Abstract
Background:
In recent times, there are reports of adrenal dysfunction in whole spectrum of liver disease. Adrenal insufficiency (AI) has been shown to correlate with progression of liver disease. Hence this study was conducted to assess adrenal function in subjects with acute liver disease (ALD), chronic liver disease (CLD) and post liver transplantation (LT).
Material and Methods:
This study included 25 healthy controls, 25 patients of ALD, 20 subjects of CLD with Child-Pugh stage A (CLD-1) and 30 with Child-Pugh stage B or C (CLD-2), and 10 subjects with LT. All subjects were assessed clinically, biochemically and for adrenal functions.
Results:
AI was present in 9 (34.6%) patients with ALD, 20 (40%) patients with CLD and 4 (40%) in subjects with LT. AI was more common in CLD-2 (18 patients – 60%) than CLD-1 (2 patients – 10%). All patients with chronic liver disease had significantly lower basal cortisol (8.8±4.8, P=0.01), stimulated cortisol (18.2±6.3, P <0.00001) and incremental cortisol (9.4±4.6, P <0.00001) as compared to controls. There was increase in percentage of subjects with adrenal dysfunction with progression of liver disease as assessed by Child-Pugh staging. AI was predicted by lower levels of serum protein, serum albumin, total cholesterol and HDL cholesterol and higher levels of serum bilirubin and INR. Adrenal functions showed recovery following liver transplantation.
Conclusions:
AI forms important part of spectrum of acute and chronic liver disease. Deterioration of synthetic functions of liver disease predicts presence of AI, and these patients should be evaluated for adrenal dysfunction periodically.
Keywords: Acute liver disease, adrenal insufficiency, chronic liver disease, cortisol, liver transplantation
INTRODUCTION
Liver diseases are common all over the world as well as in India; and the prevalence of liver diseases is likely to increase in future.[1] Among the various functions of the liver one function is metabolism of hormones. Thus liver diseases have been shown to be associated with various endocrine disturbances.[2] Liver is a synthetic storehouse for precursors of all adrenal hormones as well as cortisol binding globulin (CBG). Hence it is not surprising that adrenal dysfunction has been reported in various spectra of liver diseases.[3] Cortisol is synthesized in adrenal gland and circulates in free and bound form with CBG.[2] In recent times, there are reports of adrenal dysfunction in whole spectrum of liver disease.[3,4,5,6,7] Acute liver failure is associated with increased circulating endotoxins and pro-inflammatory mediators with reduced levels of apoprotein-1/HDL,[4] which is quite similar to clinical state of sepsis and results in diseased adrenal state, leading to introduction of new term “hepatoadrenal syndrome”.[4] Also in stable cirrhosis, a state of adrenal insufficiency (AI) has been shown which correlates with progression of stage of cirrhosis.[5] Finally, AI has also been reported in up to 92% of post liver transplant patients in early post operative period.[4] Beneficial effects of corticosteroid therapy during sepsis and shock in cirrhosis has also been reported[4,6,7] but one recent randomized controlled trial has shown no benefit.[5] We conducted this study at a tertiary care centre to assess adrenal function in subjects with acute liver disease (ALD), chronic liver disease and post liver transplantation.
MATERIALS AND METHODS
This study was conducted as a cross sectional study at the Army Hospital (Research and Referral), New Delhi in the period May 2010 to Dec 2011. Subjects with ALD, chronic liver disease (CLD) and post liver transplant state (LT) were recruited from outpatient department and inpatient ward of gastroenterology department. ALD was diagnosed on the basis of presence of hyperbilirubinemia, raised transaminases, absence of past history of liver disease, normal hepatic echotexture on ultrasonography of abdomen, and absence of evidence of portal hypertension. CLD was diagnosed on the basis of evidence of deranged liver function of more than six months duration and/or evidence of portal hypertension on ultrasonography or upper geastrointestinal endoscopy. These subjects were classified as per Child-Pugh criteria. Patients on steroids or those who had received the last dose of steroids within last three months or had alcoholic liver disease were excluded from this study. Twenty five healthy controls were enrolled from endocrine department. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Institutional Human Ethics Committee at Army Hospital (Research and Referral). Written informed consent was obtained from all patients/controls. All subjects were assessed clinically, biochemically and for adrenal functions.
