Abstract
Pneumococcus is an important human pathogen, highly antibiotic resistant and a major cause of bacterial meningitis worldwide. Better prevention requires understanding the drivers of pneumococcal infection incidence and antibiotic susceptibility. Although respiratory viruses (including influenza) have been suggested to influence pneumococcal infections, the underlying mechanisms are still unknown, and viruses are rarely considered when studying pneumococcus epidemiology. Here, we propose a novel mathematical model to examine hypothetical relationships between Streptococcus pneumoniae meningitis incidence (SPMI), acute viral respiratory infections (AVRIs) and antibiotic exposure. French time series of SPMI, AVRI and penicillin consumption over 2001–2004 are analysed and used to assess four distinct virus–bacteria interaction submodels, ascribing the interaction on pneumococcus transmissibility and/or pathogenicity. The statistical analysis reveals strong associations between time series: SPMI increases shortly after AVRI incidence and decreases overall as the antibiotic-prescription rate rises. Model simulations require a combined impact of AVRI on both pneumococcal transmissibility (up to 1.3-fold increase at the population level) and pathogenicity (up to threefold increase) to reproduce the data accurately, along with diminished epidemic fitness of resistant pneumococcal strains causing meningitis (0.97 (0.96–0.97)). Overall, our findings suggest that AVRI and antibiotics strongly influence SPMI trends. Consequently, vaccination protecting against respiratory virus could have unexpected benefits to limit invasive pneumococcal infections.
Keywords: antibiotic resistance fitness cost, mathematical model, statistical model, Streptococcus pneumonia meningitis, virus–bacteria interaction, influenza
1. Introduction
Streptococcus pneumoniae is a Gram-positive coccal bacterium that commonly colonizes the human nasopharynx, and is a leading cause of infectious disease and deaths worldwide, responsible for 1.6 million deaths per year [1,2]. In developed countries, S. pneumoniae is the main cause of bacterial meningitis at any age [3]. In the USA, it is responsible for 50 per cent of meningitides in infants three months to 2 years old and more than 70 per cent of adults more than 40 years old [4]. Lethality fluctuates between 10 and 33 per cent depending on age, with neurological relapses for 30 per cent of cases all ages combined [4,5].
Over the past decades, the spread of antibiotic-resistant pneumococcal strains has made meningitis treatment more difficult, and generally increased the morbidity and mortality associated with severe pneumococcal infections [6]. The high antibiotic-resistance rates in these bacteria have led to policies of limiting antibiotic use and encouraging anti-bacterial vaccination, resulting in important changes of pneumococcal infection ecology [7–9].
Although much attention has been focused on pneumococcal meningitis, its epidemiological dynamics are not fully understood. For example, the seasonal fluctuations of S. pneumoniae meningitis incidence (SPMI) that peak during the winter remain poorly understood [10]. Understanding factors driving these dynamics is crucial to optimize public health programmes devoted to their prevention, for example, anti-pneumococcus vaccine programmes or limiting antibiotic prescriptions.
From an ecological point of view, the dynamics of transmission and evolution of S. pneumoniae in host populations are likely to be influenced by the bacteria's environment. The latter includes population drug exposure [11–13] and co-circulating species [14,15]. Potential interaction mechanisms between community respiratory viruses and S. pneumoniae have been proposed, based on incidence time-series analysis and animal in vivo studies [16,17]. The underlying hypotheses rely on within-host pathophysiological respiratory virus–bacterium interactions that could influence S. pneumoniae colonization, transmission and disease [10,16,18–21]. However, the underlying in vivo biological mechanisms are still unclear and cannot be directly inferred [10].
In this study, we propose a new mathematical model of pneumococcal colonization and meningitis infection to explore the hypotheses that community respiratory virus infections influence the epidemiology of pneumococcal meningitis by increasing strain transmissibility or pathogenicity. To assess these hypotheses, we first analysed chronological association in French epidemiological data in the pre-vaccine era, and we then fitted distinct mechanistic submodels to observational time series.
2. Methods
(a). Data
We used three distinct datasets over the 2001–2004 period in France: the SPMI, the incidence of acute viral respiratory infection (AVRI) and antibiotic-exposure rates (ATB; figure 1). Those data were provided by three different surveillance systems.
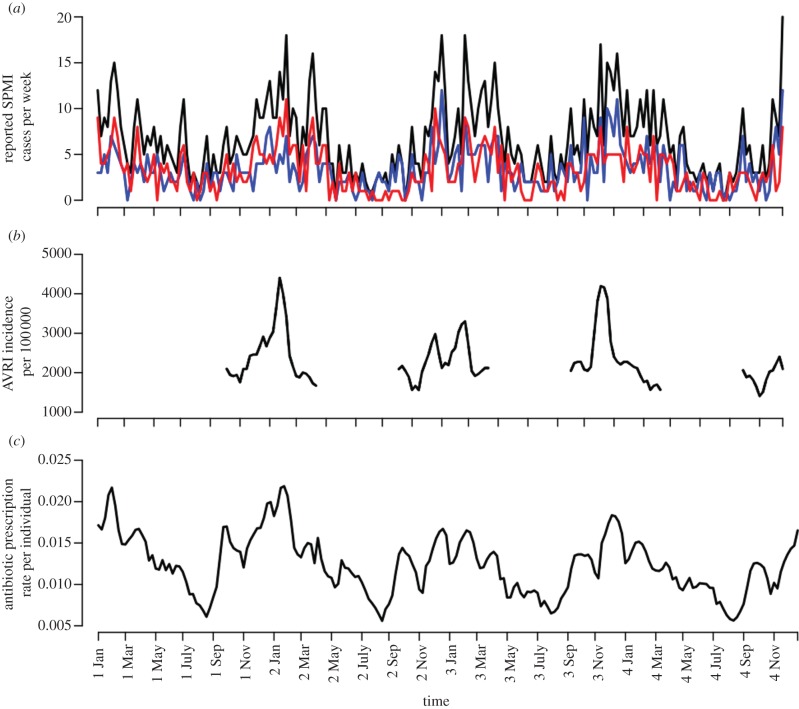
Figure 1.
