification of a number of highly potent compounds against several cancerous cell lines (i.e. 23, 27, and 29; GI50 ≤ 5 nM). These results indicate that oxygenation at C-7 and C-10 of the taxoid family is not necessary for biological activity. They also suggest that some of the reported compounds warrant further investigation aiming at their development as personalized medicines for the treatment of certain cancers.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. D.H. Huang and L. Pasternack for NMR spectroscopic, Dr. G. Siuzdak for mass spectrometric, and Dr. R. K. Chadha for X-ray crystallographic assistance. Financial support for this work was provided by the Skaggs Institute for Research and the National Institutes of Health, USA (Grant AI055475). We are grateful to Merck for a postdoctoral fellowship (to R.A.V.). We also thank Armong Chen, Fujian South Pharmaceutical Co., Ltd. (Shanghai ParlingPharmTech Co., Ltd) for generous gifts of 10-deacetylbaccatin III (III) and lactam V, the National Cancer Institute (NCI) for performing the biological experiments and obtaining the data reported in this publication, and Drs. Doug Smallwood and Mohammed Nayel of the NCI for assistance and helpful discussions.
ABBREVIATIONS
- DAST
(diethylamino)sulfurtrifluoride
- TES
triethylsilyl
- MS
molecular sieves
- THF
tetrahydrofuran
- 4-DMAP
4-(dimethylamino)pyridine.
- KHMDS
potassium bis(trimethylsilyl)amide
- NFSi
N-fluorobenzenesulfonimide
- DMF
N,N-dimethylformamide
- NaHMDS
sodium bis(trimethylsilyl)amide
- PTLC
preparative thin-layer chromatography
Footnotes
Electronic supplementary information (ESI) available: it includes experimental procedures, selected physical data for the synthesized compounds, and further details of biological assays. CCDC 931453, 931454, 931455 and 931456. For ESI and crystallographic data in CIF or other electronic format see DOI: …
EXPERIMENTAL
Supplementary Information: Includes experimental procedures, selected physical data for the synthesized compounds, and further details of biological assays (pdf and cif files). This material is available free of charge via the Internet at http://pubs.rsc.org.
NOTES AND REFERENCES
- 1.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 2.Nicolaou KC, Dai W-M, Guy RK. Angew. Chem., Int. Ed. Engl. 1994;33:15–44. [Google Scholar]
- 3.Medical Subject Headings, Tree Structures, 2003. U. S. Department of Health and Human Services, National Library of Medicine; 2002. pp. 193–220. [Google Scholar]
- 4.DeVita VT, Jr, Hellman S, Rosenberg SA. Cancer: Principles and Practice of Oncology. (7th edn.) 2005;1:1–1488. vol. 2, pp. 1489–2898. [Google Scholar]
- 5.Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012. pp. 1–64. [Google Scholar]
- 6. http://disease-ontology.org/
- 7.Nicolaou KC, Montagnon T. Molecules that Changed the World. Wiley-VCH: Weinheim; 2008. pp. 1–366. [Google Scholar]
- 8.Nicolaou KC, Chen JS, Dalby SM. Bioorg. Med. Chem. 2009;17:2290–2303. doi: 10.1016/j.bmc.2008.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. J. Am. Chem. Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y-F, Shi Q-W, Dong M, Kiyota H, Gu Y-C, Cong B. Chem. Rev. 2011;111:7652–7709. doi: 10.1021/cr100147u. [DOI] [PubMed] [Google Scholar]
- 11.Schiff PB, Fant J, Horwitz SB. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 12. http://www.cancer.gov/cancertopics/druginfo/paclitaxel.
- 13. http://www.cancer.gov/cancertopics/druginfo/docetaxel.
