Abstract
Context:
In Nigeria, much has been reported on the unacceptably high disease burden of Tuberculosis (TB) and Diabetes Mellitus (DM) but not the possible co-existence of these diseases.
Aim:
This study was conducted to document the co-existence of DM and TB in persons with established TB.
Settings and Design:
This was a cross-sectional study conducted at a Tertiary hospital's Directly Observed Therapy short course clinic in Lagos, South west, Nigeria.
Materials and Methods:
Three hundred and fifty one consecutive patients with TB who consented to the study participated after a written consent. Ethical approval was given by the Ethics committee of the institution. Clinical examination for documentation of anthropometric indices and biochemical evaluation for blood glucose levels were carried out.
Results:
The prevalence of DM among the patients with TB was 5.7%. About half of the diabetics were diagnosed (2.8%) at the screening. The mean age of the participants was 34.9 ± 13.21 years; the mean duration of symptoms of TB was 9.65 ± 9.49 months. Weight (kg) loss was the most predominant symptom occurring in 94% of the patients. There was no significant difference in the sputum positivity and duration of cough among patients with TB-DM and those with TB alone.
Conclusion:
Diabetes is an important co-morbid feature to be sought in patients with TB. This study re-echo the need to raise awareness on screening for DM in persons with TB.
Keywords: Co-morbid, diabetes mellitus, glucometer, tuberculosis
INTRODUCTION
Tuberculosis (TB) is a major cause of morbidity and mortality worldwide. In 2010 there were 8.8 million incident cases of TB with 1.1 million deaths from TB among human immunodeficiency virus (HIV) negative people and an additional 0.35 million death from HIV related TB. Nigeria is one of the 22 high burdened countries with TB.[1] Diabetes mellitus (DM) on the other hand affects an estimated 340 million people worldwide. In 2004, 3.4 million people died from DM with more than 80% of these deaths occurring in low and middle income countries. The World Health Organisation, (WHO) projected that diabetes deaths will double between 2005 and 2030.[2] The consequences of these two growing diseases are likely to be substantial.
Several studies had associated Diabetes with TB.[3,4,5,6,7,8] Richard Morton in his treatise, Pthisiologia on consumption had in 1694 stated that an association between diabetes and TB had been suggested even in Roman times.[3] Earlier studies conducted in the 1960s indicated that TB was more prevalent in individuals with DM than in those without. At that time about half of the patients had type 1 DM.[6,7] Today, with increasing prevalence of type 2 diabetes and it accounting for 90-95% of all cases of DM, the association between the two diseases is being re-evaluated.
Meta-analysis of studies on DM and TB shows that DM increases the risk of TB, regardless of different study designs, background TB incidence or geographical region of the study. It has also been shown that diabetics have impaired innate immune responses to TB.
There exists evidence that DM is an independent risk factor for lower respiratory tract infections.[9,10,11] TB occurs with an increased frequency in diabetics and causes significantly higher mortality,[9,10,11] increased reactivations of TB lesions have also been recorded in diabetics. TB also appears to aggravate DM with patients requiring increase in dosage regimen of treatment for control.[9,10,11]
However some epidemiological studies failed to demonstrate any of these associations particularly in developed countries and those with low TB prevalence.
A probable cause of increase incidence of TB in diabetics may be related to defects in host defenses and immune cell functions the immune derangements predominantly involved the cell mediated immunity. Immunologic abnormalities in diabetics include, abnormal chemotaxis, adherence, phagocytosis and microbicidal functions of polymorphonuclear leucocytes.
There is also decreased peripheral monocyte with impaired phagocytosis, poor blast transformation of lymphocytes and defective complement C3 opsonic function.[9,10,11] Multiple pulmonary physiologic abnormalities have also been documented in diabetics that contribute to delayed clearance of and spread of infection in the host. This include diminished bronchial reactivity, reduced elastic recoils and lung volumes, reduced diffusion capacity, occult mucus plugging of airways and reduced ventilator response to hypoxemia. Infection with tubercle bacilli leads to further alterations in the cytokines, monocyte–macrophages and CD4/CD8 T cell populations. The balance of the T lymphocyte subset CD4 and CD8 plays a central role in the modulation of host defenses against mycobacterial and has profound influence on the rate of regression of active pulmonary TB.[9,10,11]
The experiences from the Asian countries with increasing prevalence of DM and TB should also increase awareness among health professionals and policy makers in Nigeria especially with increasing prevalence of obesity and Type 2 DM. The high burden of TB in the country may make the double burden of disease unbearable and with dire consequences.
Most of the studies earlier mentioned are from industrialized countries with almost none coming from the Africa continent. While it is a standard practice to screen for HIV in all cases of TB, the same cannot be said of DM despite its increasing prevalence. It is therefore important to determine the prevalence of DM in TB and evaluate the possible effect of DM on the clinical presentations of TB.
The primary objective of this study is the determination of the prevalence of DM among patients with established TB. We also made an attempt at describing the clinical presentation of persons with TB who also had DM.
