Abstract
Background:
Both insulin deficiency and resistance are reported in patients with β-thalassemia major (BTM). The use of continuous blood glucose monitoring (CGM), among the different methods for early detection of glycemic abnormalities, has not been studied thoroughly in these adolescents.
Materials and Methods:
To assess the oralglucose tolerance (OGT) and 72-h continuous glucose concentration by the continuous glucose monitoring system (CGMS) and calculate homeostatic model assessment (HOMA), and the quantitative insulin sensitivity check index (QUICKI) was conducted in 16 adolescents with BTM who were receiving regular blood transfusions every 2-4 weeks and iron-chelation therapy since early childhood.
Results:
Sixteen adolescents with BTM (age: 19.75 ± 3 years) were investigated. Using OGTT, (25%) had impaired fasting blood (plasma) glucose concentration (BG) (>5.6 mmol/L). 2-h after the glucose load, one of them had BG = 16.2 mmol/L (diabetic) and two had impaired glucose tolerance (IGT) (BG > 7.8 and <11.1 mmol/L). Monitoring the maximum (postprandial) BG using CGMS,4 adolescents were diagnosed with diabetes (25%) (BG >11.1 mmol/L) and 9 with IGT (56%). HOMA and QUICKI revealed levels <2.6 (1.6 ± 0.8) and >0.33 (0.36 ± 0.03), respectively, ruling out significant insulin resistance in these adolescents. There was a significant negative correlation between the β-cell function (B%) on one hand and the fasting and the 2-h BG (r=−0.6, and − 0.48, P < 0.01, respectively) on the other hand. Neither fasting serum insulin nor c-peptide concentrations were correlated with fasting BG or ferritin levels. The average and maximum blood glucose levels during CGM were significantly correlated with the fasting BG (r = 0.68 and 0.39, respectively, with P < 0.01) and with the BG at 2-hour after oral glucose intake (r = 0.87 and 0.86 respectively, with P < 0.001). Ferritin concentrations were correlated with the fasting BG and the 2-h blood glucose levels in the OGTT (r = 0.52, and r = 0.43, respectively, P < 0.01) as well as with the average BG recorded by CGM (r = 0.75, P < 0.01).
Conclusion:
CGM has proven to be superior to OGTT for the diagnosis of glycemic abnormalities in adolescents with BTM. Defective β-cell function rather than insulin resistance appeared to be the cause for these abnormalities.
Keywords: β-Thalassemia major, continuous glucose monitoring, diabetes mellitus, impaired fasting glucose, impaired glucose tolerance, oral glucose tolerance
INTRODUCTION
In patients with β-thalassemia major (BTM), hyper-transfusion therapy has dramatically increased the duration and quality of life but has been associated with chronic iron overload, and frequently complicated by the development of diabetes mellitus (DM) or impaired glucose tolerance (IGT). DMis still responsible for significant morbidity and mortality in thalassemic patients. Prevalence has been reported to range from 2.3% to 24%.[1,2,3,4]
Increased intestinal iron absorption appears to increase the iron load in these patients. In β-thalassemia, iron absorption is regulated by tissue hypoxia, erythropoietin excretion and ineffective erythropoiesis of an enormously expanded and active bone marrow. The latter leads to over expression of growth differentiation factor (GDF15), which inhibits hepcidin synthesis, increasing the rate of iron absorption even in the presence of iron overload. Bone marrow erythroid activity (BMA) and ineffective erythropoiesis further disturb iron homeostasis by increasing plasma iron turnover, outpouring catabolic iron (NTBI) which exceeds the iron-carrying capacity of transferrin and circulates in plasma in high levels. Plasma iron turnover is increased many folds releasing considerable amounts of NTBI.[5,6,7]
The etiology of DM in BTM is suggested to be due to the effect of iron overload on the different tissues controlling the carbohydrate homeostatic mechanisms; including the pancreas and liver. However, controversy about the etiology of this glycemia abnormality still exists. Both insulin deficiency and insulin resistance are reported in patients with BTM. Suggested risk factors for development of DM in patients with BTM include old age, increased amount of blood transfusion, high serum ferritin level, family history of DM, hepatic impairment, and genetic modifiers of iron overload.[8,9,10,11,12,13]
