Abstract
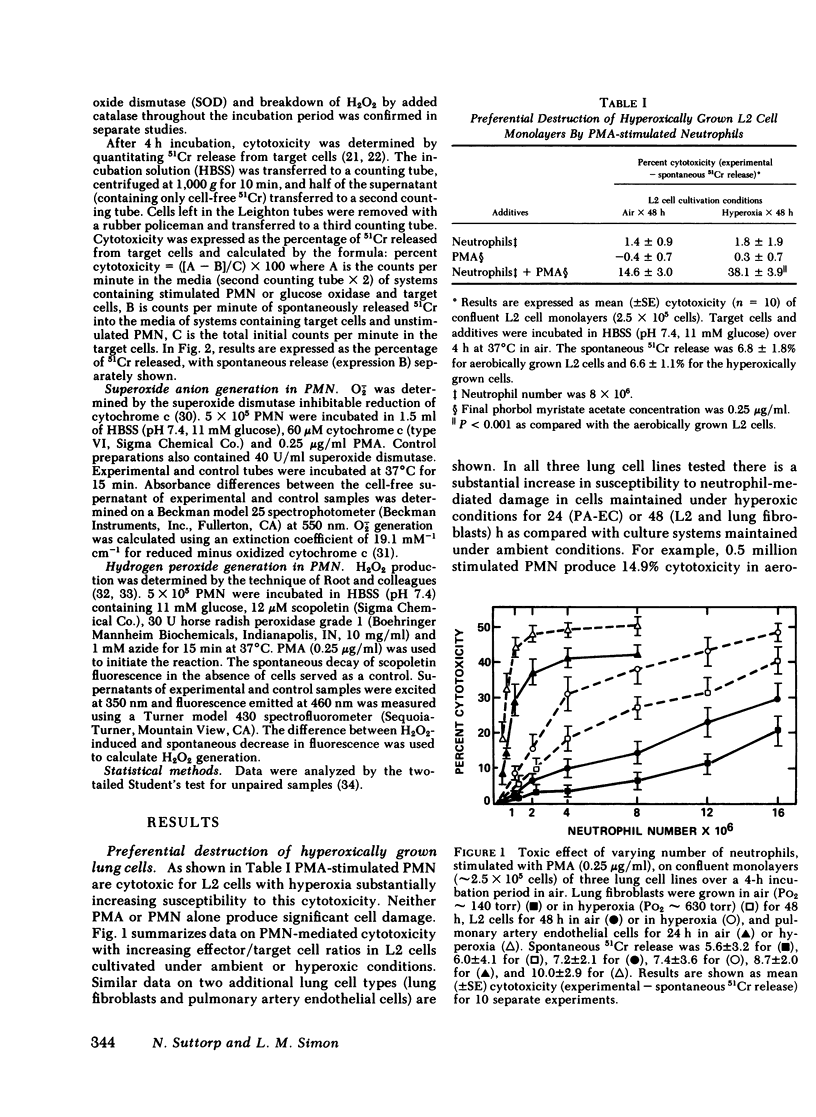
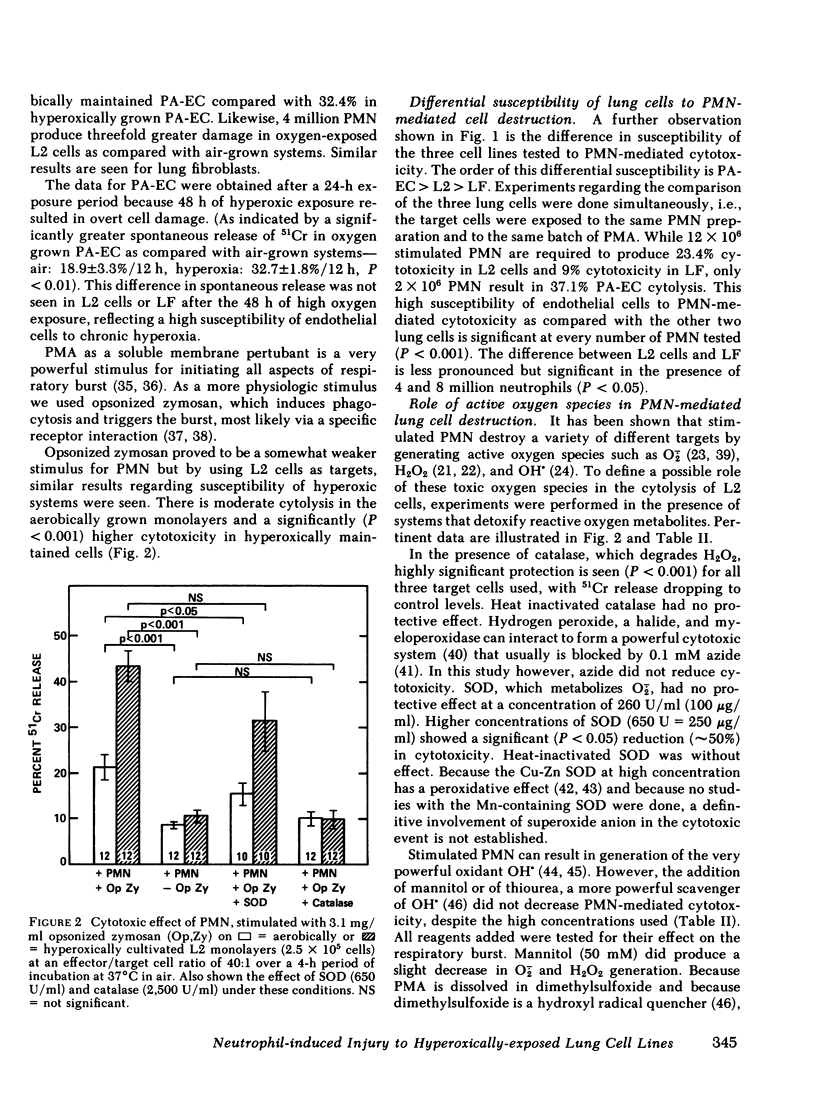
The oxidant damage of lung tissue during in vivo hyperoxic exposure appears to be amplified by neutrophils that release toxic amounts of oxygen metabolites. In our studies cloned lung epithelial cells (L2 cells), lung fibroblasts, and pulmonary artery endothelial cells were cultured under either ambient (Po2 ∼ 140 torr) or hyperoxic (Po2 ∼ 630 torr) conditions for 48 h (24 h for endothelial cells). After cultivation, phorbol myristate acetate- or opsonized zymosan-stimulated neutrophils were added to the cultivated monolayers for 4 h, and lung cell damage was quantitated using 51Cr release as an index. The data show that stimulated neutrophils are able to injure the three lung cell lines tested, with endothelial cells being highly susceptible to this injury and L2 cells being slightly more susceptible than lung fibroblasts. The studies also demonstrate that all three lung cell lines exposed to sustained hyperoxia are more susceptible to neutrophil-mediated cytotoxicity than their time-matched air controls. Hydrogen peroxide was the main toxic oxygen metabolite because catalase (2,500 U/ml) completely protected the target cells. Equivalent quantities of hydrogen peroxide generated by glucose oxidase instead of by neutrophils gave a similar degree of target cell injury. Superoxide dismutase at high concentrations (250 μg/ml) provided some protection. Other systems that detoxify oxygen metabolites were without protective effect. These findings indicate that the increase in susceptibility of lung cells to neutrophil-mediated oxidant damage is a toxic effect of hyperoxia on lung cells. This specific manifestation of oxygen damage provides insight into the integration between primary mechanisms (oxygen exposure) and secondary mechanisms (release of oxygen metabolites by neutrophils) with respect to the cellular basis for pulmonary oxygen toxicity.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badwey J. A., Curnutte J. T., Robinson J. M., Lazdins J. K., Briggs R. T., Karnovsky M. J., Karnovsky M. L. Comparative aspects of oxidative metabolism of neutrophils from human blood and guinea pig peritonea: magnitude of the respiratory burst, dependence upon stimulating agents, and localization of the oxidases. J Cell Physiol. 1980 Dec;105(3):541–545. doi: 10.1002/jcp.1041050319. [DOI] [PubMed] [Google Scholar]
- Balin A. K., Goodman B. P., Rasmussen H., Cristofalo V. J. The effect of oxygen tension on the growth and metabolism of WI-38 cells. J Cell Physiol. 1976 Oct;89(2):235–249. doi: 10.1002/jcp.1040890207. [DOI] [PubMed] [Google Scholar]