Observed S. pneumoniae meningitis reported cases (SPMI), acute viral respiratory infections (AVRI) and antibiotic use over seasons 2001–2004. (a) Weekly total (black curve), antibiotic-susceptible (blue) and -resistant SPMI (red), from surveillance within the CNRP [22]. Isolates with MICs more than 0.06 μg ml−1 were considered to be penicillin-non-susceptible, designated herein by ‘non-susceptible’ or ‘resistant’. (b) Weekly AVRI incidence rate during winter seasons, acquired from Euroflu Network (collected only from October to March, epidemiological weeks 1–15 and 40–52 of each year). (c) Weekly antibiotic prescription rate per individual. Antibiotic use was based on β-lactam reimbursement data from the French National Health Insurance [23]. The datasets covered 208 weeks for SPMI and antibiotic use, and 97 weeks of AVRI observation. Weeks ending in a new month are indicated on the x-axis.
(i). Pneumococcal meningitis
Weekly SPMI came from surveillance within the French National Centre of Reference for Pneumococci (CNRP) [22], which systematically collects data on pneumococcal meningitis in collaboration with 22 regional observatories for pneumococci, covering around 60 per cent of French cases [24]. Streptococcus pneumoniae isolates with minimal inhibitory concentrations (MICs) more than 0.06 μg ml−1 were considered to be penicillin-non-susceptible strains. In this study, both terms ‘non-susceptible’ or ‘resistant’ were used to design equally these strains. In France, during the 2001–2004 period, 85–95 per cent of the penicillin-non-susceptible strains responsible for diseases were also macrolide-resistant [25].
(ii). Winter viruses
Weekly AVRI incidence rate was acquired from the Euroflu Network (http://www.euroflu.org/cgi-files/graphs_public.cgi). French data were collected through the Groupe Regional d'Observation de la Grippe (GROG), a network of more than 500 general practitioners reporting for AVRI all over the country (see http://grog.org/presentation.html for more information on the GROG network). Data were extracted from the web page using GraphClick® (http://www.arizona-software.ch/graphclick). The Euroflu AVRI data are available only during winter season for epidemiological weeks 1–15 and 40–52. Additionally, no data were available at the country level for the 2000–2001 winter season. In total, 97 weeks of observation were available over the 2001–2004 period.
(iii). Antibiotics
In France, the National Health Insurance refunds medical care provided by physicians in private practice, community clinics and hospitals to everyone. The antibiotic data used consisted of all ambulatory β-lactam prescribed, dispensed by outpatient pharmacies and reimbursed by two main French National Health Insurance agencies (CNAM-TS and RSI, covering 85% of the French population) from 2001 to 2004 [23]. Because antibiotics are prescription drugs in France, these data are nearly exhaustive. In the following, β-lactam, penicillin and antibiotics were used equally to design this dataset.
(iv). Population
Population demographic data for the different years came from the French National Institute of Statistics and Economical Studies (http://www.insee.fr/en/default.asp).
(b). Statistical analysis of the data
Weekly SPMIs were modelled using a generalized estimating equations (GEE) approach, with a Poisson distribution [26]. This model enables specification of overdispersion, and a first-order autoregressive structure that accounts for the autocorrelation of the weekly SPMI within each year and assumes the independence of the years. Data were subjected to univariate and multivariate analyses. For all weekly time lags k in [–10,10], regressions were defined by:
where M(w) represents the SPMI at week w; ‘pop’ is the population estimate for the considered year; ‘season’ is a periodic trigonometric function and RX(w − k) represents antibiotic use or AVRI, at week (w − k), after removing seasonality using a trigonometric function of period 52 weeks:
for week w, where X(w) represents the antibiotic use (ATB) or AVRI time series, RX(w) their weekly respective residuals and a, b, c the regression coefficients.
To determine which time-delay range has the strongest association, separate lagged Poisson regressions between E[M(w)] and RATB(w − k) or RAVRI(w − k) were estimated for different k-values, −10 ≤ k ≤ 10, and t-test for the regression coefficients was performed [27].
Multivariate Poisson regressions were also performed and compared using the quasi-likelihood information criterion (QIC) [28].
The GEE model was successively tested for total, susceptible and resistant SPMI, using ATB and AVRI as covariates.
The regressions were computed using the GENMOD procedure in SAS.
(c). Mathematical modelling
We developed a new mathematical model of pneumococcal colonization and meningitis infection, and used it to identify the mechanisms responsible for the chronological associations among AVRI, antibiotic use and SPMI. A series of dynamic submodels in which pneumococcal transmissibility and pathogenicity could change as a function of earlier AVRI were formulated. The models were then fitted to SPMI data to assess their explanatory power.
We built a deterministic susceptible–colonized–susceptible model of S. pneumoniae transmission in the French community under β-lactam exposure (outlined in figure 2), using French-context parameters (table 1). We used demographic data from the French National Institute of Statistics and Economical Studies (http://www.insee.fr/en/default.asp) for the birth and mortality rates.
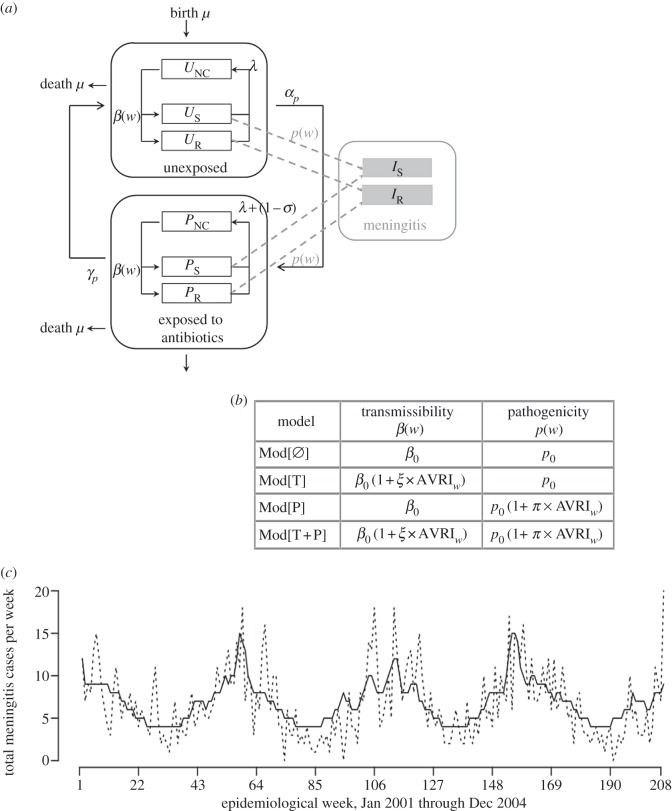
Figure 2.