Biochemical parameters
Patient's fasting blood samples were collected and analyzed for hematological parameters (complete blood count with platelet counts), liver function tests [Serum bilirubin, aspartate transaminases (AST)/alanine transaminases (ALT), serum protein, serum albumin and international normalized ratio (INR)]; blood glucose, serum creatinine, serum lipid profile, and electrolytes (serum sodium and potassium).
Hormonal assessment
Patient's blood was collected in fasting state and serum was separated and stored at -80°C. Adrenocorticotropin (ACTH) stimulation test was performed in all subjects with 250 μg of ACTH given intramuscularly and serum samples were collected after 60 minutes.[8] Serum cortisol was measured by radioimmunoassay kits provided by Immunotech, Beckman Coulter Company, France on Startec SR300 automated radioimmunoassay system. Normal range of serum cortisol was 5.0-25.0 μg/dl with intra-assay and inter-assay coefficient of variation 5.8 and 9.2% respectively. AI was defined by basalcortisol levels <3 μg/dl or a peak cortisol response <18 μg/dl[8,9] and relative adrenal insufficiency (RAI) by increment of <9 μg/dl after ACTH administration as per Critical Illness Related Corticosteroid Insufficiency (CIRCI) criteria.[10]
Statistical analysis
Statistical analysis was carried out using EPI INFO 3.5.3 (CDC, Atlanta, GA, USA). Data were presented as mean ± SD or number (%) unless specified. All parametric data were analysed by student's t-test. If Barlett's Chi-square test for equality of population variances was <0.05, then Kruskal-Wallis test was applied. All non parametric data were analysed by Chi-square test. If value in any cell was <5 than Fisher exact test was used. A P value of < 0.05 was considered statistically significant.
