Abstract
Data on calcification rate of coral and crustose coralline algae were used to test the proton flux model of calcification. There was a significant correlation between calcification (G) and the ratio of dissolved inorganic carbon (DIC) to proton concentration ([DIC] : [H+] ratio). The ratio is tightly correlated with [CO32−] and with aragonite saturation state (Ωa). An argument is presented that correlation does not prove cause and effect, and that Ωa and [CO32−] have no basic physiological meaning on coral reefs other than a correlation with [DIC] : [H+] ratio, which is the driver of G.
Keywords: coral, crustose coralline algae, calcification, proton flux, aragonite saturation state, ocean acidification
Most previous reports conclude that CO32− is the primary substrate for calcification in corals and crustose coralline algae (CCA), and assume that under increasing conditions of ocean acidification (OA) the effect of declining [CO32−] is the primary factor responsible for the observed decline in calcification rate (G). Nearly every scientific report concerning the effects of OA on coral reefs describes changes in G as a function of [CO32−] or its surrogate aragonite saturation state (Ωa). The recent report by Comeau et al. [1] challenges this paradigm by demonstrating that both HCO3− and CO32− are involved in calcification of the reef coral Porites rus and the crustose coralline algae (CCA) Hydrolithon onkodes. The effect of changes in aqueous CO2, which is the third component of total dissolved inorganic carbon (DIC), was not tested in these experiments. Their data analysis can be taken a step further by investigating the possible importance of seawater hydrogen ion concentration [H+] and [DIC] on the calcification process. These data were derived from incubation experiments, and thus describe net material flux between the calcifying organism and the water column. These data do not tell us what is actually happening within the ‘black box’ of the calcifier. Correlation of G with bulk seawater [CO32−] does not prove that [CO32−] controls G. In all their growth experiments, [HCO3−] was by far the highest potential source of inorganic carbon. Without showing active transport and use of CO32− from the seawater pool, the relationship of [CO32−] to G has no clear physiological meaning. The efflux of H+ from the calcifying organism influences speciation of bulk water [CO32−]. Also, net calcification rate as reported in these experiments does not disentangle the relative contributions of gross calcification and dissolution rates [2]. Measurement of gross calcification is difficult, but might offer an explanation for differences in calcification response of various taxa to OA [3]. Advanced physiological techniques [4] are presently providing insights into processes within coral tissue and in the calcifying space between the skeleton and coral tissues. The various forms of inorganic carbon in seawater rapidly interconvert. DIC is the sum of CO32−, HCO3− and aqueous CO2 concentration. The ratio [DIC] divided by [H+] as presented by Jokiel [5,6] can be viewed as the relative availability of reactant (inorganic carbon) in relation to concentration of inhibitory calcification waste product (protons) in the bulk water surrounding the calcifier. Analysis of existing data suggests that a mechanism influencing the net calcification rate is diffusion limitation of excess H+ from the coral into the water column. The resulting ‘proton flux model’ states that the lowered calcification rate observed in corals under increasing conditions of OA can be attributed to higher [H+] in the seawater, with consequent decrease in the efflux of waste H+ from calcifiers through the boundary layer.
