Abstract
Human serum paraoxonase 1 (PON1) is an enzyme with esterase activity, and is physically bound to high-density lipoproteins (HDL). It plays a key role in the action of HDL toward protection of lipoprotein and biological membrane against oxidative damage. It may have a protective role against atherosclerosis by virtue of its action on hydrolyzing lipid peroxides and preventing accumulation of phospholipids in oxidized low-density lipoprotein (LDL). PON1 is hypothesized to be an indicator of the risk of atherosclerosis and coronary artery disease development. Numerous studies have implicated PON1 activity in relation to various endocrine disorders. The current article reviews the clinical perspectives of PON1 activity with regards to obesity, diabetes mellitus with its complications, and dyslipidemia.
Keywords: Diabetes mellitus, dyslipidemia, high-density lipoprotein, obesity, paraoxonase
INTRODUCTION
Paraoxonase (aryldialkylphosphatase) is an enzyme having both paraoxonase and aryl esterase activity.[1,2] It hydrolyzes aromatic carboxylic acid esters and certain organophosphorous pesticides, especially paraoxon and nerve gas.[3,4] Plasma paraoxonase (PON) activity in human population demonstrates polymorphic distribution due to an amino acid substitution in the active site of the enzyme, giving rise to low-, intermediate-, or high-activity isoenzymes.[5,6,7,8] The polymorphic variation in serum PON activity may affect the metabolism of organophosphates in individuals at high risk of acute organophosphorous intoxication or of organophosphorous-induced delayed polyneuropathy.[9]
Human PONs are high-density lipoprotein (HDL) associated enzymes. PON is located in a subfraction of HDL containing apo A-I and clusterin (Apo J). PON is anchored to HDL by its hydrophobic N terminal end and Apo A-I.[10,11] PON1 and PON3 are expressed in the liver. After secretion into blood, they bind to HDL particles;[12,13] PON2 is expressed widely in a number of tissues but is not present in the blood.[14] The genes encoding the PON family (PON1, PON2, and PON3) are all located on the long arm of chromosome 7 (7q21.3-q22.1).[15]
PARAOXONASE: ACTIONS AND ALTERATION IN VARIOUS STATES
It plays a significant role in delaying/inhibiting the oxidation of both low-density lipoprotein (LDL) and HDL particles.[16,17] By virtue of its actions like prevention of accumulation of lipid peroxides in LDL, stimulation of breakdown by hydrolysis of lipid peroxides, and protection against lipoprotein oxidation, PON1 has a protective role against atherosclerosis and cardiovascular disease Figures 1 and 2.[14,15,18] PON2 and PON3 both have antioxidant properties and hydrolyze aromatic and long-chain aliphatic lactones, but lack paraoxonase or arylesterase activities.[19,20] Similar to PON1, PON3 too protects against LDL oxidation and inflammation,[13] reflecting its atheroprotective effect. Shih et al. reported that elevated PON3 expression significantly decreases atherosclerotic lesion formation and adiposity in male mice,[21] signifying the important role of PON3 in protection against obesity and atherosclerosis.
Figure 1.
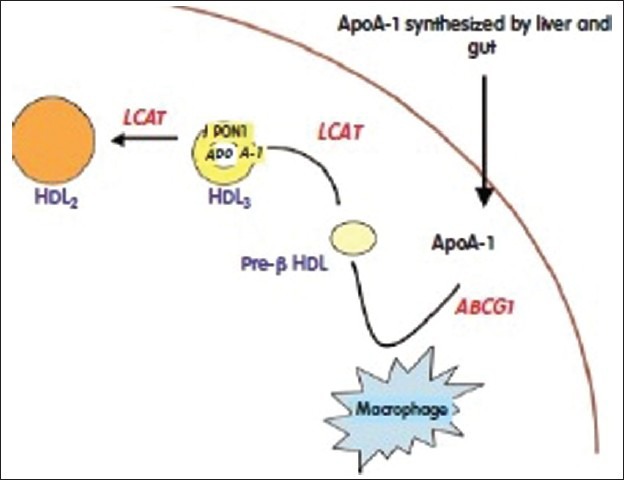
High-density lipoproteins (HDL) is maturated from pre-βmigrating HDL to HDL3 by the action of lecithin-cholesterol acyltransferase and then to HDL2 which is large and rich in paraoxonase 1
Figure 2.
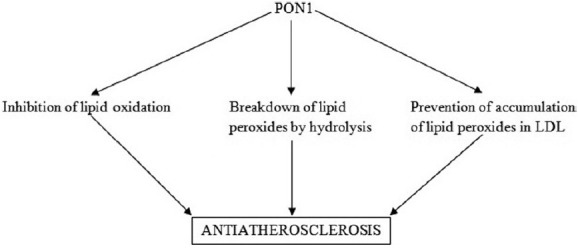
Anti-atherosclerotic properties of PON1. (PON1: Paraoxonase 1, LDL: Low-density lipoprotein)
PON1 activity is decreased in patients with diabetes mellitus (DM),[22] cardiovascular complications,[23] hypercholesterolemia, and renal failure.[24] Some environmental, pharmacological or other factors may change the activity of PON1. The factors that induce the activity of PON1 are drugs such as statins, fibrates, aspirin, glucocorticoids, and phenobarbital, which are classical inducers of PON1 activity.[1,25,26,27] Some environmental chemicals abolish its activity.[28] PON1 activity is also decreased by smoking, alcohol, fat-rich diet, and aging.[1,2,3,4,12,13,14,15,16,17,25,26,27,28,29,30,31,32] Few studies had shown that PON1 activity is slightly higher in the female gender.[7,27]
PARAOXONASE 1 ACTIVITY IN OBESITY
Obesity is associated with several alterations in the lipid metabolism, leading to changes in lipoprotein levels and composition,[33,34] and greater risk of cardiovascular disease. Oxidative stress is increased in obese subjects compared with healthy controls[35] Figure 3. Ferretti et al. reported significantly lower PON activity in obese subjects compared with control subjects.[36] A relationship was found between HDL-PON and lipid hydroperoxides in HDL and LDL of control and obese subjects, which suggests that subjects with lower PON activity are more exposed to oxidative damage Figure 4. In obese adults, diminished levels of PON1 activity is correlated with low levels of HDL-cholesterol.[36,37] Body mass index is an independent predictor of PON1 activity.[38] In another study involving childhood subjects, there was decreased PON1 activity in obese compared with normal weight peers.[39] The aryl esterase activity had significant positive correlation with adipokine levels and negative correlation with leptin concentrations. These results confirm that obesity is associated with oxidative damage of lipoproteins and may explain the increased cardiovascular risk in obese people. Orlistat treatment in obese subjects increases PON1 activity and PON/HDL ratio besides altering the lipid profile.[40]
Figure 3.
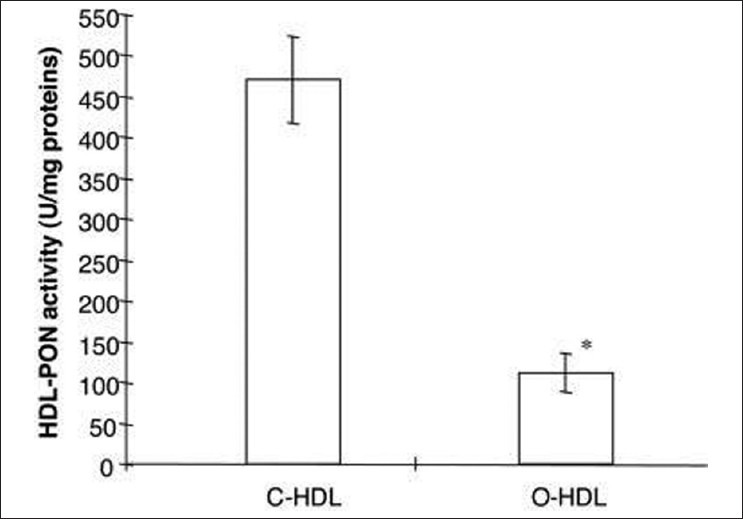
Values of paraoxonase activity in high-density lipoproteins isolated from plasma of control and obese subjects
Figure 4.