MATERIALS AND METHODS
This study took place at the outpatient clinic of the Direct Observed short course treatment for TB at a tertiary health care facility in Lagos and the duration was for 12-months. The study was approved by the Research and Ethic committee of the Hospital. Informed and written consent were obtained from the study patients. The sample size was calculated using the Yamaro Yamane formula nf = n/(1 + n/N)[12] where nf is the desired sample size when study population is less than 10,000 and n the desired sample size when population is greater than 10,000 and N the estimate of population size. N in our study was the number of patients on the Directly Observed treatment register at commencement which was 280. n = Z2pq/d2 where z is the standard estimate and = 1.96, P is prevalence given as 0.5 in this study, q = 1-p and d is precision at 0.05. The calculated nf adjusted for 80% response rate was 202 which is the minimum sample size. Thus 351 consecutive patients with physician diagnosed TB (both pulmonary and extrapulmonary) who consented were recruited into the study. The patients were interviewed with a structured questionnaire which sought information on the socio-demographic characteristics of the patients, (age sex, occupation, marital status) duration of symptoms, their presenting complaints and prior history of DM.
Fasting blood glucose was done in all patients with a glucometer. DM was diagnosed if fasting blood glucose was equal to or greater than 126 mg/dl in accordance with WHO guidelines.[13] Physical assessment that was done included measurement of weight (kg) and height in meters and also the body mass indices calculated.
The sputum smear result, HIV status, and the Tuberculin test were reviewed from the Case Folders. The data were analyzed using SPSS version 17. All quantitative data were expressed as mean ± SD The comparison of means was done using the student t-test while qualitative variables were compared using the Chi square. P value less than 0.05 was taken as significant.
RESULTS
A total of 351 patients with established TB participated in the study. This comprises 108 (30.7%) retreatment cases and 243 (69.3%) new cases. The age range of the patients was between 15 and 80 years with the mean age 34.9 ± 13.21 years while the mean age of patients with TB and DM was 41.5 ± 13.9 years. The mean duration of symptoms (in months) was 9.65 ± 9.49 months.
Among 351 TB patients, DM was present in 20 (5.7%) while HIV was present in 17 (4.8%) Out of the 20 patients with DM, about half of them[10] were newly diagnosed with DM. The mean duration of DM, in the previously diagnosed group was 4.67 ± 1.54 years. Patients with TB and DM were older than those with only TB and the difference was statistically significant. (P < 0.05). There was no statistically significant difference in the duration of cough among those with and without DM. (P > 0.05) other socio-demographic and clinical characteristics of the patients are shown in Table 1.
Table 1.
Demographic characteristics of respondents
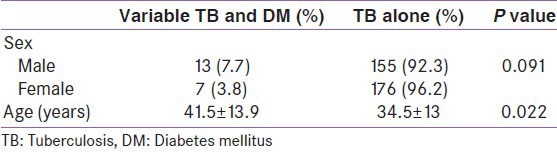
The most predominant symptom of TB was weight loss which was present in 330 (94%) of the patients. Chronic cough was present in 292 (83.2%), 190 (54.1%) had night sweat while 134 (38.2%) had hemoptysis.
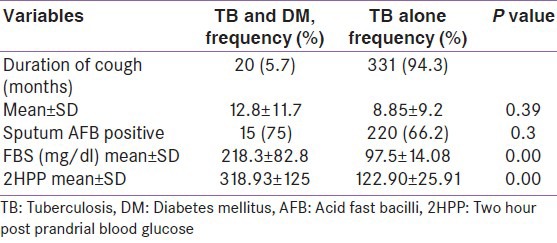
About 3/4 of the patients with TB-DM were smear positive compared with 66.2% of those with TB alone but this did not have any statistical significance as the P value was greater than 0.05. Thus, there was also no significant difference in the sputum smear positivity of persons with TB alone and those with TB and DM. Other features such as the mean fasting blood sugar as well as the two hours post prandial values are shown in Table 2.
Table 2.
Clinical presentations of respondents
DISCUSSION
In our study, the DM prevalence among patients with established TB of 5.7% is comparable with the documented prevalence rate of DM in Lagos State (6%).[14]
The prevalence of 5.7% in our study is much lower than the findings of Restrepo et al. in the United States where the epidemic of Type 2 DM prompted them to explore associaton between DM and TB on the south Texas-Mexico border and they found a self-reported DM prevalence of 27.8% among Texans with TB and 17.8% of Mexicans with TB which significantly exceeded the national self-reported rates for both countries.[15] In Indonesia, DM is strongly associated with TB with a prevalence of 13.2% (60 out of 454 patients with TB had diabetes compared with 3.2% of the control subjects).[16] The lower prevalence reported in our study may be related to the lower national prevalence of diabetes among our people when compared with these other countries. Although the patients with TB in our report do not appear to have a higher prevalence when compared with the state figures, it is worthy of note that about half of the diabetics were diagnosed during this study. This therefore emphasizes the importance of routine screening for DM in all patients with TB. The already fairly well-established TB infrastructure and health personnel could also serve to improve early detection of DM.
