Abstract
This is a large cohort analysis in severely burned pediatric children to determine whether C-reactive protein (CRP) can be used as a predictor for severe infection or sepsis. Nine-hundred eighteen pediatric burn patients were enrolled in this study. CRP values were measured throughout acute hospitalization and for up to 6 months postburn. Demographic data, incidence of infection, surgical interventions and other relevant clinical information was compiled from medical records. We performed an extensive literature search to identify models that other groups have developed to determine the effects of CRP levels postburn to assess the value of these parameters as predictors of sepsis or severe infection. Statistical analysis was performed using ANOVA and regression analysis where appropriate. Three-hundred fifteen female and 603 male pediatric patients were enrolled in this study. Average total body surface area (TBSA) burn was 45±23%, with full thickness burn over 32±27% TBSA, and patients were 7±6 years old. CRP values significantly correlated with burn size, survival and gender. Significantly higher levels of CRP were found in large burns, in non-survivors, and in females, p<0.05. Using various described models to determine whether CRP levels change before and after an event can predict sepsis or severe infection, we found that CRP cannot predict severe infection or sepsis. Although CRP is a marker of the inflammatory response postburn, CRP fails to predict infection or sepsis in severely burn patients.
Keywords: Burn, infection, sepsis, mortality, CRP, pediatric, biomarker
Introduction
Severe burn injury leads to hypermetabolic and catabolic responses driven by the inflammatory response [1]. Hypermetabolism is accompanied by skeletal muscle protein catabolism, adipose tissue lipolysis, increased energy expenditure and other physiologic alterations, which can lead to compromised organ function [1]. These alterations can lead to immune dysfunction, increased susceptibility to infection and sepsis. Currently, sepsis is one of the major causes of death in burn patients. Recently, we found that 30-50% of the deaths are due to sepsis [2], therefore, it would be ideal to have a biomarker to predict the risk of developing sepsis or severe infection. Early identification of patients with the greatest risk would result in more aggressive clinical interventions at an early stage.
Plasma C-reactive protein (CRP) is a biomarker commonly used to assess the inflammatory response, and increases are associated with increased inflammation, infection, or sepsis [3]. For example, CRP plasma concentrations exceeding 8 mg/dl can distinguish the inflammatory response due to infection from other types of inflammation [4], indicating that CRP levels can be predictive of infection. Neely, et al. [5] showed that a rise in CRP serum levels significantly predicted the incidence of major infection 2.3 days before sepsis occurred. In a follow-up study [6], the same authors evaluated CRP and PCT, and found that PCT did not correlate and predict sepsis; however, a rise in CRP of 1.5 mg/dl indicated sepsis and a rise of 3 mg/dl indicated severe bacterial or fungal infection [6]. A study by Lavrentieva, et al. [3] showed that CRP did not correlate with sepsis incidence. Controversy exists regarding conflicting results from studies that investigated the effect of CRP as the marker of severe infections after a burn [4,7,8]. A rise in CRP greater than 10 mg/dl was associated with life-threatening systemic infection. The authors concluded that an increase of 5 mg/dl has the best indication of sepsis in the pediatric burn population.
Given the controversy, the question whether CRP can serve as a predictor for severe infection or sepsis remains unanswered. Therefore, our objective was to investigate in a large clinical trial whether CRP can be used as a predictor for severe infections or sepsis in severely burned children.
Materials and methods
Nine-hundred eighteen pediatric patients were enrolled in this study between 1998 and 2010. Patients underwent standard burn treatment. They were resuscitated according to the Galveston formula with 5000 ml/m² total body surface area (TBSA) burned + 2000 ml/m² TBSA lactated Ringer’s solution given in increments over the first 24 hours. Within 48 hours of admission, all patients underwent total burn wound excision and the wounds were covered with autograft. Any remaining open areas were covered with homograft. After the first operative procedure, patients were taken back to the operation theater when donor sites were healed. This procedure was repeated until all open wound areas were covered with autologous skin. The study was approved by the Institutional Review Board of the University of Texas Medical Branch and informed consent was obtained from patients, parents, or legal guardians prior to enrollment.
All patients underwent standard of care, including prophylactic antibiotic treatment (Vancomycin for Gram positive and Zosyn for Gram negative organisms).