RESULTS
In this study, we studied 25 patients of ALD; 20 patients of CLD with preserved liver functions (CLD-1) which included Child-Pugh stage A and 30 patients with more advanced CLD (CLD-2) which included Child-Pugh stage B or C; and 10 patients who were post-liver transplant (LT). Most of the subjects with ALD were acute viral hepatitis (18 patients), however etiology could not be assessed in all cases. Among 50 subjects with CLD, 29 were hepatitis B virus (HBV) related cirrhosis, eight were hepatitis C virus (HCV) related cirrhosis, three had HBV and HCV co-infection and 10 had cryptogenic cirrhosis. Liver transplantations were performed for HBV relatedcirrhosis in five patients, post cryptogenic liver disease in three patients and HCV cirrhosis in two patients. Basic parameters of all subjects are given in Table 1.
Table 1.
Basic parameters of various groups
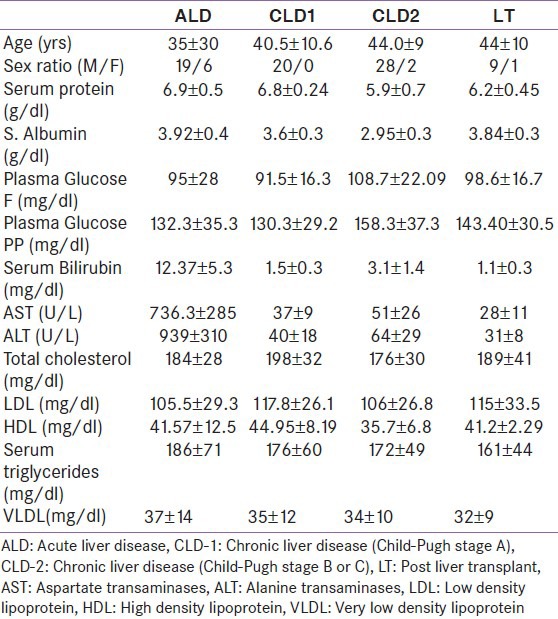
Acute liver disease
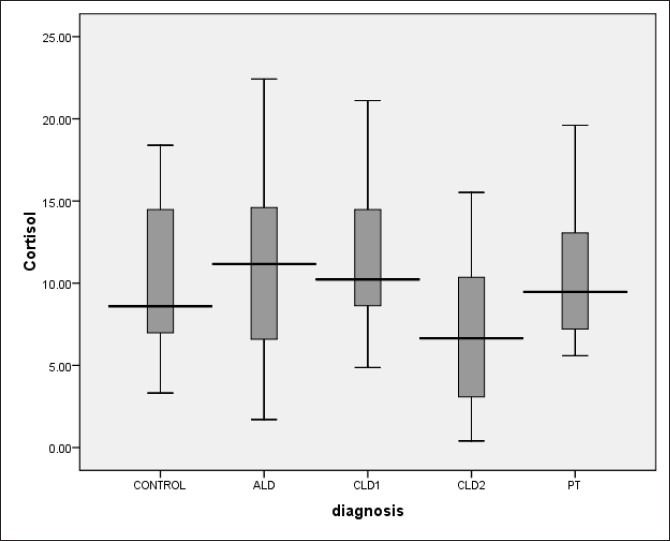
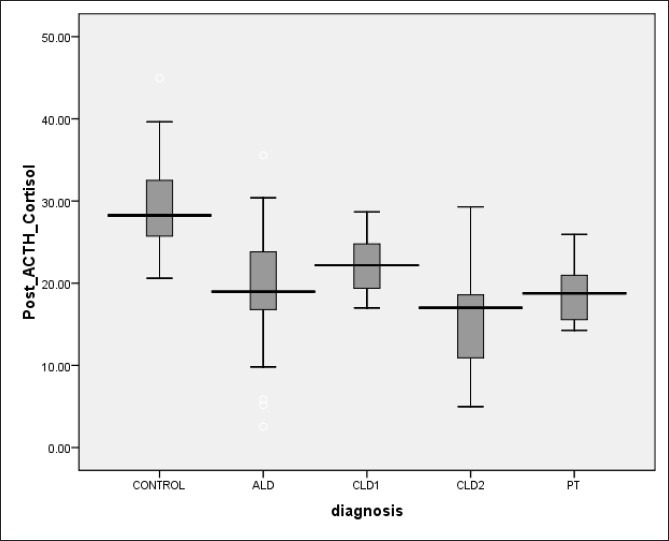
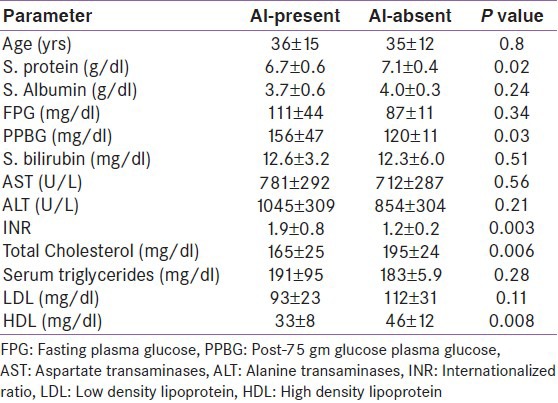
Among subjects with ALD, 9 (34.6%) patients were diagnosed AI [Table 2]. Basal cortisol values were similar in ALD and control group. Stimulated and incremental cortisol after ACTH was significantly lower in ALD group as compared to healthy controls [Figures 1 and 2]. RAI was more common in patients with ALD according to cortisol increment criteria [Table 2]. In acute hepatitis group patients with AI had lower levels of serum protein, total cholesterol and HDL cholesterol, and higher post-prandial glucose and presence of coagulopathy [Table 3].
Table 2.
Adrenal functions in subjects with liver diseases compared and controls
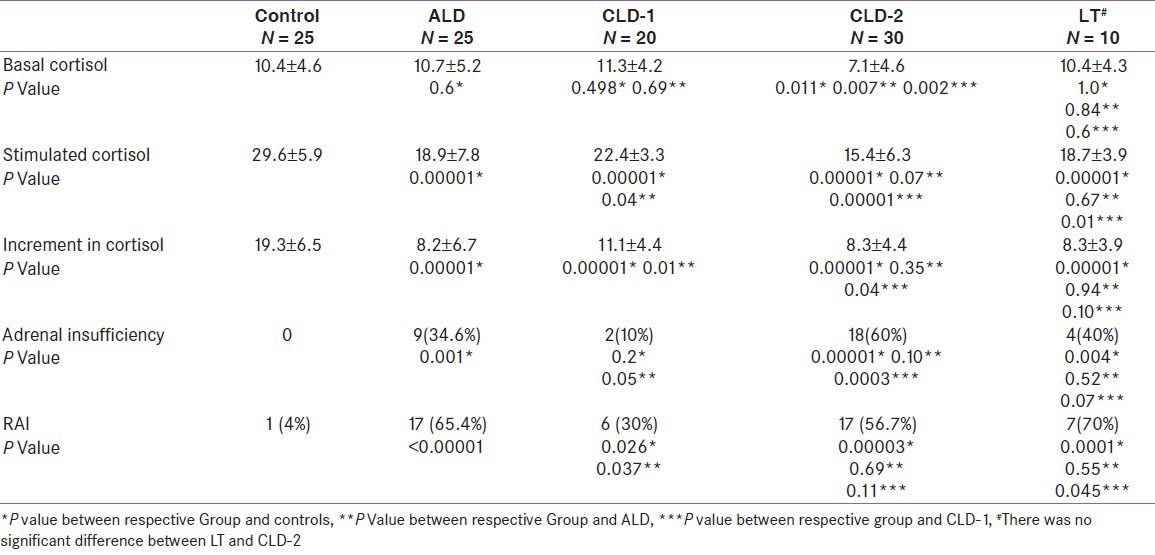
Figure 1.