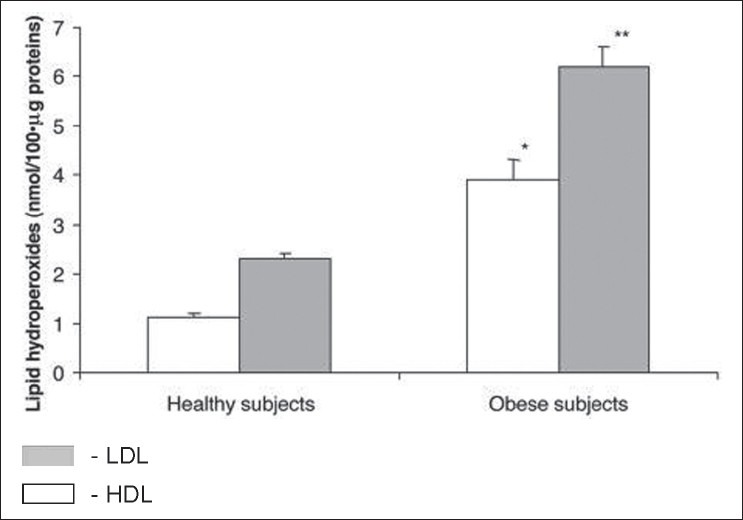
Levels of lipid hydroperoxides in high-density lipoproteins (HDL) and low-density lipoprotein (LDL) isolated from plasma of control and obese subjects. Data are expressed as the mean ± SEM. *P< 0.001 vs. lipid hydroperoxides in HDL from control subjects; **P< 0.001 vs. levels of hydroperoxides in LDL from control subjects
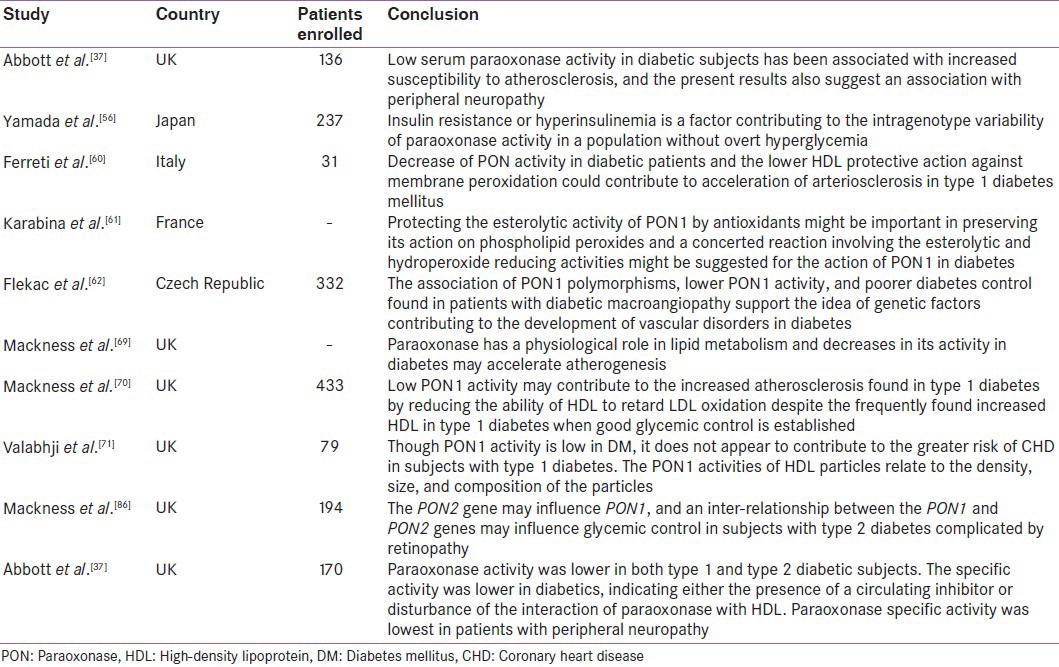
Correlations between leptin levels[38] as well as adiponectin levels and PON1 activity have been previously proven in obese adults[41] Figure 5. There are several mechanisms of adipokines influencing PON1 activity. Beltowski found that rats treated with leptin had decreased PON1 activity.[42] These mechanisms may be the following: leptin as a hydrophobic peptide can bind to HDL[43] and inhibit directly the PON1 enzyme. On the other hand, leptin enhances oxidative stress[44,45] through the generation of reactive oxygen species (ROS)[42] and stimulates the secretion of inflammatory cytokines[46] and other acute phase proteins which have diminishing effect on PON1 enzyme activity by the inhibitory effect on hepatic PON1 synthesis.[47] Leptin also enhances the production of serum amyloid A protein[48] which can replace apolipoprotein-A1 (apo A-I) in HDL. Apo A-I plays a major role in stabilizing the structure of PON1. Leptin may have modulatory effect through the alteration of the lipid content in HDL particles;[49] inverse correlation has been observed between leptin, HDL, and apo A-I in human subjects.[50] Adiponectin might accelerate the reverse cholesterol transport and increase apo A-I-mediated cholesterol efflux through enhancing HDL assembly in the liver.[51,52] Another study in male mice has demonstrated that elevated PON3 expression significantly decreases atherosclerotic lesion formation and adiposity.[21] Women with polycystic ovarian syndrome (PCOS) and obesity were found to have high insulin resistance and significantly lower PON1 levels.[53,54] These findings suggest that decreased PON1 activity in PCOS patients might contribute to increased insulin resistance and attendant atherosclerotic heart disease. Table 1 gives the summary of studies demonstrating the relationship between lower PON activity in obesity and associated increase in cardiovascular risk. Analysis of the studies on PON1 activity in obesity leads to the conclusion that obesity is associated with 50–60% reduction in PON activity, accompanied by significant fall in LDL and increase in HDL. This reflects in higher atherosclerotic potential. Studies have not yet analyzed the corresponding fall in PON with the incremental body mass index.
Figure 5.
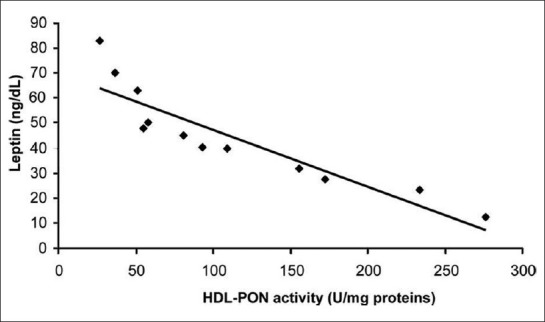
Correlation between the levels of HDL-PON activity and leptin in the plasma of obese subjects (r= −0.90; P< 0.001; n= 12)
Table 1.
Summary of studies mentioning low paraoxonase 1 activity in obesity and associated increase in cardiovascular risk
PARAOXONASE 1 ACTIVITY IN DIABETES MELLITUS
PON1 activity has been studied in patients with metabolic syndrome and insulin resistance, and some contradictions were found between the studies performed in non-diabetic subjects. In non-diabetic Swiss population, the significantly reduced serum PON1 concentrations and activities were associated with metabolic syndrome defined according to the World Health Organization (WHO) guidelines.[55] Yamada et al. have shown that there was a positive correlation between the Homeostasis Model Assessment (HOMA) index and HDL-corrected PON1 activity in non-diabetic Japanese subjects.[56] In another study on Turkish population, PON1 activities were not different between non-diabetic subjects with and without metabolic syndrome.[57] Additionally, Beer et al.[58] found that PON1 activities and concentrations were not different in diabetic patients compared to subjects with impaired fasting glucose and controls, although postprandial hyperlipemia was associated with changes in serum PON1 in diabetic subjects. In the same study, significantly low serum PON1 concentrations in the postprandial period were demonstrated, attributed to postprandial hypertriglyceridemia, whereas the decrease in PON1 activity was not statistically significant. There was no difference in the postprandial PON1 response between diabetic and non-diabetic groups. In another study,[59] PON1 activity was not significantly altered compared with normoglycemic controls, although oxidized LDL levels were significantly higher in glucose-intolerant and newly diagnosed diabetic subjects.[59] All these studies suggest that PON1 activity loss may occur later in the course of diabetes mellitus and hyperglycemia, rather than in the stage of insulin resistance.
Serum PON1 activity is significantly decreased in type 1 and type 2 diabetics compared to the healthy control subjects.[60,61,62] Ferretti et al. reported significantly lower PON1 activity in type 1 diabetic patients compared to healthy controls. They hypothesized that the decreased ability of HDL to protect erythrocyte membranes could be related to lipid composition of HDL and to this low PON1 enzyme activity[61] Figure 6. Diabetes is associated with oxidative damage.[63] The higher plasma levels of lipid peroxidation products in diabetic patients[64] are ascribed to higher susceptibility of plasma lipoproteins to oxidation[65,66] and decrease of plasma antioxidant defenses.[67] Altered myocardial substrate/ fat metabolism leads to diabetic cardiomyopathy.[68] Apart from decreased PON activity,[37,69,70] abnormal HDL composition and altered HDL subclasses distribution are also found in type 1 diabetes patients.[71,72]
Figure 6.