The relationship between glucose concentrations in interstitial fluid (ISF) and blood has generated great interest due to its importance in minimally invasive and non-invasive techniques for measuring blood glucose. The relationship between glucose levels in dermal ISF, and glucose levels in capillary and venous blood measured simultaneously was studied. Glucose levels in the three compartments exhibited high correlations both when individual subjects were considered separately and when data from all subjects were combined. No significant time lag during glucose excursions was observed among the ISF, and capillary and venous glucose levels.[14] Therefore, it has been demonstrated recently that the continuous glucose monitoring system (CGMS) is a useful and valid tool in defining glucose metabolism affected by secondary forms of glucose derangements. The advantages of CGMS are that it can help to identify fluctuations and trends that would otherwise go unnoticed with other standard methods and that these measurements are during real life of the subject.[15,16]
Rimondi et al., used CGMS in a small number (n = 6) of thalassemic patients with abnormal glucose homeostasis after an oral glucose tolerance test (OGTT). In five patients, the CGMS confirmed the IGT. This study suggested that the use of CGMS is a useful method to detect the variability of glucose fluctuations and offers the opportunity for better assessment of glucose homeostasis in TM patients[17]
The aim of the work was to assess oral glucose tolerance (OGT) and the 72-h continuous blood glucoseconcentrations during normal (usual) life-style (natural and various carbohydrate loads) andin adolescents with BTM, measure their fasting insulin secretion and calculate their HOMA and QUIKI indices and correlate these findings with serum ferritin concentration and hepatic functions in adolescents with BMT.
MATERIALS AND METHODS
Sixteen adolescents (age between 14 and 22 years) with BTM on regular blood transfusion and iron chelation therapy attending the BTM were randomly recruited (randomly including every third patient with TM attending the clinic) from the Paediatric and Endocrinology and Haematology outpatient clinics in Hamad Medical Center (HMC) and Al Amal Hospitals. Thalassemic patients with hepatic impairment, or history of other systemic or endocrine abnormalities were excluded.
The study has been approved by the ethical committee of Hamad medical center (HMC) and informed consents obtained from all the patients and their parents before including in the study. Patients with hepatic impairment, family history of DM, or other systemic illness were excluded from the study.
All adolescents were assessed clinically and the following lab investigations performed in a fasting venous sample at 8 AM: Serum insulin, C-peptide, and ferritin and plasma glucose levels. A standard OGTT was performed [0 and 2 h BG using 1.75 g of glucose/kg (max 75 g)]. Every patient was supplied with a glucometer (one touch ultra-machine, which uses the glucose oxidase principle for measuring capillary BG) and asked to measure BG before meals and snacks and record the values in the CGMS for better calibration. Meanwhile, a CGMS (Medtronic type) (which continuously measures glucose in the ISF every 5 minutes) was inserted. These glucose concentrations (by CGMS and glucometer) were downloaded after 3 days and interpreted using Medtronic software. The diagnosis of glycemia status whether normal, diabetic, IFG, IGT was done according to American diabetes association criteria.[15] As plasma, serum and IF glucose levels exhibit high correlations and in the absence of published criteria for diagnosing glycemic abnormalities by CGMS, the same criteria were applied for both the OGTT results as well as the CGMS data.[14,18]
Serum insulin levels were measured by a chemi-luminescent immunoassay method on ADVIA Centaur analyser using a commercial kit (ADVIA Centaur IRI). Lower and upper detection limits were 0.5 and 300 μUI/ml (3-1800 pmol/l), respectively. The intra-and inter-assay CV ranges were 3.3-4.6% and 2.6-5.9%, respectively. C-peptide concentrations were measured using (C-peptide ELISA kit) in the fasting state. The DAI C-PEPTIDE is a quantitative solid phase ELISA. (Cortez Diagnostics, Inc., USA).