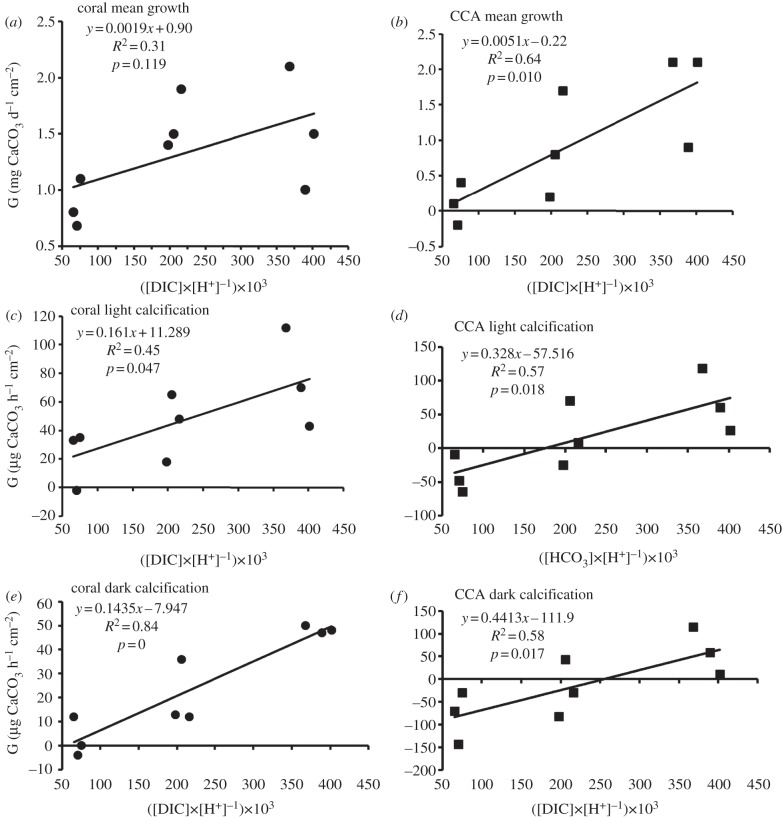
In order to test these hypotheses, the data from Comeau et al. [1] were used to calculate [DIC], [H+] and the ratio of [DIC] to [H+] (tables 1 and 2). Calcification rate (G) was plotted against the [DIC] : [H+] ratio for each of their six incubation experiments (figure 1). Coral and CCA hourly light and dark calcification rates (figure 1c–f) showed a significant relationship to the [DIC] : [H+] ratio. Mean growth over a two-week period showed a significant trend for CCA (figure 1b), but this trend was not significant for mean coral growth (figure 1a). The same pattern was found by Comeau et al. [1] in relation to [CO32−] and [HCO3−]. The results are consistent with the concept that calcification (G) is controlled by the [DIC] : [H+] ratio. The relatively weak correlation during rapid coral calcification in the light (figure 1c) outweighed the high correlation for the coral in the dark (figure 1e) to produce a non-significant relationship with mean coral growth measured over the integrated two-week period (figure 1a). These data emphasize the important role played by HCO3− and H+ in daylight when corals are undergoing rapid photosynthesis as well as rapid calcification [6].
Table 1.
Data from Comeau et al. [1] used in the calculations.
| CO32− | HCO3− | [HCO3−] | [CO32−] | [CO2] | pCO2 | AT | |||
|---|---|---|---|---|---|---|---|---|---|
| conditions | conditions | (μmol kg−1) | (μmol kg−1) | (μmol kg−1) | (μatm) |
Ωa | T (°C) | (μmol kg−1) |
pHT |
| low CO32− | high HCO3− | 2243 | 75 | 56 | 2108 | 1.2 | 28 | 2424 | 7.44 |
| med HCO3− | 1695 | 85 | 27 | 1047 | 1.4 | 28 | 1910 | 7.62 | |
| low HCO3− | 1025 | 82 | 13 | 503 | 1.3 | 28 | 1258 | 7.8 | |
| medium CO32− | high HCO3− | 2287 | 223 | 19 | 733 | 3.6 | 28 | 2814 | 7.91 |
| med HCO3− | 1731 | 227 | 11 | 401 | 3.7 | 28 | 2289 | 8.04 | |
| low HCO3− | 1069 | 203 | 5 | 188 | 3.3 | 28 | 1612 | 8.19 | |
| high CO32− | high HCO3− | 2334 | 384 | 11 | 435 | 6.2 | 28 | 3224 | 8.13 |
| med HCO3− | 1802 | 381 | 7 | 257 | 6.1 | 27 | 2712 | 8.25 | |
| low HCO3− | 1195 | 365 | 3 | 120 | 5.8 | 28 | 2114 | 8.41 |
Table 2.