All patients underwent the same nutritional treatment according to a standardized protocol. The intake was calculated as 1500 kcal/m² body surface + 1500 kcal/m² area burn as previously published [1]. The nutritional route of choice in our patient population was enteral nutrition via a duodenal (Dobhoff) or nasogastric tube. Parenteral nutrition was only given in rare instances if the patient could not tolerate tube feeds.
In our institution, we prospectively collect blood samples from all the patients involved in a research study every Monday and Thursday, before every operation and with each study, between 5 and 8 AM. Biopsies for quantitative cultures are collected every Monday, Wednesday, and Friday and the organisms present are identified and quantified. The serum/plasma samples used in this study were prospectively collected and stored at -80°C until analyzed.
Relevant clinical information, such as demographic data, operation dates, and infection episodes was abstracted from medical records. Severe infection is defined as the detection of greater than 105 colony forming units of bacteria and/or fungus per gram tissue.
CRP values were measured during the acute hospital stay and up to 6 months after the initial injury, using a nephelometric technique on a BNII plasma protein analyzer (Dade Behring/Siemens Health Care, Wilmington, DE). Briefly, serum or plasma collected from these patients at different time points was incubated with antisera containing specific human CRP antibodies and the formation of agglutinates was monitored for 6 minutes. The rate of formation of agglutinates was used to determine the concentration of CRP protein from a standard curve. To evaluate whether CRP can predict infection and sepsis postburn, we used previously published models. Statistical analysis was performed using regression and ANOVA where appropriate.
Results
Nine-hundred eighteen patients were enrolled in this study. The total body surface area burned (mean ± standard deviation) was 45±23%, with full thickness burn over 32±27% of TBSA, and patients were 7±6 years old.
We first sought to validate CRP values and described models by correlating CRP levels with burn size, gender, surgical intervention and mortality.
CRP values correlate with the burn size (Figure 1). Massive burns (>80% TBSA) had the highest CRP values both acutely and up to 6 months postburn. The elevation in CRP was significant for a longer period of time in the >80% burn group than in the smaller burn sizes, beginning 8-10 days following burn injury, p<0.05.
Figure 1.
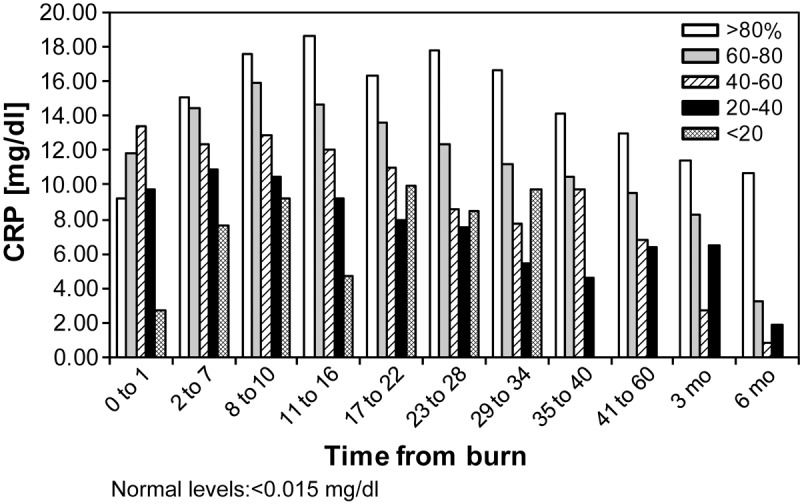
C-reactive protein values over time by burn size: C-reactive protein values correlate with burn size. Massive burns have significantly and persistently higher C-reactive protein levels beginning 2-7 days postburn, p<0.05.
Next, we determined if CRP values are affected by gender (Figure 2). We found that CRP levels for females are higher than in males over time and statistical significance was reached at one time point at 11-16 days postburn, p<0.05.
Figure 2.
