Abstract
Environmental shifts and lifestyle changes may result in formerly adaptive traits becoming non-functional or maladaptive. The subsequent decay of such traits highlights the importance of natural selection for adaptations, yet its causes have rarely been investigated. To study the fate of formerly adaptive traits after lifestyle changes, we evaluated sexual traits in five independently derived asexual lineages, including traits that are specific to males and therefore not exposed to selection. At least four of the asexual lineages retained the capacity to produce males that display normal courtship behaviours and are able to fertilize eggs of females from related sexual species. The maintenance of male traits may stem from pleiotropy, or from these traits only regressing via drift, which may require millions of years to generate phenotypic effects. By contrast, we found parallel decay of sexual traits in females. Asexual females produced altered airborne and contact signals, had modified sperm storage organs, and lost the ability to fertilize their eggs, impeding reversals to sexual reproduction. Female sexual traits were decayed even in recently derived asexuals, suggesting that trait changes following the evolution of asexuality, when they occur, proceed rapidly and are driven by selective processes rather than drift.
Keywords: regressive evolution, trait decay, relaxed selection, asexuality
1. Introduction
Studies on how natural selection favours adaptations typically focus on the evolution of novel traits [1–4]. However, the fate of traits that no longer contribute to fitness can also highlight the importance of natural selection for adaptive traits [5–8]. Formerly adaptive traits may become non-functional, or even maladaptive, as a consequence of environmental shifts or changes in lifestyle, accompanied by changes in selective pressures [5,8]. Such traits often decay and in some cases may disappear completely.
Trait decay represents a form of regressive evolution, which Haldane suggested may be more than tenfold more common than adaptive evolution towards more complex phenotypes [4]. It can occur via different mechanisms, depending on whether the formerly adaptive trait is neutral or maladaptive in the new selective environment. In the first case, the trait would be under relaxed selection, whereby trait-affecting mutations that would have been removed by selection under the past conditions may accumulate and fix via drift [9,10]. This process of neutral mutation accumulation would be expected to proceed slowly (reviewed in [11]). In the second case, the trait would be expected to regress rapidly, driven by selection for reduced or modified trait expression [5,12,13]. However, trait decay is not necessarily accompanied by a degeneration of the associated molecular pathways. Maintenance of pathways may stem, for example, from pleiotropy, where the same gene networks function in several processes [5]. Thus, even if a character is phenotypically absent, the genetic information responsible for its development can remain quiescent, occasionally resulting in character expression.
An exceptionally large range of traits is expected to decay in asexually reproducing organisms. All higher asexual eukaryotes derive from sexual ancestors [14], and many ‘sexual traits’ (that is, traits involved in mate location and attraction, mating, or traits specific to the male sex) should become useless or maladaptive after the transition to asexuality, as demonstrated in previous studies [15–18].
Distinguishing selection from drift is a major challenge in studies of trait decay following a change in the selective environment [5,16], but the fate of sexual traits in asexuals can provide insights into both of these processes. The majority of asexual lineages produce either no males, or may produce occasional males that do not have mating opportunities or success in natural populations [19–21]. Male-specific traits are therefore not exposed to selection in asexuals, such that male trait decay would either stem from neutral mutation accumulation [22] or pleiotropic effects expressed in females.
In contrast to the male-specific traits, certain sexual traits expressed in females (for example, those involved in mate attraction) are unlikely to be neutral in asexuals. In addition to possible energy costs associated with the production of mate attraction signals, pheromones or acoustic signals are prime targets for predators and parasitoids to locate their prey [13,23]. Thus, the production of such signals should be under strong negative selection in asexuals, where mate attraction is superfluous.
Few previous studies have analysed the causes and rates of trait decay, especially in phylogenetic contexts that allow for replicated inferences concerning patterns of evolutionary change. Here, we investigate the fate of a suite of sexual traits, including male-specific traits, in five independently derived asexual stick insects in the genus Timema. Because the transitions from sexual reproduction to asexuality have occurred at different times in these lineages (some lineages are recently derived asexuals while others have been asexual for over one million years [24,25]), a temporal component can be included, with a higher level of sexual trait decay expected in old when compared with young asexual lineages. Our results show that neutral traits display little or no regression even in old asexuals, whereas (presumably) selected traits display major shifts already in young asexuals, such that reversals to sexual reproduction are unlikely, even for recently derived asexual lineages.
2. Material and methods
Five different asexual Timema species have been described based on morphological and host plant information [26–28], and previous studies revealed that these species correspond to at least seven independent transitions to asexuality, ranging in age from recent to old (table 1). We investigated sexual traits in five of the seven asexual lineages (ranked by increasing age: T. shepardi, T. douglasi ‘south’, T. genevievae, T. tahoe and T. monikensis), as well as in their closest sexual sister species (table 1).
Table 1.
Independently derived asexual Timema lineages and their sexual sister species, ranked by increasing upper age estimates (age estimates from [25], based on intra- and interspecific molecular divergences). The asexual lineages in bold were included in this study.
| asexual species | age range (1000 yr) | sexual sister species |
|---|---|---|
| T. shepardi | 100–400 | T. californicum |
| T. douglasi (‘central’) | 100–450 | T. poppensis |
| T. douglasi (‘south’) | 700–600 | T. poppensis |
| T. douglasi (‘north’) | 1000–950 | T. poppensis |
| T. genevievae | 1500–1450 | T. podura |
| T. tahoe | 450–1550 | T. bartmani |
| T. monikensis | 100–1850 | T. cristinae |
During our field collections in spring 2007–2012, we found 11 asexually produced males (see §3). For seven of these 11 males, we were able to evaluate courtship behaviour and fertilization success by pairing these males with virgin females of their respective sexual sister species. Although asexually produced males do not currently encounter sexual females in natural populations because of their non-overlapping distribution ranges, we used sexual females for these pairings because they were more likely to elicit sexual behaviour in males than the asexual females, and because we were unable to obtain copulations of asexually produced males with females of their own species, despite repeated efforts.