- Block E. R., Stalcup S. A. Depression of serotonin uptake by cultured endothelial cells exposed to high O2 tension. J Appl Physiol Respir Environ Exerc Physiol. 1981 Jun;50(6):1212–1219. doi: 10.1152/jappl.1981.50.6.1212. [DOI] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Endothelial regeneration as a marker of the differential vascular responses in oxygen-induced pulmonary edema. Lab Invest. 1974 Mar;30(3):350–357. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med. 1975 Jun 1;141(6):1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Szot S. The myeloperoxidase-hydrogen peroxide-halide system as effector of neutrophil-mediated tumor cell cytotoxicity. J Immunol. 1981 Apr;126(4):1295–1301. [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Peters-Golden M., Marsh-Salin J., Shelburne J. S. Pathologic changes in the lungs of oxygen-adapted rats: a morphometric analysis. Lab Invest. 1978 Dec;39(6):640–653. [PubMed] [Google Scholar]
- Crapo J. D., Tierney D. F. Superoxide dismutase and pulmonary oxygen toxicity. Am J Physiol. 1974 Jun;226(6):1401–1407. doi: 10.1152/ajplegacy.1974.226.6.1401. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., Johnston R. B., Jr Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood. 1976 Apr;47(4):545–554. [PubMed] [Google Scholar]
- Deneke S. M., Fanburg B. L. Normobaric oxygen toxicity of the lung. N Engl J Med. 1980 Jul 10;303(2):76–86. doi: 10.1056/NEJM198007103030204. [DOI] [PubMed] [Google Scholar]
- Douglas W. H., Kaighn M. E. Clonal isolation of differentiated rat lung cells. In Vitro. 1974 Sep-Oct;10(3-4):230–237. doi: 10.1007/BF02615237. [DOI] [PubMed] [Google Scholar]
- Fox R. B., Hoidal J. R., Brown D. M., Repine J. E. Pulmonary inflammation due to oxygen toxicity: involvement of chemotactic factors and polymorphonuclear leukocytes. Am Rev Respir Dis. 1981 May;123(5):521–523. doi: 10.1164/arrd.1981.123.5.521. [DOI] [PubMed] [Google Scholar]
- Frank L., Bucher J. R., Roberts R. J. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol Respir Environ Exerc Physiol. 1978 Nov;45(5):699–704. doi: 10.1152/jappl.1978.45.5.699. [DOI] [PubMed] [Google Scholar]
- Frank L., Massaro D. Oxygen toxicity. Am J Med. 1980 Jul;69(1):117–126. doi: 10.1016/0002-9343(80)90509-4. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981 Nov 10;256(21):10986–10992. [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habliston D. L., Whitaker C., Hart M. A., Ryan U. S., Ryan J. W. Isolation and culture of endothelial cells from the lungs of small animals. Am Rev Respir Dis. 1979 Jun;119(6):853–868. doi: 10.1164/arrd.1979.119.6.853. [DOI] [PubMed] [Google Scholar]
- Heflin A. C., Jr, Brigham K. L. Prevention by granulocyte depletion of increased vascular permeability of sheep lung following endotoxemia. J Clin Invest. 1981 Nov;68(5):1253–1260. doi: 10.1172/JCI110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. Secretion of granule constituents and increased oxidation of glucose. J Clin Invest. 1975 Oct;56(4):1053–1061. doi: 10.1172/JCI108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: chemiluminescence and peroxidation. Biochemistry. 1975 Dec 2;14(24):5299–5303. doi: 10.1021/bi00695a011. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981 Jun;126(6):2365–2369. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball R. E., Reddy K., Peirce T. H., Schwartz L. W., Mustafa M. G., Cross C. E. Oxygen toxicity: augmentation of antioxidant defense mechanisms in rat lung. Am J Physiol. 1976 May;230(5):1425–1431. doi: 10.1152/ajplegacy.1976.230.5.1425. [DOI] [PubMed] [Google Scholar]