Basal cortisol over spectrum of liver disease and control group
Figure 2.
Post adrenocorticotropin serum cortisol over spectrum of liver disease and control group
Table 3.
Clinical and biochemical predictors of adrenal insufficiency in patients with acute liver disease
Chronic liver disease
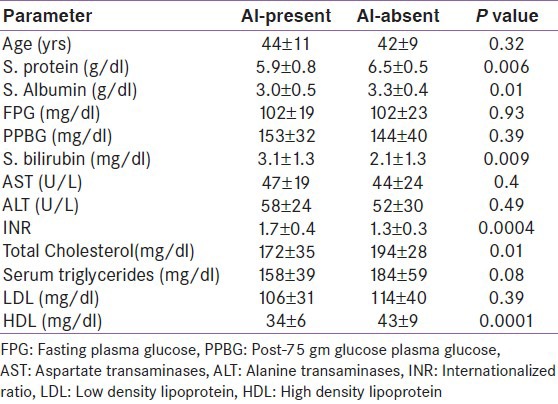
All patients with chronic liver disease had significantly lower basal cortisol (8.8 ± 4.8, P = 0.01), stimulated cortisol (18.2 ± 6.3, P < 0.00001) and incremental cortisol (9.4 ± 4.6, P < 0.00001) as compared to controls. AI was present in 20 (40%) patients. AI was more common in CLD-2 (18 patients – 60%) than CLD-1 (2 patients – 10%).
In CLD-1, there was no significant difference in basal cortisol, but stimulated and incremental cortisol was significantly lower as compared controls and ALD. Patients in CLD-2 had significantly lower basal, stimulated and incremental cortisol than controls and CLD-1, whereas only basal cortisol was significantly lower in CLD-2 compared to ALD.
Comparison of all three Child-Pugh stages of chronic liver disease revealed progressively decline in basal cortisol (11.3±4.2 vs. 7.2±5.1 vs. 6.9±4.1) and stimulated cortisol (22.4±3.3 vs. 18.1±5.9 vs. 12.7±5.6) with progression of liver disease [Figure 1 and 2]. Also percentage of AI patients increased with worsening of liver disease (10% vs. 46.7% vs. 73.3%).
In CLD group, AI was predicted by lower levels of serum protein, serum albumin, total cholesterol and HDL cholesterol and higher levels of serum bilirubin and INR [Table 4].
Table 4.
Clinical and biochemical predictors of adrenal insufficiency in patients with chronic liver disease
Post liver transplantation
AI was present in 4 (40%) patients with liver transplantation [Table 1]. Basal cortisol increased as compared to CLD- 2 and became comparable to controls. Stimulated and incremental cortisol remained low as compared to controls. However, stimulated cortisol improved significantly than CLD-2. Number of subjects with AI was also lower in subjects after liver transplantation than CLD-2.
As per CIRCI criteria prevalence of AI was maximum (70%) in this group, followed by ALD group (65.4%) and CLD group (46%).
DISCUSSION
This study revealed, firstly, high percentage of subjects with AI in ALD, CLD and in LT patients. Secondly, there was increase in percentage of subjects with adrenal dysfunction with progression of liver disease as assessed by Child-Pugh staging. And lastly, various parameters related to liver dysfunction were also predictor of AI.
Among patients with ALD, 34.6% were diagnosed to have AI in the present study. Other studies have reported AI in 33 and 62% subjects with ALD.[4,11] Harry et al,[11] reported higher number of subjects with AI because they studied subjects with liver failure compared to stable patient in our study. Presence of AI has been reported to be associated with increased mortality and morbidity.[4] High INR and post prandial plasma glucose, low serum protein, total cholesterol and HDL cholesterol were predictors of presence of AI in patients with ALD, whereas Marik et al[4], reported low HDL as the only predictor of AI.