Homeostatic model assessment (HOMA) is a widely validated clinical and epidemiological tool for estimating insulin resistance and beta cell function. It is derived from mathematical assessment of balance between hepatic glucose output and insulin secretion from fasting levels of glucose and insulin. The HOMA-IR indexis the HOMA of insulin resistance and is the product of basal glucose and insulin levels divided by 22.5 and is regarded as a simple, inexpensive and reliable surrogate measure of insulin resistance. The HOMA-B index is the HOMA of B cell function computed as the product of 20 and basal insulin levels divided by the value of basal glucose concentrations minus 3.5, thishas been proposed to be a good measure of B cell function.[19] Quantitative insulin sensitivity check index (QUIKI) correlates well with glucose clamp studies (r = 0.78), and is useful for measuring insulin sensitivity (IS), which is the inverse of insulin resistance (IR).[20] Percentbeta cell function was calculated.[21]
Statistical analysis
Data are expressed as means ± SD. To explore the factors predicting the presence of glucose values above 200 mg/dl, a logistic regression analysis was performed with the presence/absence of glucose values above 200 mg/dl at follow-up as the dependent variable. Linear regression equations were used to investigate correlations between the different variables including: Age, BMI, insulin, C-peptide, and glucose data measured by the two methods (OGTT and CGMS). Data were presented as mean ± SD and significance was accepted at P < 0.05. Excel version 2007 was used for all analysis
RESULTS
Sixteenadolescents and young adults with BTM (age 19.75 ± 3 years, 12 males and 4 females) were investigated. They were 10 males and 6 females, with mean height (157.5 ± 8.7 cm) and weight (51.4 ± 10.3 kg). Their height SDS (HtSDS) = −2.2 ± 0.9 and BMI = 20.6 ± 3. Seven out of the 14 patients were short (HtSDS<−2) and 6 were very short (HtSDS<−3). One patient had low BMI (13.2 kg/m2).
Twelve out of the 16 patients started transfusion at age of 2 years while the remaining 4 started at the age of one and half years. One patient was on packed cell transfusion (PCTx) every 5 weeks, 7 patients were receiving every3 weeks, 1 patient every 2 weeks, and the remaining 7 were on PCTxevery4 weeks. Fourteen patients were on hyper transfusion regimen (i.e., pre transfusion Hb > 9.5 g/dl and post transfusion Hb not exceeding 14 g/dl). Two patients were on medium transfusion regimen (pre transfusion Hb not less than 8.5 g/dl). Fourteen patients were on oral deferasirox, one on desferioxamine subcutaneous therapy and one patient was receiving sequential therapy desferioxamine plus deferiprone. Neither of our patients was splenecomized nor did they have hepatitis C, B or HIV.
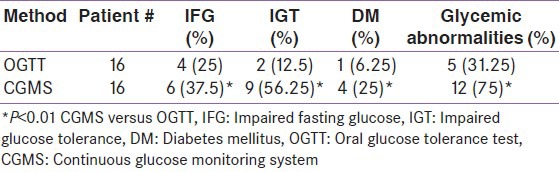
Using OGTT, 4 patients had impaired fasting BG > 5.6 mmol/L. One of them had BG = 16.2 mmol/L after 2-h (diabetic) and two patients had IGT (BG > 7.8 and < 11.1 mmol/L). Using CGMS in addition to the glucose data measured by glucometer (3-5 times/day), the maximum (postprandial) BG recorded exceeded 11.1 mmol/L in 4 patients (25%) (diabetics) and was > 7.8 but < 11.1 mmol/L in 9 cases (56%) (IGT). HOMA and QUICKI revealed levels < 2.6 and > 0.33, respectively, in most of the patients (13/16) [mean ± SD = (1.38 ± 0.8) and (0.37 ± 0.04), respectively] ruling out significant insulin resistance in these adolescents [Table 1].
Table 1.
Glycemic abnormalities diagnosed by OGTT versus CGMS in thalassemic patients
There was a significant negative correlation between the β-cell function (B%) on t one hand and the fasting and the 2-h blood glucose levels during OGTT (r = −0.6 and − 0.48, P < 0.01) on the other hand [Table 2].
Table 2.