Male courtship behaviour varies little among sexual Timema species. It is initiated by the male after climbing onto the female's dorsal surface, and involves ‘leg waving’ (rapidly kicking posterior legs to the side, whereby different species kick with either two or four posterior legs), followed by ‘antennae waving’ and copulation attempts [29]. We evaluated whether the asexually produced males used this same courtship sequence, and whether they waved the same posterior legs as their sexual sister species.
Sperm functionality in the seven asexually produced males was evaluated in crosses with virgin females of the sexual species (two per male). In addition, to test whether asexually produced males were able to also fertilize eggs when in (sperm) competition with other males, each of them was mated to two additional females, either 24 h before or after these females mated with a conspecific male. The 28 mated females were then maintained individually in plastic cups with cuttings of their host plant and allowed to lay about 40 eggs each (which takes 25–40 days). Offspring hatching from these eggs, as well as their (putative) parents, were genotyped at the four microsatellites Tim1, Tim4, Tim6 and Tim7 [30] to assess paternity. The three of the 28 females that died before they laid enough eggs (one singly and two doubly mated females) were replaced by new females mated to the dead females' mating partner(s).
To evaluate the fate of female-specific sexual traits in asexuals, we considered pheromone production and attractiveness to males, as well as the fate of the spermatheca (a pocket where sperm is stored [31]) and egg fertilization. Previous behavioural experiments provided evidence for airborne and contact pheromonal cues produced by females in the sexual species [29,32]. We therefore evaluated the attractiveness of asexual females to males of their sexual sister species at longer distances as well as upon contact.
To test whether asexual females emit pheromonal signals attractive to males, we conducted two-choice trials in horizontal Y-mazes (stem and side arms: 10 cm long × 3.5 cm diameter each; angle between side arms: 120°). By random assignment, the orifice of a side arm received a food leaf with or without a female within a mesh-covered glass tube. Females were left in the maze for at least 15 min before a male was introduced. Fifteen minutes after introducing a male, we recorded whether he had walked two-thirds of the length of the female-containing side arm. Males of the five sexual species were tested against females of three groups: (i) asexual females (of the sister-lineage to the male's species; table 1), (ii) sexual females of the males' own species and (iii) sexual females of a different species. The sexual females of a different species were used as controls; some degree of reduced attractiveness of asexual females to males of their closest sexual sister species may be expected because of between-species differences rather than because of asexuality. For each of the five sexual species, we conducted 109–211 trials (30–98 for each of the three female groups), for a total of 751 two-choice trials, with different individuals used in every trial. The proportion of trials in which males chose the female-containing tube, out of the total number of trials conducted for a given female group, was considered proportional to the ‘attractiveness’ of putative airborne pheromonal cues emitted by females.
Contact attractiveness of asexual females was tested in no-choice trials, which were conducted by introducing one male and one female into a 6 cm Petri dish and recording after 1 h whether the pair was in copula [29,33]. In each replicate, we paired a male of the five focal sexual species with a female of their asexual sister species, or with a female of their own species (with 20–106 trials per species combination). Similar to the Y-maze tests, we also used females of other sexual species as a control for reduced attractiveness because of species divergences. However, we used females from eight different sexual species instead of only one, ranging from closely to distantly related to the species of the focal males (figure 1). Mating propensity for each male species by female species combination was then estimated as the proportion of copulating pairs out of the total number of trials conducted for that combination (see the electronic supplementary material, table S1 for sample sizes per species combination).
Figure 1.
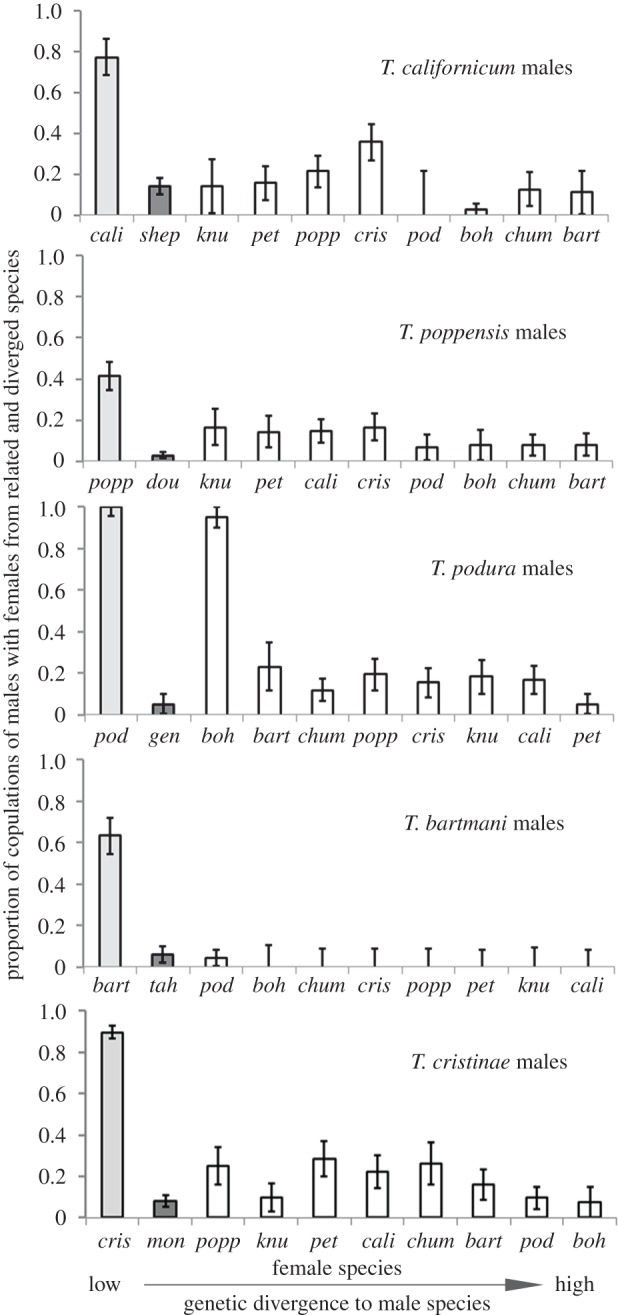
Females of asexual species are characterized by reduced sexual attractiveness to males of closely related sexual species, whereby this reduction is not solely explained by genetic divergence between species. Indicated is the proportion of copulations of sexual males with females of their own species (light grey bars), their asexual sister species (dark grey bars) and related sexual species (white bars). Female species are ordered according to increasing nuclear genetic divergence from focal males, with intraspecific matings first and matings with females from the most distantly related species last. bart, T. bartmani; boh, T. boharti; cali, T. californicum; chum, T. chumash; cris, T. cristinae; gen, T. genevievae; knu, T. knulli; pet, T. petita; pod, T. podura; popp, T. poppensis; shep, T. shepardi; tah, T. tahoe.