In this study 40% of CLD patients had AI. Various authors have reported percentage of AI ranging from 33-68% in patients with CLD.[5,7,12,13,14,15,16,17] This is due to varied etiology, severity, and different method used for the diagnosis of AI by different study. Galbois et al[12] and Tan et al,[13] noted AI in 33% and 39% of subjects with stable cirrhosis using same method and criteria as in present study. However, percentage of AI decreased to 9 and 12% when free cortisol criteria were used by them. Also with low dose short synecthan test (LDSST), Fede et al[5], found 38% AI in 101 patients with stable cirrhotic patients. Patients with chronic liver disease have low levels of CBG and low basal total cortisol, which can lead to overestimation of AI. However, diagnosis of AI based on free cortisol has not been universally standardized. Higher total bilirubin, INR, scores on Child-Pugh staging, and lower basal total cortisol, albumin, total cholesterol and LDL cholesterol were predictors of AI in our study, which has also been observed by others.[5,14,15] Mc Donald and coworkers[16] compared CLD patients (38 patients) with healthy controls and found patients with liver disease had 39% reduction in maximal increments of plasma cortisol to direct adrenal stimulation by short synecthen test (SST). In present study we observed 47% reduction in incremental response of cortisol on ACTH stimulation. Zeitz et al,[15] noted AI in 58% of their CLD patients with CRH stimulation test. This may indicate alteration of hypothalamo-pituitary axis, but they have included alcoholic liver disease in their study, which is known to affect HPA axis. On the contrary, adrenal dysfunction in subjects with ALD in present study is suggestive of primary alteration in adrenal function. Tsai et al,[14] noted AI in 51% of critically ill patients with CLD using different cut off values for diagnosis of AI (baseline cortisol < 9 μg/dl or an increment <9 μg/dl after 250 μg ACTH stimulation). Fernandez et al,[7] noted RAI in 68 % of cirrhotic patients with sepsis with AI defined as baseline cortisol concentration <15 μg/dl or an increase in plasma cortisol < 9 μg/dl with SST in patients with baseline cortisol concentration below 35 μg/dl7. Acevedo et al,[17] noted that among patients with advanced cirrhosis AI was present in 39% and was independently associated with higher mortality.
AI was noted in 40% patients with liver transplantation in present study. Marik et al,[4] evaluated early post transplant patients treated with steroid free immunosuppressant therapy and noted AI in 92% patients, however, prevalence decreased to 61% on prospective follow up with LDSST. LDSST can have lower cortisol response as compared to SST.[18] Patel et al,[19] conducted a retrospective analysis of prospectively collected data from 90 consecutive patients undergoing first elective liver transplantation. Improved post operative hemodynamics and the need for organ support in patients receiving intraoperative methylprednisolone compared to patients on steroid free regimen, indirectly supports the notion that the common finding of AI in patients with cirrhosis has clinical importance.
In our study low total and HDL cholesterol were found to be associated with presence of AI in ALD and CLD group, which has also been reported by Marik et al.[4] This can either be explained by direct decrease in substrate supply[20] or indirect effect of cytokines[21,22] or alteration in adrenal function de novo.[23] Cortisol synthesis requires cholesterol as a substrate in adrenal glands.[20] Adrenal cells may synthesize cholesterol de novo in adrenal cortex (20%) or may internalize circulating cholesterol (80%) via LDL and HDL receptors present on adrenal gland.[23] In humans traditionally LDL-C is considered as the preferred substrate for steroid synthesis[24] and HDL-C a minor substrate, but studies in rats and bovine animals have shown HDL-C as predominant source of cholesterol for cortisol synthesis.[23,25] It is known that liver is responsible for HDL-C synthesis and low HDL-C levels are known in chronic liver disease.[26] Also acute, chronic and post liver transplant states are associated with increased inflammation markers like TNF-α etc which are known to cause low HDL which may again contribute to increased AI in liver disease patients.[21] TNF-α has been shown to reduce the secretion of ACTH from the pituitary gland.[22] CLA-1 (CD36 and LIMPII Analogous-1) has been shown to be present on adrenal gland, liver, ovaries and testes and though CLA-1 binds HDL, LDL, oxidized LDL and VLDL it acts as high affinity receptors for HDL in liver.[27,28] CLA-1 mRNA is highly expressed in adrenals.[29]
Assessment of basal cortisol values in various spectra revealed higher basal cortisol values in acute hepatitis patients which then decreased progressively with progression of CLD followed by recovery in post transplant patients [Figure 1]. High basal cortisol values in acute hepatitis group can be due to acute stress response to hepatitis or release of preformed CBG into the circulation leading to high basal cortisol value akin to what is seen with thyroid functions specifically elevated thyroxin levels.[30] Values of basal cortisol decreased in CLD group with worsening of liver function, which is due to altered synthetic functions of liver followed by recovery after transplant due to improved liver function. Stimulated cortisol was lowest in CLD group (Child-Pugh score B and C) which improved after transplant [Figure 2]. Mean stimulated and incremental cortisol was lower in the complete spectrum of liver disease patients as compared to controls. This suggests that post liver transplant as liver function improves; there may also be recovery of LDL and HDL synthetic function of liver leading to recovery of adrenal function. This supports giving steroid cover during liver transplant in form of methyl prednisolone followed by steroid free immunosuppression and periodic follow up of adrenal function. In patients having evidence of RAI, patients should be treated with stress dosing of hydrocortisone and periodically evaluated for recovery of adrenal axis subsequently.