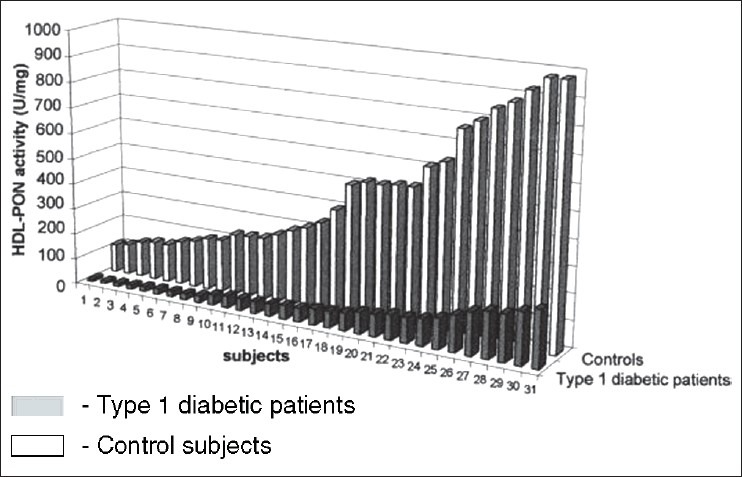
Activity of PON associated with HDL (HDL-PON) isolated from control subjects and type 1 diabetic patients. Activity is expressed as units per milligram of HDL protein
The compositional changes in HDL lead to its altered functional activities resulting in lower protection exerted by HDL from diabetic patients against LDL oxidation and a decreased capacity to induce cholesterol efflux from biological membranes.[17,73,74,75,76] Binding of PON to HDL is affected. There is also a conformational change in PON and altered availability of substrates within the hydrophobic region of HDL in which PON is active. It also gives rise to an increase in susceptibility to organophosphate poisoning.[9] Low serum PON activity cannot detoxify these compounds via hydrolysis. This leads to higher susceptibility to neural damage by these environmental toxins due to low PON activity in diabetes, resulting in an increase in organophosphate-induced delayed polyneuropathy in diabetic subjects.
There are propositions that other enzymes associated with HDL surface, such as plasma platelet-activating factor acetylhydrolase, lecithin cholesterol acetyl transferase (LCAT), and cholesteryl ester transfer protein (CETP),[77] might be involved in the alterations of the protective effect exerted by diabetic HDL against oxidative damage of biological membranes. In fact, previous studies have shown, in diabetic patients, modifications of the activities of enzymes involved in the antioxidant role of HDL, such as LCAT.[78] Moreover, a PON-independent inhibition of LDL oxidation by HDL has been observed by Graham et al.[79]
A larger proportion of the PON protein could be inactive in diabetes either because of the presence of an endogenous circulating inhibitor or perhaps because of increased glycosylation of PON. The loss of the strong correlation between PON concentration and glycation of apo A-I in healthy subjects[10] and in all the diabetic populations might indicate a disruption in the interaction between PON and the HDL particle. PON1 activity is lower in type 1 and type 2 diabetic patients, coupled with higher triglyceride levels, compared to non-diabetic control subjects although PON serum levels are not significantly different. Unlike type 1 diabetes, HDL and apo A-I levels have been found to be lower in type 2 diabetic patients.[37] There is a contradiction in the literature about the correlation between glycated hemoglobin (HbA1c) and PON1 activity. A negative correlation was found in both types of diabetes,[62] although no significant association was reported between HbA1c and PON1 activity in a study performed in type 1 diabetes patients.[60] There was also a negative correlation between PON1 activity and presence of vascular complications.[62] The lower serum PON1 activity was found in diabetic patients with macrovascular complications than in those with microvascular complications. In a recent study,[80] reduced PON1 activity in type 2 diabetic patients was found to be associated with a significant increase in the risk of cardiovascular disease (CVD) which leads to the conclusion that PON1 activity could be a predictor of CVD in type 2 diabetes.[80] Serum PON1 activity in diabetic patients with neuropathy was significantly lower than in patients without diabetic neuropathy.[80] Additionally, serum PON1 activity was decreased in patients with myocardial infarction.[81] Both PON1 and arylesterase enzyme activities were lower in diabetic foot patients compared to healthy control subjects.[82] Studies investigating the relationship between diabetes complications and PON1 polymorphisms have shown variable results. Flekac et al. reported that L55M and Q192R polymorphisms are more common in diabetes and are associated with macroangiopathy[62] Figure 7. PON1-55 MM and PON1-192QQ genotypes were associated with poorer diabetes control than LL and RR genotypes. Better diabetes control was found in patients with LL genotype than with MM, and similarly, in those with RR genotype versus QQ (P < 0.05). Chiu et al. have shown that L55M polymorphism, not Q192M, was an independent determinant for beta-cell function in glucose-tolerant Whites.[83] In Turkish population, PON1-R192 variant was found as an independent genetic risk factor more than three times in the development of complications in diabetes[84] and RR genotype may be a risk factor for cardiac complications in type 2 diabetes patients.[85] Association of LL genotype and the development of diabetic retinopathy has been reported by Mackness et al.[86] Figure 8. Oxidized LDL has been shown to be cytotoxic to retinal capillary endothelial cells and pericytes, leading to development of retinopathy in diabetes.[87] PON1 activity was significantly low in patients with non–insulin-dependent diabetes mellitus and retinopathy compared to patients without complication, but was not different between patients with and without proteinuria.[88] The authors have reported serum PON activity as one of the factors for retinopathy. Other studies have reported lower PON1 activity in diabetic subjects with nephropathy.[37,86] In another study by Abbott et al., paraoxonase activity was lower in both type 1 and type 2 diabetic subjects. This decrease was unrelated to differences in paraoxonase phenotype distribution or its serum concentration. Rather, the difference in paraoxonase activity was explained by its specific activity, which was lower in diabetics, indicating either the presence of a circulating inhibitor or disturbance of the interaction of paraoxonase with HDL, affecting its activity. Paraoxonase specific activity was lowest in patients with peripheral neuropathy.[37] Table 2 gives the summary of studies demonstrating the relationship between lower PON activity in diabetes and its complications. Analysis of the studies on PON1 activity in diabetes leads to the conclusion that diabetes is associated with 10–15% reduction in PON activity. The fall in PON1 activity is significantly more when diabetes is accompanied by microvascular complications, especially neuropathy and nephropathy resulting in higher risk for atherosclerosis.
Figure 7.
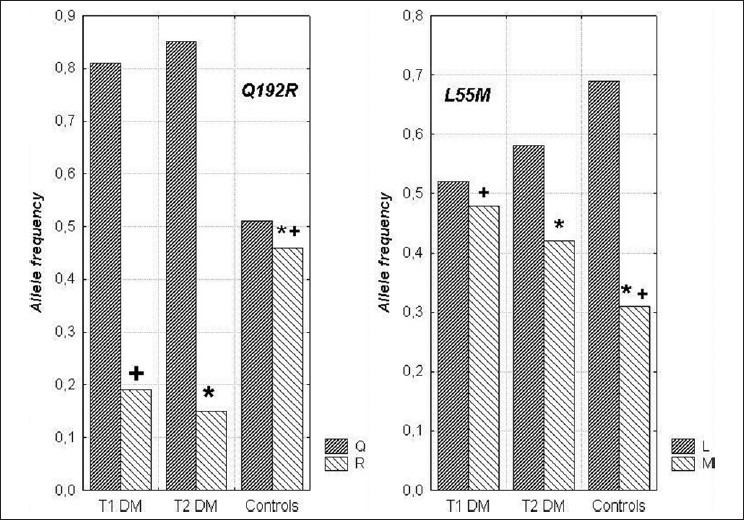
The frequencies of alleles in L55M and Q192R polymorphisms in diabetic patients and healthy subjects. Statistically significant differences (P< 0.05): +between T1DM vs. control subjects; *between T2DM vs. control subjects
Figure 8.
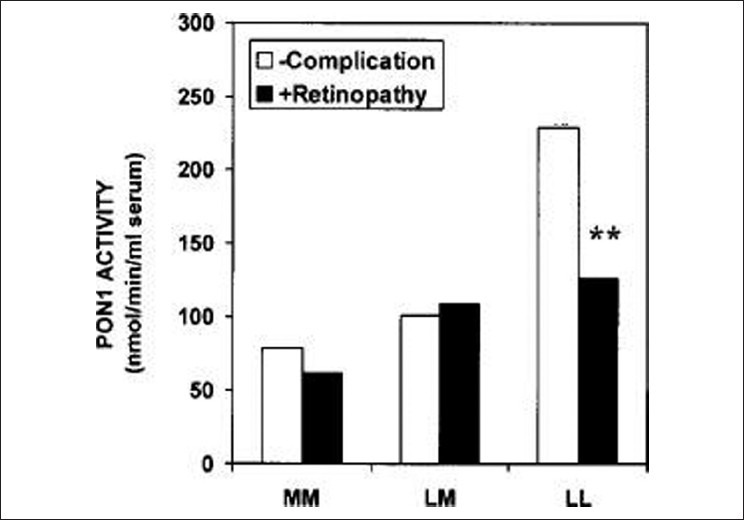
Effects of the PON1-55 genotype on serum PON1 activity in subjects with type II diabetes either with retinopathy or with no complications. LL and MM-Homozygotes, LM-Heterozygotes. **P< 0.001
Table 2.