Because a previous study [29] suggested that cuticular hydrocarbons of females may provide signals for mate discrimination in sexual Timema species, we quantified the relative amounts of six hydrocarbon components (3-, 5-, 7-, 9-, 11- and 13-methylheptacosane) on the cuticule of five to 10 virgin adult females (for methods see the electronic supplementary material). To quantify the extent of the profile shift between each asexual lineage and its sexual sister species, we calculated the average of multivariate Euclidian distances (where each hydrocarbon component defines an axis in a multi-dimensional space) between all sexual and asexual individuals of a species pair. We then compared this distance with the profile differences (also measured by Euclidian distance averages) between sexual species pairs. The removal of selection on females to produce male-destined cues may also result in asexual females producing less precise (i.e. more variable) cues. We therefore determined for each species the hydrocarbon profile variability, by calculating the average profile distance between two individuals, and compared this distance between reproductive modes.
We also tested whether eggs produced by asexual females could still be fertilized, and whether egg hatching success for asexual females was affected by copulations with males (as there could be interference between sperm and asexual embryo development). We aimed at obtaining 10 mated females per asexual species. Because very few asexual females mated during the standardized no-choice mating trials (see §3), we paired numerous asexual females with males of their sexual sister species for up to 8 h and checked regularly for copulations. The mated asexual females were maintained individually in plastic cups with cuttings of their host plant and allowed to lay eggs for approximately three weeks. The females were dissected to verify that their spermatheca contained sperm, and each female, her mating partner and four to eight of her offspring were microsatellite-genotyped to determine whether the males' sperm was included in the offspring's genome. For each asexual species, the egg hatching success of the 10 mated females was compared with the hatching success of 20 unmated females that, except for the mating trials, had been treated and maintained in the same way as the mated females.
Finally, we compared the spermathecae of the sexual and asexual Timema to test for decay of a morphological trait. Timema females have spermatheca comprising two separate pockets (figure 2) [31]. We measured the maximum length and height of both pockets of 10–13 virgin females per species on pictures taken with a Motic Images 2000USB camera on a Zeiss Stemi SV 6 stereo microscope (at 50× magnification) using Motic Images Plus v. 2.0 (Motic Group Co., Ltd.).
Figure 2.
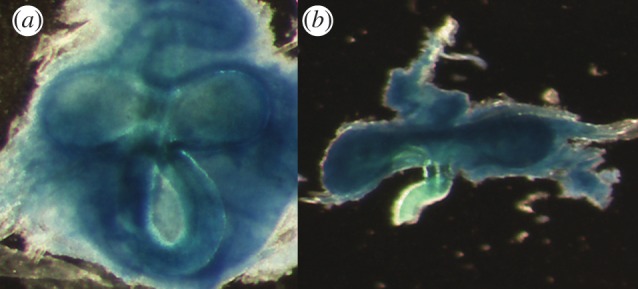
Spermatheca of (a) a sexual female (T. californicum) and (b) an asexual female (T. shepardi) at 50× magnification. (Online version in colour.)
3. Results
Males are produced at a very low rate by the asexual lineages. Out of over 5000 individuals collected in the field, only 11 were males: three T. shepardi, two T. douglasi ‘south’, five T. monikensis and one T. genevievae. No male has thus far been found for T. tahoe. We found no evidence for decay of courtship behaviour when pairing asexually produced males with females of their sexual sister species, although their small numbers precluded a quantitative assessment. The courtship sequences performed by these males were identical to those described for sexual males. Furthermore, T. shepardi and T. douglasi ‘south’ males used two posterior legs for leg waving, similar to their sexual sister species T. californicum and T. poppensis, whereas T. monikensis and T. genevievae males used four legs, matching the pattern described for T. cristinae and T. podura.