The mean age of the patients with TB and DM was higher than in those with TB alone. This is similar to the study in the United States.[15] This may be related to the fact that Type 2 DM is seen more frequently in the older age group, the fact that this study was conducted largely among adults with TB may also be a factor. In our study majority of the patients were presumed to have Type 2 DM with only 25% of the patients studied being 30 years and below.
Although the risk of TB in DM has been long recognized, Restrepo et al. in the United States found that those with TB and diabetes were more likely to have hemoptysis, pulmonary cavitations, be smear positive at diagnosis, and remain positive at the end of the first (Texas) or second (Mexico) month of treatment.[15]
The HIV prevalence in our study was 4.8% compared with 5.7% prevalence of DM despite the greater attention paid to HIV assessment in TB patients. This is similar to the finding of Gupta et al.[17] in India where they found a significantly higher preponderance of DM (31%) over HIV (8.9%). This requires further exploration in our locality to define the potential significance of this finding.
Some of the limitations of our study include the fact that the study was conducted within a hospital setting and may therefore not truly represent the true prevalence of the conditions in the community. It is also important to note that this study was a crossectional survey and there was no follow up to determine the response to treatment and relapse rate between the two groups.
However, it is our hope that the findings from this preliminary report will serve as a basis for a more prospective large scale study in the future to determine the association between TB and DM in our population.
CONCLUSION
The prevalence of DM in patients with TB in our series was 5.7%. About half of the TB-DM patients in our study were diagnosed for the first time during this study and therefore it is recommended that diabetes screening should be incorporated into the routine assessment of all patients with TB in our environment. The rising burden of DM may adversely affect TB control and effective utilization of the TB control program could be beneficial in early detection and treatment of DM.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Geneva: World Health Organization; 2010. [Last accessed on 2011 Mar 25]. Global tuberculosis report. Available from : http:// whqlibdoc.who.int/publications/2010/9789241564069_eng.pdf . [Google Scholar]
- 2.WHO|Diabetes. [Last accessed on 2011 Aug 05]. Available from: http://www.who.int/mediacentre/factsheets/fs312/en .
- 3.Richard Morton. London: Smith and Watford; 1694. Phthisiologia: Or a Treatise of Consumption; p. 88. [Google Scholar]
- 4.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: A systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Root H. The association of diabetes and tuberculosis. NEJM. 1934;210:1–13. [Google Scholar]
- 6.Boucot KR, Dillon ES, Cooper DA, Meier P, Richardson R. Tuberculosis among diabetics: The Philadelphia survey. Am Rev Tuberc. 1952;65:1–50. [PubMed] [Google Scholar]
- 7.Stevenson CR, Forouhi NG, Roglic G, Williams BG, Lauer JA, Dye C, et al. Diabetes and tuberculosis: The impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234. doi: 10.1186/1471-2458-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: Convergence of two epidemics. Lancet Infect Dis. 2009;9:737–46. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMahon MM, Bistrian BR. Host defenses and susceptibility to infection in patients with diabetes mellitus. Infect Dis Clin North Am. 1995;9:1–9. [PubMed] [Google Scholar]
- 10.Koziel H, Koziel MJ. Pulmonary complications of diabetes mellitus. Pneumonia. Infect Dis Clin North Am. 1995;9:65–96. [PubMed] [Google Scholar]
- 11.Gupta A, Shah A. Tuberculosis and diabetes: Appraisal. Ind J Tub. 2000;47:3–8. [Google Scholar]
- 12.Israel GD. Determining Sample Size. University of Florida: IFAS; 2002. [Last accessed on 2010 July 04]. Available from: http://edis.ifas.ufl.edu/PD006] webcite . [Google Scholar]
- 13.Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia. Report of a WHO/IDF consultation. Diabet Med. 2008;25:1151–6. [Google Scholar]
- 14.State wide hypertension and diabetes screening. The Lagos State programme. [Last accessed on 2012 July 24]. Available from http://www.lsmoh.com/programme_info.php?programme_id=26 .
- 15.Restrepo BI, Fisher-Hoch SP, Crespo JG, Whitney E, Perez A, Smith B, et al. Type 2 diabetes and tuberculosis in a dynamic bi-national border population. Epidemiol Infect. 2007;135:483–91. doi: 10.1017/S0950268806006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alisjahbana B, van Crevel R, Sahiratmadja E, den Heijer M, Maya A, Istriana E, et al. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int J Tuberc Lung Dis. 2006;10:696–700. [PubMed] [Google Scholar]
- 17.Gupta S, Shenoy VP, Bairy I, Srinivasa H, Mukhopadhyay C. Diabetes mellitus and HIV as co-morbidities in tuberculosis patients of rural south India. J Infect Public Health. 2011;4:140–4. doi: 10.1016/j.jiph.2011.03.005. [DOI] [PubMed] [Google Scholar]