Correlation analysis between variables
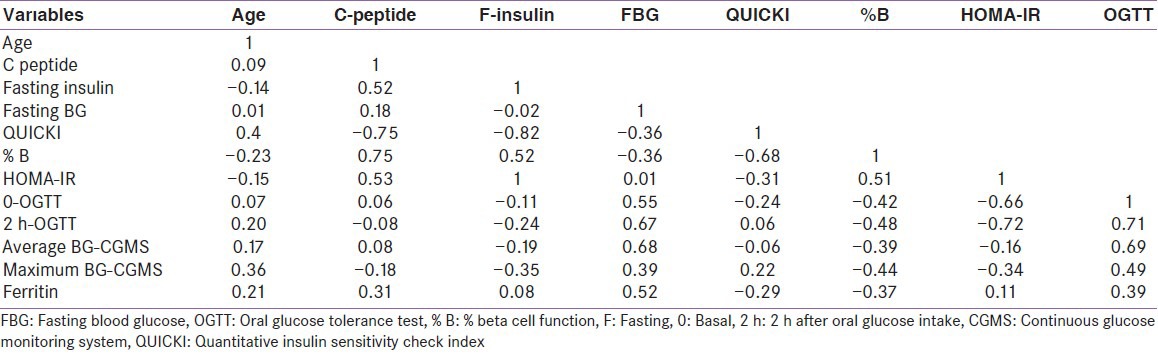
During continuous glucose monitoring (CGM), the average and maximum (postprandial) BG were positively correlated with the fasting BG (r = 0.69 and 0.6, respectively, with P < 0.01) and with the 2-hour glucose level of the OGTT (r = 0.87 and 0.86 respectively, with P <0.01). Fasting serum insulin and c-peptide concentrations did not correlate with fasting BG or ferritin levels [Table 2]. The statistical power of diagnosing glycemic abnormalities using CGMS versus OGTT = 100% at a confidence level of 0.05.
Serum ferritin concentrations were positively correlated with the fasting and 2-h BG in the OGTT (r = 0.69, 0.43, respectively, P < 0.01) as well as with the average and the maximum BG recorded by the CGMS (r = 0.75, and 0.64, respectively, with P < 0.01). Ferritin concentrations were negatively correlated with the β-cell function (r = −0.41, P < 0.01) [Figure 1].
Figure 1.
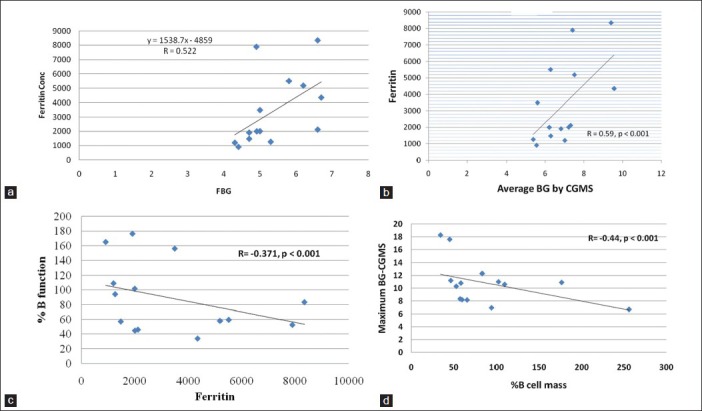
(a) Association between serum ferritin concentrations and fasting blood glucose (r=0.522, P<0.001), (b) Association between serum ferritin concentrations and average blood glucose by CGMS (r=0.59, P<0.001), (c) Association between ferritin concentration and % B cell function (r=−0.371, P<0.01), (d) Association between maximum blood glucose concentrations and % B cell function (r=−0.44, P<0.001)
DISCUSSION
Worldwide, DM and IGT are still prevalent complications in patients with BTM with hyper-transfusion therapy. DM is responsible for significant morbidity and mortality in these patients.[22,23,24,25] CGMS is an FDA-approved device that records blood sugar levels throughout the day and night. It has the advantage of measuring glucose concentrations under the usual (day-to-day) real-life conditions of the patient. This CGM can help identify fluctuations and trends that would otherwise go unnoticed with standard OGTT, intermittent finger stick and HbA1c measurements.