Each asexually produced male fathered offspring when mated to virgin females of his sexual sister species, indicating that these males still produce functional sperm. The hatching success among eggs from these crosses was low (mean ± s.d.: 29.5 ± 15.2%), but it is not possible to assess whether low hatching success is a consequence of poor sperm quality or hybrid breakdown, given that at least the older sexual–asexual species pairs have diverged for over a million years (table 1). Asexually produced males fathered no offspring, however, when these males copulated with a sexual female that also mated with a male of her own species. Microsatellite genotyping revealed that not a single offspring produced by these doubly mated females was fathered by a male of the asexual lineages, independently of whether this male was the female's first or second mating partner. The hatching rate among eggs produced by these doubly mated females (mean ± s.d.: 42.1 ± 16.7%) was also significantly higher than the hatching rate of eggs from females mated only to an asexually produced male (F1,26 = 4.3, p = 0.047).
In contrast to the maintained male traits, we found evidence for decay for each of the investigated female sexual traits. Females of all five asexual lineages were significantly less attractive to males than sexual females at both long range (mediated by a volatile signal) and upon contact. In a Y-maze, males approached the asexual females significantly less often than the sexual females of their own species (Fisher's exact tests, all p < 0.05). This loss of attractiveness was not solely due to genetic divergence between sexual and asexual species, as asexual females were as unattractive to males as females of diverged sexual species for four of the five asexual lineages tested (T. douglasi ‘south’, T. genevievae, T. tahoe and T. monikensis; Fisher's exact tests, all p > 0.4). Only females of the youngest asexual, T. shepardi, were more attractive to males of their sexual sister species than females of a diverged sexual species (Fisher's exact test: p = 0.041).
Reduced attractiveness of asexual females to sexual males was even more pronounced in no-choice mating trials. Males of four of the five sexual species courted and copulated significantly less often with females of their asexual sister species than with sexual females, irrespective of the genetic divergence between the male and the sexual female (all p < 0.01; figure 1). For the fifth sexual species, T. bartmani, the proportion of copulations with females of the asexual T. tahoe was comparable with the proportion of copulations with females of the closest sexual species, T. podura (figure 1). Furthermore, asexual females actively resisted many copulation attempts from males by moving their abdomen away (T.S. 2007–2012, personal observation) while this behaviour is only rarely observed among sexual females [29] (T.S. 2007–2012, personal observation).
Consistent with the decayed attractiveness of asexual females, asexual females displayed hydrocarbon profiles more variable than and distinct from those of females of their sexual sister species. The hydrocarbon profile divergences between females of the young asexual T. shepardi and its sexual sister T. californicum were significant but relatively small (MANOVA; Wilks's λ = 0.2, F1,18 = 6.7, p < 0.001), while profile divergences were considerable for all other sexual–asexual species pairs (MANOVA; Wilks's λ: 0.001–0.1, all p < 0.0001). Within each of the sexual–asexual species pairs, the profiles were more variable among asexual than sexual females (paired t-test: t4 = 4.3, p = 0.01; figure 3).
Figure 3.
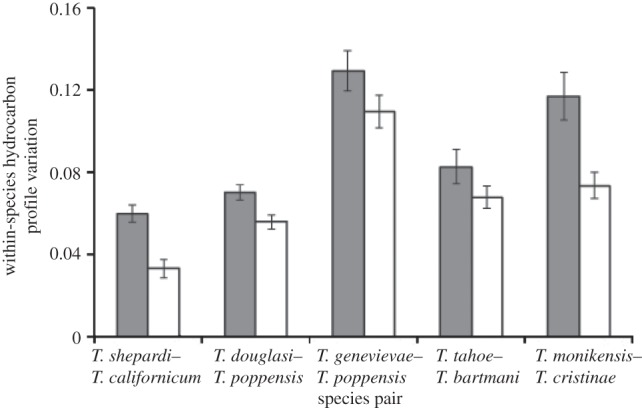
Within-species hydrocarbon profile variation (measured as the average profile distance between two individuals) for asexual Timema lineages (grey bars) and their sexual sister species (white bars).
As in a previous study of spermathecae in sexual and asexual wasps [34], spermathecal morphology clearly differed between the sexual and asexual Timema. The two spermathecae pockets of virgin sexual females were spherical, whereas they were flattened in asexual females (reflected by significantly different length/width ratios between sexual and asexual females within each species pair; F1,100 = 9.5, p = 0.003). Within each sexual–asexual species pair, the two spermatheca pockets were more asymmetric for asexual females (F1,100 = 5.3, p = 0.02), possibly reflecting decreased developmental stability in asexuals. Even though males generally transferred sperm when copulating with asexual females (we found spermatozoa in the spermatheca of 44 of the 50 mated asexual females), we found no evidence for fertilization of asexually produced eggs. Out of 322 offspring genotyped, none had any genetic contribution from the males. The hatching rate of eggs laid by asexual females was also not affected by whether the females had copulated with a male (F1,98 = 0.48; p = 0.42).
4. Discussion
We investigated the fate of sexual traits in five independently derived asexual stick insect lineages, including old and recently derived ones. At least four of five lineages are still able to produce functional males, although these males, given the lack of partners for reproduction, appear to have zero fitness in natural populations. Sexual Timema species are characterized by an XX : XO sex determination system (males are the heterogametic sex, carrying only a single X chromosome [30]). Most likely, male development in the Timema asexuals follows an accidental loss of an X chromosome during oogenesis, whereby the existence of such asexually produced males indicates that the molecular pathways underlying male development remain functional.