- 14.Lyseng-Williamson KA, Fenton C. Drugs. 2005;65:2513–2531. doi: 10.2165/00003495-200565170-00007. [DOI] [PubMed] [Google Scholar]
- 15.Arroyo PA, Pérez RU, Díez BC, Feijóo MAF, Magdalena JH, Hernández MAC. Cancer Treat. Rev. 2011;37:105–110. [Google Scholar]
- 16.Samaranayake G, Neidigh KA, Kingston DGI. J. Nat. Prod. 1993;56:884–898. doi: 10.1021/np50096a012. [DOI] [PubMed] [Google Scholar]
- 17.Ojima I, Duclos O, Zucco M, Bissery M-C, Combeau C, Vrignaud P, Riou JF, Lavelle F. J. Med. Chem. 1994;37:2602–2608. doi: 10.1021/jm00042a013. [DOI] [PubMed] [Google Scholar]
- 18.Ojima I, Bounaud P-Y, Takeuchi C, Pera P, Bernacki RJ. Bioorg. Med. Chem. Lett. 1998;8:189–194. doi: 10.1016/s0960-894x(97)10218-9. [DOI] [PubMed] [Google Scholar]
- 19.Dubois J, Thoret S, Guéritte F, Guénard D. Tetrahedron Lett. 2000;41:3331–3334. [Google Scholar]
- 20.Cheng Q, Oritani T, Horiguchi T. Tetrahedron. 2000;56:1667–1679. [Google Scholar]
- 21.Iimura S, Uoto K, Ohsuki S, Chiba J, Yoshino T, Iwahana M, Jimbo T, Terasawa H, Soga T. Bioorg. Med. Chem. Lett. 2001;11:407–410. doi: 10.1016/s0960-894x(00)00682-x. [DOI] [PubMed] [Google Scholar]
- 22.Kant J, Schwartz WS, Fairchild C, Gao Q, Huang S, Long BH, Kadow JF, Langley DR, Farina V, Vyas D. Tetrahedron Lett. 1996;37:6495–6498. [Google Scholar]
- 23.Ojima I, Kuduk SD, Pera P, Veith JM, Bernacki RJ. J. Med. Chem. 1997;40:279–285. doi: 10.1021/jm9606711. [DOI] [PubMed] [Google Scholar]
- 24.Querolle O, Dubois J, Thoret S, Roussi F, Montiel-Smith S, Guéritte F, Guénard D. J. Med. Chem. 2003;46:3623–3630. doi: 10.1021/jm030770w. [DOI] [PubMed] [Google Scholar]
- 25.Ojima I, Fumero-Oderda CL, Kuduk SD, Ma Z, Kirikae F, Kirikae T. Bioorg. Med. Chem. 2003;11:2867–2888. doi: 10.1016/s0968-0896(03)00181-0. [DOI] [PubMed] [Google Scholar]
- 26.Querolle O, Dubois J, Thoret S, Roussi F, Guéritte F, Guénard D. J. Med. Chem. 2004;47:5937–5944. doi: 10.1021/jm0497996. [DOI] [PubMed] [Google Scholar]
- 27.Ke B, Qin Y, Zhao F, Qu Y. Bioorg. Med. Chem. Lett. 2008;18:4783–4785. doi: 10.1016/j.bmcl.2008.07.101. [DOI] [PubMed] [Google Scholar]
- 28.Lu H-F, Xie C, Chang J, Lin G-Q, Sun X. Eur. J. Med. Chem. 2011;46:1743–1748. doi: 10.1016/j.ejmech.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 29.Samaranayake G, Magri NF, Jitrangsri C, Kingston DGI. J. Org. Chem. 1991;56:5114–5119. [Google Scholar]
- 30.Wahl A, Guéritte-Voegelein F, Guénard D, Le Goff M-T, Potier P. Tetrahedron. 1992;48:6965–6974. [Google Scholar]
- 31.Chen S-H, Huang S, Wei J, Farina V. Tetrahedron. 1993;49:2805–2828. [Google Scholar]
- 32.Pulicani J-P, Hervé B, Bourzat J-D, Commerçon A. Tetrahedron Lett. 1994;35:9709–9712. [Google Scholar]
- 33.Chen S-H, Farina V, Huang S, Gao Q, Golik J, Doyle TW. Tetrahedron. 1994;50:8633–8650. [Google Scholar]
- 34.Chen S-H, Huang S, Gao Q, Golik J, Farina V. J. Org. Chem. 1994;59:1475–1484. [Google Scholar]
- 35.Yu C, Liu Z. Tetrahedron Lett. 1997;38:4133–4136. [Google Scholar]
- 36.Johnson RA, Dobrowolski PJ, Nidy EG, Gebhard I, Qualls SJ, Wicnienski NA, Kelly RC, Pitts TW, Lopes NM, DeKoning TF, Mattern SJ, Weed SD, McGovren JP, Chidester CG. J. Med. Chem. 1997;40:2810–2812. doi: 10.1021/jm9703350. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Zhang H-B, Liu J-K, Su J, Li Y, Yao Z-J, Zhao Q-S. Tetrahedron Lett. 2011;52:139–142. [Google Scholar]