Outline and description of the four tested models. (a) Pneumococcal transmission model. The population was divided into several compartments, according to S. pneumoniae colonization status and antibiotic exposure, with U, P and I, respectively, representing unexposed, penicillin-exposed and meningitis-infected compartments; NC, S and R subscripts designating, respectively, non-colonized, colonized with susceptible and resistant strains. Uncolonized individuals (UNC, PNC) could become colonized at rate β(w) per contact with colonized individuals (US, UR, PS, PR) independently of antibiotic exposure or antibiotic resistance of colonizing strains. β(w) is defined as a time-varying function depending on β0, the natural pneumococcal transmission rate and AVRI incidence over time. Independently of their carriage status, individuals could be exposed during an average time 1/γP to antibiotics at frequency αP, for which the population weekly antibiotic exposure rate was used (for all weeks w, αP(w) = ATB(w)). Decolonization could result either from natural immunity (after average time 1/λ) or antibiotic consumption (with σ = (σS, σR) being the probability of decolonization depending on colonizing strain susceptibility). Finally, meningitis infection (IS, IR) could occur in carriers at rate p(w), regardless of the level of susceptibility to penicillin. (b) Description of the four distinct models of SPMI–AVRI interactions. Because the link between AVRI and SPMI fluctuations has been ascribed to different factors, AVRI effects were modelled within transmission (T, i.e. pneumococcal transmissibility or acquisition) and pathogenicity (P) parameters. (c) Observed (dashed line) global SPMI (January 2001–December 2004) versus the simulated one (solid line), using the median set of parameters obtained with the best-fitting model Mod[T+P]. Note that parameters were estimated using SPMI data only from weeks when AVRI were available. The simulation for the whole period is presented here, using an extrapolated AVRI incidence rate when not available.
Table 1.
Model parameters.
| parameter | notation | value |
|---|---|---|
| birth rate, death rate | μ | 0.00025 individual−1 week−1 |
| infectious contact rate: | ||
| intrinsic pneumococcal transmission | β0 | estimated |
| impact of AVRI on transmission | ξ | estimated |
| time-varying transmission | β(w) = f(β0, w) | |
| colonization duration: | ||
| for susceptible strains | 1/λS | three weeks |
| for resistant strains | 1/λR | estimated |
| antibiotic exposure duration | 1/γP | 8 days |
| decolonization after one week of antibiotic use: | ||
| probability for susceptible strains | 1 − σS | 1 |
| probability for resistant strains | 1 − σR | 0 |
| antibiotic exposure rate | αP(w) | ATB(w) |
| pathogenicity in colonized individuals: | ||
| natural probability of meningitis | p0 | estimated |
| impact of AVRI on pathogenicity | π | estimated |
| time-varying pathogenicity | p(w) = g(p0, w) | |
| fitness cost for resistant strains | fR/S | estimated |
Model compartments were structured with respect to colonization status and antibiotic exposure (figure 2a). Uncolonized individuals (NC) could become colonized at rate β through contacts with colonized individuals. Two stages of S. pneumoniae penicillin susceptibility, corresponding to different levels of MIC, were modelled: susceptible (S) for strains with MIC < 0.06 μg ml−1; and intermediate or resistant (R) for strains with MIC ≥ 0.06 μg ml−1. Emergence of resistance or increase of its level was not considered in the model. Independently of their carriage status, individuals could be unexposed (UNC, US, UR) or exposed (PNC, PS, PR) to antibiotics.
Because antibiotic exposure varies over the year, we used the observed time series ATB(w) to parametrize the rates of exposure each week w of the period considered: αP(w) = ATB(w). In the absence of antibiotic use, natural immunity was defined as inducing carriage clearance in colonized individuals with an average of three weeks in the population [29–32]. The impact of antibiotic exposure, characterized by σ, depended on the colonizing strain's levels of resistance: antibiotics cleared susceptible bacteria in one week on average (σS = 0), but had no effect on colonization with resistant bacteria (σR = 1). Antibiotic exposure duration was assumed to have a mean of 1/γP = 8 days.
The duration of colonization by susceptible strains was fixed (1/λS = 3 weeks on average). Possible epidemic fitness differences between penicillin-susceptible and -resistant pneumococcal strains were included in the model by introducing different clearance rates for each strain. Resistance-associated fitness cost was defined as the mean ratio of carriage durations between resistant and susceptible strains: fR/S = λS/λR. Other parameters being equal, this quantity is equivalent to the ratio of resistant to susceptible pneumococci reproductive numbers. fR/S was numerically estimated to maximize the log-likelihood of the model to reproduce observed susceptible and resistant SPMI data.
Meningitis infection (I) could occur in all pneumococcal carriers. We assumed that the natural probability p0 of developing meningitis was constant throughout carriage duration and was independent of antibiotic susceptibility. In France, vaccination with a pneumococcal conjugate vaccine (PCV) was introduced for children in 2003. Because vaccination recommendations were restrictive before 2005 [33], immunization was considered to be marginal during the period of study, and PCV was not considered in the model.
Four interaction submodels were derived, assuming different mechanisms of virus-induced modified transmissibility and pathogenicity (figure 2):
— MOD[Ø], in which AVRI has no impact on SPMI
- — MOD[T], for which we assumed that, during AVRI epidemic periods, transmission of pneumococcal strains to non-carriers could be promoted when either these non-carriers or the pneumococcal carriers were infected with the virus. The pneumococcal transmission rate at week w was modelled as
where β0 is the intrinsic pneumococcal transmission rate, AVRI(w) is the virus-infection incidence in the population at week w and ξ is a factor representing the impact of a respiratory virus infection on pneumococcal strains transmission, reflecting either a modification of the chance of transmission, or of the rate of acquisition.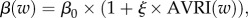
- — MOD[P], for which we assumed that the probability of colonized individuals developing meningitis at week w, p(w) (=P[meningitis|colonized]) could be increased in people with AVRI:
where π is a factor representing the impact of a respiratory virus infection on the pathogenicity of pneumococcal strains and p0 the natural pneumococcal pathogenicity.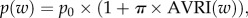
— MOD[T + P], which combined the assumed impacts on transmissibility and pathogenicity of models MOD[T] and MOD[P].
The reported time series of AVRI from the GROG were directly used in the model to parameterize the rate of AVRI per individual each week. Reporting fidelity to AVRI was assumed to be equal to 100 per cent.