We excluded alcoholic liver disease patients from this study because alcohol is known to affect HPA axis, hence may lead to under or over estimation of AI according to method used. In present study most of patients were stable and not critically ill, leading to lesser confounding factors in AI estimation. In our study, we used cortisol level cut off as 18 μg/dl after SST and basal cortisol values <3 μg/dl as diagnosis of AI as these cut offs are most validated.[31,32] LDSST has limitation of over estimation of AI in stressful conditions. Basal cortisol values alone also fail to detect all AI cases when compared with gold standard test insulin tolerance test (ITT).[33] ITT was not preferred because of associated morbidity. Salivary cortisol values (free cortisol) still need standardization,[32] and were not available in our institute. SST has been used in critically ill and non-critically ill patients and has been reported to have reliable results.[34] Though we have evaluated our patient with CIRCI criteria for comparison with other studies; we have not used CIRCI criteria to define AI in our study as most of CLD and post transplant patients were stable. Also, only 7 out of 25 patients in ALD group were sick enough to be considered as critically ill.
Our study had some limitations. Firstly, we assessed AI by estimating total cortisol and liver diseases are known to decrease CBG levels and thus it might have led to over estimation of AI in this group. Secondly, AI couldn't be classified as primary or secondary as we did not measure plasma ACTH levels due to local constraints. However, altered adrenal functions seen with ALD with less than four weeks of disease duration suggest primary adrenal involvement as adrenal function get altered after four weeks in secondary adrenal insufficiency. Adrenal involvement has been reported in HCV[35] and HBV[36] infection. Thirdly, as this was a cross sectional study we could not assess factors which could have predicted mortality or morbidity.
In conclusion, AI forms important part of spectrum of acute and chronic liver disease. Deterioration of synthetic functions of liver disease predicts presence of AI, and these patients should be evaluated for adrenal dysfunction periodically. Adrenal function worsens with progression of liver disease. Steroid replacement in CLD patients at time of stress and critical illness may be beneficial.[4,6,7] Also presence of AI may predict survival of CLD patients. Though adrenal function shows recovery with liver transplant, AI is still common and requires periodic assessment. Further studies are needed to establish role of steroids in improving outcome of liver disease patients, also standardization of criteria for diagnosis of AI in setting of liver disease needs to be established.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Acharya SK, Madan K, Dattagupta S, Panda SK. Viral hepatitis in India. Natl Med J India. 2006;19:203–17. [PubMed] [Google Scholar]
- 2.Levine A, Zagoory-Sharon O, Feldman R, Lewis JG, Weller A. Measuring cortisol in human psychobiological studies. Physiol Behave. 2007;90:43–53. doi: 10.1016/j.physbeh.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 3.Weiser JN, Do YS, Feldman D. Synthesis and Secretion of Corticosteroid Binding Globulin by Rat Liver. J Clin Invest. 1979;63:461–7. doi: 10.1172/JCI109323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marik P, Gayoski T, Starzl T. The hepatoadrenal syndrome: A common yet Un-recognized clinical condition. Crit Care Med. 2005;33:1254–9. doi: 10.1097/01.ccm.0000164541.12106.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fede G, Spadaro L, Tomaselli T, Privitera G, Piro S, Rabuazzo AM, et al. Assessment of adrenocortical reserve in stable patients with cirrhosis. J Hepatol. 