The maintenance of male developmental pathways under asexuality seems to be widespread, as occasional production of males has been reported in a range of asexual lineages (e.g. Potamopyrgus antipodarum snails [35], Saga pedo crickets [36], thrips [21] and most asexual hymenopterans [37]). Notably, males are not only produced in recently derived asexual linages but have even been found in darwinulid ostracods [38] and oribatid mites [20], groups that may have been asexual for over 100 million years [39,40]. Tests for trait decay in asexually produced males have been conducted in hymenopterans with endosymbiont-induced parthenogenesis [37,41,42], where males are produced by asexual females cured of their endosymbionts [43]. With few exceptions [44], these males appear fully functional, produce viable sperm [37,41,45,46] and sometimes even father similar numbers of offspring as sexual males [47,48] when mated to females from related sexual strains or species. Only in exceptional instances, notably in ‘ancient’ asexuals, has male functionality appeared to have decayed, as suggested by the lack of sperm in the darwinulid males [49] and the production of non-functional spermatophores by males in the oribatid mites [20].
In Timema, we found that asexually produced males only successfully fathered offspring when the sole mating partner of sexual females. They fathered no offspring in situations of sperm competition with sexual males. Whether this effect is due to sperm from asexual males performing poorly in situations of sperm competition or to cryptic female choice remains to be investigated. Independently of the mechanism, this result is unlikely to stem from sexual trait decay in these males. Rather, it could indicate the improved competitiveness of the sexual males' sperm, driven by ongoing intra-sexual conflict, relative to sperm properties present several thousand generations earlier and ‘frozen’ as such in the asexuals’ genomes.
Males in a minority of asexual lineages (notably asexuals recently derived from cyclical parthenogenetic ancestors such as Daphnia waterfleas and aphids) successfully mate with females of related sexual lines and thereby generate new asexual lineages (‘contagious parthenogenesis’ [50,51]). Contagious parthenogenesis can account for the maintenance of male developmental pathways in asexuals, given the mating success of asexually produced males. However, there is currently little evidence that contagious parthenogenesis occurs in natural populations of species outside the cyclical parthenogens [50,52]. In the absence of mating success for males produced by asexual females, the maintenance of male functionality could stem from three non-exclusive mechanisms. First, male developmental and physiological pathways may consist almost exclusively of components with pleiotropic effects on both sexes. When, by some rare accident, the male developmental pathway is triggered, it would therefore still generate functional males. Second, significant levels of trait decay may typically occur via selective processes rather than by drift; it may well be that neutral decay would require hundreds of millions of years, a time frame rarely reached by asexual lineages [53]. Finally, the development of functional males may indicate that lineages presumed to be asexual have some low level of cryptic sex. However, although formally demonstrating the lack of sexual reproduction in a lineage is challenging [53], this explanation is unlikely in groups that display the genomic signatures of asexuality, such as Timema [25,30,54], and is difficult to reconcile with decay of sexual traits in females, reported in cases with functional males [37] (this study).
Indeed, all female sexual traits investigated in Timema displayed significant shifts in asexual females relative to the sexual sister species. Spermatheca morphology and the ability to fertilize eggs seem to have decayed since the abandonment of the formerly sexual lifestyle. The decay of long- and short-distance mate attraction in asexuals, in addition to potential mate recognition cues being more variable in asexual when compared with sexual females, is also consistent with decayed sexual signal production. However, if copulating with males incurs costs to asexual females, then traits that decrease the probability of matings may be selectively favoured, and decreased mating probability may stem from signals with novel compositions, rather than from reduced signal expression.
Although more extensive sexual decay may be predicted for old when compared with young asexuals, we found little effect of the age of Timema asexuals on sexual trait expression. For male traits, considered to be neutral, we found no regression in any of the asexuals, whereas for female traits, the effect sizes of the trait shifts were mostly unrelated to how long a given lineage has been asexual. The sole exception is attractiveness to sexual males, where only females of the youngest asexual, T. shepardi, appeared to be somewhat attractive to males of their sexual sister (T. californicum). This lack of strong differences between young and old asexuals may indicate that decay of female sexual traits is generally selectively favoured, such that it would spread rapidly enough to occur even in recently derived asexuals (younger than 100 000 years). Consistent with this view, studies of other asexual lineages, most of them representing very recently derived asexuals, have reported reduced mate attraction and mating propensity of asexual when compared with sexual females. Thus, females of Drosophila mercatorum parthenogenetic strains showed reduced mating propensity compared with females of sexual strains [15]. In bushcrickets, where males of sexual species attract females with songs, the asexual species Poecilimon intermedius has lost phonotaxis [18,55]. Female mating behaviour is also strongly reduced or lost in many species with endosymbiont-induced parthenogenesis [41,42,47] (but see [43]). Although Potamopyrgus snails, where females appear to play a passive role in mate finding and copulation, may represent an exception to this pattern [56], these parallel and rapid losses of different female traits involved in mate attraction and mating suggest that selective mechanisms, rather than drift, are driving trait changes.
In conclusion, we find parallel decay of female sexual traits and the maintenance of male traits across independently derived asexual Timema lineages. The lack of decay for male traits may stem from pleiotropy, or from these traits only regressing via drift, which may require more than a million years (i.e. the approximate age of the oldest Timema asexuals) to generate strong phenotypic effects. Sexual trait decay in females was apparently not affected by how long a lineage has been asexual, suggesting that trait shifts caused by asexuality, if they occur, proceed rapidly and are probably more driven by selective processes than by drift.
Data accessibility
Data archived in the Dryad repository under doi:10.5061/dryad.sj6p8.