(d). Statistical inference and model fitting
Model compartments were initialized to describe a population in which penicillin-resistant pneumococcal rates were equivalent to the French 2001 data (CNRP) with more than 50 per cent of penicillin-non-susceptible strains [25]. Global pneumococcal carriage was set to 10 per cent on average, with a rate of non-susceptible strains initialized to 55 per cent. The meningitis infection compartments (I) were initialized by directly using meningitis observed incidence at the beginning of the study period.
The model was numerically simulated over the 2001–2004 period. To study the role of AVRI, the parameters of the four interaction models (MOD[Ø], MOD[T], MOD[P] and MOD[T + P]) were estimated to obtain the best-fitting model with the observed susceptible and resistant SPMI data, using a Markov chain Monte Carlo (MCMC) algorithm. Only the 97 weeks during which AVRI incidence was available were used for the calculation of the likelihood. The Metropolis–Hasting algorithm was used to sample over the parameter space. For each model, parameters were selected to maximize the Poisson log-likelihood for the susceptible and resistant meningitis time series (with expression detailed in electronic supplementary material, §S3). Posterior estimates were obtained using 3 × 106 MCMC iterations with sampling every 1000 and a burn-in of 100 000 iterations to allow for the chain convergence. Uninformative priors were used, and several starting points were tested. The best-fitting model was determined using the deviance information criterion (DIC; table 2). This criterion has the advantage of taking into account the model complexity associated with the number of parameters. The selected model is the one that presents the lowest DIC.
Table 2.
Posterior median estimates (95% credibility interval) for the four fitted models. Four distinct models of SPMI–AVRI interactions were tested: Mod[Ø], AVRI had no influence whatsoever on SPMI; Mod[T], AVRI affected only pneumococcal transmission, whereas pathogenicity remained constant; Mod[P], AVRI influenced only pneumococcal pathogenicity; and Mod[T+P], AVRI could affect both pneumococcal pathogenicity and transmission (transmissibility or acquisition). Here, β0 defines natural pneumococcal transmission rate, ξ defines virus impact on pneumococcal transmission, p0 defines the natural pathogenicity rate, π defines virus impact on pathogenicity and fR/S defines resistance-associated fitness cost, which was defined as the mean ratio of carriage duration between susceptible and resistant strains fR/S = λS/λR. In the best-fitting model (highlighted in grey), resulting S. pneumoniae transmission β(w) and pathogenicity p(w) ranged, respectively, from 0.36 to 0.47 per week per person and from 6 × 10–7 to 1.8 × 10–6.
 |
aWe used the following expression of the deviance information criterion: DIC = −4 × LL + 2 × max(LL) , where LL is the mean of the log-likelihood and max(LL) the mode value for the MCMC sample. Model minimizing the DIC was preferred.
The model and estimation framework were programmed in C. The R statistical software (www.r-project.org) was used for the statistical analysis of the model outputs and graphics. The equations of the model are provided in the electronic supplementary material, §S2.
3. Results
(a). French data and statistical analysis
The data from January 2001 to December 2004, corresponding to a total of 208 weeks for SPMI and β-lactam use, and 97 weeks of AVRI observation, are given in figure 1. A total of 1383 SPMI episodes were reported. All three time series (SPMI, AVRI and ATB) present a 52-week seasonality with epidemics peaking during the winter.
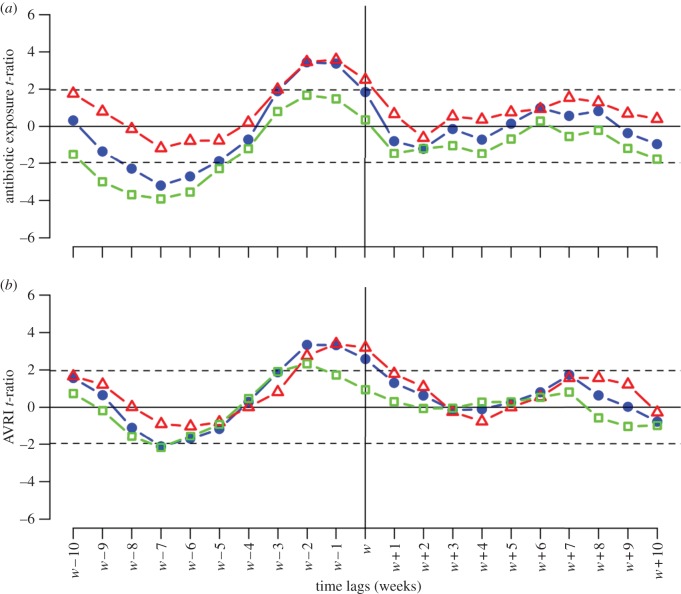
Figure 3 shows plotted t-ratios between SPMI and residual ATB or residual AVRI, computed for all time lags k between −10 and +10 weeks. Detailed estimates are provided in the electronic supplementary material, §S1.
Figure 3.
Statistical association between SPMI and AVRI or antibiotic exposure. Antibiotic and AVRI time series were detrended, and seasonality removed to create ‘residual time series’. Lagged regression coefficient t-ratios between numbers of meningitis cases and residual antibiotic or residual AVRI are plotted for all lags k between weeks −10 and +10. (a) SPMI association with antibiotics. Total (blue circles) and resistant meningitides (red triangles) are positively significantly linked to antibiotic use at weeks −1 and −2. Long-term relationships were observed between total and susceptible meningitides (green squares) and antibiotic use, with a negative significant link for weeks −5 to −8, with a peak at −7. (b) SPMI association with AVRI. Regardless of strain susceptibility, increased SPMI was significantly associated with high AVRI incidence during the same and the preceding 2 weeks, indicating positive short-term dependency. Negative long-term dependency (week −7) was observed for total and susceptible meningitides, but associations were only marginally significant. Like the antibiotics analysis, resulting t-ratios were markedly asymmetric.
As shown in figure 3, the t-ratios plotted versus the time lag between AVRI or antibiotic and SPMI were highly asymmetric (none of the t-ratios for the forward lags was significant), suggesting that the SPMI during a given week is linked to AVRI incidence or antibiotic consumption over the previous weeks, but not to those of subsequent weeks. This asymmetry reinforces the plausibility of direct links among AVRI and antibiotic consumption and SPMI. By contrast, a symmetrical plot would have indicated that common confounders acted simultaneously on the different factors.