2011;54:243–50. doi: 10.1016/j.jhep.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 6.Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al-Abdulkareem A, et al. Low-dose hydrocortisone in patients with cirrhosis and septic shock: A randomized controlled trial. CMAJ. 2010;182:1971–7. doi: 10.1503/cmaj.090707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández J, Escorsell A, Zabalza M, Felipe V, Navasa M, Mas A, et al. Adrenal insufficiency in patients with cirrhosis and septic shock: Effect of treatment with hydrocortisone on survival. Hepatology. 2006;44:1288–95. doi: 10.1002/hep.21352. [DOI] [PubMed] [Google Scholar]
- 8.Stewart PM, Corrie J, Seckl JR, Edwards CR, Padfield PL. A rational approach for assessing the hypothalamo-pituitary-adrenal axis. Lancet. 1988;1:1208–10. doi: 10.1016/s0140-6736(88)92020-x. [DOI] [PubMed] [Google Scholar]
- 9.Hägg E, Asplund K, Lithner F. Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin Endocrinol. 1987;26:221–6. doi: 10.1111/j.1365-2265.1987.tb00780.x. [DOI] [PubMed] [Google Scholar]
- 10.Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348:727–34. doi: 10.1056/NEJMra020529. [DOI] [PubMed] [Google Scholar]
- 11.Harry R, Auzinger G, Wendon J. The clinical importance of adrenal insufficiency in acute hepatic dysfunction. Hepatology. 2002;36:395–402. doi: 10.1053/jhep.2002.34514. [DOI] [PubMed] [Google Scholar]
- 12.Galbois A, Rudler M, Massard J, Fulla Y, Bennani A, Bonnefont-Rousselot D, et al. Assessment of adrenal function in cirrhotic patients: salivary cortisol should be preferred. J Hepatol. 2010;52:839–45. doi: 10.1016/j.jhep.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Tan T, Chang L, Woodward A, McWhinney B, Galligan J, Macdonald GA, et al. Characterising adrenal function using directly measured plasma free cortisol in stable severe liver disease. J Hepatol. 2010;53:841–8. doi: 10.1016/j.jhep.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Tsai MH, Peng YS, Chen YC, Liu NJ, Ho YP, Fang JT, et al. Adrenal Insufficiency inPatients with Cirrhosis: Severe Sepsis and Septic Shock. Hepatology. 2006;43:673–81. doi: 10.1002/hep.21101. [DOI] [PubMed] [Google Scholar]
- 15.Zietz B, Lock G, Plach B, Drobnik W, Grossmann J, Schölmerich J, et al. Dysfunction of the hypothalamic-pituitary-glandular axes and relation to Child-Pugh classification in male patients with alcoholic and virus-related cirrhosis. Eur J Gastroenterol Hepatol. 2003;15:495–501. doi: 10.1097/01.meg.0000059115.41030.e0. [DOI] [PubMed] [Google Scholar]
- 16.Mc Donald JA, Handelsman DJ, Dilworth P, Conway AJ, Mccaughan GW. Hypothalamic-pituitary adrenal function in end stage non- alcoholic liver disease. J Gastroenterol Hepatol. 1993;8:247–53. doi: 10.1111/j.1440-1746.1993.tb01195.x. [DOI] [PubMed] [Google Scholar]
- 17.Acevedo J, Fernandez J, Castro M, Roca D, Gines P, Arroyo V, et al. Impact of relative adrenal insufficiency on circulatory function and mortality in advanced cirrhosis. J Hepatol. 2011;54:S61–208. [Google Scholar]
- 18.Fede G, Spadaro L, Tomaselli T, Privitera G, Germani G, Tsochatzis E. Adreno-cortical dysfunction in liver disease: A systematic review. Hepatology. 2012;55:1282–91. doi: 10.1002/hep.25573. [DOI] [PubMed] [Google Scholar]
- 19.Patel S, Broomhead R, Burroughs AK, Mallett SV, O’Beirne J. Effect of intra-operative methylprednisolone on post liver transplant (LT) intensive care unit (ITU) course - further evidence for the existence of hepato-adrenal syndrome? J Hepatol. 2010;52(Supp 1):S197. [Google Scholar]
- 20.Yaguchi H, Tsutsumi K, Shimono K, Omura M, Sasano H, Nishikawa T, et al. Involvement of high density lipoprotein as substrate cholesterol for steroidogenesis by bovine adrenal fasciculo-reticularis cells. Life Sci. 1998;62:1387–95. doi: 10.1016/s0024-3205(98)00077-0. [DOI] [PubMed] [Google Scholar]