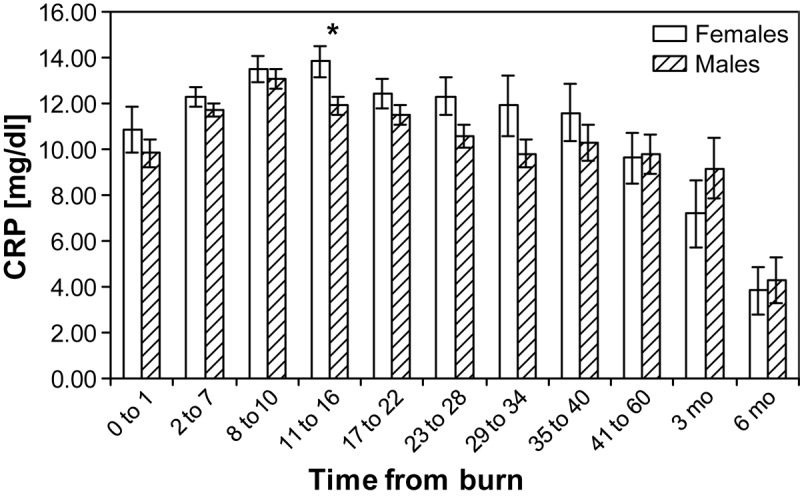
Gender differences in C-reactive protein values over time: females have higher C-reactive protein levels over time, but significance is reached at 11-16 days postburn, p<0.05.
In a subgroup of patients who never had a severe infection, we determined the effect of surgical intervention on the CRP levels. CRP values the day before surgery and up to 7 days afterward were compared. Surgery increased CRP values and the levels slowly decreased over time, but not significantly (Figure 3). For this group of patients we found that the average CRP values were about 10 mg/dl, but the individual responses were highly variable.
Figure 3.
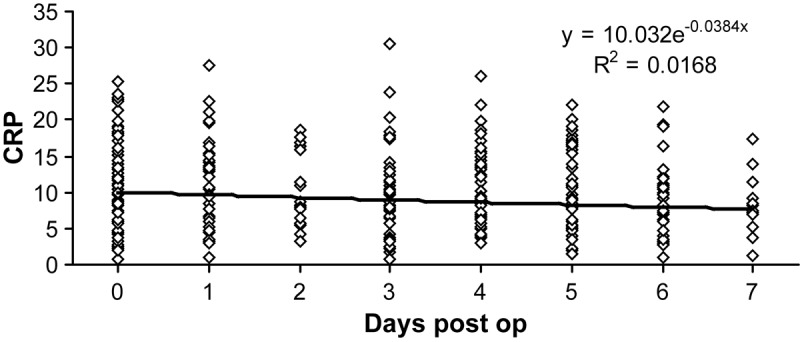
C-reactive protein values before and after surgery in patients who never had a severe infection: surgery causes initial increase in C-reactive protein values, which slowly decrease over time.
We did find a significant difference in the CRP levels over time between survivors and non-survivors starting at 2 days postburn (Figure 4). Survivors had lower CRP levels at every time point except 0 to 1 (p<0.05). Regression analysis revealed a strong correlation between CRP values and survival. We found that if a patient had CRP values greater than 20 mg/dl at day 11-16 after burn injury, there was a 50% chance the patient will die.
Figure 4.
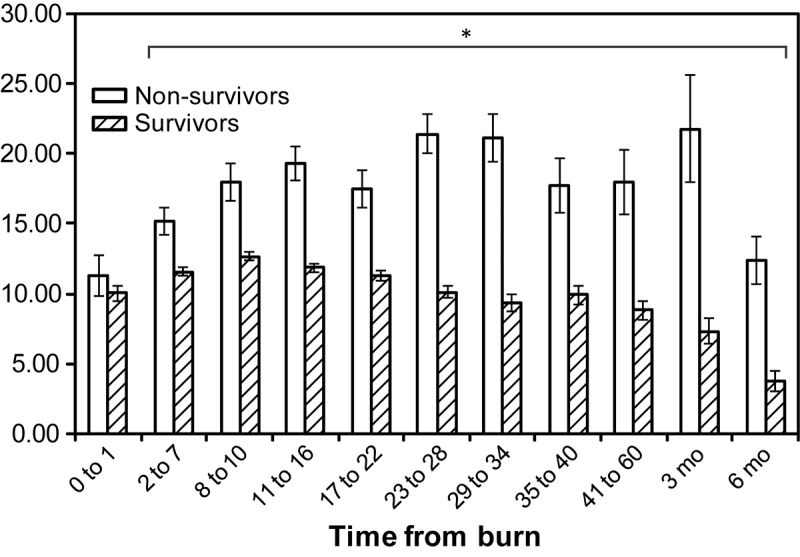
Comparison of C-reactive protein values in survivors and non-survivors: non-survivors have significantly higher C-reactive protein values (p<0.05) than survivors, beginning at day 2 after burn.