References
- 1.Cracraft J. 1990. The origin of evolutionary novelties: pattern and process at different hierarchical levels. In Evolutionary innovations (ed. Nitecki MH.), pp. 21–44 Chicago, IL: University of Chicago Press [Google Scholar]
- 2.Brakefield PM. 2011. Evo-devo and accounting for Darwin's endless forms. Phil. Trans. R. Soc. B 366, 2069–2075 10.1098/rstb.2011.0007 (doi:10.1098/rstb.2011.0007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moczek AP. 2008. On the origins of novelty in development and evolution. BioEssays 30, 432–447 10.1002/bies.20754 (doi:10.1002/bies.20754) [DOI] [PubMed] [Google Scholar]
- 4.Moczek AP, Sultan S, Foster S, Ledon-Rettig C, Dworkin I, Nijhout HF, Abouheif E, Pfennig DW. 2011. The role of developmental plasticity in evolutionary innovation. Proc. R. Soc. B 278, 2705–2713 10.1098/rspb.2011.0971 (doi:10.1098/rspb.2011.0971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong DW, Kane TC, Culver DC. 1995. Vestigialization and loss of nonfunctional characters. Annu. Rev. Ecol. Syst. 26, 249–268 10.1146/annurev.es.26.110195.001341 (doi:10.1146/annurev.es.26.110195.001341) [DOI] [Google Scholar]
- 6.Wiens JJ. 2001. Widespread loss of sexually selected traits: how the peacock lost its spots. Trends Ecol. Evol. 16, 517–523 10.1016/s0169-5347(01)02217-0 (doi:10.1016/s0169-5347(01)02217-0) [DOI] [Google Scholar]
- 7.Porter ML, Crandall KA. 2003. Lost along the way: the significance of evolution in reverse. Trends Ecol. Evol. 18, 541–547 10.1016/s0169-5347(03)00244-1 (doi:10.1016/s0169-5347(03)00244-1) [DOI] [Google Scholar]
- 8.Lahti DC, Johnson NA, Ajie BC, Otto SP, Hendry AP, Blumstein DT, Coss RG, Donohue K, Foster SA. 2009. Relaxed selection in the wild. Trends Ecol. Evol. 24, 487–496 10.1016/j.tree.2009.03.010 (doi:10.1016/j.tree.2009.03.010) [DOI] [PubMed] [Google Scholar]
- 9.Lande R. 1978. Evolutionary mechanisms of limb loss in tetrapods. Evolution 32, 73–92 10.2307/2407411 (doi:10.2307/2407411) [DOI] [PubMed] [Google Scholar]
- 10.Lahti DC. 2006. Persistence of egg recognition in the absence of cuckoo brood parasitism: pattern and mechanism. Evolution 60, 157–168 10.1554/05-052.1 (doi:10.1554/05-052.1) [DOI] [PubMed] [Google Scholar]
- 11.Teotónio H, Rose MR. 2000. Variation in the reversibility of evolution. Nature 408, 463–465 10.1038/35044070 (doi:10.1038/35044070) [DOI] [PubMed] [Google Scholar]
- 12.Prout T. 1964. Observations on structural reduction in evolution. Am. Nat. 98, 239–249 10.1086/282323 (doi:10.1086/282323) [DOI] [Google Scholar]
- 13.Zuk M, Rotenberry JT, Tinghitella RM. 2006. Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol. Lett. 2, 521–524 10.1098/rsbl.2006.0539 (doi:10.1098/rsbl.2006.0539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell G. 1982. The masterpiece of nature: the evolution and genetics of sexuality, p. 635 Berkeley, CA: University of California Press [Google Scholar]
- 15.Carson HL, Chang LS, Lyttle TW. 1982. Decay of female sexual-behavior under parthenogenesis. Science 218, 68–70 10.1126/science.218.4567.68 (doi:10.1126/science.218.4567.68) [DOI] [PubMed] [Google Scholar]
- 16.Dorken ME, Neville KJ, Eckert CG. 2004. Evolutionary vestigialization of sex in a clonal plant: selection versus neutral mutation in geographically peripheral populations. Proc. R. Soc. Lond. B 271, 2375–2380 10.1098/rspb.2004.2875 (doi:10.1098/rspb.2004.2875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pannebakker BA, Beukeboom LW, van Alphen JJ, Brakefield PM, Zwaan BJ. 2004. The genetic basis of male fertility in relation to haplodiploid reproduction in Leptopilina clavipes (Hymenoptera: Figitidae). Genetics 168, 341–349 10.1534/genetics.104.027680 (doi:10.1534/genetics.104.027680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehmann GUC, Siozios S, Bourtzis K, Reinhold K, Lehmann AW. 2011. Thelytokous parthenogenesis and the heterogeneous decay of mating behaviours in a bushcricket (Orthopterida). J. Zool. Syst. Evol. Res. 49, 102–109 10.1111/j.1439-0469.2010.00588.x (doi:10.1111/j.1439-0469.2010.00588.x) [DOI] [Google Scholar]