Summary of studies demonstrating low paraoxonase 1 activity in diabetes and its complications
PARAOXONASE 1 ACTIVITY IN DYSLIPIDEMIA
The positive correlation between PON1, HDL cholesterol, and apo A-I is well known.[37] Saha et al. found correlations with triglycerides and apoB as well as HDL.[89] PON genotype is a major determinant of serum lipid and lipoprotein concentrations, particularly HDL-associated parameters.[90] The rise in apo A-I, which plays a protective role against atherosclerosis, was associated with an increase in serum PON1 concentration. On the other hand, human apo A-II is proatherogenic.[91] In vitro displacement of PON1 by human apo A-II at physiological concentrations could explain the observation that PON1 was mostly found in HDL particles containing apo A-I but not apo A-II, and thus the lack of anti-atherogenic properties of apo A-II–enriched HDL.[92,93] Studies have demonstrated susceptibility of HDL to atherogenic modifications like glycation and homocysteinylation, induced in vitro in the absence of PON1 activity.[94,95] Zech et al. have reported that PON1 may first bind to smaller HDL3 particles and then transform into a larger HDL2 particle.[96] Studies performed with both gel filtration and electron microscopic measurements confirmed this hypothesis and PON1 would seem to be present in larger sized HDL2 particle.[97,98,99]
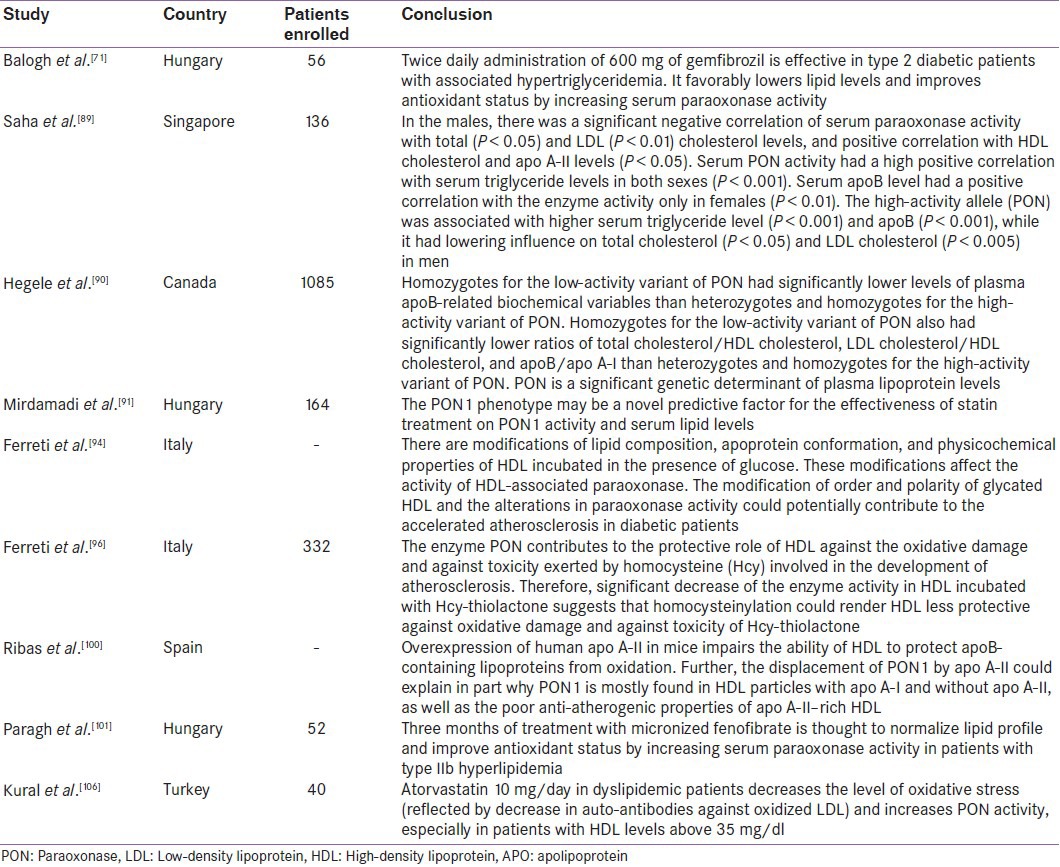
Some studies have demonstrated that lipid-lowering treatments improve PON1 serum activity. In a recent study[100] performed in 164 patients with type IIb hypercholesterolemia, 3 months of statin treatment (atorvastatin 10 mg/day, simvastatin 10/20 mg/day, and fluvastatin 80 mg/day) significantly increased the PON activity in the three statin-treated groups compared to controls. Paragh et al. reported that 3-month treatment with micronized fenofibrate 200 mg/day in patients with coronary heart disease and type IIb hyperlipidemia significantly increased serum PON activity (P < 0.05) and improved the antioxidant status.[101] Opposite to this, in another study on normolipidemic rats, fenofibrate treatment dose-dependently decreased plasma PON1 activity by 20–40%[102] Figure 9. In type 2 diabetic patients, gemfibrozil (GEM) 600 mg/bid for 3 months increased PON1 activity,[103] although no difference was observed in PON1 activity with GEM (600 mg/bid) and bezafibrate (400 mg/day) treatments for 8 weeks in type IIb hyperlipidemic patients.[104] Macan et al.[105] reported that after GEM treatment, plasma PON1 activity significantly reduced in rats on high-sucrose diet or on control diet compared to rats on diet-only therapy. In conclusion, the decreasing effect of fenofibrate on PON1 activity may be potentially an adverse effect, which could be masked by the positive changes in plasma lipid profile. Statin intake too is shown to promote PON to decrease LDL oxidation.[106] A recent study has demonstrated that though carriers of CYP2C19*2 alleles exhibited lower levels of platelet inhibition by clopidogrel and higher on-treatment platelet aggregation than noncarriers, there was no significant difference in platelet aggregation among PON1 Q192R genotypes.[107] This leads to the conclusion that PON1 (Q192R) polymorphism does not appear to be a significant determinant of clopidogrel response. Paraoxonase detoxifies homocysteine thiolactone in human blood and is thus hypothesized to delay the development of atherosclerosis. In Tunisian population, coronary artery disease was associated with increased homocysteine levels. The low PON1 activity was associated with the PON1 192RR genotypes corresponding with the severity of CAD.[108]
Figure 9.
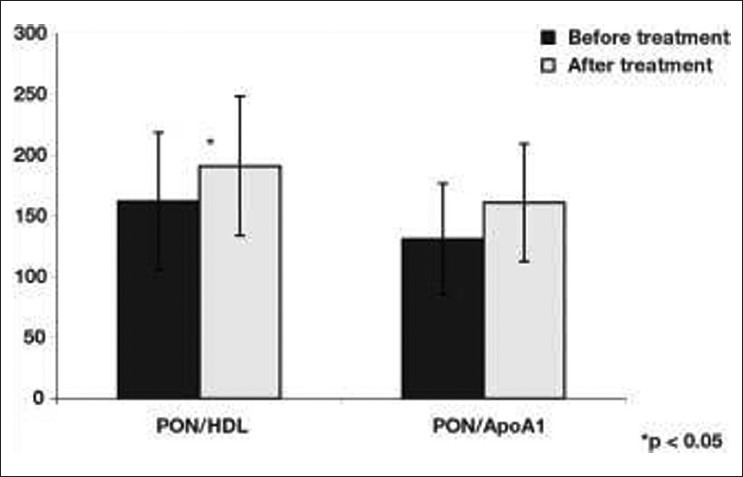
PON/HDL and PON/apo A-I ratio changes before and after micronized fenofibrate treatment. *P< 0.05
PON1 gene therapy may play a role in the management of dyslipidemia and liver diseases in the future. Injection of plasmid containing the human PON1 gene via rat tail vein could prevent dyslipidemia and hepatic lipid accumulation by increasing antioxidant superoxide dismutase (SOD) and decreasing the serum levels of LDL, total cholesterol, triglyceride, and malondialdehye (MDA). These results indicate that gene therapy with plasmid coated DNA/PON1 might be an effective treatment for hyperlipidemia and liver diseases like hepatosteatosis.[109] Table 3 provides the summary of studies demonstrating the relationship between lower PON activity in dyslipidemia and the effect of hypolipidemic agents on serum PON activity. The summary of studies on paraoxonase activity in dyslipidemia depicts 50–60% higher LDL and 8–10% lower HDL cholesterol in obese subjects, contributing to higher cardiovascular risk. Treatment with hypolipidemic agents results in improvement in PON1 activity.