(i). Acute viral respiratory infection versus Streptococcus pneumoniae meningitis incidence
We found that, regardless of pneumococcal strain susceptibility, SPMI followed AVRI incidence by up to two weeks, indicative of positive short-term dependency (p < 0.01). Negative long-term dependency (w−7) was observed for total and susceptible meningitides, but associations were only marginally significant.
(ii). Antibiotic versus Streptococcus pneumoniae meningitis incidence
Total and antibiotic-resistant SPMIs were also positively linked with antibiotic consumption over the two preceding weeks. By contrast, total and antibiotic-susceptible SPMI were negatively significantly linked to penicillin consumption over the preceding five to nine weeks (p < 0.02), indicating long-term dependency.
(iii). Multivariate analysis
In countries with high antibiotic exposure, such as France, antibiotic use is strongly associated with AVRI dynamics, meaning that the short-term SPMI–antibiotic association could be an artefact of the data, a hypothesis consistent with our multivariate analysis results. Including residual RAVRI(w−2) or RATB(w−2) in the multivariate analyses of total, susceptible and resistant meningitides gave similar QIC, indicating that AVRI could act as a confounder in the short-term SPMI–antibiotic association (see the electronic supplementary material, §S1).
(b). Mathematical modelling and mechanisms testing
For each of the four tested models, posterior estimates of the parameters are given in table 2. Among the four possibilities, we found that the best fit was obtained when both transmissibility and risk of infection were allowed to depend on observed AVRI (table 2).
For the selected model, the posterior median estimates were ξ = 8.7 (95% credibility interval (4.6–14.4)) for virus impact on pneumococcal transmission/acquisition; π = 92 (28–361) for virus impact on pathogenicity; and fR/S = 0.97 (0.969–0.975) for the antibiotic-resistance-associated fitness cost estimate. In a typical simulation using the median posterior estimates, pneumococcal transmissibility ranged from 0.36 to 0.47 per week per person (reaching a 1.3-fold increase) and pneumococcal pathogenicity ranged from 6×10–7 to 1.8×10–6 (a threefold increase). Although low, the resistance-associated fitness cost was significantly lower than 1, suggesting that resistant strains are slightly less competitive than susceptible ones.
Lastly, one can directly interpret the posterior estimates of ξ and π at the individual level (see the electronic supplementary material, §S2 for details on the mathematical development and interpretation). From that point of view, the model predicts that AVRI substantially increases a colonized patient's absolute risk of meningitis (by a factor of 90) and also increases the individual's risk of acquisition/transmissibility of pneumococci (by a factor of 9).
The 2001–2004 SPMI simulated using the selected model and the median estimated parameters are depicted in figure 2c (see also the electronic supplementary material, figure S2), along with the observed SPMI over the same period.
4. Discussion
Pneumococcal meningitis incidence (SPMI) was found to be highly associated with the incidence of AVRIs and with antibiotic use: SPMI increased shortly after AVRI incidence (the two following weeks) and decreased overall as the antibiotic-prescription rate rose (six to seven weeks after). In addition, our modelling study discarded the hypothesis of a unique interaction between AVRI and pneumococci. Model simulations required a combined AVRI impact on both pneumococcal transmissibility and pathogenicity to reproduce the data accurately, along with diminished epidemic fitness of resistant pneumococcal strains causing meningitis.
Vaccination with PCV has been shown to widely modify pneumococcal epidemiology [7,9]. In France, PCV vaccination was initiated in 2003, targeting a specific population (at-risk children less than 2 years old), with recommendations extended in 2006 to every child less than 2 years old. In the same period, a campaign to decrease antibiotic consumption was launched [23]. In this study, we focused on years 2001–2004, in order to obtain stable meningitis ecology, with a limited effect, if any, of vaccination.
For clarity purposes, the model developed here used several simplifications regarding S. pneumoniae epidemiology. First, the model was not age-stratified. Pneumococcal colonization is known to decrease with age [34], and children have generally higher rates of pneumococcal meningitis and antibiotic consumption. Although this simplification should not affect our findings on the association between AVRI and SPMI, extending the model and examining the effect of age on such an association will be of interest in the future. Second, the model included only penicillin resistance, neglecting other drug resistances (and multiresistances). In France, most penicillin-resistant pneumococcal strains also carry a macrolide resistance gene. The inclusion in the model of the 2001–2004 macrolide exposure rates, in addition to the β-lactams ones, would increase the co-selection of resistant strains under antibiotic exposure [35]. Therefore, it is predictable that the estimated epidemic fitness difference between susceptible and resistant S. pneumoniae would be increased to compensate for this selective advantage.
The model and data used here did not allow estimating any reporting fidelity in AVRI. The AVRI reporting rate was therefore assumed to be equal to 100 per cent in the model. In practice, reporting fidelity in AVRI is usually imperfect. This simplification should not impact the significance of our conclusions regarding the role of AVRI on pneumococcus epidemiology. It would, however, affect the amplitude of this effect: a lower reporting rate would reduce the quantitative estimation of ξ and π by the same rate. The presented estimates can therefore be interpreted as a maximization of AVRI's impact on pneumococcal pathogenicity and transmissibility.
Some data limitations should also be mentioned. Two independent surveillance systems report viral respiratory infections in France: the Sentinelle network, which reports influenza-like syndrome incidence; and the GROG surveillance programme, which combines virological testing and sentinel indicators to report AVRI (including influenza-like illness and respiratory syncytial virus, RSV) [36]. In order to include AVRI with the largest definition, data from the GROG network were used here. As mentioned earlier, during the study period, the GROG network reported only viral infections occurring during winter seasons. As a consequence, our statistical and inference analyses were restricted to these periods, excluding summertime incidences and limiting the power of our analysis. Furthermore, other respiratory viral pathogens, not reported by the GROG, together with other environmental factors, such as air pollution, daily light or temperature, were not included in this study. Despite these limitations, the short-term associations found between AVRI and SPMI are in accordance with previous findings [10,17,37].
High incidences of bacterial respiratory infections during influenza pandemics have been reported in the past [19,38], and an increased incidence of these infections (e.g. pneumonia) in potentially coming pandemics is of concern [39]. The temporal relationship among circulating winter virus infections (influenza virus, RSV) and invasive pneumococcal disease has also been reported in several studies [10,17–20,37]. Herein, we highlight two distinct mechanisms underlying such associations and suggest that the existence of a unique interaction mechanism is very unlikely.