- 38.Gao F, Yang Z-K, Chen Q-H, Chen X-G, Wang F-P. Org. Biomol. Chem. 2012;10:361–366. doi: 10.1039/c1ob06535a. [DOI] [PubMed] [Google Scholar]
- 39.Fried J, Sabo EF. J. Am. Chem. Soc. 1954;76:1455–1456. [Google Scholar]
- 40.Bartlett PA, Otake A. J. Org. Chem. 1995;60:3107–3111. [Google Scholar]
- 41.Isanbor C, O’Hagan D. J. Fluorine Chem. 2006;127:303–319. [Google Scholar]
- 42.Kirk KL. J. Fluorine Chem. 2006;127:1013–1029. [Google Scholar]
- 43.O’Hagan D. J. Fluorine Chem. 2010;131:1071–1081. [Google Scholar]
- 44.Zimmer LE, Sparr C, Gilmour R. Angew. Chem., Int. Ed. 2011;50:11860–11871. doi: 10.1002/anie.201102027. [DOI] [PubMed] [Google Scholar]
- 45. http://dtp.nci.nih.gov/ [Google Scholar]
- 46.Paquette LA, Crich D, Fuchs PL, Molander G. Encyclopedia of Reagents for Organic Synthesis. 2nd edn. John Wiley & Sons; 2009. pp. 1–12094. (14 volumes). [Google Scholar]
- 47.Albeit this particular conversion was previously unknown, Kant et al. from Bristol-Myers Squibb Research Institute observed similar undesired side-product while preparing C-10 Taxol® analogs. Kant J, O’Keeffe WS, Chen S-H, Farina V, Fairchild C, Johnston K, Kadow JF, Long BH, Vyas D. Tetrahedron Lett. 1994;35:5543–5546.
- 48.Denis J-N, Greene AE. J. Am. Chem. Soc. 1988;110:5917–5919. [Google Scholar]
- 49.Intermediate 5d, under warmer temperature and prolonged treatment, is prone to rearrangement, producing taxyunansin-related [37] motif 6 through A-ring contraction, as previously reported by Kingston [29], Liu [35], and Chen [50–51]. In the presence of moisture 5d hydrolyzes, forming a mixture of two diastereomericsulfinamides (7 or 7′ were observed exclusively in the reaction between 13-acetylbaccatin III with DAST) [51]: Chen S-H, Huang S, Wei J, Farina V. J. Org. Chem. 1993;58:4520–4521.
- 50.Chen S-H, Huang S, Farina V. Tetrahedron Lett. 1994;35:41–44. [Google Scholar]
- 51.Roth GP, Marshall DR, Chen S-H. Tetrahedron Lett. 1995;36:1609–1612. [Google Scholar]
- 52.No cyclopropane-containing compound was isolated in the reaction, see: Johnson RA, Nidy EG, Dobrowolski PJ, Gebhard I, Qualls SJ, Wicnienski NA, Kelly RC. Tetrahedron Lett. 1994;35:7893–7896. see also refs [49–51].
- 53.Gerstenberger MRC, Haas A. Angew. Chem., Int. Ed. Engl. 1981;20:647–667. [Google Scholar]
- 54.Lal GS, Pez GP, Syvret RG. Chem. Rev. 1996;96:1737–1755. doi: 10.1021/cr941145p. [DOI] [PubMed] [Google Scholar]
- 55.Ma J-A, Cahard D. Chem. Rev. 2004;104:6119–6146. doi: 10.1021/cr030143e. [DOI] [PubMed] [Google Scholar]
- 56.Ma J-A, Cahard D. J. Fluorine Chem. 2007;128:975–996. [Google Scholar]
- 57.Nyffeler PT, Durón SG, Burkart MD, Vincent SP, Wong C-H. Angew. Chem., Int. Ed. 2005;44:192–212. doi: 10.1002/anie.200400648. [DOI] [PubMed] [Google Scholar]
- 58.Chen’s group reported the formation of α-fluorinated enone (at C-12, related to 9) together with similar dienone by using Yarovenko’s reagent (ClFHCCF2NEt2): Chen S-H, Fairchild C, Mamber SW, Farina V. J. Org. Chem. 1993;58:2927–2928.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.