Table 3.
Summary of studies demonstrating low paraoxonase 1 activity in dyslipidemia and effect of hypolipidemic agents on serum paraoxonase activity
CONCLUSION
PON1, by virtue of its antioxidant property, prevents the formation of oxidized LDL and protects phospholipids in HDL from oxidation by inactivating LDL-derived oxidized phospholipids. Serum PON1 levels decrease in states of high oxidative stress like metabolic syndrome, obesity, uncontrolled diabetes, and dyslipidemia. Serum PON1 activity was found to be lower in diabetic patients with macrovascular and microvascular complications. PON1 level is positively correlated with HDL cholesterol and negatively correlated with LDL cholesterol. Numerous polymorphisms in PON1 gene have been described; however, there is still not enough evidence to support the existence of relationship between metabolic disorders and specific polymorphism in PON1 gene.
ACKNOWLEDGMENTS
All the authors would extend their heartfelt thanks to Dr. Jagadeesh Tangudu, M Tech, MS, PhD, and Mrs. Sowmya Jammula, M Tech, for their immense and selfless contribution toward manuscript preparation, language editing, and final approval of text.[110]
Footnotes
Source of Support: Nil
Conflict of Interest: No
REFERENCES
- 1.Aldridge WN. Serum esterases. I. Two types of esterase (A and B) hydrolyzing P-nitrophenyl acetate, propionate and butyrate, and a method for their determination. Biochem J. 1953;53:110–7. doi: 10.1042/bj0530110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorenson RC, Primo-Parmo SL, Kuo CL, Adkins S, Lockridge O, La Du BN. Reconsideration of the catalytic center and mechanism of mammalian paraoxonase/arylesterase. Proc Natl Acad Sci U S A. 1995;92:7187–91. doi: 10.1073/pnas.92.16.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldridge WN. Serum esterases. II. An enzyme hydrolysing diethyl P-nitrophenyl phosphate (E600) and its identity with the A-esterase of mammalian sera. Biochem J. 1953;53:117–24. doi: 10.1042/bj0530117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa LG, Furlong CE, editors. Norwell; MA: Kluwer Academic publishers; 2002. Paraoxonase (PON1) in health and disease: Basic and clinical aspects. [Google Scholar]
- 5.Adkins S, Gan KN, Mody M, La Du BN. Molecular basis for the polymorphic forms of human serum paraoxonase/arylesterase: Glutamine or arginine at position 191, for the respective A or B allozymes. Am J Hum Genet. 1993;52:598–608. [PMC free article] [PubMed] [Google Scholar]
- 6.Humbert R, Adler DA, Disteche CM, Hassett C, Omiecinski CJ, Furlong CE. The molecular basis of the human serum paraoxonase activity polymorphism. Nat Genet. 1993;3:73–6. doi: 10.1038/ng0193-73. [DOI] [PubMed] [Google Scholar]
- 7.Mueller RF, Hornung S, Furlong CE, Anderson J, Giblett ER, Motulsky AG. Plasma paraoxonase polymorphism: A new enzyme assay, population, family, biochemical and linkage studies. Am J Hum Genet. 1983;35:393–408. [PMC free article] [PubMed] [Google Scholar]
- 8.Bayrak T, Bayrak A, Demirpençe E, Kilinç K. Purification and kinetic properties of rabbit liver paraoxonase 1. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1791–5. doi: 10.1016/j.jchromb.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Li W-F, Costa LG, Furlong CE. Serum paraoxonase status: A major factor in determining resistance to organophosphates. J Toxicol Environ Health. 1993;40:337–46. doi: 10.1080/15287399309531798. [DOI] [PubMed] [Google Scholar]
- 10.La Du BN, Novais J. Human serum organophosphatase: Biochemical characteristics and polymorphic inheritance. In: Reiner E, Aldridge WN, Hoskin FCG, editors. Enzymes hydrolysing organophosphorus compounds. Chichester, UK: Ellis-Horwood; 1989. pp. 41–52. [Google Scholar]
- 11.Furlong CE, Costa LG, Hassett C, Richter RJ, Sunderstrom JA, Adler DA, et al. Human and rabbit paraoxonases: Purification, cloning, sequencing, mapping and role of polymorphism in organophosphate detoxication. Chem Biol Interact. 1993;37:35–48. doi: 10.1016/0009-2797(93)90023-r. [DOI] [PubMed] [Google Scholar]
- 12.Mackness MI, Hallam SD, Peard T, Warner S, Walker CH. The separation of sheep and human serum “A”-esterase activity into the lipoprotein fraction by ultracentrifugation. Comp Biochem Physiol B. 1985;82:675–7. doi: 10.1016/0305-0491(85)90506-1. [DOI] [PubMed] [Google Scholar]
- 13.Reddy ST, Wadleigh DJ, Grijalva V, Ng C, Hama S, Gangopadhyay A, et al. Human paraoxonase-3 is an HDL associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler Thromb Vasc Biol. 2001;21:542–7. doi: 10.1161/01.atv.21.4.542. [DOI] [PubMed] [Google Scholar]
- 14.Mochizuki H, Scherer SW, Xi T, Nickle DC, Majer M, Huizenga JJ, et al. Human PON2 gene at 7q21.3: Cloning, multiple mRNA forms, and missense polymorphisms in the coding sequence. Gene. 1998;213:149–57. doi: 10.1016/s0378-1119(98)00193-0. [DOI] [PubMed] [Google Scholar]
- 15.Primo-Parmo SL, Sorenson RC, Teiber J, La Du BN. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics. 1996;33:498–507. doi: 10.1006/geno.1996.0225. [DOI] [PubMed] [Google Scholar]
- 16.Draganov DI, La Du BN. Pharmacogenetics of paraoxonase: A brief review. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:79–88. doi: 10.1007/s00210-003-0833-1. [DOI] [PubMed] [Google Scholar]
- 17.Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991;286:152–4. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- 18.Mackness MI, Mackness B, Durrington PN, Connelly PW, Hegele RA. Paraoxonase: Biochemistry, genetics and relationship to plasma lipoproteins. Curr Opin Lipidol. 1996;7:69–76. doi: 10.1097/00041433-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, Nvab M, et al. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem. 2001;276:44444–9. doi: 10.1074/jbc.M105660200. [DOI] [PubMed] [Google Scholar]
- 20.Draganov D, Stetson PL, Watson C, Billecke S, La Du BN. Rabbit serum paraoxonase-3 (PON3) is a high-density lippoprotein-associated lactonase and protects lipoprotein against oxidation. J Biol Chem. 2000;275:33435–42. doi: 10.1074/jbc.M004543200. [DOI] [PubMed] [Google Scholar]
- 21.Shih DM, Xia YR, Wang XP, Wang SS, Bourquard N, Fogelman AM, et al. Decreased obesity and atherosclerosis in human paraoxonase 3 transgenic mice. Circ Res. 2007;100:1200–7. doi: 10.1161/01.RES.0000264499.48737.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscler, Thromb Vasc Biol. 2001;21:473. doi: 10.1161/01.atv.21.4.473. [DOI] [PubMed] [Google Scholar]
- 23.Mackness B, Durrington P, McElduff P, Yarnell J, Azam N, Watt M, et al. Low paraoxonase activity predicts coronary events in the Caerphilly prospective study. Circulation. 2003;107:2775–9. doi: 10.1161/01.CIR.0000070954.00271.13. [DOI] [PubMed] [Google Scholar]
- 24.Paragh G, Seres I, Balogh Z, Varga Z, Karpati I, Matyus J, et al. The serum paraoxonase activity in patients with chronic renal failure and hyperlipidemia. Nephron. 1998;80:166–70. doi: 10.1159/000045161. [DOI] [PubMed] [Google Scholar]
- 25.Aviram M, Rosenblat M, Bisgaier CL, Newton RS. Atorvastatin and gemfibrozil metabolites, but not the parent drugs, are potent antioxidants against lipoprotein oxidation. Atherosclerosis. 1998;138:271–80. doi: 10.1016/s0021-9150(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 26.Blatter-Garin MC, Kalix B, De Pre S, James RW. Aspirin use is associated with higher concentrations of the antioxidant enzyme, paraoxonase-1. Diabetologia. 2003;46:593–4. doi: 10.1007/s00125-003-1065-0. [DOI] [PubMed] [Google Scholar]