AVRIs are known to increase the risk of invasive bacterial infection [16]. Several biological mechanisms have been suggested to explain such phenomena. First, the idea that daily light and temperature exposure modifications altered host immune responses was advanced to explain the seasonal variations of infection incidences [40]. This hypothesis does not agree with our statistical results. Such a mechanism would tend to influence AVRI and SPMI simultaneously. Herein, the time-series relationships were strongly asymmetric. Second, virus infection might facilitate pneumococcal infection by damaging the airway mucosa and epithelial lining [10,41,42], increasing pneumococcal adherence to epithelia [41,43] and/or impairing innate immune responses to pneumococcal infections [42,44–46].
However, our results suggest that AVRI-induced increased pathogenicity of pneumococci is not enough to explain the observed trends of pneumococcal meningitis. The AVRI should also modify between-host interactions by emphasizing S. pneumoniae's transmissibility. Such an increased epidemic potential could actually reflect two distinct mechanisms. First, AVRI infection could enhance the transmission probability of the bacteria in colonized individuals (and therefore increase pneumococcus's reproductive number) by increasing bacterial load in the host or creating new transmission routes. Second, virus infection could increase host susceptibility to pneumococcal colonization (e.g. enhance the acquisition risk) in AVRI-infected individuals. Our model did not allow discriminating between the two. A more complex model, in which the dynamics of virus infection in the population is explicitly modelled, will be necessary in the future to explore in details this enhancing effect on pneumococcal transmissibility. To date, this phenomenon of ‘cloud humans’, in which virus-infected individuals transmit more bacteria, has been little studied in human populations [47,48].
Based on the influenza-like illness incidence reported by the Sentinelles network, it is probable that influenza viruses are responsible for the second winter peak shape of AVRI. Here, the lack of virological information impeded the separate quantification of the role of the distinct viruses on pneumococcal epidemiology. However, assessing the role of the viruses individually will be of great interest in the future.
A distinct but related aspect is that of the significant resistance-associated fitness cost. In this study, antibiotic-driven selection on its own was not sufficient to reproduce the observed trends, and significantly lower fitness for resistant pneumococci was found (estimated resistance-associated fitness cost of 0.97 (0.969–0.975)). Reduced transmissibility of antibiotic-resistant pneumococcal strains has already been suggested [49,50], but biological evidence in humans is still lacking. Although resistance-associated fitness cost was a secondary consideration here, this parameter is of importance in measuring the selective power associated with antibiotic exposure pressure. Our finding provides some insights into the influence of antibiotic exposure on the selection of circulating pneumococcal strains: susceptible strains would be disadvantaged under antibiotic exposure but become more epidemic than resistant strains when antibiotic use declines. A possible consequence of fewer antibiotic prescriptions might be an increased incidence of severe pneumococcal infections caused by antibiotic-susceptible strains.
The results presented here bring to light, at the epidemiologic level, the potential means for respiratory viruses and S. pneumoniae interactions. They suggest that respiratory viruses actually increase the incidence of invasive pneumococcal infections by both enhancing S. pneumoniae dissemination in human populations and increasing the risk of invasive infections in colonized individuals. This finding could be of importance when considering priorities for anti-bacterial and anti-viral vaccine policies, as it may lead to an indirect public health impact of anti-viral vaccines conferring immunity against influenza (and in the near future RSV) on the spread of S. pneumoniae strains and on meningitis risk [51]. This impact will probably have to be considered when elaborating vaccine combination strategies aimed at further decreasing SPMI.
Acknowledgements
The authors thank Sophie Pepin (CNAM-TS), Michel Leroy (RSI) and the French National Health Insurance for providing the data on β-lactam reimbursements. They also thank Anne Cori, Anne Thiebaut, Elisabeth Delarocque-Astagneau, Margarita Pons-Salort and Matthieu Domenech de Celles for their comments on the manuscript.
Funding statement
L.O. was supported by a joint grant from the Centre National de la Recherche Scientifique (CNRS), the Institut National de la Sante Et de la Recherche Médicale (INSERM) and the Institut National de la Recherche en Informatique et Automatique (INRIA). L.O. was also supported by a Sanofi-Aventis grant. This study has received funding from the French government's Investissement d'Avenir programme, Laboratoire d'Excellence ‘Integrative Biology of Emerging Infectious Diseases’ (grant no. ANR-10-LABX-62-IBEID).
References
- 1.WHO 2007. Pneumococcal conjugate vaccine for childhood immunization. Wkly Epidemiol. Rec. 82, 93–104 [PubMed] [Google Scholar]
- 2.Swartz MN. 2004. Bacterial meningitis: a view of the past 90 years. N. Engl. J. Med. 351, 1826–1828 10.1056/NEJMp048246 (doi:10.1056/NEJMp048246) [DOI] [PubMed] [Google Scholar]