We sought to find out if CRP values can be used as a predictor for sepsis or severe infection. The CRP values before and after a documented infection were compiled. The percent of patients with documented infections was compared with patients who never had infection. We set the threshold change at discrete values between 0 and 20 (in increments of 1), as previously described [3]. We found that none of the thresholds could predict infection or sepsis. When we compared CRP changes of never infected patients to patients with infection or septic complications, even a change of 5, 10, 15 or 20 mg/dl was not indicative of infection. There was no apparent relationship between the CRP values and when a severe infection occurs (Figure 5).
Figure 5.
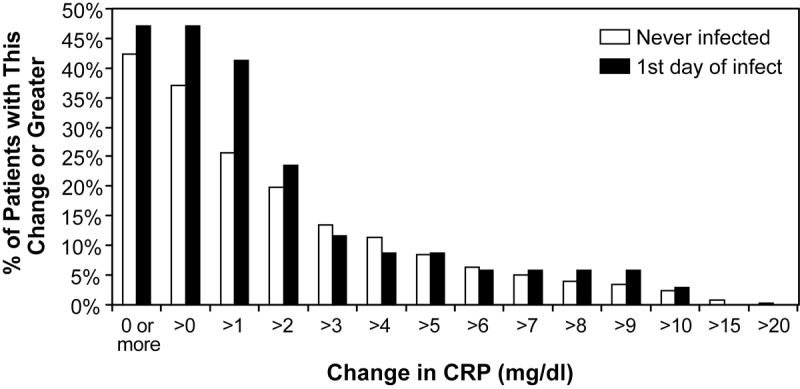
Change in C-reactive protein values before and after a documented infection of >105 cfu/gram tissue: discrete increments in the change in C-reactive protein levels before and after infection failed to predict infectious episodes or sepsis. There are no significant differences between groups, thus C-reactive protein has no predictive value.
Discussion
Increasing bacterial antibiotic resistance and fungal invasions represent the major contributors to morbidity and mortality in severely burned children [8]. We believe the early identification of major infections or sepsis and initiation of focused treatment may improve hospital outcome of this patient population. In clinical practice at our institution, when signs of infection are present, very early different antibiotic strategies are initiated. Antibiotic treatment is adjusted during the hospital course in order to prevent the occurrence of sepsis. A special focus on this patient throughout the hospital course by the burn attending is warranted.
A useful tool to identify patients at risk for major infection or sepsis would be a serum biomarker that can be determined in an easy fashion all over the world and is easily available. CRP, as a general marker for the inflammatory response, is an acute phase protein synthesized and released by the liver. CRP is dramatically increased postburn, but decreases over the hospital course. There is conflicting data in the literature whether CRP can be used as a biomarker to predict major infection or sepsis. The study by Neely, et al. [5,6] showed the change in CRP of 1.5, 3 or 10 mg/dl can predict sepsis and major infection. However, studies by Lavrentiva, et al. [3] showed that CRP cannot be used as a predictor for sepsis. These were all small clinical studies therefore we conducted this large cohort study to determine whether CRP is a biomarker that can be used for the prediction of infection or sepsis. We found that CRP cannot predict severe infection or sepsis; however, we found that CRP correlates significantly with burn size. Larger burns had higher CRP values. Furthermore, we found that females have higher CRP levels than males [9]. We also showed that survivors have lower CRP levels during the hospital course beginning on day two compared to non-survivors. Our data implies that CRP is not a predictor of infection, however, is a good marker that reflects the homeostasis of the burned patient [10,11].
We did not investigate the predictive value of PCT therefore we cannot make any statements about this parameter. In addition, we previously conducted a study in which we found that the cytokine expression profile at admit can identify patients at risk to develop sepsis throughout hospital course and to die from the septic complication [9]. Based on this data, we initiated a prospective clinical trial in which we obtained serum/plasma from our pediatric burn patients at admit and analyzed the cytokine expression profile to validate our model in a large patient population. This would be a very similar applicable approach as CRP because cytokines can be analyzed throughout the world.