- 27.Ali B, Zhang Q, Lim YK, Feng D, Retnam L, Lim SK. Expression of major HDL associated antioxidant PON1 is gender dependent and regulated during inflammation. Free Radic Biol Med. 2003;34:824–9. doi: 10.1016/s0891-5849(02)01436-3. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalvo MC, Gil F, Hernandez AF, Villanueva E, Pla A. Inhibition of paraoxonase activity in human liver microsomes by exposure to EDTA, metals and mercurials. Chem Biol Interact. 1997;105:169–79. doi: 10.1016/s0009-2797(97)00046-x. [DOI] [PubMed] [Google Scholar]
- 29.Nishio E, Watanabe Y. Cigarette smoke extracts inhibits plasma paraoxonase activity by modification of the enzyme's free thiols. Biochem Biophys Res Commun. 1997;236:289–93. doi: 10.1006/bbrc.1997.6961. [DOI] [PubMed] [Google Scholar]
- 30.Debord J, Dantoine T, Bollinger JC, Abraham MH, Verneuil B, Merle L. Inhibition of arylesterase by aliphatic alcohols. Chem Biol Interact. 1998;113:105–15. doi: 10.1016/s0009-2797(98)00018-0. [DOI] [PubMed] [Google Scholar]
- 31.de Roos NM, Schouten EG, Scheek LM, van Tol A, Katan MB. Replacement of dietary saturated fat with trans fat reduces serum paraoxonase activity in healthy men and women. Metabolism. 2002;51:1534–7. doi: 10.1053/meta.2002.36305. [DOI] [PubMed] [Google Scholar]
- 32.Seres I, Paragh G, Deschene E, Fulop T, Khalil A. Study of factors influencing the decreased HDL associated PON1 activity with aging. Exp Gerontol. 2004;39:59–66. doi: 10.1016/j.exger.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Tarcin O. Paraoxonase-1 and coronary heart diseases. Marmara Med J. 2011;24:59–63. [Google Scholar]
- 34.Kota SK, Jammula S, Kota SK, Krishna SVS, Meher LK, Rao ES, et al. Nutraceuticals in Pathogenic Obesity; Striking the Right Balance between Energy Imbalance and Inflammation. J Med Nutr Nutraceut. 2012;1:63–76. [Google Scholar]
- 35.Mutlu-Turkoglu U, Oztezcan S, Telci A, Orhan Y, Aykac-Toker G, Sivas A, et al. An increase in lipoprotein oxidation and endogenous lipid peroxides in serum of obese women. Clin Exp Med. 2003;2:171–4. doi: 10.1007/s102380300002. [DOI] [PubMed] [Google Scholar]
- 36.Ferretti G, Bacchetti T, Moroni C, Savino S, Liuzzi A, Balzola F, et al. Paraoxonase activity in high-density lipoproteins: A comparison between healthy and obese females. J Clin Endocrinol Metab. 2005;90:1728–33. doi: 10.1210/jc.2004-0486. [DOI] [PubMed] [Google Scholar]
- 37.Abbott CA, Mackness MI, Kumar S, Boulton AJ, Durrington PN. Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler Thromb Vasc Biol. 1995;15:1812–8. doi: 10.1161/01.atv.15.11.1812. [DOI] [PubMed] [Google Scholar]
- 38.Bajnok L, Seres I, Varga Z, Jeges S, Peti A, Karanyi Z, et al. relationship of serum resistin level to traits of metabolic syndrome and serum paraoxonase 1 activity in a population with a broad range of body mass index. Exp Clin Endocrinol Diabetes. 2008;116:592–9. doi: 10.1055/s-2008-1065350. [DOI] [PubMed] [Google Scholar]
- 39.Koncsos P, Seres I, Harangi M, Illyes I, Jozsa L, Gonczi F, et al. Human Paraoxonase-1 Activity in Childhood Obesity and Its Relation to Leptin and Adiponectin Levels. Pediatr Res. 2010;67:309–13. doi: 10.1203/PDR.0b013e3181c9fb66. [DOI] [PubMed] [Google Scholar]
- 40.Audikovszky M, Pados G, Seres I, Harangi M, Fulop P, Katona E, et al. Orlistat increases serum paraoxonase activity in obese patients. Nutr Metab Cardiovasc Dis. 2007;17:268–73. doi: 10.1016/j.numecd.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Bajnok L, Csongradi E, Seres I, Varga Z, Jeges S, Peti A, et al. Relationship of adiponectin to serum paraoxonase 1. Atherosclerosis. 2008;197:363–7. doi: 10.1016/j.atherosclerosis.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Beltowski J, Wo´jcicka G, Jamroz A. Leptin decreases plasma paraoxonase 1 (PON1) activity and induces oxidative stress: The possible novel mechanism for proatherogenic effect of chronic hyperleptinemia. Atherosclerosis. 2003;170:21–9. doi: 10.1016/s0021-9150(03)00236-3. [DOI] [PubMed] [Google Scholar]
- 43.Holub M, Zwiauer K, Winkler C, Dillinger-Paller B, Schuller E, Schober E, et al. Relation of plasma leptin to lipoproteins in overweight children undergoing weight reduction. Int J Obes Relat Metab Disord. 1999;23:60–6. doi: 10.1038/sj.ijo.0800759. [DOI] [PubMed] [Google Scholar]
- 44.Wu B, Fukuo K, Suzuki K, Yoshino G, Kazumi T. Relationships of systemic oxidative stress to body fat distribution, adipokines and inflammatory markers in healthy middle-aged women. Endocr J. 2009;56:773–82. doi: 10.1507/endocrj.k08e-332. [DOI] [PubMed] [Google Scholar]
- 45.Aviram M, Rosenblat M, Billecke S, Erogul J, Sorenson R, Bisgaier CL, et al. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic Biol Med. 1999;26:892–904. doi: 10.1016/s0891-5849(98)00272-x. [DOI] [PubMed] [Google Scholar]
- 46.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 47.Feingold KR, Memon RA, Moser AH, Grunfeld C. Paraoxonase activity in the serum and hepatic mRNA levels decrease during the acute phase response. Atherosclerosis. 1998;139:307–15. doi: 10.1016/s0021-9150(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 48.Liang CP, Tall AR. Transcriptional profiling reveals global defects in energy metabolism, lipoprotein, and bile acid synthesis and transport with reversal by leptin treatment in ob/ob mouse liver. J Biol Chem. 2001;276:49066–76. doi: 10.1074/jbc.M107250200. [DOI] [PubMed] [Google Scholar]
- 49.Sorenson RC, Bisgaier CL, Aviram M, Hsu C, Billecke S, La Du BN. Human serum Paraoxonase/Arylesterase's retained hydrophobic N-terminal leader sequence associates with HDLs by binding phospholipids: Apolipoprotein A-I stabilizes activity. Arterioscler Thromb Vasc Biol. 1999;19:2214–25. doi: 10.1161/01.atv.19.9.2214. [DOI] [PubMed] [Google Scholar]
- 50.Rainwater DL, Comuzzie AG, VandeBerg JL, Mahaney MC, Blangero J. Serum leptin levels are independently correlated with two measures of HDL. Atherosclerosis. 1997;132:237–43. doi: 10.1016/s0021-9150(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 51.Verges B, Petit JM, Duvillard L, Dautin G, Florentin E, Galland F, et al. Adiponectin is an important determinant of apoA-I catabolism. Arterioscler Thromb Vasc Biol. 2006;26:1364–9. doi: 10.1161/01.ATV.0000219611.50066.bd. [DOI] [PubMed] [Google Scholar]
- 52.Tsubakio-Yamamoto K, Matsuura F, Koseki M, Oku H, Sandoval JC, Inagaki M, et al. Adiponectin prevents atherosclerosis by increasing cholesterol efflux from macrophages. Biochem Biophys Res Commun. 2008;375:390–4. doi: 10.1016/j.bbrc.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Dursun P, Demirtaş E, Bayrak A, Yaralı H. Decreased serum paraoxonase 1 (PON1) activity: An additional risk factor for atherosclerotic heart disease in patients with PCOS? Hum Reprod. 2006;21:104–8. doi: 10.1093/humrep/dei284. [DOI] [PubMed] [Google Scholar]
- 54.Fenkci IV, Serteser M, Fenkci S, Kose S. Paraoxonase levels in women with polycystic ovary syndrome. J Reprod Med. 2007;52:879–83. [PubMed] [Google Scholar]
- 55.Garin MC, Kalix B, Morabia A, James RW. Small, dense lipoprotein particles and reduced paraoxonase-1 in patients with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90:2264–9. doi: 10.1210/jc.2004-1295. [DOI] [PubMed] [Google Scholar]