Calcification data from Comeau et al. [1] and calculated values for the [DIC] : [H+] ratio.
| conditions | [DIC] | [H+] | [DIC] : [H+] ratio | mean coral G | mean CCA G | coral G (light) | coral G (dark) | CCA G (light) | CCA G (dark) |
|---|---|---|---|---|---|---|---|---|---|
| (μmol kg−1) | (nmol kg−1) | ([DIC] × [H+]−1) × 103 | (mg CaCO3 d−1 cm−2) | (mg CaCO3 d−1 cm−2) | (μg CaCO3 h−1 cm−2) | (μg CaCO3 h−1 cm−2) | (μg CaCO3 h−1 cm−2) | (μg CaCO3 h−1 cm−2) | |
| low CO32− | 2374 | 36.31 | 65 | 0.8 | 0.1 | 33 | 12 | −9 | −70 |
| 1807 | 23.99 | 75 | 1.1 | 0.4 | 35 | 0 | −65 | −30 | |
| 1120 | 15.85 | 71 | 0.68 | −0.2 | −2 | −4 | −48 | −143 | |
| medium CO32− | 2529 | 12.30 | 206 | 1.5 | 0.8 | 65 | 36 | 70 | 43 |
| 1969 | 9.12 | 216 | 1.9 | 1.7 | 48 | 12 | 8 | −30 | |
| 1277 | 6.46 | 198 | 1.4 | 0.2 | 18 | 13 | −25 | −83 | |
| high CO32− | 2729 | 7.41 | 368 | 2.1 | 2.1 | 112 | 50 | 118 | 115 |
| 2190 | 5.62 | 389 | 1 | 0.9 | 70 | 47 | 60 | 58 | |
| 1563 | 3.89 | 402 | 1.5 | 2.1 | 43 | 48 | 26 | 11 |
Figure 1.
Plots of [DIC] to [H+] ratio versus calcification rate (G) for (a,c,e) the coral Porites rus and (b,d,f) the crustose coralline algae (CCA) Hydrolithon onkodes using data from Comeau et al. [1]. (a,b) Net calcification measured by buoyant weight over two-week incubations; (c,d) net hourly calcification in the light measured by the alkalinity anomaly technique; (e,f) net hourly calcification in the dark measured by the alkalinity anomaly technique.
Plotting the data for the [DIC] : [H+] ratio against either CO32− or Ωa provides another useful insight (figure 2). It is apparent that [CO32−] is correlated with the ratio of [DIC] to [H+], which explains its correlation with G. Likewise, Ωa is essentially a function of [CO32−], and therefore will also correlate with G. The physical chemist's concept of Ωa has been essential to our understanding of global changes in the carbonate chemistry of the sea. The vertical and horizontal distribution of Ωa in the past, present and future will continue to be the subject of extensive research. Originally, the correlation between Ωa and G along with the expansion of OA research led biologists away from the classic physiological concepts [7], with acceptance of a physical chemistry concept that has no intrinsic relationship to coral metabolites. Biologists involved in OA studies have widely used Ωa as the independent variable related to coral calcification, based on this empirical correlation. The use of the [DIC] : [H+] ratio is based on measurement of the most important materials involved in calcification and thus is relevant to understanding basic physiology. Therefore, the [DIC] : [H+] ratio is conceptually preferable to the physical chemistry concept of Ωa in describing G. This is especially important if we wish to draw conclusions about G from cases where Ωa is decoupled from [H+] as occurs in the palaeo-ocean over timescales greater than 10 000 years [8]. The practice of plotting G versus Ωa will probably continue because it is convenient to relate a primary biological response of corals (G) to the primary physical chemistry measurement describing ocean chemistry (Ωa), especially in modelling the future changes on coral reefs. The nature of seawater carbonate chemistry is that some of the parameters are strongly correlated with each other and some correlate with calcification rate. However, correlation does not prove cause and effect, and we must keep in mind the fact that parameters such as Ωa and [CO32−] have no basic physiological meaning on coral reefs other than a correlation with [DIC] : [H+] ratio, which is the driver of G. Certain tropical calcifying species such as the coral Pocillopora damicornis and calcified alga Halimeda macroloba appear to be insensitive to OA, whereas others show dramatic changes in calcification rate [9]. With increasing OA, the decrease in [CO32−] is accompanied by a large increase in [HCO3−] and [H+], so organisms with effective morphological and metabolic means of dissipating H+ while increasing uptake of HCO3− [6] can maintain high rates of G as the [DIC] : [H+] ratio of seawater decreases.