- 19.Scali V. 1968. Biologia riproduttiva del Bacillus rossius (Rossi) nei dintorni di Pisa con particolare riferimento all'influenza del fotoperiodo. Atti Soc. Tosc. Sc. Nat. 75, 108–139 [Google Scholar]
- 20.Taberly G. 1988. Recherches sur la parthénogenèse thélytoque de deux espèces d'acariens oribates: Trhypochthonius tectorum (Berlese) et Platynothrus peltifer (Koch). IV. Observations sur les mâles ataviques. Acarologia 29, 95–107 [Google Scholar]
- 21.Mirab-Balou M, Chen X-X. 2010. First description of the male of the wheat thrips, Anaphothrips obscurus (Thysanoptera: Thripidae). Zootaxa 2540, 65–68 [Google Scholar]
- 22.Masel J, King OD, Maughan H. 2007. The loss of adaptive plasticity during long periods of environmental stasis. Am. Nat. 169, 38–46 10.1086/510212 (doi:10.1086/510212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuk M, Kolluru GR. 1998. Exploitation of sexual signals by predators and parasitoids. Q. Rev. Biol. 73, 415–438 10.1086/420412 (doi:10.1086/420412) [DOI] [Google Scholar]
- 24.Law JH, Crespi BJ. 2002. The evolution of geographic parthenogenesis in Timema walking-sticks. Mol. Ecol. 11, 1471–1489 10.1046/j.1365-294X.2002.01547.x (doi:10.1046/j.1365-294X.2002.01547.x) [DOI] [PubMed] [Google Scholar]
- 25.Schwander T, Henry L, Crespi BJ. 2011. Molecular evidence for ancient asexuality in Timema stick insects. Curr. Biol. 21, 1129–1134 10.1016/j.cub.2011.05.026 (doi:10.1016/j.cub.2011.05.026) [DOI] [PubMed] [Google Scholar]
- 26.Sandoval CP, Carmean DA, Crespi BJ. 1998. Molecular phylogenetics of sexual and parthenogenetic Timema walking-sticks. Proc. R. Soc. Lond. B 265, 589–595 10.1098/rspb.1998.0335 (doi:10.1098/rspb.1998.0335) [DOI] [Google Scholar]
- 27.Vickery VR, Sandoval CP. 1999. Two new species of Timema (Phasmoptera: Timematodea: Timematidae), one parthenogenetic, in California. J. Orthoptera Res. 8, 41–43 10.2307/3503424 (doi:10.2307/3503424) [DOI] [Google Scholar]
- 28.Vickery VR, Sandoval CP. 2001. Description of three new species of Timema (Phasmoptera: Timematodea: Timematidae) and notes on three other species. J. Orthoptera Res. 10, 53–61 10.1665/1082-6467(2001)010[0053:DOTNSO]2.0.CO;2 (doi:10.1665/1082-6467(2001)010[0053:DOTNSO]2.0.CO;2) [DOI] [Google Scholar]
- 29.Arbuthnott D, Crespi BJ. 2009. Courtship and mate discrimination within and between species of Timema walking-sticks. Anim. Behav. 78, 53–59 10.1016/j.anbehav.2009.02.028 (doi:10.1016/j.anbehav.2009.02.028) [DOI] [Google Scholar]
- 30.Schwander T, Crespi BJ. 2009. Multiple direct transitions from sexual reproduction to apomictic parthenogenesis in Timema stick insects. Evolution 63, 84–103 10.1111/j.1558-5646.2008.00524.x (doi:10.1111/j.1558-5646.2008.00524.x) [DOI] [PubMed] [Google Scholar]
- 31.Tilgner EH, Kiselyova TG, McHugh JV. 1999. A morphological study of Timema cristinae Vickery with implications for the phylogenetics of Phasmida. Dtsche. Entomol. Z. 46, 149–162 10.1002/mmnd.19990460203 (doi:10.1002/mmnd.19990460203) [DOI] [Google Scholar]
- 32.Nosil P, Crespi BJ, Gries R, Gries G. 2007. Natural selection and divergence in mate preference during speciation. Genetica 129, 309–327 10.1007/s10709-006-0013-6 (doi:10.1007/s10709-006-0013-6) [DOI] [PubMed] [Google Scholar]
- 33.Nosil P, Crespi BJ, Sandoval CP. 2002. Host-plant adaptation drives the parallel evolution of reproductive isolation. Nature 417, 440–443 10.1038/417440a (doi:10.1038/417440a) [DOI] [PubMed] [Google Scholar]
- 34.Kraaijeveld K, Franco P, Reumer BM, van Alphen JJ. 2009. Effects of parthenogenesis and geographic isolation on female sexual traits in a parasitoid wasp. Evolution 63, 3085–3096 10.1111/j.1558-5646.2009.00798.x (doi:10.1111/j.1558-5646.2009.00798.x) [DOI] [PubMed] [Google Scholar]
- 35.Neiman M, Larkin K, Thompson AR, Wilton P. 2012. Male offspring production by asexual Potamopyrgus antipodarum, a New Zealand snail. Heredity 109, 57–62 10.1038/hdy.2012.13 (doi:10.1038/hdy.2012.13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baur H. 2010. Das Männchen von Saga pedo. Mainz, Germany: Jahrestagung der Deutschen Gesellschaft für Orthopterologie.