In this study, we demonstrated high prevalence of glycemic abnormalities in adolescents with BTM that differ by the different methods used. CGMS diagnosed glycemic abnormalitiesin75% of our adolescents with BTM, whereas OGTT diagnosed 25% only. DM was diagnosed in 25% of our patients during CGMS whereas only one diabetic patient was diagnosed using OGTT. CGMS appears to be more sensitive and superior to the conventional OGTT or HbA1c concentrations. In support to this view, CGMS has proved to be superior to the conventional methods of glucose monitoring in diabetic and non-diabetic patients.[26,27,28]
Controversy about the etiology of this glycemic abnormality in BTM still exists. Insulin deficiency and/or insulin resistance are reported by different authors in patients with thalassemia major with variable risk factors.[1,2,3,4,5,6,7,8,9,10,11,12] The majority of our patients with BTM and glycemic abnormalities (13/16) did not show significant insulin resistance using HOMA and QUICKI. In support of our findings, insulin deficiency rather than insulin resistance was reported by many other authors.[8,9,10]
In our study, the ferritin level was positively correlated with the fasting BG, 2-h BG in the OGTT as well as with the average, and the maximum BG recorded by the CGMS. Moreover, ferritin concentrations were negatively correlated with the β-cell function. These data proved that poor chelation, and subsequently hemosiderosis of the pancreas and other organs appear to be a major cause for glycemic abnormalities in our patients with BTM. A single injection of iron nanoparticles in mice induced inflammation with significantly increased levels of pro-inflammatory cytokines (IL-1, TNF-alpha, and IL-6).[29] Recent data demonstrated increased circulating levels of IL-1α, TNF-α and IL-6 in patients with BTM which may explain the gradual and progressive deterioration of organs that may include the beta cells of the pancreas.[30]
Decreased insulin secretion in response to iron overload has been supported by animal experiments. The effect of iron overload on function of pancreatic islet cells was studied in four groups in Wistar rats. Group A received repeated intraperitoneal (i.p.) injections of ferric nitrilotriacetate (FeNTA); group B received the equivalent dose of Na2 NTA; Group C received i.p. injection of diethylenetriaminepentaaceticacid in addition to FeNTA; and Group D rats were untreated controls. Glucose tolerance tests were performed at the beginning, 5th week, and 10th week. At the 10th week, the levels of plasma glucose at 2 hours after glucose load in groups A and C were higher than those in groups B and D (P = 0.043); the granules of insulin in β-cells of group A were decreased obviously, the area of islets of group A was smaller than those of other groups (P = 0.000). Iron overload might influence glycometabolism. The β-cells’ capability to secrete insulin was obviously decreased.[31] A longitudinal study in thalassemic children showed progressive decrease in the beta cell function with age.[32]
However, three of our adolescents with BTM showed insulin resistance state (HOMA and QUIKI), one of them had DM, one had IGT during CGM and the third did not have any glycemic abnormality during CGM or OGTT. Other authors suggested that insulin resistance precedes the glycemic abnormalities. This state of insulin resistance may overwork the beta cell function and in addition to iron toxicity, lead to IGT and DM later.[14,15,16]
Three of our patients with BTM and increased insulin resistance had hepatomegaly, with ultrasonographic evidence of iron overload and high serum ferritin concentrations denoting a chronic form of liver siderosis. During a study of pathogenetic mechanisms in the hepatic cirrhosis of thalassemia major, 16 liver biopsies were examined by electron microscopy. Ultrastructural studies of liver cells during iron overload have shown electron-dense iron as lysosomal hemosiderin, and as lysosomal and cell-sap ferritin. Ferritin molecules have been shown within lysosomes in a specific pattern in relationship with regularly arranged lamellae. Increased insulin resistance is frequently associated with chronic liver disease and is a pathophysiological feature of hepatogenous diabetes.[33,34]