Figure 2.
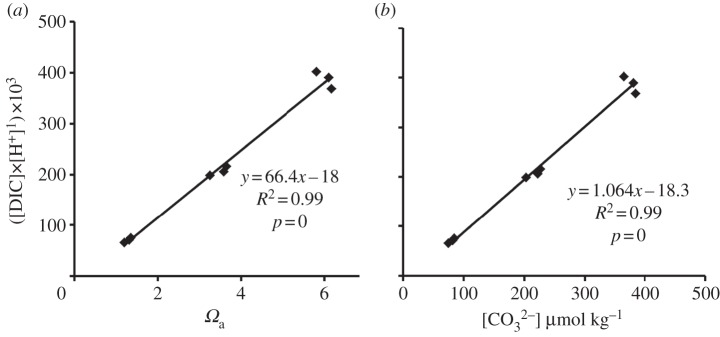
Relationship between (a) Ωa and [DIC] : [H+], and (b) [CO32−] and [DIC] : [H+], using data from Comeau et al. [1].
Acknowledgements
I thank the Pacific Climate Change Cooperative for their support in this work. S. Comeau and two anonymous reviewers provided valuable comments.
Footnotes
The accompanying reply can be viewed at http://dx.doi.org/10.1098/rspb.2013.1153.
References
- 1.Comeau S, Carpenter RC, Edmunds PJ. 2013. Coral reef calcifiers buffer their response to ocean acidification using both bicarbonate and carbonate. Proc. R. Soc. B 280, 20122374. 10.1098/rspb.2012.2374 (doi:10.1098/rspb.2012.2374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ries J. 2011. Acid ocean cover-up. Nat. Clim. Change 1, 294–295 10.1038/nclimate1204 (doi:10.1038/nclimate1204) [DOI] [Google Scholar]
- 3.Rodolfo-Metalpa R, et al. 2011. Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat. Clim. Change 1, 308–312 10.1038/nclimate1200 (doi:10.1038/nclimate1200) [DOI] [Google Scholar]
- 4.Venn A, Tambutté E, Holcomb M, Laurent J, Allemand D, Tambutté S. 2013. Impact of seawater acidification on pH at the tissue-skeleton interface and calcification in reef corals. Proc. Natl Acad. Sci. USA 110, 1634–1639 10.1073/pnas.1216153110 (doi:10.1073/pnas.1216153110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jokiel PL. 2011. Ocean acidification and control of reef coral calcification by boundary layer limitation of proton flux. Bull. Mar. Sci. 87, 639–657 10.5343/bms.2010.1107 (doi:10.5343/bms.2010.1107) [DOI] [Google Scholar]
- 6.Jokiel PL. 2011. The reef coral two compartment proton flux model: a new approach relating tissue-level physiological processes to gross corallum morphology. J. Exp. Mar. Biol. Ecol. 409, 1–12 10.1016/j.jembe.2011.10.008 (doi:10.1016/j.jembe.2011.10.008) [DOI] [Google Scholar]
- 7.Roleda MY, Boyd PW, Hurd CL. 2012. Before ocean acidification: calcifier chemistry lessons. J. Phycol. 48, 840–843 10.1111/j.1529-8817.2012.01195.x (doi:10.1111/j.1529-8817.2012.01195.x) [DOI] [PubMed] [Google Scholar]
- 8.Hönisch B, et al. 2012. The geological record of ocean acidification. Science 335, 1058–1063 10.1126/science.1208277 (doi:10.1126/science.1208277) [DOI] [PubMed] [Google Scholar]
- 9.Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. 2013. The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point . Limnol. Oceanogr. 58, 388–398 10.4319/lo.2013.58.1.0388 (doi:10.4319/lo.2013.58.1.0388) [DOI] [Google Scholar]