- 3.Weisfelt M, de Gans J, van der Poll T, van de Beek D. 2006. Pneumococcal meningitis in adults: new approaches to management and prevention. Lancet Neurol. 5, 332–342 10.1016/S1474-4422(06)70409-4 (doi:10.1016/S1474-4422(06)70409-4) [DOI] [PubMed] [Google Scholar]
- 4.Thigpen MC, et al. 2011. Bacterial meningitis in the United States, 1998–2007. N. Engl. J. Med. 364, 2016–2025 10.1056/NEJMoa1005384 (doi:10.1056/NEJMoa1005384) [DOI] [PubMed] [Google Scholar]
- 5.Levy C, Varon E, Bingen E, Picard C, de La Rocque F, Aujard Y, Cohen R. 2008. [Pneumococcal meningitis in children in France: 832 cases from 2001 to 2007]. Arch. Pediatr. 15(Suppl. 3), S111–S118 10.1016/S0929-693X(08)75493-9 (doi:10.1016/S0929-693X(08)75493-9) [DOI] [PubMed] [Google Scholar]
- 6.Whitney CG, et al. 2000. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 343, 1917–1924 10.1056/NEJM200012283432603 (doi:10.1056/NEJM200012283432603) [DOI] [PubMed] [Google Scholar]
- 7.Whitney CG, et al. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348, 1737–1746 10.1056/NEJMoa022823 (doi:10.1056/NEJMoa022823) [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein JA, et al. 2008. Impact of a 16-community trial to promote judicious antibiotic use in Massachusetts. Pediatrics 121, e15–e23 10.1542/peds.2007-0819 (doi:10.1542/peds.2007-0819) [DOI] [PubMed] [Google Scholar]
- 9.Tsai CJ, Griffin MR, Pekka Nuorti J, Grijalva CG. 2008. Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States. Clin. Infect. Dis. 46, 1664–1672 10.1086/587897 (doi:10.1086/587897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talbot TR, Poehling KA, Hartert TV, Arbogast PG, Halasa NB, Edwards KM, Schaffner W, Craig AS, Griffin MR. 2005. Seasonality of invasive pneumococcal disease: temporal relation to documented influenza and respiratory syncytial viral circulation. Am. J. Med. 118, 285–291 10.1016/j.amjmed.2004.09.016 (doi:10.1016/j.amjmed.2004.09.016) [DOI] [PubMed] [Google Scholar]
- 11.Goossens H. 2009. Antibiotic consumption and link to resistance. Clin. Microbiol. Infect. 15(Suppl. 3), 12–15 10.1111/j.1469-0691.2009.02725.x (doi:10.1111/j.1469-0691.2009.02725.x) [DOI] [PubMed] [Google Scholar]
- 12.Guillemot D, Varon E, Bernede C, Weber P, Henriet L, Simon S, Laurent C, Lecoeur H, Carbon C. 2005. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin G-nonsusceptible Streptococcus pneumoniae. Clin. Infect. Dis. 41, 930–938 10.1086/432721 (doi:10.1086/432721) [DOI] [PubMed] [Google Scholar]
- 13.Weinberger DM, Malley R, Lipsitch M. 2011. Serotype replacement in disease after pneumococcal vaccination. Lancet 378, 1962–1973 10.1016/S0140-6736(10)62225-8 (doi:10.1016/S0140-6736(10)62225-8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keeling MJ, Rohani P. 2008. Modeling infectious diseases in humans and animals. Princeton, NJ: Princeton University Press [Google Scholar]
- 15.Vasco DA, Wearing HJ, Rohani P. 2007. Tracking the dynamics of pathogen interactions: modeling ecological and immune-mediated processes in a two-pathogen single-host system. J. Theor. Biol. 245, 9–25 10.1016/j.jtbi.2006.08.015 (doi:10.1016/j.jtbi.2006.08.015) [DOI] [PubMed] [Google Scholar]
- 16.Short KR, Habets MN, Hermans PW, Diavatopoulos DA. 2012. Interactions between Streptococcus pneumoniae and influenza virus: a mutually beneficial relationship? Future Microbiol. 7, 609–624 10.2217/fmb.12.29 (doi:10.2217/fmb.12.29) [DOI] [PubMed] [Google Scholar]
- 17.Murdoch DR, Jennings LC. 2009. Association of respiratory virus activity and environmental factors with the incidence of invasive pneumococcal disease. J. Infect. 58, 37–46 10.1016/j.jinf.2008.10.011 (doi:10.1016/j.jinf.2008.10.011) [DOI] [PubMed] [Google Scholar]
- 18.Kuster SP, Tuite AR, Kwong JC, McGeer A, Fisman DN. 2011. Evaluation of coseasonality of influenza and invasive pneumococcal disease: results from prospective surveillance. PLoS Med. 8, e1001042. 10.1371/journal.pmed.1001042 (doi:10.1371/journal.pmed.1001042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brundage JF. 2006. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect. Dis. 6, 303–312 10.1016/S1473-3099(06)70466-2 (doi:10.1016/S1473-3099(06)70466-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Henriques Normark B. 2010. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J. Infect. Dis. 202, 1287–1295 10.1086/656333 (doi:10.1086/656333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis BM, Aiello AE, Dawid S, Rohani P, Shrestha S, Foxman B. 2012. Influenza and community-acquired pneumonia interactions: the impact of order and time of infection on population patterns. Am. J. Epidemiol. 175, 363–367 10.1093/aje/kwr402 (doi:10.1093/aje/kwr402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perrocheau A, Scarlett G, Laurent E. 2004. Épidémiologie des méningites bactériennes en France en 2002: Méningites bactériennes communautaires [epidemiology of bacterial meningitis in France in 2002: bacterial meningitis]. La Revue du Praticien 54, 945–950 [PubMed] [Google Scholar]
- 23.Sabuncu E, David J, Bernede-Bauduin C, Pepin S, Leroy M, Boelle PY, Watier L, Guillemot D. 2009. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002–2007. PLoS Med. 6, e1000084. 10.1371/journal.pmed.1000084 (doi:10.1371/journal.pmed.1000084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrocheau A, Doyle A, Bernillon P, Varon E. 2006. Estimation du nombre total de méningites à pneumocoque de l'enfant, par la méthode capture-recapture à 3 sources, France, 2001–2002. Bull. Epidémiol. Hebdomadaire 2–3, 16–18 [Google Scholar]
- 25.Varon E, Gutmann L.2001–2004. Centre National de Référence des Pneumocoques. Rapport d'activité. See http://www.invs.sante.fr/Espace-professionnels/Centres-nationaux-de-reference/Rapports-d-activites-et-liens .