- 56.Yamada A, Shoji T, Tahara H, Emoto M, Nishizawa Y. Effect of insulin resistance on serum paraoxonase activity in a nondiabetic population. Metabolism. 2001;50:805–11. doi: 10.1053/meta.2001.24215. [DOI] [PubMed] [Google Scholar]
- 57.Tabur S, Torun AN, Sabuncu T, Turan MN, Celik H, Ocak AR, et al. Nondiabetic metabolic syndrome and obesity do not affect serum paraoxonase and arylesterase activities but do affect stress and inflammation. Eur J Endocrinol. 2010;162:535–41. doi: 10.1530/EJE-09-0732. [DOI] [PubMed] [Google Scholar]
- 58.Beer S, Moren X, Ruiz J, James RW. Postprandial modulation of serum paraoxonase activity and concentration in diabetic and non-diabetic subjects. Nutr Metab Cardiovasc Dis. 2006;16:457–65. doi: 10.1016/j.numecd.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Kopprasch S, Pietzsch J, Kuhlisch E, Graessler J. Lack of association between paraoxonase 1 activities and increased oxidized low-density lipoprotein levels in impaired glucose tolerance and newly diagnosed diabetes mellitus. J Clin Endocrinol Metab. 2003;88:1711–6. doi: 10.1210/jc.2002-021561. [DOI] [PubMed] [Google Scholar]
- 60.Ferretti G, Bacchetti T, Busni D, Rabini RA, Curatola G. Protective effect of paraoxonase activity in high-density lipoproteins against erythrocyte membranes peroxidation: A comparison between healthy subjects and type 1 diabetic patients. J Clin Endocrinol Metab. 2004;89:2957–62. doi: 10.1210/jc.2003-031897. [DOI] [PubMed] [Google Scholar]
- 61.Karabina SA, Lehner AN, Franke E, Parthasara S, Santanam N. Oxidative inactivation of paraoxonase—implications in diabetes mellitus and atherosclerosis. Biochim Biophys Acta. 2005;1725:213–21. doi: 10.1016/j.bbagen.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 62.Flekac M, Skrha J, Zidkova K, Lacinova Z, Hilgertova J. Paraoxonase 1 gene polymorphisms and enzyme activities in diabetes mellitus. Physiol Res. 2008;57:717–26. doi: 10.33549/physiolres.931285. [DOI] [PubMed] [Google Scholar]
- 63.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 64.Nourooz-Zadeh J, Tajaddini-Sarmadi J, McCarthy S, Betteridge DJ, Wolff SP. Elevated levels of authentic plasma hydroperoxides in NIDDM. Diabetes. 1995;44:1054–8. doi: 10.2337/diab.44.9.1054. [DOI] [PubMed] [Google Scholar]
- 65.Beaudeux JL, Guillausseau PJ, Peynet J, Flourie F, Assayag M, Tielmans D, et al. Enhanced susceptibility of low density lipoprotein to in vitro oxidation in type 1 and type 2 diabetic patients. Clin Chim Acta. 1995;239:131–41. doi: 10.1016/0009-8981(95)06106-n. [DOI] [PubMed] [Google Scholar]
- 66.Rabini RA, Fumelli P, Galassi R, Dousset N, Taus M, Ferretti G, et al. Increased susceptibility to lipid oxidation of low density lipoproteins and erythrocyte membranes from diabetic patients. Metabolism. 1994;43:1470–4. doi: 10.1016/0026-0495(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 67.Santini SA, Marra G, Giardina B, Cotroneo P, Mordente A, Martorana GE, et al. Defective plasma antioxidant defenses and enhanced susceptibility to lipid peroxidation in uncomplicated IDDM. Diabetes. 1997;46:1853–8. doi: 10.2337/diab.46.11.1853. [DOI] [PubMed] [Google Scholar]
- 68.Kota SK, Kota SK, Jammula S, Panda S, Modi KD. Effect of diabetes on alteration of metabolism in cardiac myocytes: therapeutic implications. Diabetes Technol Ther. 2011;13:1155–60. doi: 10.1089/dia.2011.0120. [DOI] [PubMed] [Google Scholar]
- 69.Mackness MI, Harty D, Bhatnagar D, Winocour PH, Arrol S, Ishola M, et al. Serum paraoxonase activity in familial hypercholesterolaemia and insulin-dependent diabetes mellitus. Atherosclerosis. 1991;86:193–9. doi: 10.1016/0021-9150(91)90215-o. [DOI] [PubMed] [Google Scholar]
- 70.Mackness B, Durrington PN, Boulton AJ, Hine D, Mackness MI. Serum paraoxonase activity in patients with type 1 diabetes compared to healthycontrols. Eur J Clin Invest. 2002;32:259–64. doi: 10.1046/j.1365-2362.2002.00977.x. [DOI] [PubMed] [Google Scholar]
- 71.Valabhji J, McColl A-J, Schachter M, Dhanjil S, Richmond W, Elkeles RS. High density lipoprotein composition and paraoxonase activity in type 1 diabetes. Clin Sci (Lond) 2001;101:659–70. [PubMed] [Google Scholar]
- 72.Colhoun HM, Taskinen MR, Otvos JD, Van Den Berg P, O’Connor J, Van Tol A. Relationship of phospholipid transfer protein activity to HDL and apolipoprotein B-containing lipoproteins in subjects with and without type 1 diabetes. Diabetes. 2002;51:3300–5. doi: 10.2337/diabetes.51.11.3300. [DOI] [PubMed] [Google Scholar]
- 73.Gowri MS, Van der Westhuyzen DR, Bridges SR, Anderson JW. Decreased protection by HDL from poorly controlled type 2 diabetic subjects against LDL oxidation may be due to the abnormal composition of HDL. Arterioscler Thromb Vasc Biol. 1999;19:2226–33. doi: 10.1161/01.atv.19.9.2226. [DOI] [PubMed] [Google Scholar]
- 74.Fievet C, Theret N, Shojaee N, Duchateau P, Castro G, Ailhaud G, et al. Apolipoprotein A-I-containing particles and reverse cholesterol transport in IDDM. Diabetes. 1992;41:81–5. doi: 10.2337/diab.41.2.s81. [DOI] [PubMed] [Google Scholar]
- 75.Mackness MI, Arrol S, Abbott CA, Durrington PN. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis. 1993;104:129–135. doi: 10.1016/0021-9150(93)90183-u. [DOI] [PubMed] [Google Scholar]
- 76.Cavallero E, Brites F, Delfly B, Nicolaiew N, Decossin C, De Geitere C, et al. Abnormal reverse cholesterol transport in controlled type II diabetic patients. Studies on fasting and postprandial LpA-I particles. Arterioscler Thromb Vasc Biol. 1995;15:2130–5. doi: 10.1161/01.atv.15.12.2130. [DOI] [PubMed] [Google Scholar]
- 77.Winocour PH, Durrington PN, Bhatnagar D, Ishola M, Arrol S, Mackness M. Abnormalities of VLDL, IDL, and LDL characterize insulin-dependent diabetes mellitus. Arterioscler Thromb. 1992;12:920–8. doi: 10.1161/01.atv.12.8.920. [DOI] [PubMed] [Google Scholar]
- 78.Weight MJ, Coetzee HS, Smuts CM, Marais MP, Maritz JS, Hough FS, et al. Lecithin: Cholesterol acyltransferase activity and high density lipoprotein subfraction composition in type 1 diabetic patients with improving metabolic control. Acta Diabetol. 1993;30:159–65. doi: 10.1007/BF00572861. [DOI] [PubMed] [Google Scholar]
- 79.Graham A, Hassall DG, Rafique S, Owen JS. Evidence for a paraoxonase independent inhibition of low density lipoprotein oxidation by high density lipoprotein. Atherosclerosis. 1997;135:193–204. doi: 10.1016/s0021-9150(97)00162-7. [DOI] [PubMed] [Google Scholar]
- 80.Poh R, Muniandy S. Paraoxonase 1 activity as a predictor of cardiovascular disease in type 2 diabetes. Southeast Asian J Trop Med Public Health. 2010;41:1231–46. [PubMed] [Google Scholar]
- 81.McElveen J, Mackness MI, Colley CM, Peard T, Warner S, Walker CH. Distribution of paraoxon hydrolysing activity in the serum of patients after myocardial infarction. Clin Chem. 1986;32:671–3. [PubMed] [Google Scholar]
- 82.Lixandru D, Mohora M, Coman A, Stoian I, van Gils C, Aerts P, et al. Diet and paraoxonase 1 enzymatic activity in diabetic foot patients from Romania and Belgium: Favorable association of high flavonoid dietary intake with arylesterase activity. Ann Nutr Metab. 2010;56:294–301. doi: 10.1159/000298879. [DOI] [PubMed] [Google Scholar]