- Kistler G. S., Caldwell P. R., Weibel E. R. Development of fine structural damage to alveolar and capillary lining cells in oxygen-poisoned rat lungs. J Cell Biol. 1967 Mar;32(3):605–628. doi: 10.1083/jcb.32.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The biology and pathology of oxygen radicals. Ann Intern Med. 1978 Jul;89(1):122–127. doi: 10.7326/0003-4819-89-1-122. [DOI] [PubMed] [Google Scholar]
- McCormick J. R., Harkin M. M., Johnson K. J., Ward P. A. Suppression by superoxide dismutase of immune-complex--induced pulmonary alveolitis and dermal inflammation. Am J Pathol. 1981 Jan;102(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Arrick B. A., Murray H. W., DeSantis N. M., Cohn Z. A. Tumor cell anti-oxidant defenses. Inhibition of the glutathione redox cycle enhances macrophage-mediated cytolysis. J Exp Med. 1981 Apr 1;153(4):766–782. doi: 10.1084/jem.153.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson P. A., Matalon S., Farhi L. E. An ultrastructural study of alveolar permeability to cytochrome C in the rabbit lung: effect of exposure to 100% oxygen at one atmosphere. Am J Pathol. 1981 Jan;102(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Nilsson R., Pick F. M., Bray R. C. EPR studies on reduction of oxygen to superoxide by some biochemical systems. Biochim Biophys Acta. 1969 Oct 7;192(1):145–148. doi: 10.1016/0304-4165(69)90022-1. [DOI] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffin T. A., Simon L. M., Braun D., Theodore J., Robin E. D. Impairment of phagocytosis by moderate hyperoxia (40 to 60 per cent oxygen) in lung macrophages. Lab Invest. 1980 Jun;42(6):622–626. [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981 Jul 25;256(14):7094–7096. [PubMed] [Google Scholar]
- Repine J. E., White J. G., Clawson C. C., Holmes B. M. Effects of phorbol myristate acetate on the metabolism and ultrastructure of neutrophils in chronic granulomatous disease. J Clin Invest. 1974 Jul;54(1):83–90. doi: 10.1172/JCI107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rister M., Baehner R. L. The alteration of superoxide dismutase, catalase, glutathione peroxidase, and NAD(P)H cytochrome c reductase in guinea pig polymorphonuclear leukocytes and alveolar macrophages during hyperoxia. J Clin Invest. 1976 Nov;58(5):1174–1184. doi: 10.1172/JCI108570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin E. D. Basic aspects of pulmonary dysfunction as related to hemotherapy. Bibl Haematol. 1980;(46):210–219. doi: 10.1159/000430559. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J. A. H2O2 release from human granulocytes during phagocytosis. Relationship to superoxide anion formation and cellular catabolism of H2O2: studies with normal and cytochalasin B-treated cells. J Clin Invest. 