- 37.Bordenstein SR. 2003. Symbiosis and the origin of species. In Insect symbiosis (eds Bourtzis K, Miller TA.), pp. 283–304 New York, NY: CRC Press [Google Scholar]
- 38.Smith RJ, Kamiya T, Horne DJ. 2006. Living males of the ‘ancient asexual’ Darwinulidae (Ostracoda: Crustacea). Proc. R. Soc. B 273, 1569–1578 10.1098/rspb.2005.3452 (doi:10.1098/rspb.2005.3452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martens K, Rossetti G, Horne DJ. 2003. How ancient are ancient asexuals? Proc. R. Soc. Lond. B 270, 723–729 10.1098/rspb.2002.2270 (doi:10.1098/rspb.2002.2270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heethoff M, Domes K, Laumann M, Maraun M, Norton RA, Scheu S. 2007. High genetic divergences indicate ancient separation of parthenogenetic lineages of the oribatid mite Platynothrus peltifer (Acari, Oribatida). J. Evol. Biol. 20, 392–402 10.1111/j.1420-9101.2006.01183.x (doi:10.1111/j.1420-9101.2006.01183.x) [DOI] [PubMed] [Google Scholar]
- 41.Pannebakker BA, Schidlo NS, Boskamp GJ, Dekker L, van Dooren TJ, Beukeboom LW, Zwaan BJ, Brakefield PM, van Alphen JJ. 2005. Sexual functionality of Leptopilina clavipes (Hymenoptera: Figitidae) after reversing Wolbachia-induced parthenogenesis. J. Evol. Biol. 18, 1019–1028 10.1111/j.1420-9101.2005.00898.x (doi:10.1111/j.1420-9101.2005.00898.x) [DOI] [PubMed] [Google Scholar]
- 42.Kremer N, Charif D, Henri H, Bataille M, Prevost G, Kraaijeveld K, Vavre F. 2009. A new case of Wolbachia dependence in the genus Asobara: evidence for parthenogenesis induction in Asobara japonica. Heredity 103, 248–256 10.1038/hdy.2009.63 (doi:10.1038/hdy.2009.63) [DOI] [PubMed] [Google Scholar]
- 43.Stouthamer R, Luck RF, Hamilton WD. 1990. Antibiotics cause parthenogenetic Trichogramma (Hymenoptera/Trichogrammatidae) to revert to sex. Proc. Natl Acad. Sci. USA 87, 2424–2427 10.1073/pnas.87.7.2424 (doi:10.1073/pnas.87.7.2424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gottlieb Y, Zchori-Fein E. 2001. Irreversible thelytokous reproduction in Muscidifurax uniraptor. Entomol. Exp. Appl. 100, 271–278 10.1046/j.1570-7458.2001.00874.x (doi:10.1046/j.1570-7458.2001.00874.x) [DOI] [Google Scholar]
- 45.Zchori-Fein E, Roush RT, Hunter MS. 1992. Male production induced by antibiotic treatment in Encarsia formosa (Hymenoptera: Aphelinidae), an asexual species. Experientia 48, 102–105 10.1007/BF01923619 (doi:10.1007/BF01923619) [DOI] [Google Scholar]
- 46.Zchori-Fein E, Zeidan FM, Gottlieb Y, Rosen D. 1995. Parthenogenesis-inducing microorganisms in Aphytis (Hymenoptera: Aphelinidae). Insect Mol. Biol. 4, 173–178 10.1111/j.1365-2583.1995.tb00023.x (doi:10.1111/j.1365-2583.1995.tb00023.x) [DOI] [PubMed] [Google Scholar]
- 47.Pijls JWAM, Van Steenbergen HJ, van Alphen JJ. 1996. Asexuality cured: the relations and differences between sexual and asexual Apoanagyrus diversicornis. Heredity 76, 506–513 10.1038/hdy.1996.73 (doi:10.1038/hdy.1996.73) [DOI] [Google Scholar]
- 48.Arakaki N, Noda H, Yamagishi K. 2000. Wolbachia-induced parthenogenesis in the egg parasitoid Telenomus nawai. Entomol. Exp. Appl. 96, 177–184 10.1046/j.1570-7458.2000.00693.x (doi:10.1046/j.1570-7458.2000.00693.x) [DOI] [Google Scholar]
- 49.Schön I, Rossetti G, Martens K. 2009. Darwinulid ostracods: ancient asexual scandals or scandalous gossip? In Lost sex: the evolutionary biology of parthenogenesis (eds Schön I, Martens K, Van Dijk PJ.), pp. 217–240 Dordrecht, The Netherlands: Springer Academic Publishers [Google Scholar]
- 50.Simon JC, Delmotte F, Rispe C, Crease T. 2003. Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals. Biol. J. Linn. Soc. 79, 151–163 10.1046/j.1095-8312.2003.00175.x (doi:10.1046/j.1095-8312.2003.00175.x) [DOI] [Google Scholar]
- 51.Paland S, Colbourne JK, Lynch M. 2005. Evolutionary history of contagious asexuality in Daphnia pulex. Evolution 59, 800–813 [PubMed] [Google Scholar]
- 52.Sandrock C, Schirrmeister BE, Vorburger C. 2011. Evolution of reproductive mode variation and host associations in a sexual–asexual complex of aphid parasitoids. BMC Evol. Biol. 11, 348. 10.1186/1471-2148-11-348 (doi:10.1186/1471-2148-11-348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schurko AM, Neiman M, Logsdon JM. 2009. Signs of sex: what we know and how we know it. Trends Ecol. Evol. 24, 208–217 10.1016/j.tree.2008.11.010 (doi:10.1016/j.tree.2008.11.010) [DOI] [PubMed] [Google Scholar]
- 54.Maderspacher F. 2011. Asexuality: the insects that stick with it. Curr. Biol. 21, R495–R497 10.1016/j.cub.2011.06.010 (doi:10.1016/j.cub.2011.06.010) [DOI] [PubMed] [Google Scholar]
- 55.Lehmann GU, Strauss J, Lakes-Harlan R. 2007. Listening when there is no sexual signalling? Maintenance of hearing in the asexual bushcricket Poecilimon intermedius. J. Comp. Physiol. A 193, 537–545 10.1007/s00359-007-0209-y (doi:10.1007/s00359-007-0209-y) [DOI] [PubMed] [Google Scholar]
- 56.Nelson AE, Neiman M. 2011. Persistent copulation in asexual female Potamopyrgus antipodarum: evidence for male control with size-based preferences. Int. J. Evol. Biol. 2011, 439046. 10.4061/2011/439046 (doi:10.4061/2011/439046) [DOI] [PMC free article] [PubMed] [Google Scholar]