In the present large cohort study, we determined that CRP has significant correlations to burn size and mortality. We also found that the changes of CRP values do not reflect and do not predict the incidence of major infection or sepsis. Therefore, CRP can be used as a biomarker for homeostasis of a burned patient, but does not serve as predictor for major infections and/or sepsis.
Conclusions
In summary, we found that CRP measurements are a parameter to be used for modeling response postburn, but they cannot predict severe infection or sepsis. Although CRP by itself does not have a predictive value for sepsis or severe infection, but in conjunction with other tools, such as the cytokine expression profile, hormones and other proteins may be more helpful for the overall evaluation of severely burned patients.
Acknowledgements
The authors thank David L. Chinkes, PhD, for his constructive comments and contribution on this manuscript. This study was supported by grants from the Shriners Hospitals for Children (84080, 71008, 8660, and 79135) National Institutes of Health (R01-GM56687, T32-GM008256, P50-GM60338, R01-GM087285-01), National Institute on Disability and Rehabilitation Research (H133A020102), Canadian Institutes of Health Research (123336), and Physician’s Services Incorporated Foundation: Health Research Grant Program. CCF is an ITS career development scholar supported, in part, by NIH KL2RR029875 and NIH UL1RR029876.
Disclosure of conflict of interest
Authors declare no conflicts of interest.
References
- 1.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, Branski LK, Gauglitz GG, Mlcak RP, Herndon DN. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams FN, Herndon DN, Hawkins HK, Lee JO, Cox RA, Kulp GA, Finnerty CC, Chinkes DL, Jeschke MG. The leading causes of death after burn injury in a single pediatric burn center. Crit Care. 2009;13:R183. doi: 10.1186/cc8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavrentieva A, Kontakiotis T, Lazaridis L, Tsotsolis N, Koumis J, Kyriazis G, Bitzani M. Inflammatory markers in patients with severe burn injury. What is the best indicator of sepsis? Burns. 2007;33:189–194. doi: 10.1016/j.burns.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Barati M, Alinejad F, Bahar MA, Tabrisi MS, Shamshiri AR, Bodouhi NO, Karimi H. Comparison of WBC, ESR, CRP and PCT serum levels in septic and non-septic burn cases. Burns. 2008;34:770–774. doi: 10.1016/j.burns.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Neely AN, Smith WL, Warden GD. Efficacy of a rise in C-reactive protein serum levels as an early indicator of sepsis in burned children. J Burn Care Rehabil. 1998;19:102–105. doi: 10.1097/00004630-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Neely AN, Fowler LA, Kagan RJ, Warden GD. Procalcitonin in pediatric burn patients: an early indicator of sepsis? J Burn Care Rehabil. 2004;25:76–80. doi: 10.1097/01.BCR.0000105095.94766.89. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Xu W, Shi J. [The changes and clinical significance of serum CRP, C3, Tf, and PA in the early postburn stage] . Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi. 1997;13:361–364. [PubMed] [Google Scholar]
- 8.Sachse C, Machens HG, Felmerer G, Berger A, Henkel E. Procalcitonin as a marker for the early diagnosis of severe infection after thermal injury. J Burn Care Rehabil. 1999;20:354–360. doi: 10.1097/00004630-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Finnerty CC, Herndon DN, Chinkes DL, Jeschke MG. Serum cytokine differences in severely burned children with and without sepsis. Shock. 2007 Jan;27:4–9. doi: 10.1097/01.shk.0000235138.20775.36. [DOI] [PubMed] [Google Scholar]
- 10.Finnerty CC, Ju H, Spratt H, Victor S, Jeschke MG, Hegde S, Bhavnani SK, Luxon BA, Brasier AR, Herndon DN. Proteomics improves the prediction of burns mortality: results from regression spline modeling. Clin Transl Sci. 2012;5:243–9. doi: 10.1111/j.1752-8062.2012.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeschke MG, Gauglitz GG, Finnerty CC, Kraft R, Mlcak RP, Herndon DN. Survivors Versus Nonsurvivors Postburn: Differences in Inflammatory and Hypermetabolic Trajectories. Ann Surg. 2013 doi: 10.1097/SLA.0b013e31828dfbf1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