1977 Dec;60(6):1266–1279. doi: 10.1172/JCI108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Hydroxyl radical generation by polymorphonuclear leukocytes measured by electron spin resonance spectroscopy. J Clin Invest. 1979 Dec;64(6):1725–1729. doi: 10.1172/JCI109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U. S., Clements E., Habliston D., Ryan J. W. Isolation and culture of pulmonary artery endothelial cells. Tissue Cell. 1978;10(3):535–554. doi: 10.1016/s0040-8166(16)30347-0. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Evidence for the role of superoxide radicals in neutrophil-mediated cytotoxicity. Immunology. 1979 Jun;37(2):301–309. [PMC free article] [PubMed] [Google Scholar]
- Simon L. M., Axline S. G., Robin E. D. The effect of hyperoxia on phagocytosis and pinocytosis in isolated pulmonary macrophages. Lab Invest. 1978 Dec;39(6):541–546. [PubMed] [Google Scholar]
- Simon L. M., Liu J., Theodore J., Robin E. D. Effect of hyperoxia, hypoxia, and maturation on superoxide dismutase activity in isolated alveolar macrophages. Am Rev Respir Dis. 1977 Feb;115(2):279–284. doi: 10.1164/arrd.1977.115.2.279. [DOI] [PubMed] [Google Scholar]
- Simon L. M., Raffin T. A., Douglas W. H., Theodore J., Robin E. D. Effects of high oxygen exposure on bioenergetics in isolated type II pneumocytes. J Appl Physiol Respir Environ Exerc Physiol. 1979 Jul;47(1):98–103. doi: 10.1152/jappl.1979.47.1.98. [DOI] [PubMed] [Google Scholar]
- Simon L. M., Robin E. D., Raffin T., Theodore J., Douglas W. H. Bioenergetic pattern of isolated type II pneumocytes in air and during hypoxia. J Clin Invest. 1978 May;61(5):1232–1239. doi: 10.1172/JCI109039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. M., Robin E. D., Theodore J. Differences in oxygen-dependent regulation of enzymes between tumor and normal cell systems in culture. J Cell Physiol. 1981 Sep;108(3):393–400. doi: 10.1002/jcp.1041080313. [DOI] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Spikes J. D., Swartz H. M. International conference on singlet oxygen and related species in chemistry and biology: review and general discussion. Photochem Photobiol. 1978 Oct-Nov;28(4-5):921–933. doi: 10.1111/j.1751-1097.1978.tb07041.x. [DOI] [PubMed] [Google Scholar]
- Turino G. M., Rodriguez J. R., Greenbaum L. M., Mandl I. Mechanisms of pulmonary injury. Am J Med. 1974 Sep;57(3):493–505. doi: 10.1016/0002-9343(74)90142-9. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. Oxygen effect on lung cells. Arch Intern Med. 1971 Jul;128(1):54–56. [PubMed] [Google Scholar]
- Weiss S. J., Rustagi P. K., LoBuglio A. F. Human granulocyte generation of hydroxyl radical. J Exp Med. 1978 Feb 1;147(2):316–323. doi: 10.1084/jem.147.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. The role of superoxide in the destruction of erythrocyte targets by human neutrophils. J Biol Chem. 1980 Oct 25;255(20):9912–9917. [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]