CGM discloses early and frequent hyperglycemia in non-diabetic patients with BTM. Intensive glucose monitoring during the late childhood and adolescence appears to be an efficient method in screening for hyperglycemia and could be a valuable guide to initiating insulin therapy and to further investigate outcomes in BTM.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Lassman MN, Genel M, Wise JK, Hendler R, Felig P. Carbohydrate homeostasis and pancreatic islet cell function in thalassemia. Ann Intern Med. 1974;80:65–9. doi: 10.7326/0003-4819-80-1-65. [DOI] [PubMed] [Google Scholar]
- 2.Saudek CD, Hemm RM, Peterson CM. Abnormal glucose tolerance in beta-thalassemia major. Metabolism. 1977;26:43–52. doi: 10.1016/0026-0495(77)90126-3. [DOI] [PubMed] [Google Scholar]
- 3.Gabutti V, Piga A. Results of long-term iron-chelating therapy. ActaHaematol. 1996;95:26–36. doi: 10.1159/000203853. [DOI] [PubMed] [Google Scholar]
- 4.Arrigo T, Crisafulli G, Meo A, Sturiale M, Lombardo F, Miceli M, et al. Glucose tolerance, insulin secretion and peripheral sensitivity in thalassaemia major. J PediatrEndocrinol Metab. 1998;11:863–6. [PubMed] [Google Scholar]
- 5.Hershko C, Link G, Cabantchik I. Pathophysiology of iron overload. Ann NY AcadSci. 1998;850:191–201. doi: 10.1111/j.1749-6632.1998.tb10475.x. [DOI] [PubMed] [Google Scholar]
- 6.Kattamis C, Lazaropoulou C, Delaporta P, Apostolakou F, Kattamis A, Papassotiriou I. Disturbances of biomarkers of iron and oxidant-antioxidant homeostasis in patients with β-thalassemia intermedia. Pediatr Endocrinol Rev. 2011;8:256–62. [PubMed] [Google Scholar]
- 7.Scott MD. H2O2 injury in β thalassemic erythrocytes: Protective role of catalase and the preoxidant effects of GSH. Free Radical Biol Med. 2006;40:1264–72. doi: 10.1016/j.freeradbiomed.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 8.De Sanctis V, Zurlo MG, Senesi E, Boffa C, Cavallo L, Di Gregorio F. Insulin dependent diabetes in thalassemia. Arch Dis Child. 1988;63:58–62. doi: 10.1136/adc.63.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suvarna J, Ingle H, Deshmukh CT. Insulin resistance and beta cell function in chronically transfused patients of thalassemia major. Indian Pediatr. 2006;43:393–400. [PubMed] [Google Scholar]
- 10.Cario H, Holl RW, Debatin KM, Kohne E. Insulin sensitivity and beta-cell secretion in thalassaemia major with secondary haemochromatosis: Assessment by oral glucose tolerance test. Eur J Pediatr. 2003;162:139–46. doi: 10.1007/s00431-002-1121-7. [DOI] [PubMed] [Google Scholar]
- 11.Chern JP, Lin KH, Lu MY, Lin DT, Lin KS, Chen JD, et al. Abnormal glucose tolerance in transfusion-dependent beta-thalassemic patients. Diabetes Care. 2001;24:850–4. doi: 10.2337/diacare.24.5.850. [DOI] [PubMed] [Google Scholar]
- 12.Dmochowski K, Finegood DT, Francombe W, Tyler B, Zinman B. Factors determining glucose tolerance in patients with thalassemia major. J Clin Endocrinol Metab. 1993;77:478–83. doi: 10.1210/jcem.77.2.8345055. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg ED. Iron loading in humans: A risk factor for enhanced morbidity and mortality. J Nut Envir Med. 2007;16:43–51. [Google Scholar]
- 14.Thennadil SN, Rennert JL, Wenzel BJ, Hazen KH, Ruchti TL, Block MB. Comparison of glucose concentration in interstitial fluid, and capillary and venous blood during rapid changes in blood glucose level. Diabetes Technol Ther. 2001;3:357–65. doi: 10.1089/15209150152607132. [DOI] [PubMed] [Google Scholar]
- 15.Khammar A, Stremler N, Dubus JC, Gross G, Sarles J, Reynaud R. [Value of continuous glucose monitoring in screening for diabetes in cystic fibrosis. Arch Pediatr. 2009;16:1540–6. doi: 10.1016/j.arcped.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Franzese A, Valerio G, Buono P, Spagnuolo MI, Sepe A, Mozzillo E, et al. Continuous glucose monitoring system in the screening of early glucose derangements in children and adolescents with cystic fibrosis. J Pediatr Endocrinol Metab. 2008;21:109–16. doi: 10.1515/jpem.2008.21.2.109. [DOI] [PubMed] [Google Scholar]
- 17.Rimondi F, Banin P, Gamberini MR, De Sanctis V. The continuous glucose monitoring system (CGMS) in patients with beta-thalassemia major and impaired glucose homeostasis: Preliminary results. Pediatr Endocrinol Rev. 2008;6:190–2. [PubMed] [Google Scholar]