- 83.Chiu KC, Chuang LM, Chu A, Lu J, Hu J, Fernando S. Association of paraoxonase 1 polymorphism with beta-cell function: A case of molecular heterosis. Pancreas. 2004;28:e96–103. doi: 10.1097/00006676-200405000-00021. [DOI] [PubMed] [Google Scholar]
- 84.Altuner D, Suzen SH, Ates I, Koc GV, Aral Y, Karakaya A. Are PON1 Q/R 192 and M/L 55 polymorphisms risk factors for diabetes complications in Turkish population? Clin Biochem. 2011;44:372–6. doi: 10.1016/j.clinbiochem.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 85.Ergun MA, Yurtcu E, Demirci H, Ilhan MN, Barkar V, Yetkin I, et al. PON1 55 and 192 gene polymorphisms in type 2 diabetes mellitus patients in a Turkish Population. Biochem Genet. 2011;49:1–8. doi: 10.1007/s10528-010-9376-6. [DOI] [PubMed] [Google Scholar]
- 86.Mackness B, Durrington PN, Abuashia B, Boulton AJ, Mackness MI. Low paraoxonase activity in type II diabetes mellitus complicated by retinopathy. Clin Sci(Lond) 2000;98:355–63. [PubMed] [Google Scholar]
- 87.Lyons TJ, Li W, Wells-Knecht MC, Jokl R. Toxicity of mildly modified low-density lipoproteins to cultured retinal capillary endothelial cells and pericytes. Diabetes. 1994;43:1090–5. doi: 10.2337/diab.43.9.1090. [DOI] [PubMed] [Google Scholar]
- 88.Ikeda Y, Suehiro T, Inoue M, Nakauchi Y, Morita T, Arii K, et al. Serum paraoxonase activity and its relationship to diabetic complications in patients with non-insulin dependent diabetes mellitus. Metabolism. 1998;47:598–602. doi: 10.1016/s0026-0495(98)90246-3. [DOI] [PubMed] [Google Scholar]
- 89.Saha N, Roy AC, Teo SH, Tay JSH, Ratnam SS. Influence of serum paraoxonase polymorphism on serum lipids and lipoproteins. Clin Genet. 1991;40:277–82. doi: 10.1111/j.1399-0004.1991.tb03096.x. [DOI] [PubMed] [Google Scholar]
- 90.Hegele RA, Brunt JH, Connelly PW. A polymorphism of the para-oxonase gene associated with variation in plasma lipoproteins in a genetic isolate. Arterioscler Thromb Vasc Biol. 1995;15:89–95. doi: 10.1161/01.atv.15.1.89. [DOI] [PubMed] [Google Scholar]
- 91.Mirdamadi HZ, Sztanek F, Derdak Z, Seres I, Harangi M, Paragh G. The human paraoxonase-1 phenotype modifies the effect of statins on paraoxonase activity and lipid parameters. Br J Clin Pharmacol. 2008;66:366–74. doi: 10.1111/j.1365-2125.2008.03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kota SK, Jammula S, Kota SK, Krishna SVS, Meher LK, Modi KD. Nutraceuticals in Dyslipidemia Management. J Med Nutr Nutraceut. 2013;2:26–40. [Google Scholar]
- 93.Escola-Gil JC, Marzal-Casacuberta A, Julve-Gil J, Ishida BY, Ordonez-Lianos J, Chan L, et al. Human apolipoprotein A-II is a proatherogenic molecule when its expressed in transgenic mice at a level similar to that in humans: Evidence of a potentially relevant species-specific interaction with diet. J Lipid Res. 1998;39:457–62. [PubMed] [Google Scholar]
- 94.Ferretti G, Bacchetti T, Marchionni C, Caldarelli L, Curatola G. Effect of glycation of high density lipoproteins on their physicochemical properties and on paraoxonase activity. Acta Diabetol. 2001;38:163–9. doi: 10.1007/s592-001-8074-z. [DOI] [PubMed] [Google Scholar]
- 95.Ferretti G, Bacchetti T, Marotti E, Curatola G. Effect of homocysteinylation on human high-density lipoproteins: A correlation with paraoxonase activity. Metabolism. 2003;52:146–51. doi: 10.1053/meta.2003.50033. [DOI] [PubMed] [Google Scholar]
- 96.Zech R, Rockseisen M, Kluge K, Dewald K, Armstrong VW, Chemnitius JM. Lipoproteins and hydrolysis of organophosphorus compounds. Chem Biol Interact. 1993;87:85–94. doi: 10.1016/0009-2797(93)90028-w. [DOI] [PubMed] [Google Scholar]
- 97.Blatter MC, James RW, Messmer S, Barja F, Pometta D. Identification of a distinct human high-density lipoprotein sub-species defined by a lipoprotein-associated protein, K-45: Identity of K-45 with paraoxonase. Eur J Biochem. 1993;211:871–9. doi: 10.1111/j.1432-1033.1993.tb17620.x. [DOI] [PubMed] [Google Scholar]
- 98.Kelso GJ, Stuart WD, Richter RJ, Furlong CE, Jordan-Starck TC, Harmony JA. Apolipoprotein J is associated with paraoxonase in human plasma. Biochemistry. 1994;33:832–9. doi: 10.1021/bi00169a026. [DOI] [PubMed] [Google Scholar]
- 99.Kontush A, Chantepie S, Chapman MJ. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler Thromb Vasc Biol. 2003;23:1881–8. doi: 10.1161/01.ATV.0000091338.93223.E8. [DOI] [PubMed] [Google Scholar]
- 100.Ribas V, Sanchez-Quesada JL, Anton R, Camacho M, Julve J, Escola- Gil JC, et al. Human apolipoprotein A-II enrichement displaces paraoxonase from HDL and impairs its antioxidant properties. Circ Res. 2004;95:789–97. doi: 10.1161/01.RES.0000146031.94850.5f. [DOI] [PubMed] [Google Scholar]
- 101.Paragh G, Seres I, Harangi M, Balogh Z, Illyes L, Boda J, et al. The effect of micronized fenofibrate on paraoxonase activity in patients with coronary heart disease. Diabetes Metab. 2003;29:613–8. doi: 10.1016/s1262-3636(07)70077-0. [DOI] [PubMed] [Google Scholar]
- 102.Beltowski J, Wojcicka G, Mydlarczyk M, Jamroz A. The effect of peroxisome proliferator-activated receptors (PPAR) alpha agonist, fenofibrate, on lipid peroxidation, total antioxidant capacity, and plasma paraoxonase 1 (PON1) activity. J Physiol Pharmacol. 2002;53:463–75. [PubMed] [Google Scholar]
- 103.Balogh Z, Seres I, Harangi M, Kovacs P, Kakuk GY, Paragh GY. Gemfibrozil increases paraoxonase activity in type 2 diabetic patients. A new hypothesis of the beneficial action of fibrates? Diabetes Metab. 2001;27:604–10. [PubMed] [Google Scholar]
- 104.Durrington PN, Mackness MI, Bhatnagar D, Julier K, Prais H, Arrol S, et al. Effects of two different fibric acid derivatives on lipoproteins, cholesterol ester transfer, fibrinogen, plasminogen activator inhibitor and paraoxonase activity in type IIb hyperlipoproteinemia. Atherosclerosis. 1998;138:217–25. doi: 10.1016/s0021-9150(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 105.Macan M, Vrkic N, Vrdoljak AL, Radic B, Bradamante V. Effects of high sucrose diet, gemfibrozil, and their combination on plasma paraoxonase 1 activity and lipid levels in rats. Acta Biochim Pol. 2010;57:321–6. [PubMed] [Google Scholar]
- 106.Kural BV, Orem C, Uydu HA, Alver A, Orem A. The effects of lipid-lowering therapy on paraoxonase activities and their relationships with the oxidant-antioxidant system in patients with dyslipidemia. Coron Artery Dis. 2004;15:277–83. doi: 10.1097/01.mca.0000135221.32523.a1. [DOI] [PubMed] [Google Scholar]
- 107.Kreutz RP, Nystrom P, Kreutz Y. Influence of paraoxonase-1 Q192R and cytochrome P450 2C19 polymorphisms on clopidogrel response. Clin Pharmacol. 2012;4:13–20. doi: 10.2147/CPAA.S27822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kerkeni M, Addad F, Chauffert M, Chuniaud L, Miled A, Trivin F, et al. Hyperhomocysteinemia, paraoxonase activity and risk of coronary artery disease. Clin Biochem. 2006;39:821–5. doi: 10.1016/j.clinbiochem.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 109.Fu AL, Wu SP. Single intravenous injection of plasmid DNA encoding human paraoxonase-1 inhibits hyperlipidemia in rats. Biochem Biophys Res Commun. 2010;397:257–62. doi: 10.1016/j.bbrc.2010.05.095. [DOI] [PubMed] [Google Scholar]
- 110.Shih DM, Xia YR, Yu JM, Lusis AJ. Temporal and tissue-specific patterns of Pon3 expression in mouse: In situ hybridization analysis. Adv Exp Med Biol. 2010;660:73–87. doi: 10.1007/978-1-60761-350-3_8. [DOI] [PMC free article] [PubMed] [Google Scholar]