- 21.Bornstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med. 2009;360:2328–39. doi: 10.1056/NEJMra0804635. [DOI] [PubMed] [Google Scholar]
- 22.Gaillard RC, Turnill D, Sappino P, Muller AF. Tumor necrosis factor alpha inhibits the hormonal response of the pituitary gland to hypothalamic releasing factors. Endocrinology. 1990;127:101–6. doi: 10.1210/endo-127-1-101. [DOI] [PubMed] [Google Scholar]
- 23.Borkowski AJ, Levin S, Delcroix C, Mahler A, Vera Verhaset V. Blood cholesterol and hydrocortisone production in man: Quantitative aspects of the utilization of circulating cholesterol by the adrenals at rest and under adrenocorticotropin stimulation. J Clin Invest. 1967;46:797–811. doi: 10.1172/JCI105580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gwynne JT, Strauss JF., III The role of lipoprotein in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev. 1982;3:299–329. doi: 10.1210/edrv-3-3-299. [DOI] [PubMed] [Google Scholar]
- 25.Landschulz KT, Pathak RK, Rigotti A. Regulation of scavenger receptor, class B, type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J Clin Invest. 1996;98:984–95. doi: 10.1172/JCI118883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fatima M, Ranjha FA. Dyslipidemia in chronic liver disease. Pak J Med Health Sci. 2007;1:103–5. [Google Scholar]
- 27.Calvo D, Gómez-Coronado D, Lasunción MA, Vega MA. CLA-1 is an 85-kD plasma membrane glycoprotein that acts as a high-affinity receptor for both native (HDL, LDL, and VLDL) and modified (OxLDL and AcLDL) lipoproteins. Arterioscler Thromb Vasc Biol. 1997;17:2341–9. doi: 10.1161/01.atv.17.11.2341. [DOI] [PubMed] [Google Scholar]
- 28.Llera-Moya M, Connelly MA, Drazul D, Klein SM, Favari E, Yancey PG, et al. Scavenger receptor class B type 1 affects cholesterol homeostasis by magnifying cholesterol flux between cells and HDL. J Lipid Res. 2001;42:1969–78. [PubMed] [Google Scholar]
- 29.Liu J, Heikkila P, Meng QH, Kahri AI, Tikkanen MJ, Voutilainen R, et al. Expression of low and high density lipoprotein receptorgenes in human adrenals. Eur J Endocrinol. 2000;142:677–82. doi: 10.1530/eje.0.1420677. [DOI] [PubMed] [Google Scholar]
- 30.Malik R, Hodgson H. The relationship between the thyroid gland and the liver. QJM. 2002;95:559–69. doi: 10.1093/qjmed/95.9.559. [DOI] [PubMed] [Google Scholar]
- 31.Stewart PM, Corrie J, Seckl JR, Edwards CR, Padfield PL. A rational approach for assessing the hypothalamo-pituitary-adrenal axis. Lancet. 1988;1:1208–10. doi: 10.1016/s0140-6736(88)92020-x. [DOI] [PubMed] [Google Scholar]
- 32.Wiebke A. The Approach to the Adult with Newly Diagnosed Adrenal Insufficiency. J Clin Endocrinol Metab. 2009;94:1059–67. doi: 10.1210/jc.2009-0032. [DOI] [PubMed] [Google Scholar]
- 33.Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab. 1998;83:2350–4. doi: 10.1210/jcem.83.7.4980. [DOI] [PubMed] [Google Scholar]
- 34.Kazlauskaite R, Evans AT, Villabona CV, Abdu TA, Ambrosi B, Atkinson AB, et al. Corticotropin tests for hypothalamic-pituitary- adrenal insufficiency: A meta-analysis. J Clin Endocrinol Metab. 2008;93:4245–53. doi: 10.1210/jc.2008-0710. [DOI] [PubMed] [Google Scholar]
- 35.Tran HA, Song S, Lojewski RJ, Reeves GE. Exacerbation of hepatitis C induced subclinical hypoadrenalism by Interferon-alpha2beta: A case report. Cases J. 2008;1:157. doi: 10.1186/1757-1626-1-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Somlo F, Berry GR. Extrahepatic manifestations of hepatitis B virus infection: Addison's disease and myelofibrosis in a patient with persistent hepatitis B surface antigenemia. Can J Infect Dis. 1993;4:139–44. doi: 10.1155/1993/393861. [DOI] [PMC free article] [PubMed] [Google Scholar]