- 18.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song Y, Manson JE, Tinker L, Howard BV, Kuller LH, Nathan L, et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: The women's health initiative observational study. Diabetes Care. 2007;30:1747–52. doi: 10.2337/dc07-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: A simple, accurate method of assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 21.Hermans MP, Levy JC, Morris RJ, Turner RC. Comparison of tests of ß-cell function across a range of glucose tolerance from normal to diabetes. Diabetes. 1999;48:1779–86. doi: 10.2337/diabetes.48.9.1779. [DOI] [PubMed] [Google Scholar]
- 22.Gerli GC, Beretta L, Bianchi M, Pellegatta A, Agostoni A. Erythrocyte superoxide dismutase, catalase and glutathione peroxidase activities in beta-thalassemia (major and minor) Scand J Hamatol. 1980;25:87–92. doi: 10.1111/j.1600-0609.1981.tb01370.x. [DOI] [PubMed] [Google Scholar]
- 23.Chakraborty D, Bhattachayya M. Antioxidant defense status of red blood cells of patients with β-thalassemiaand Eβ thalassemia. Clin Chem Acta. 2001;305:123–9. doi: 10.1016/s0009-8981(00)00428-9. [DOI] [PubMed] [Google Scholar]
- 24.Kassab-Chekir A, Laradi S, Ferchichi S, Hajkhelil A, Feki M, Amri F, et al. Oxidant antioxidant status and metabolic data in patients with beta thalassemia. Clin Chem Acta. 2003;338:79–86. doi: 10.1016/j.cccn.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Walter PB, Fung EB, Kililea DW, Jiang Q, Huder M, Madden J, et al. Oxidative stress and inflammation in iron-overload patients with β-thalassemia or sickle cell disease. Br J Haematol. 2006;135:254–63. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radermecker RP, Sultan A, Piot C, Remy AS, Avignon A, Renard E. Continuous glucose monitoring as a tool to identify hyperglycaemia in non-diabetic patients with acute coronary syndromes. Diabet Med. 2009;26:167–70. doi: 10.1111/j.1464-5491.2008.02643.x. [DOI] [PubMed] [Google Scholar]
- 27.Raccah D, Sulmont V, Reznik Y, Guerci B, Renard E, Hanaire H, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: The RealTrend study. Diabetes Care. 2009;32:2245–50. doi: 10.2337/dc09-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salardi S, Zucchini S, Santoni R, Ragni L, Gualandi S, Cicognani A, et al. The glucose area under the profiles obtained with continuous glucose monitoring system relationships with HbA (lc) in pediatric type 1 diabetic patients. Diabetes Care. 2002;25:1840–4. doi: 10.2337/diacare.25.10.1840. [DOI] [PubMed] [Google Scholar]
- 29.Tavazzi D, Duca L, Graziadei G, Comino A, Fiorelli G, Capellini MD. Membrane-bound iron contributes to oxidative damage of β-thalassemia intermedia. Br J Haematol. 2000;112:48–50. doi: 10.1046/j.1365-2141.2001.02482.x. [DOI] [PubMed] [Google Scholar]
- 30.Mahachoklertwattana P, Yimsumruay T, Poomthavorn P, Chuansumrit A, Khlairit P. Acute effects of blood transfusion on growth hormone and insulin-like growth factor-1 levels in children with thalassemia. Horm Res Paediatr. 2011;75:240–5. doi: 10.1159/000321189. [DOI] [PubMed] [Google Scholar]
- 31.Chen M, Feng T, Yang B, Tian H. [Effect of iron overload on function of pancreatic β cells in rats. Sheng Wu Yi Xue Gong Cheng XueZaZhi. 2009;26:1088–93. [PubMed] [Google Scholar]
- 32.Soliman AT, el Banna N, alSalmi I, Asfour M. Insulin and glucagon responses toprovocation with glucose and arginine in prepubertal children with thalassemia major before and after long-term blood transfusion. J Trop Pediatr. 1996;42:291–6. doi: 10.1093/tropej/42.5.291. [DOI] [PubMed] [Google Scholar]
- 33.Porter R, Fitzsimons DW. The liver in thalassaemia major: Ultra-structural observations. In: Iancu TC, Neustein HB, Landing BH, editors. Ciba Foundation Symposium 51-Iron Metabolism. Chapter 14. Ciba foundation. California, USA: Wiley Publication; 2008. [Google Scholar]
- 34.Labropoulou-Karatza C, Goritsas C, Fragopanagou H, Repandi M, Matsouka P, Alexandrides T. High prevalence of diabetes mellitus among adult β-thalassaemic patients with chronic hepatitis C. Eur J Gastroenterol Hepatol. 1999;11:1033–6. doi: 10.1097/00042737-199909000-00014. [DOI] [PubMed] [Google Scholar]