- 26.Fouillet A, Rey G, Jougla E, Frayssinet P, Bessemoulin P, Hemon D. 2007. A predictive model relating daily fluctuations in summer temperatures and mortality rates. BMC Public Health 7, 114. 10.1186/1471-2458-7-114 (doi:10.1186/1471-2458-7-114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubert B, Watier L, Garnerin P, Richardson S. 1992. Meningococcal disease and influenza-like syndrome: a new approach to an old question. J. Infect. Dis. 166, 542–545 10.1093/infdis/166.3.542 (doi:10.1093/infdis/166.3.542) [DOI] [PubMed] [Google Scholar]
- 28.Pan W. 2001. Akaike's information criterion in generalized estimating equations. Biometrics 57, 120–125 10.1111/j.0006-341X.2001.00120.x (doi:10.1111/j.0006-341X.2001.00120.x) [DOI] [PubMed] [Google Scholar]
- 29.Ekdahl K, Ahlinder I, Hansson HB, Melander E, Molstad S, Soderstrom M, Persson K. 1997. Duration of nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae: experiences from the south Swedish pneumococcal intervention project. Clin. Infect. Dis. 25, 1113–1117 10.1086/516103 (doi:10.1086/516103) [DOI] [PubMed] [Google Scholar]
- 30.Melegaro A, Gay NJ, Medley GF. 2004. Estimating the transmission parameters of pneumococcal carriage in households. Epidemiol. Infect. 132, 433–441 10.1017/S0950268804001980 (doi:10.1017/S0950268804001980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cauchemez S, Temime L, Valleron AJ, Varon E, Thomas G, Guillemot D, Boelle PY. 2006. S. pneumoniae transmission according to inclusion in conjugate vaccines: Bayesian analysis of a longitudinal follow-up in schools. BMC Infect. Dis. 6, 14. 10.1186/1471-2334-6-14 (doi:10.1186/1471-2334-6-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hogberg L, Geli P, Ringberg H, Melander E, Lipsitch M, Ekdahl K. 2007. Age- and serogroup-related differences in observed durations of nasopharyngeal carriage of penicillin-resistant pneumococci. J. Clin. Microbiol. 45, 948–952 10.1128/JCM.01913-06 (doi:10.1128/JCM.01913-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Institut de veille sanitaire (InVS) 2006. Calendrier vaccinal 2006. Bull. Epidémiol. Hebdomadaire 29–30, 212 [Google Scholar]
- 34.Regev-Yochay G, Raz M, Dagan R, Porat N, Shainberg B, Pinco E, Keller N, Rubinstein E. 2004. Nasopharyngeal carriage of Streptococcus pneumoniae by adults and children in community and family settings. Clin. Infect. Dis. 38, 632–639 10.1086/381547 (doi:10.1086/381547) [DOI] [PubMed] [Google Scholar]
- 35.Opatowski L, Temime L, Varon E, Leclerc R, Drugeon H, Boelle PY, Guillemot D. 2008. Antibiotic innovation may contribute to slowing the dissemination of multiresistant Streptococcus pneumoniae: the example of ketolides. PLoS ONE 3, e2089. 10.1371/journal.pone.0002089 (doi:10.1371/journal.pone.0002089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hannoun C, Dab W, Cohen JM. 1989. A new influenza surveillance system in France: the Ile-de-France ‘GROG’. I. Principles and methodology. Eur. J. Epidemiol. 5, 285–293 10.1007/BF00144828 (doi:10.1007/BF00144828) [DOI] [PubMed] [Google Scholar]
- 37.Jansen AG, Sanders EA, Van Der Ende A, Van Loon AM, Hoes AW, Hak E. 2008. Invasive pneumococcal and meningococcal disease: association with influenza virus and respiratory syncytial virus activity Epidemiol. Infect. 136, 1448–1454 10.1017/S0950268807000271 (doi:10.1017/S0950268807000271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klugman KP, Chien YW, Madhi SA. 2009. Pneumococcal pneumonia and influenza: a deadly combination. Vaccine 27(Suppl. 3), C9–C14 10.1016/j.vaccine.2009.06.007 (doi:10.1016/j.vaccine.2009.06.007) [DOI] [PubMed] [Google Scholar]
- 39.Palacios G, et al. 2009. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS ONE 4, e8540. 10.1371/journal.pone.0008540 (doi:10.1371/journal.pone.0008540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dowell SF, Whitney CG, Wright C, Rose CE, Jr, Schuchat A. 2003. Seasonal patterns of invasive pneumococcal disease. Emerg. Infect. Dis. 9, 573–579 10.3201/eid0905.020556 (doi:10.3201/eid0905.020556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Brien KL, Walters MI, Sellman J, Quinlisk P, Regnery H, Schwartz B, Dowell SF. 2000. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clin. Infect. Dis. 30, 784–789 10.1086/313772 (doi:10.1086/313772) [DOI] [PubMed] [Google Scholar]
- 42.McCullers JA. 2006. Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 19, 571–582 10.1128/CMR.00058-05 (doi:10.1128/CMR.00058-05) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Sluijs KF, et al. 2006. Involvement of the platelet-activating factor receptor in host defense against Streptococcus pneumoniae during postinfluenza pneumonia. Am. J. Physiol. Lung Cell Mol. Physiol. 290, L194–199 10.1152/ajplung.00050.2005 (doi:10.1152/ajplung.00050.2005) [DOI] [PubMed] [Google Scholar]
- 44.Sun K, Metzger DW. 2008. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat. Med. 14, 558–564 10.1038/nm1765 (doi:10.1038/nm1765) [DOI] [PubMed] [Google Scholar]
- 45.van der Sluijs KF, Nijhuis M, Levels JH, Florquin S, Mellor AL, Jansen HM, van der Poll T, Lutter R. 2006. Influenza-induced expression of indoleamine 2,3-dioxygenase enhances interleukin-10 production and bacterial outgrowth during secondary pneumococcal pneumonia. J. Infect. Dis. 193, 214–222 10.1086/498911 (doi:10.1086/498911) [DOI] [PubMed] [Google Scholar]
- 46.Nakamura S, Davis KM, Weiser JN. 2011. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J. Clin. Invest. 121, 3657–3665 10.1172/JCI57762 (doi:10.1172/JCI57762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bassetti S, et al. 2005. Dispersal of Staphylococcus aureus into the air associated with a rhinovirus infection. Infect. Control Hosp. Epidemiol. 26, 196–203 10.1086/502526 (doi:10.1086/502526) [DOI] [PubMed] [Google Scholar]
- 48.Sherertz RJ, Bassetti S, Bassetti-Wyss B. 2001. ‘Cloud’ health-care workers. Emerg. Infect. Dis. 7, 241–244 10.3201/eid0702.700241 (doi:10.3201/eid0702.700241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trzcinski K, Thompson CM, Gilbey AM, Dowson CG, Lipsitch M. 2006. Incremental increase in fitness cost with increased beta-lactam resistance in pneumococci evaluated by competition in an infant rat nasal colonization model. J. Infect. Dis. 193, 1296–1303 10.1086/501367 (doi:10.1086/501367) [DOI] [PubMed] [Google Scholar]
- 50.Dagan R, Barkai G, Givon-Lavi N, Sharf AZ, Vardy D, Cohen T, Lipsitch M, Greenberg D. 2008. Seasonality of antibiotic-resistant Streptococcus pneumoniae that causes acute otitis media: a clue for an antibiotic-restriction policy J. Infect. Dis. 197, 1094–1102 10.1086/528995 (doi:10.1086/528995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCullers JA. 2011. Preventing and treating secondary bacterial infections with antiviral agents. Antivir. Ther. 16, 123–135 10.3851/IMP1730 (doi:10.3851/IMP1730) [DOI] [PMC free article] [PubMed] [Google Scholar]